Abstract
The present study evaluated the nutritional value and antimicrobial activities of Thalassiosira weissflogii and Tetraselmis sp. concentrates. The study also ascertained the effect of microalgae supplemented diets on the growth and survival of Penaeus vannamei post larvae (PL 18, mean weight: 19.714 ± 1.62 mg). Microalgae concentrates were prepared by flocculation, and the proximate composition showed no significant difference in the crude protein content between these species (T. wesissflogii: 43.07 ± 1.78%; Tetraselmis sp. 42.11 ± 2.55%; p > 0.05). However, the crude lipid content of T. weissflogii was significantly (p < 0.05) higher (20.11 ± 1.02%) than that of Tetraselmis sp. (10.56 ± 0.27%). Significantly higher (p < 0.05) polyunsaturated fatty acid was found in Tetraselmis sp. compared to T. weissflogii. Significantly higher inhibition against Vibrio parahaemolyticus was shown by the Tetraselmis extract compared to that of T. weissflogii. Further, a 42-day feeding trial was conducted with three different inclusions of T. weissflogii (THA) and Tetraselmis sp. (TET) concentrates in P. vannamei nursery diet (0, 0.5, 1, and 1.5 g kg−1 of diet). Significantly higher (p < 0.05) average body weight (ABW) was observed in TET0.5, TET1.0, TET1.5, THA1.0 and THA1.5 compared to the control. The highest ABW was recorded in TET1.0 (0.96 ± 0.02 g), which was significantly higher than all other treatments. Significantly higher (p < 0.05) weight gain was observed in TET1.0 (0.94 ± 0.02 g) compared to the rest of the diets. Significantly higher (p < 0.05) average daily gain was observed in TET1.0 (22.48 ± 0.55 mg day−1). Haematological parameters of P. vannamei fed with microalgae concentrates were higher than those of the control group. The gut microbial analysis showed a significant reduction in total Vibrio count in the animals fed with 1% and 1.5% Tetraselmis or Thalassiosira compared to other treatments. These results indicated the beneficial effect of growth and better antimicrobial property to withstand against the common pathogenic microbe and thus indicating the beneficial effect in the early life stages of shrimp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shrimp is a highly traded seafood commodity farmed commercially in at least sixty countries. However, shrimp aquaculture has often been hampered due to mass mortality caused by infectious bacterial and viral diseases, which cause heavy economic loss to the farmers (Thornber et al. 2020; Patil et al. 2021). Most of the problems are encountered during the initial period of first 30 days of stocking the seed. With an effective nursery cycle, overall productivity can be significantly improved by increasing survival and growth rates during the early stages of life (Wasielesky et al. 2013). The nursery-rearing system has become a crucial phase in sustainable shrimp production in many shrimp-farming countries since it enhances productivity and profitability (Crab et al. 2012; Anand et al. 2021). Further, the short duration of the final grow-out phase allows the execution of more rearing cycles each year, which results in more significant economic gain for the farmer (Anand et al. 2021).
Although it is possible to manage higher stocking densities and increase the number of crops per year in shrimp farms through nursery cycles, the health of the shrimps in the nursery system is crucial. The diseases were treated with antibiotics, and the incidence of antibiotic residues coupled with multiple drug-resistant bacteria has become an emerging problem in shrimp aquaculture (Thornber et al. 2020) which is one of the reasons for many shrimp export rejections from India (Geetha et al. 2020). Hence, ensuring the health of the shrimp in the nursery phase is one of the major researchable areas getting attention among the aquaculture researchers.
Microalgae metabolites, including antioxidants, anticoagulants, anti-inflammatory agents, antimicrobials, and anticancer agents, have demonstrated strong biological activity (Kiran and Venkata Mohan 2021; Sandeep et al. 2022). Thus, it is an excellent ingredient for fish and shrimp feed due to their favourable biochemical composition, shape, size, palatability, digestibility, and cell wall composition (Radhakrishnan et al. 2016). Microalgae are the natural food for early life stages of shrimp and fish and hence inclusions of these algae enhance the nutritional value and palatability of the feed while also helping in the economic and sustainable front. Providing proteins with a balanced ratio of the essential amino acids, omega-3 fatty acids, and pigments is an additional significant advantage of using microalgae as fish feed. Several studies have shown that adding microalgae to fish and shrimp diets improves the growth and nutritional qualities (Ju et al. 2012; Chen et al. 2016; Ansari et al. 2021; Sandeep et al. 2022). Some microalgae are rich in long-chain polyunsaturated fatty acids, mainly eicosapentaenoic (EPA), docosahexaenoic, and arachidonic acid, which improve and stabilise nutrition, promote growth and appetites, and are considered essential for high survival rate and normal development of larval and juvenile fish, crustacean, and mollusc (Shan and Lin 2014; Sandeep et al. 2019).
Microalgae have developed several defensive and adaptive strategies to survive in a highly competitive environment, with widely fluctuating chemical and physical parameters, synthesising a tremendous diversity of compounds (Ghasemi et al. 2004). Microalgae pigments possess antimicrobial activity (Hajimahmoodi et al. 2010; Bhattacharjya et al. 2020; Sandeep et al. 2022), and extracts of green algae, diatoms, and dinoflagellates produce a variety active antibacterial activity in vitro (Katircioglu et al. 2006). The antimicrobial properties against pathogenic microbes help to control diseases in the culture systems when the particular microalgae are incorporated into shrimp diet. There were few studies regarding the partial replacement of fish meal and fish oil by microalgae biomass in the diet of shrimps (Ju et al. 2012; Allen et al. 2019). Adding microalgae biomass to the diet will enhance shrimp’s growth and overall health (Basri et al. 2015). Similarly, the inclusion of microalgae concentrates as a dietary source revealed a positive effect on the eastern oyster, Crassostrea virginica (Rikard and Walton 2012); winged pearl oyster, Pteria penguin (Wassnig and Southgate 2016); and giant clam, Tridacna noae (Southgate et al. 2017).
However, conclusive studies on the effect of dietary microalgae concentrate in P. vannamei post larvae are not there. The present study evaluates the nutritional composition, antioxidant properties, and anti-Vibrio properties of microalgae concentrates on understanding the underlying mechanisms of the effect of microalgae on the shrimp diet. Moreover, the present study holistic approach was used to substantiate the beneficial effect through the haematological parameters and gut microbiology of P. vannamei fed with different levels of different microalgae concentrations.
Materials and methods
Ethical statement
The experiments in the present study were carried out according to the guidelines of the Committee for Control and Supervision of Experiments on Animals, a statutory committee of the Department of Animal Husbandry and Dairying, Ministry of Fisheries, Animal Husbandry and Dairying, Govt. of India. Further, the approval was obtained from the Institutional Animal Ethics Committee of the ICAR-Central Institute of Brackishwater Aquaculture, Chennai, India.
Microalgae cultures
The microalgae used in the present study, Thalassiosira weissflogii and Tetraselmis sp., were isolated from the Muttukadu estuarine ecosystem, Tamil Nadu, India, and were maintained in the microalgae repository of ICAR-Central Institute of Brackishwater Aquaculture, Chennai (Sandeep et al. 2019; 2021). They are axenic cultures maintained under laboratory conditions. The pure isolates were grown in Conway (Walne 1966) medium in the indoor microalgae laboratory at 24 ± 1 °C in a light intensity of 4000–5000 lx. Photoperiod was fixed at 14:8-h light and dark periods.
