Abstract
Sex development is a multi-step process involving determination, differentiation, and maintenance of the gonads, which culminates in producing fertile sperm and eggs. In teleosts, candidate genes mastering this process can be thermosensitive before or just at the beginning of differentiation, the sensitive period in fish. The knowledge of these mechanisms may be useful for the production of monosex populations in aquaculture. Here we investigated the influence of temperature on survival, growth, expression profiles of genes involved in early sexual development, and sex ratio. 5-day-old (undifferentiated) larvae were reared at 26, 28 and 31 °C for 90 days, and expression of genes involved in testis- (wt1b and ar) and ovary-determining (amh and foxl2) pathways, and vasa, were analysed four times from 20 to 90 days post-hatching, and the resulting sex ratios were estimated. Only foxl2 showed a thermosensitive transcription at 20 dph, decreasing at the highest temperature. None of the male-bias genes were regulated by temperature. Still, both wt1b and ar were upregulated in some individuals long after sex differentiation, suggesting the importance of testis-skewed genes not only at testis establishment but also at later stages. The sex ratio was not affected by temperature. Our study also revealed an unusual vasa expression profile before and after gonadal differentiation; in some fish increasing expression values were recorded while others presented low vasa expression even after differentiation. Our results support a strict genetic control in the sex determination of tambaqui, despite thermosensitivity of some genes involved in its sexual development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The establishment of the sex of an individual depends on the close relationship between the mechanisms of sex determination and gonad differentiation. Among vertebrates, fish are the most diverse group regarding both processes, which generally occurs in species-specific and even strain-specific ways (Kossack and Draper 2019; Nagahama et al. 2021). In aquaculture species, the manipulation of reproduction and sex ratio are important features to optimize fish production (Taranger et al. 2010; Martínez et al. 2014).
In short, teleost present genetic (GSD) and environmental (ESD) sex determination systems, with the possibility of mixed systems (Nagahama et al. 2021). In species displaying ESD, there are no consistent genetic differences between males and females and the sex ratio is rarely 1:1 (Valenzuela et al. 2003; Sarre et al. 2004; Valenzuela 2008). In these cases, the sex can be influenced by temperature, pH, photoperiod, hypoxia, density, and social interactions that somehow overlap the genetic background (reviewed by Fernandino et al. 2013). One of the most important environmental factors driving sex differentiation in fish is temperature (temperature-dependent sex determination, TSD). In such cases, the water temperature during the phase of undifferentiated gonads permanently and irreversibly influences the phenotypic sex of sensitive individuals (Valenzuela et al. 2003; Ospina-Álvarez and Piferrer 2008). Even in some GSD species the exposure to extreme temperatures can alter the sex by epigenetic regulation (Shao et al. 2014; Miyoshi et al. 2020; Geffroy et al. 2021).
It is assumed that the thermal master switch, which directs the undifferentiated gonads to follow the male or female pathway, is the main gene(s) activated during the thermosensitive period that drives specific responses during this developmental time window (Shen and Wang 2014). Candidates for this role are genes expressed prior to, or exactly at, the onset of the sensitive period. Genes such as hsd11b2, gr1, ar, sox9, sox8, fgf9, amh (mis), wt1 and dmrt