Bacterial cultures
The three pathogenic bacteria used in the present study were collected from culture repository of AAHED (Aquatic animal health and environment division), ICAR-CIBA (Central Institute of Brackishwater Aquaculture), Chennai, India. Vibrio campbelli LB1 (Kumar et al. 2021), V. harveyi SB1, and V. parahaemolyticus AMR2021S14 were used in the study. The bacterial strains were isolates collected from shrimps during disease outbreaks in shrimp hatcheries and farm.
Flocculation of microalgae to make concentrate
A preliminary dose assessment trial was conducted to evaluate the flocculation efficiencies of different alkalis (NaOH, KOH, and NH4OH) at different concentrations (0.1, 0.5, 1, 10, and 20 mM). Based on the study, the best alkali with the optimum dose was used to flocculate Thalassiosira weissflogii and Tetraselmis sp. cultures. The flocculation efficiency was calculated at 30 min for 3 h after the chemical treatment. The cultures were subjected to intense aeration immediately after adding alkalis for 2 min to allow the pH to stabilise. The flocculation efficiency was calculated by applying the formula of Gerde et al. (2014).
where A is the OD at 750 nm of the sample, and B is the OD at 750 nm of reference.
Microalgae concentrate obtained after flocculation was deflocculated with 0.1 N HCl by reducing the elevated pH. The number of cells in the culture and flocculated concentrates was estimated using a haemocytometer. The concentrates were kept at 4 °C until use.
Nutrient profiling
The microalgal biomass was harvested in the log phase of the growth and freeze-dried. The proximate composition (g/100 g) of experimental diets and microalgae was analysed according to the standard procedures of AOAC (2005). For moisture analysis, (the initial microalgal sample, final shrimp samples, and diets) samples were oven dried at 105 °C until a constant weight was achieved. The micro-Kjeldahl method (FOSS, Kjeltec™ 8400 analyser, USA) was followed to estimate the crude protein (CP), and lipid was estimated by solvent extraction using Soxhlet’s apparatus (SOCS PLUS Six Place Fully Automatic Solvent Extraction System, Pelican, India). Samples were incinerated at 550 °C for 5 h in a muffle furnace to determine the total ash (TA) content. The crude fibre (CF) content of fat-free samples was performed through acid digestion followed by alkaline digestion (Fibre Cap™ 2021, FOSS, USA). Then the digested sample was incinerated in a muffle furnace at 550 °C for 5 h. The subtraction method was employed to calculate nitrogen-free extract (NFE) content of the experimental diets. The proximate composition was calculated based on the following formulae:
where 0.1 is the normality of acid, 0.014 is the molecular mass of nitrogen, and 6.25 is the constant relationship between N and animal protein of sample.
where EE is the ether extract.
where NFE is the nitrogen-free extract, CP is the crude protein (g/100g), CL is the crude lipid (g/100g), CF is the crude fibre (g/100g), and TA is the total ash (g/100g).
The total protein of microalgae biomass was determined following Lowry’s method (Lowry et al. 1951) and lipids by Bligh and Dyer method (Bligh and Dyer 1959), and the respective fatty acid methyl esters were prepared and extracted into petroleum ether. Analysis of methyl esters was performed by a gas chromatograph (GC2014 Shimadzu, Japan) on an RTX wax capillary column (100 m length × 0.25 mm I.D × 0.2 µm film thickness). The quantity of fatty acids (mg kg−1) was calculated according to Aziz et al. (2012).
Antioxidant properties
Total phenolic content
Estimation of the total phenolic content (TPC) of the sample was done by Folin–Ciocalteau (FC) assay described by Singleton and Rossi (1985) with minor modifications as per Sivaramakrishnan et al. (2017). Briefly, 200 µl of the extracted sample was mixed with 200 µl FC reagent (0.5 N) followed by 1.6 ml 7.5% Na2CO3 and incubated for 2 h in the dark at room temperature. The absorbance was measured at 765 nm using a UV–visible spectrophotometer. Gallic acid was standard, and the phenolic contents were expressed as gallic acid equivalents (mgGAE g−1) of macroalgae extract.
Total flavonoid content
The flavonoid content of the microalgae extract was determined by the spectrophotometric method of Zishen et al. (1999) with slight modification as per Sivaramakrishnan et al. (2017). Briefly, 0.5 ml of sample was mixed with 0.3 ml 15% sodium nitrite, 0.6 ml 10% ammonium chloride hexahydrate, and 3 ml of 1N sodium hydroxide after 5 min. Absorbance was immediately measured at 510 nm. Flavonoid content was expressed as Rutin equivalents of extract (mg RE g−1).
Total antioxidant activity
The total antioxidant activity of methanolic extracts was quantified by Prieto et al. (1999) method with slight modification as per Sivaramakrishnan et al. (2017). Briefly, 0.2 mg/ml of samples in different aliquots was mixed with 1 ml of each of the three reagent solutions (0.6 M sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The samples were incubated at 95 °C for 90 min in a water bath. Absorbance was measured at 695 nm. Total antioxidant activity was expressed as milligrammes of ascorbic acid per gramme of extract.
Antibacterial assay
The microalgal biomass was harvested in the log phase of the growth and freeze-dried. About 25 g freeze-dried microalgal powder was percolated in 60% alcohol (100 ml) and ground with a mortar and pestle. The extraction was repeated until the solvent became colourless. Subsequently, the extract was dried under vacuum at 45 °C to remove the solvent. The dried extract was collected and stored at − 20 °C until used. Bacterial strains Vibrio harveyi, Vibrio campbellii, and Vibrio parahaemolyticus were obtained from the culture collection of Aquatic Animal Health Division, ICAR-CIBA, Chennai, India. The bacterial strains were cultured in nutrient broth (Hi Media, India) for 12 h. Turbidity was adjusted to 0.5 McFarland Standards (107 CFU/ml) and confirmed by spectrophotometric reading at 600 nm. McFarland Standards were used to standardise numbers of bacteria when required by a procedure or for the susceptibility test. The antibacterial activity of the methanolic extracts of microalgae was tested against selected bacteria by agar well diffusion method (Holder and Boyce 1994) using nutrient agar media (Hi Media, India). Both methanol and DMSO-dissolved microalgae extracts (50 µl each) were loaded separately into the wells in duplicate. Likewise, negative control (solvent alone) and positive control (streptomycin) were also prepared in duplicate. The plates with bacterial inoculums and extracts were incubated for 24 h at 37 °C. Zones of inhibition formed after treatment with extracts were measured using a Hi Antibiotic Zone Scale (HiMedia Laboratories).
Preparation of formulated feed
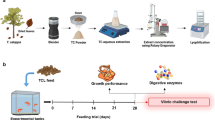
Seven iso‐nitrogenous and iso‐energetic experimental diets were prepared (Table 1) with different levels of microalgae concentrates as additives at three different inclusion levels of Tetraselmis (0.5%, 1%, and 1.5%) and T. weissflogii (0.5%, 1%, and 1.5%). The pH of the microalgae concentrates was brought to the neutral pH with 0.1N HCl before including in the feed. All the dry solid ingredients were ground separately into a fine powder, passed through a 100-μm sieve, and further homogenised. The dough was steam-cooked and cooled. Then vitamin and mineral mixtures were added to this dough and were homogenised again for uniformity.
Experimental animals
Post larvae (PL 18) of P. vannamei were stocked at 100 PL/100 L tank (mean weight: 19.72 ± 1.62 mg). The Penaeus vannamei post-larvae were procured from a private hatchery (Approved hatchery by Coastal Aquaculture Authority, Chennai, India) from Tamil Nadu, India. The SPF P. vannamei post-larvae (PL 18) were screened again for all OIE-listed pathogens in Aquatic Animal Health Laboratory, ICAR-CIBA, Chennai, and healthy PLs were used for the experiment. The animals were kept in a flow through system during the entire period of the experiment. The water temperature during the experiment was 28.55 ± 1.97 °C, which was measured at 11:00 h on every alternate day. The feeding trial was conducted for 42 days. Animals were fed with seven iso‐nitrogenous and iso‐energetic experimental diets prepared with different levels of microalgae concentrates as additives: Tetraselmis (0.5%, 1%, and 1.5%) and T. weissflogii (0.5%, 1%, and 1.5%).
Growth performances of P. vannamei post larvae
All the animals were individually weighed at the beginning and the end of the experiment. Average body weight (ABW), total biomass, percentage weight gain (WG %), specific growth rate (SGR), average daily gain (ADG), and survival were estimated using the formulae mentioned by Steffens (1989).
Haematology
In order to estimate the total haemoglobin count (THC), granular haemoglobin (GH), and nongranular haemoglobin (NGH) counts, haemolymph was obtained from the animals at the end of a 42-day feeding trial. This was performed through the ventral sinus of the first abdominal segment. The proportions of GH, which had both large granular haemocytes (LGHs) and small granular haemocytes (SGHs), and NGH, which had both large nongranular haemocytes (LNGHs) and small nongranular haemocytes (SNGHs), were estimated in accordance with prior studies (Ananda Raja et al. 2017).
Gut microbiology
According to the process prescribed by Kumar et al. (2017), gut samples were collected and used for microbial investigation. Six animals from each treatment (chosen at random, two from each replication) were taken at the end of the feeding trial, anaesthetized with clove oil (50 L L−1), and then transferred to ice. The aseptically removed gut was weighed and homogenised in a normal saline solution (NSS; 0.89%). Then samples were serially diluted (tenfold) in NSS, and 100 µl of appropriate dilutions was plated on nutrient agar having 1.5% w/v NaCl and 2% agar concentration for total plate count and thiosulfate citrate bile salt sucrose agar for total Vibrio count. The gut samples were also processed on starch agar (De et al. 2015) and peptone gelatin agar (Bairagi et al. 2002) for amylolytic and proteolytic bacterial counts, respectively. These culture plates were incubated at 30 °C for 48 h. Colonies in the range of 30 to 300 counted and expressed as colony forming unit (CFU/g for gut).
Statistical analysis
Statistical analyses were performed using R statistical software (version 4.0.4). Data were expressed as mean ± SD. The independent sample t test was performed to check the significance (p < 0.05) of difference in mean growth characteristics, haematological parameters, and gut microbial counts using R ‘stats’ package. One-way analysis of variance, followed by Tukey’s HSD post hoc tests, was performed to test the significance (p < 0.05) of the differences in mean of various parameters separately. All the plots were created using ‘ggplot2’ package (Wickham 2016) of R statistical software (ver. 4.0.5).
Results
Flocculation of microalgae
Flocculation trials on T. weissflogii and Tetraselmis sp. revealed that the highest flocculation efficiency (89% and 82%, respectively) was shown by 10 mM and 20 mM NaOH after 30 min (Figs. 1 and 2). Further, no significant difference was found in the flocculation efficiency between 10 and 20 mM NaOH from 30 to 180 min. However, the flocculation efficiency was increased in both concentrations along with time. The results revealed that 10 mM NaOH could help in quick flocculation of T. weissflogii and Tetraselmis sp. Moreover, the flocculation efficiency of NaOH is significantly higher than that of KOH at 10 mM and 20 mM at different time intervals. The results also showed that NH4OH did not induce flocculation in all the tested concentrations in both the microalgae.
Nutritional composition of microalgae
The proximate composition of T. weissflogii and Tetraselmis sp. concentrates showed that the microalgae biomass is rich in protein (43.07% and 42.11%, respectively) and the values were not significant between them (Fig. 3). However, T. weisfloii has significantly higher lipid content than Tetraselmis sp. (20.11% vs 10.56%; p < 0.05). No significant difference was observed for other proximate parameters between the two microalgae concentrates. Fatty acid profile analysis indicates that Tetraselmis sp. had more than double the concentration of polyunsaturated fatty acids (PUFA) (Table 2) compared to T. weissflogii (56.32% vs 25.24%; p < 0.05). On the contrary, saturated fatty acid was significantly higher in T. weisssflogii (34.56%) than in Tetraselmis sp. (25.73%). The higher percentage of PUFA in Tetraselmis sp. is mainly attributed to the presence of linoleic and alpha-linolenic acids. In T. weissflogii, the PUFA percentage is mainly contributed by EPA presence.
Antioxidant properties of microalgae
The TPC of T. weissflogii was significantly higher than that of Tetraselmis sp. (45.83 ± 1.17 mg GAE g−1 vs. 36.18 ± 2.91 mg GAE g−1; p < 0.05) (Fig. 4). However, no significant difference was found in the total flavonoid content (TFC) of T. weissflogii (6.56 ± 0.62 mg RE g−1) and Tetraselmis sp. (7.26 ± 0.51 mg RE g−1). In the case of total antioxidant activity, the values of T. weissflogii (4.9 ± 0.09 mg RE g−1) are significantly higher than that of Tetraselmis sp. (3.39 ± 0.99 mg RE g−1).
Anti-Vibrio properties of microalgae extracts
Methanolic extracts of T. weissflogii and Tetraselmis sp. showed antimicrobial activity against three pathogenic bacteria: V. harveyi, V. parahaemolyticus, and V. campbellii on the disc-diffusion assay (Table 3). The Tetraselmis extract showed a significantly (p < 0.05) higher level of antimicrobial activity against V. parahaemolyticus (16.94 ± 1.05 mm) compared to that of T. weissflogii against the same pathogen (13.59 ± 0.3). The Tetraselmis extract showed better antimicrobial activity against V. harveyi also (16.58 ± 0.59 mm) as compared to that of T. weissflogii extract against same pathogen (15.66 ± 0.62 mm); however, the difference was nonsignificant (Table 3). Interestingly, T. weissflogii extract showed higher antimicrobial activity against V. campbellii (14.47 ± 1.19 mm) than Tetraselmis extract (13.4 ± 0.48 mm).
Growth of P. vannamei fed with microalgae incorporated diet
Post larvae (PL 18) fed with microalgae incorporated diet THA1.0 showed significantly higher survival rates compared to other treatments (Fig. 5(d)). Results revealed significantly higher (p < 0.05) ABW in treatments fed with TET0.5, TET1.0, TET1.5, THA1.0, and THA1.5 compared to control (Fig. 5(a)). The highest ABW was noticed in animals fed with TET1.0 (0.96 ± 0.02 g), followed by TET1.5 (0.89 ± 0.03 g), which was significantly higher than the rest. Significantly higher (p < 0.05) WG was observed in TET1.0 (0.94 ± 0.02 g) compared to all other treatments, including the control (Fig. 5(b)). The WG % also followed the pattern of WG showing significantly higher value in TET1.0 (4919.87 ± 473.36), than the rest of the treatments (Fig. 5(c)).
Growth parameters of P. vannamei fed with microalgae incorporated diet (a: average body weight; b: weight gain; c: weight gain (%); d: survival (%); e: average daily gain; f: specific growth rate). Data are shown as mean ± SD (n = 3); mean values with different alphabets differ significantly (p < 0.05); ABW, average body weight; WG, weight gain; ADG, average daily gain; SGR, specific growth rate
Significantly higher (p < 0.05) ADG was observed in TET1.0 (22.48 ± 0.55 mg day−1) compared to that of all other treatments, including control followed by TET1.5 (20.82 ± 0.59 mg day−1), THA1.0 (19.69 ± 0.59 mg day−1), and THA1.5 (19.42 ± 0.44 mg day−1). Lower ADG was observed in THA0.5 and control (Fig. 5(e)). SGR of TET1.0 was significantly higher (p < 0.05) than that of other treatments (9.32 ± 0.23). The lowest SGR was noticed in THA0.5 (8.39 ± 0.17); however, the difference was non-significant with that of the control (Fig. 5(f)).
Haematological parameters
The THC was significantly higher (p < 0.05) in shrimps fed with THA1.0 (7.40 ± 0.54 × 107) than in the rest of the treatments (Supplementary Fig. 1a). THCs of animals in TET0.5, TET1.0, THA1.0, and THA1.5 were significantly (p < 0.05) higher than those of the control (4.30 ± 0.10 × 107). The SNGHs were observed in animals fed with TET1.0 followed by animals fed with THA1.0 (Supplementary Fig. 1b). Higher levels of LNGHs were observed in animals fed with TET1.0 and THA1.0 (Supplementary Fig. 1c); however, there was no significant difference among the treatments. The SGH showed a significant increase (p < 0.05) in shrimps fed with TET1.0 and THA1.0 compared to other treatments (Supplementary Fig. 1d). The highest SGH was observed in animals fed with THA1.0 (4.30 ± 0.57 × 107). There was a significant increase (p < 0.05) in LGHs in all treatments compared to the control (Supplementary Fig. 1e). Higher LGH was observed in THA1.0 (0.96 ± 0.33 × 107) and THA1.5 (0.96 ± 0.84 × 107). The lowest LGH value was observed in the control group.
There was no significant difference among control, TET0.5, TET1.0, and THA1.5 in the percentage occurrence of SNGH (Supplementary Fig. 1f). The lowest percentage occurrence of SNGH was observed in THA1.0 followed by TET1.5. The highest percentage occurrence of LNGH was observed in TET1.0 (Supplementary Fig. 1g). However, the differences were not significant between the treatments. The percentage occurrence of SGH was significantly lower in THA1.5 compared to that of all other treatments (Supplementary Fig. 1h). The % of LGH was significantly higher (p < 0.05) in shrimps fed with TET1.5 and THA1.0 compared to other treatments (Supplementary Fig. 1i). The lowest percentage occurrence of LGH was observed in control.
Gut microbiology
An increase in the total microbial count was observed in treatments with microalgal supplementation compared to control except in THA1.5 (Supplementary Fig. 2a). The highest level of microbial counts were observed in THA1.0 which was significantly higher (p < 0.05) compared to control, TET0.5, TET1.0, and THA1.5. The lowest gut bacterial count was observed in THA1.5, which was non-significant that of control. The increasing inclusion levels of Tetraselmis and T. weissfloggi (THA) were found to decrease the level of Vibrios in the gut. Significant reduction (p < 0.05) in gut Vibrio count was noticed in animals fed with TET1.0, TET1.5, THA1.0, and THA1.5 as compared to control, TET0.5, and THA0.5 (Supplementary Fig. 2b). The lowest Vibrio count was observed in THA1.5, followed by TET1.5 and TET1.0. There was no significant difference between the gut Vibrio counts of control, TET0.5, and THA0.5. In the case of the proteolytic bacterial count, there was a significant increase (p < 0.05) in TET1.0, TET1.5, and THA1.0 compared to the control (Supplementary Fig. 2c). The lowest gut proteolytic count was noticed in control. However, there was no significant difference among the treatments that received microalgal supplementation. The amylolytic counts showed a reduction in TET0.5, TET1.0, and TET1.5 compared to the control; however, there was no significant difference between the treatments (Supplementary Fig. 2d). Compared to the control, a significant reduction (p < 0.05) of gut amylolytic count was noticed in THA0.5 and THA1.0. The lowest gut amylolytic count was observed in THA0.5, whereas the highest gut amylolytic count was observed in control.
Discussion
Microalgal concentrates have been proven to be a viable alternative for live microalgae in hatcheries to reduce the risk associated in maintaining mass culture like the sudden crash culture. However, due to its small size, harvesting microalgae is still a challenge in aquaculture production systems (Vandamme et al. 2010). In the present study, chemical flocculation using 10 mM NaOH was successfully used in concentrating cultures of T. weissflogii and Tetraselmis sp. Earlier researchers also reported the efficiency of strong alkali to flocculate microalgae by altering pH (Vandamme et al. 2012; Li et al. 2020).
The nutritional composition of microalgae largely depends on many factors like growth stage, culture conditions (nutrients, temperature, light, etc.), strain difference, and species. As far as aquaculture is concerned, the nutritional quality of microalgae biomass is an essential factor. The present study estimated the protein content in T. weissflogii as 43.07%. The superior nutritional quality of T. weissflogii is already reported (Kiatmetha et al. 2011; Sandeep et al. 2019). The protein content in Tetraselmis sp. was (42.11%) similar to the values reported in previous studies (Abiusi et al. 2014) and higher than the values reported by D’Souza and Kelly (2000). The lipid content of T. weissflogii (20.11%) was similar to the earlier reports (Sandeep et al. 2019). The levels of PUFA observed in Tetraselmis sp. (56.32%) in the present study are higher than the earlier reports (Guzmán et al. 2010). In the present study, fatty acid profiling of T. weissflogii revealed higher EPA content (17.77% of total fatty acids) which was in consonant with the observations recorded in the earlier studies on different species of Thalassiosira sp. (Thompson and Harrison 1992; Sandeep et al. 2019). In the present study, the EPA content of Tetraselmis sp. was 8.39% of total FA, which is higher than the values reported by some of the earlier studies in Tetraselmis suecica (D’Souza and Kelly 2000; Abiusi et al. 2014). The clear understanding of nutritional composition of these microalgae helps in their inclusion in shrimp feed as additives.
Microalgae provide various health benefits due to their high levels of polyunsaturated fatty acids, phytosterols, sulfated polysaccharides, pigments, and phenolic compounds (Ghasemi et al. 2011). Earlier studies have described their polyphenolic and antioxidant components (Hajimahmoodi et al. 2010). The antioxidant properties are essential to understand the potential of a microalgae species to be used as a nutraceutical in aquaculture. Chemical properties like total phenolic, total flavonoid, and total antioxidant activity are essential to screen the potential microalgae for application in aquaculture as a nutraceutical. The methanolic microalgae extracts were used in the present study to screen the properties as per the earlier reports (Widowati et al. 2017). The present study recorded a higher total phenolic content from the methanolic extract of T. weissflogii (45.83 ± 1.17 mg GAE g−1), which was higher than the earlier report (Bhattacharjya et al. 2020). However, there are reports on high total phenolic content in microalgae, Scenedesmus rubescens, up to 48.57 ± 3.99 mg GAE g−1 (Morowvat and Ghasemi 2016). The total phenolic content of Tetraselmis sp. in the present study was 36.18 ± 1.9 mg GAE g−1, which is higher than the earlier report (Widowati et al. 2017). The total flavonoid content of the methanolic extract of Tetraselmis sp. in the present study was 7.26 ± 0.05 mg RE g−1 which is higher than the values reported earlier (Haoujar et al. 2019). The total flavonoid content of the methanolic extract of T. weissflogii in the present study was 6.56 ± 0.62 mg RE g−1, which is higher than the values reported by Bhattacharjya et al. (2020). The reasons for higher phenolic and flavonoid may be indicated.
Many microalgae have antibacterial properties besides their rich nutritional composition. Microalgae produce a wide range of bioactive secondary metabolites, either stored in the cell or excreted in the surrounding environment after the exponential and stationary growth phase ends (Bhuvaneswari et al. 2013; Tavakoli et al. 2021). Research is being done to uncover natural molecules to help in limiting the misuse of commercial antibiotics because extended use may lead to the development of resistant bacterial strains coupled with antibiotic residues in the final produce. The extract of Thalassiosira sp. showed inhibition zones of 12 mm, 13 mm, and 17 mm for E. coli, S. aureus, and B. subtilis, respectively (Bhattacharjya et al. 2020). Similarly, in the present study, the methanolic extract of T. weissflogii showed a zone of inhibition against V. harveyi (15.66 ± 0.18 mm), V. campbellii (14.47 ± 0.78 mm), and V. parahaemolyticus (14.59 ± 0.29 mm). Moreover, the study by Jusidin et al. (2022) revealed that the hydrophilic chemicals in T. weissflogii extract have antibiotic activity against the highly virulent V. harveyi. The antibacterial properties of Tetraselmis sp. were studied against different pathogenic bacteria (Guzmán et al. 2019; Widowati et al. 2021). In the present study, the zone of inhibition shown by methanolic extract of Tetraselmis sp. was 13.58 ± 0.26 mm against V. harveyi, 12.40 ± 0.67 mm against V. campbellii, and 12.94 ± 0.73 mm against V. parahaemolyticus. Due to the presence of phenolic and flavonoid contents, bioactive compounds in microalgae are effective scavengers of intracellular free radicals. Earlier studies showed potent antibacterial activity of methanolic extract of Chlorella vulgaris, whereas the extracts were rich in phenolic and flavonoid contents (Pradhan et al. 2021). The present study clearly showed the better antibacterial properties of Tetraselmis sp. against V. parahaemolyticus and V. harveyi compared to T. weissflogii. The results suggest the benefits of the incorporation of Tetraselmis sp. and T. weissflogii in P. vannamei nursery diet.
The present study demonstrated that animals fed with the concentrate of 1% Tetraselmis sp. attained the highest body weight (Table 3). Furthermore, all the growth characteristics were significantly higher in the treatment fed with Tetraselmis sp. incorporated diet. Similarly, Sharawy et al. (2020) showed that including Tetraselmis suecica (7.5 g kg−1) improved the growth and nutritional quality of P. vannamei. This may be due to the various properties attributed to Tetraselmis sp., such as high protein, lipid, PUFA, antioxidant properties, and anti-vibrio properties (Widowati et al. 2017; Guzmán et al. 2019). The addition of Thalassiosira weissflogii biomass or concentrate to the diet of P. vannamei has not been documented in any published literature. The most important hurdle in utilising Thalassiosira biomass is the constraints in harvesting, and this was addressed in the present study by concentrating the microalgae biomass by flocculation. The results of the present study revealed the potential of dietary microalgae concentrates in growth of the P. vannamei post larvae and would open the avenues for next-generation nursery diets with these algae as functional ingredients for growth.
The present study showed that haematological parameters such as THC, SNGH, and LNGH were significantly higher in shrimps fed with microalgal concentrates of Thalassiosira and Tetraselmis compared to the control. In crustaceans, particularly in shrimps, the haemocytes perform many vital functions related to the growth and well-being of the animals, and largely the levels are attributed to the physiological condition of the shrimps (Song and Hsieh 1994; Cheng and Chen 2001). Hence, in the present study, the supplementation of microalgae concentrates in the diet might have enhanced the health condition of shrimps due to the presence of bioactive compounds present and the nutritional quality of the microalgae. Many earlier researchers reported an increase in haematological characteristics. Raji et al. (2018) showed an increase in haematological parameters (WBC and RBC) in African catfish (Clarias gariepinus) fed with a diet supplemented with Arthrospira platensis and Chlorella vulgaris. Prabhu et al. (2018) also reported an increase in total haemocyte count in Penaeus vannamei fed with Syzygium cumini leaf powder. The haematological parameters indicated the beneficial effects on important haematological parameters and further supported the growth findings observed in this study.
The dietary application of microalgae in shrimps has shown positive effects on growth, gut microbiology, and disease resistance (Wang et al. 2017; Liu et al. 2022). Sharawy et al. (2020) showed that the total heterotrophic bacterial count was significantly enhanced in P. vannamei fed with feed containing dried Tetraselmis suecica. Gut microbiology affects aquatic organisms’ health by influencing digestion, nutrient assimilation, immunity, biological antagonism, and anti-aging (Li et al. 2018). The present study showed a significant increase in total gut bacterial count in all treatments fed with microalgae supplemented diet except THA1.5 compared to the control. Moreover, the present study showed a significant reduction in total Vibrio count in the animals fed with 1% and 1.5% microalgae (both Tetraselmis and Thalassiosira) compared to other treatments. Previous studies documented the anti-Vibrio properties of these microalgae (Regunathan and Wesley 2004; Quin et al. 2013; Dash et al. 2017; Widowati et al. 2021). The result on antibacterial assays of microalgae extracts against V. harveyi, V. campbellii, and V. parahaemolyticus in the present study supports the findings of gut microbiology. Hence, the dietary application of Tetraselmis and Thalassiosira might have helped to reduce the Vibrio load in the gut of P. vannamei in the present study. The results of this study clearly elucidated the antimicrobial property of these microalgae and this would be helpful to the commercial hatchery operators for maintaining the post larval and nursery stages using these potential antimicrobial agents present in these microalgae.
Conclusion
The results of the present study give the baseline information about the benefits of including microalgae concentrates in shrimp nursery feed in terms of growth and health. The study also revealed the various properties of T. weissflogii and Tetraselmis sp. to use as feed additives in the penaeid shrimp diet. As the nursery phase of P. vannamei is getting more important in the present farming regime, a nursery feed with nutraceutical properties would ensure a healthier and better crop for the farmer. The feed which can provide nutrition and health benefits in the early stages of shrimp can reduce the occurrence of disease in the initial months of the culture which will ensure an enhanced productivity and would pave the way for sustainability in the shrimp aquaculture. Moreover, the results of the study can be an opening for the new avenues of functional feeds in the early life stages of shrimp larval feeds containing the potential microalgae concentrates.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abiusi F, Sampietro G, Marturano G, Biondi N, Rodolfi L, D’Ottavio M, Tredici MR (2014) Growth, photosynthetic efficiency, and biochemical composition of Tetraselmis suecica F&M-M33 grown with LEDs of different colors. Biotechnol Bioeng 111(5):956–964. https://doi.org/10.1002/bit.25014
Allen KM, Habte-Tsion HM, Thompson KR, Filer K, Tidwell JH, Kumar V (2019) Freshwater microalgae (Schizochytrium sp) as a substitute to fish oil for shrimp feed. Sci Rep 9(1):1–10. https://doi.org/10.1038/s41598-019-41020-8
Ambasankar K, Dayal JS, Vasagam KK, Sivaramakrishnan T, Sandeep KP, Panigrahi A, Raja RA, Burri L, Vijayan KK (2022) Growth, fatty acid composition, immune-related gene expression, histology and haematology indices of Penaeus vannamei fed graded levels of Antarctic krill meal at two different fishmeal concentrations. Aquaculture 553:738069. https://doi.org/10.1016/j.aquaculture.2022.738069
Anand PS, Aravind R, Biju IF, Balasubramanian CP, Antony J, Saranya C, Christina L, Rajamanickam S, Panigrahi A, Ambasankar K, Vijayan KK (2021) Nursery rearing of Indian white shrimp, Penaeus indicus: optimization of dietary protein levels and stocking densities under different management regimes. Aquaculture 542:736807. https://doi.org/10.1016/j.aquaculture.2021.736807
Ananda Raja R, Sridhar R, Balachandran C, Palanisammi A, Ramesh S, Nagarajan K (2017) Pathogenicity profile of Vibrio parahaemolyticus in farmed Pacific white shrimp, Penaeus vannamei. Fish Shellfish Immunol 67:368–381. https://doi.org/10.1016/j.fsi.2017.06.020
Ansari FA, Guldhe A, Gupta SK, Rawat I, Bux F (2021) Improving the feasibility of aquaculture feed by using microalgae. Environ Sci Pollut Res 28(32):43234–43257. https://doi.org/10.1007/s11356-021-14989-x
AOAC (2005) Official methods of analysis of the Association of Analytical Chemists International. Official Methods: Gaithersburg, MD, USA.
Aziz NA, Azlan A, Ismail A, MohdAlinafiah S, Razman MR (2012) Quantitative determination of fatty acids in marine fish and shellfish from warm water of straits of Malacca for nutraceutical purposes. Biomed Res Int 284329:1–12. https://doi.org/10.1155/2013/284329
Bairagi A, Ghosh KS, Sen SK, Ray AK (2002) Enzyme producing bacterial flora isolated from fish digestive tracts. Aquacult Int 10:109–121. https://doi.org/10.1023/A:1021355406412
Basri NA, Shaleh SRM, Matanjun P, Noor NM, Shapawi R (2015) The potential of microalgae meal as an ingredient in the diets of early juvenile Pacific white shrimp, Litopenaeus vannamei. J Appl Phycol 27(2):857–863. https://doi.org/10.1007/s10811-014-0383-6
Bhattacharjya R, Marella TK, Tiwari A, Saxena A, Singh PK, Mishra B (2020) Bioprospecting of marine diatoms Thalassiosira, Skeletonema and Chaetoceros for lipids and other value-added products. Bioprocess Technol 318:124073. https://doi.org/10.1016/j.biortech.2020.124073
Bhuvaneswari GR, Shukla SP, Makesh M, Thirumalaiselvan S, Sudhagar SA, Kothari DC, Singh A (2013) Antibacterial activity of spirulina (Arthospira platensis geitler) against bacterial pathogens in Aquaculture. Isr J Aquac Bamidgeh 932:1–8
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917. https://doi.org/10.1139/o59-099
Chen YY, Chen JC, Tayag CM, Li HF, Putra DF, Kuo YH, Bai JC, Chang YH (2016) Spirulina elicits the activation of innate immunity and increases resistance against Vibrio alginolyticus in shrimp. Fish Shellfish Immunol 55:690–698. https://doi.org/10.1016/j.fsi.2016.06.042
Cheng W, Chen JC (2001) Effects of intrinsic and extrinsic factors on the haemocyte profile of the prawn. Macrobrachium Rosenbergii Fish and Shellfish Immunol 11(1):53–63. https://doi.org/10.1006/fsim.2000.0293
Crab R, Defoirdt T, Bossier P, Verstraete W (2012) Biofloc technology in aquaculture: beneficial effects and future challenges. Aquaculture 356–357:351–356. https://doi.org/10.1016/j.aquaculture.2012.04.046
Dash P, Avunje S, Tandel RS, Sandeep KP, Panigrahi A (2017) Biocontrol of luminous vibriosis in shrimp aquaculture: a review of current approaches and future perspectives. Rev Fisheries Sci Aquaculture 25(3):245–255. https://doi.org/10.1080/23308249.2016.1277973
De D, Ghoshal TK, Ananda Raja R, Kumar S (2015) Growth performance, nutrient digestibility and digestive enzyme activity in Asian seabass, Lates calcarifer juveniles fed diets supplemented with cellulolytic and amylolytic gut bacteria isolated from brackishwater fish. Aquac Res 46(7):1688–1698. https://doi.org/10.1111/are.12325
D’Souza FM, Kelly GJ (2000) Effects of a diet of a nitrogen-limited alga (Tetraselmis suecica) on growth, survival and biochemical composition of tiger prawn (Penaeus semisulcatus) larvae. Aquaculture 181(3–4):311–329. https://doi.org/10.1016/S0044-8486(99)00231-8
FAO (2022) The State of World Fisheries and Aquaculture 2020. Towards Blue Transformation. FAO, Rome.
Geetha R, Ravisankar T, Patil PK, Avunje S, Vinoth S, Sairam CV, Vijayan KK (2020) Trends, causes, and indices of import rejections in international shrimp trade with special reference to India: a 15-year longitudinal analysis. Aquacult Int 28(3):1341–1369. https://doi.org/10.1007/s10499-020-00529-w
Gerde JA, Yao L, Lio J, Wen Z, Wang T (2014) Microalgae flocculation: impact of flocculant type, algae species and cell concentration. Algal Res 3:30–35. https://doi.org/10.1016/J.ALGAL.2013.11.015
Ghasemi Y, Yazdi MT, Shafiee A, Amini M, Shokravi S, Zarrini G (2004) Parsiguine, a novel antimicrobial substance from Fischerella ambigua. Pharm Biol 42(4–5):318–322. https://doi.org/10.1080/13880200490511918
Ghasemi Y, Rasoul-Amini S, Fotooh-Abadi E (2011) The biotransformation, biodegradation, and bioremediation of organic compounds by microalgae. J Phycol 47(5):969–980. https://doi.org/10.1111/j.1529-8817.2011.01051.x
Guzmán HM, de la Jara VA, Duarte LC, Presmanes KF (2010) Estimate by means of flow cytometry of variation in composition of fatty acids from Tetraselmis suecica in response to culture conditions. Aquacult Int 18(2):189–199. https://doi.org/10.1007/s10499-008-9235-1
Guzmán F, Wong G, Román T, Cárdenas C, Alvárez C, Schmitt P, Albericio F, Rojas V (2019) Identification of antimicrobial peptides from the microalgae Tetraselmis suecica (Kylin) Butcher and bactericidal activity improvement. Mar Drugs 17(8):453. https://doi.org/10.3390/md17080453
Hajimahmoodi M, Faramarzi MA, Mohammadi N, Soltani N, Oveisi MR, Nafissi-Varcheh N (2010) Evaluation of antioxidant properties and total phenolic contents of some strains of microalgae. J Appl Phycol 22(1):43–50. https://doi.org/10.1007/s10811-009-9424-y
Haoujar I, Cacciola F, Abrini J, Mangraviti D, Giuffrida D, Oulad El Majdoub Y, Kounnoun A, Miceli N, Fernanda Taviano M, Mondello L, Rigano F (2019) The contribution of carotenoids, phenolic compounds, and flavonoids to the antioxidative properties of marine microalgae isolated from Mediterranean Morocco. Molecules 24(22):4037. https://doi.org/10.3390/molecules24224037
Holder IA, Boyce ST (1994) Agar well diffusion assay testing of bacterial susceptibility to various antimicrobials in concentrations non-toxic for human cells in culture. Burns 20(5):426–429. https://doi.org/10.1016/0305-4179(94)90035-3
Ju ZY, Deng DF, Dominy W (2012) A defatted microalgae (Haematococcus pluvialis) meal as a protein ingredient to partially replace fish meal in diets of Pacific white shrimp (Litopenaeus vannamei, Boone, 1931). Aquaculture 354:50–55. https://doi.org/10.1016/j.aquaculture.2012.04.028
Jusidin MR, Othman R, Shaleh SRM, Ching FF, Senoo S, Oslan SNH (2022) In vitro antibacterial activity of marine microalgae extract against Vibrio harveyi. Appl Sci 12(3):1148
Katircioglu H, Beyatli Y, Aslim B, Yüksekdag Z, Atici T (2006) Screening for antimicrobial agent production of some microalgae in freshwater. The Internet Journal of Microbiology 2(2):1–9. https://ispub.com/IJMB/2/2/9098
Kiatmetha P, Siangdang W, Bunnag B, Senapin S, Withyachumnarnkul B (2011) Enhancement of survival and metamorphosis rates of Penaeus monodon larvae by feeding with the diatom Thalassiosira weissflogii. Aquacult Int 19(4):599–609. https://doi.org/10.1007/s10499-010-9375-y
Kiran BR, Venkata Mohan S (2021) Microalgal cell biofactory: therapeutic, nutraceutical and functional food applications. Plants 10(5):836. https://doi.org/10.3390/plants10050836
Kumar S, Anand PSS, De D, Deo AD, Ghoshal TK, Sundaray JK, Ponniah AG, Jithendran KP, Raja RA, Biswas G, Lalitha N (2017) Effects of biofloc under different carbon sources and protein levels on water quality, growth performance and immune responses in black tiger shrimp Penaeus monodon (Fabricius. Aquac Res 48(3):1168–1182. https://doi.org/10.1111/are.12958
Kumar S, Kumar CB, Rajendran V, Abishaw N, Anand PS, Kannapan S, Nagaleekar VK, Vijayan KK, Alavandi SV (2021) Delineating virulence of Vibrio campbellii: a predominant luminescent bacterial pathogen in Indian shrimp hatcheries. Sci Rep 11(1):15831
Li E, Xu C, Wang X, Wang S, Zhao Q, Zhang M, Qin JG, Chen L (2018) Gut microbiota and its modulation for healthy farming of Pacific white shrimp Litopenaeus vannamei. Rev Fisheries Sci Aquaculture 26(3):381–399. https://doi.org/10.1080/23308249.2018.1440530
Li S, Hu T, Xu Y, Wang J, Chu R, Yin Z, Mo F, Zhu L (2020) A review on flocculation as an efficient method to harvest energy microalgae: mechanisms, performances, influencing factors and perspectives. Renew Sustain Energy Rev 131:110005. https://doi.org/10.1016/j.rser.2020.110005
Liu L, Cai X, Ai Y, Li J, Long H, Ren W, Huang A, Zhang X, Xie ZY (2022) Effects of Lactobacillus pentosus combined with Arthrospira platensis on the growth performance, immune response, and intestinal microbiota of Litopenaeus vannamei. Fish Shellfish Immunol 120:345–352. https://doi.org/10.1016/j.fsi.2021.12.005
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Morowvat MH, Ghasemi Y (2016) Evaluation of antioxidant properties of some naturally isolated microalgae: identification and characterization of the most efficient strain. Biocatal Agric Biotechnol 8:263–269. https://doi.org/10.1016/j.bcab.2016.09.010
Patil PK, Geetha R, Ravisankar T, Avunje S, Solanki HG, Abraham TJ, Vinoth SP, Jithendran KP, Alavandi SV, Vijayan KK (2021) Economic loss due to diseases in Indian shrimp farming with special reference to Enterocytozoon hepatopenaei (EHP) and white spot syndrome virus (WSSV). Aquaculture 533:736231. https://doi.org/10.1016/j.aquaculture.2020.736231
Prabhu DL, Chandrasekar S, Ambashankar K, Dayal JS, Ebeneezar S, Ramachandran K, Kavitha M, Vijayagopal P (2018) Effect of dietary Syzygium cumini leaf powder on growth and non-specific immunity of Litopenaeus vannamei (Boone 1931) and defense against virulent strain of Vibrio parahaemolyticus. Aquaculture 489:9–20. https://doi.org/10.1016/j.aquaculture.2018.01.041
Pradhan B, Patra S, Dash SR, Nayak R, Behera C, Jena M (2021) Evaluation of the antibacterial activity of methanolic extract of Chlorella vulgaris Beyerinck [Beijerinck] with special reference to antioxidant modulation. Future J Pharma Sci 7(1):1–11. https://doi.org/10.1186/s43094-020-00172-5
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341. https://doi.org/10.1006/abio.1999.4019
Quin JG, Trent DA, Zhang W, Franco C (2013) Discovery of antimicrobial activities of a marine diatom Thalassiosira rotula. African J Microbiol Res 7(50):5687–5696. https://doi.org/10.5897/AJMR12.2183
Radhakrishnan S, Belal IEH, Seenivasan C, Muralisankar T, Bhavan PS (2016) Impact of fishmeal replacement with Arthrospira platensis on growth performance, body composition and digestive enzyme activities of the freshwater prawn, Macrobrachium rosenbergii. Aquacult Rep 3:35–44. https://doi.org/10.1016/j.aqrep.2015.11.005
Raji AA, Alaba PA, Yusuf H, Bakar NHA, Taufek NM, Muin H, Alias Z, Milow P, Razak SA (2018) Fishmeal replacement with Spirulina platensis and Chlorella vulgaris in African catfish (Clarias gariepinus) diet: effect on antioxidant enzyme activities and haematological parameters. Res Vet Sci 119:67–75. https://doi.org/10.1016/j.rvsc.2018.05.013
Regunathan C, Wesley SG (2004) Control of Vibrio spp in shrimp hatcheries using the green algae Tetraselmis suecica. Asian Fisheries Sci 17:147–158. https://doi.org/10.33997/j.afs.2004.17.2.006
Rikard FS, Walton WC (2012) Use of microalgae concentrates for rearing oyster larvae, Crassostrea virginica. Mississippi–Alabama Sea Grant Publication No.: MASGP-12, 48
Sandeep KP, Kumaraguru Vasagam KP, Kumararaja P, Syama Dayal J, Sreekanth GB, Ambasankar K, Vijayan KK (2019) Microalgal diversity of a tropical estuary in south India with special reference to isolation of potential species for aquaculture. J Coast Conserv 23(1):253–267. https://doi.org/10.1007/s11852-018-0655-4
Sandeep KP, Avunje S, Dayal JS, Balasubramanian CP, Sawant PB, Chadha NK, Ambasankar K, Vijayan KK (2021) Efficiency of different microalgae as monospecific and bispecific diets in larval rearing of Penaeus indicus with special reference to growth, nutrient composition and antimicrobial activity of microalgae. Aquac Res 52(11):5146–5154
Sandeep KP, Angel JRJ, Sivaramakrishnan T, Sudhin S, Suganya N, Ananda Raja R, Kumar S, Sherly T, Dayal JS, Balasubramanian CP, Sawant PB, Shekhar MS, Chadha NK, Ambasankar K (2022) Effect of dietary C-Phycocyanin on growth, survival, haematology, immune response, gut microbiome and disease resistance of Pacific white shrimp. Penaeus Vannamei Aquaculture Res 53(17):6292–6309. https://doi.org/10.1111/are.16102
Shan X, Lin M (2014) Effects of algae and live food density on the feeding ability, growth and survival of miiuy croaker during early development. Aquaculture 428:284–289. https://doi.org/10.1016/j.aquaculture.2014.03.021
Sharawy ZZ, Ashour M, Abbas E, Ashry O, Helal M, Nazmi H, Kelany M, Kamel A, Hassaan M, Rossi W Jr, El-Haroun E (2020) Effects of dietary marine microalgae, Tetraselmis suecica, on production, gene expression, protein markers and bacterial count of Pacific white shrimp Litopenaeus vannamei. Aquac Res 51(6):2216–2228. https://doi.org/10.1111/are.14566
Singleton VL, Rossi JA (1985) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Viticulture 16:144–158
Sivaramakrishnan T, Swain S, Saravanan KRKS, Sankar K, Roy SD, Biswas L (2017) In vitro antioxidant and free radical scavenging activity and chemometric approach to reveal their variability in green macroalgae from south Andaman Coast of India. Turk J Fish Aquat Sci 17:639–648. https://doi.org/10.4194/1303-2712-v17_3_20
Song YL, Hsieh YT (1994) Immunostimulation of tiger shrimp (Penaeus monodon) hemocytes for generation of microbicidal substances: analysis of reactive oxygen species. Dev Comp Immunol 18(3):201–209. https://doi.org/10.1016/0145-305X(94)90012-4
Southgate PC, Braley RD, Militz TA (2017) Ingestion and digestion of micro-algae concentrates by veliger larvae of the giant clam, Tridacna noae. Aquaculture 473:4430–4448. https://doi.org/10.1016/j.aquaculture.2017.02.032
Steffens W (1989) Principles of fish nutrition. Halsted Press, New York, p 384
Tavakoli S, Hong H, Wang K, Yang Q, Gahruie HH, Zhuang S, Li Y, Liang Y, Tan Y, Luo Y (2021) Ultrasonic-assisted food-grade solvent extraction of high-value added compounds from microalgae Spirulina platensis and evaluation of their antioxidant and antibacterial properties. Algal Res 60:102493
Thompson PA, Guo MX, Harrison PJ, Whyte JN (1992) Effects of variation in temperature on the fatty acid composition of eight species of marine phytoplankton. J Phycol 28(4):488–497. https://doi.org/10.1111/j.0022-3646.1992.00488.x
Thornber K, Verner-Jeffreys D, Hinchliffe S, Rahman MM, Bass D, Tyler CR (2020) Evaluating antimicrobial resistance in the global shrimp industry. Rev Aquac 12(2):966–986
Vandamme D, Foubert I, Meesschaert B, Muylaert K (2010) Flocculation of microalgae using cationic starch. J Appl Phycol 22(4):525–530
Vandamme D, Foubert I, Fraeye I, Meesschaert B, Muylaert K (2012) Flocculation of Chlorella vulgaris induced by high pH: role of magnesium and calcium and practical implications. Biores Technol 105:114–119. https://doi.org/10.1016/j.biortech.2011.11.105
Walne PR (1966) Experiments in the large scale culture of the larvae of Ostrea edulis. Fishery investigations (Great Britain. Ministry of Agriculture, Fisheries and Food) 25(4):1–53
Wang Y, Li M, Filer K, Xue Y, Ai Q, Mai K (2017) Evaluation of Schizochytrium meal in microdiets of Pacific white shrimp (Litopenaeus vannamei) larvae. Aquac Res 48(5):2328–2336
Wasielesky WJ, Froes C, Foes G, Krummenauer D, Lara G, Poersch L (2013) Nursery of Litopenaeus vannamei reared in a biofloc system: the effect of stocking densities and compensatory growth. J Shellfish Res 32:799–806. https://doi.org/10.2983/035.032.0323
Wassnig M, Southgate PC (2016) The effects of stocking density and ration on survival and growth of winged pearl oyster (Pteria penguin) larvae fed commercially available micro-algae concentrates. Aquaculture Reports 4:17–21. https://doi.org/10.1016/j.aqrep.2016.05.004
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag
Widowati I, Zainuri M, Kusumaningrum HP, Hardivillier Y, Leignel V, Bourgougnon N, Mouget JL (2021) Growth of shrimp infected by Vibrio, fed with formulated feed with inclusions of Dunaliella salina and Tetraselmis chuii extracts. Aqua Aqua Conserv Legislation 14(2):981–987
Widowati I, Zainuri M, Kusumaningrum HP, Susilowati R, Hardivillier Y, Leignel V, Bourgougnon N, Mouget JL (2017) Antioxidant activity of three microalgae Dunaliella salina, Tetraselmis chuii and Isochrysis galbana clone Tahiti. In: IOP Conference Series: Earth and Environmental Science 55(1):012067. IOP Publishing
Zishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559. https://doi.org/10.1016/S0308-8146(98)00102-2
Acknowledgements
The authors thank the Director of the Central Institute of Brackishwater Aquaculture (ICAR-CIBA) for supporting the present study. The authors are thankful to Dr. R. Vidya, Scientist, ICAR-CIBA, Chennai, for her help in providing microbial cultures for antibacterial study. The authors also thank the Indian Council of Agricultural Research (ICAR) for providing financial support to carry out this study.
Funding
This work was funded by Indian Council of Agricultural Research (ICAR), New Delhi, for the project “Novel approaches for development and improvement of sustainable shrimp and fish feeds” Grant Number: FISHCIBASIL201800800136. Fund was received by Ambasankar, K.
Author information
Authors and Affiliations
Contributions
Sandeep KP, Chadha NK, and Ambasankar K designed the study. Sandeep KP, Sivaramakrishnan T, and Sudhin S conducted the experiments and collected the data. Sandeep KP, Sudheer NS, Raymond JAJ, Syama Dayal J, and Balasubramanian CP analysed and interpreted the data, and Ambasankar K supervised the study. Aananda Raja R analysed the haematological parameters Sujeet K analysed the gut microbiology. Sandeep KP wrote the manuscript. Balasubramanian CP, Sivaramakrishnan T, Paramita BS, and Ambasankar K revised the manuscript. All authors edited and approved the final manuscript for submission to the journal.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sandeep, K.P., Sivaramakrishnan, T., Sudhin, S. et al. Influence of dietary microalgal concentrates on growth, survival and health status of Penaeus vannamei. Aquacult Int 31, 2883–2903 (2023). https://doi.org/10.1007/s10499-023-01114-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01114-7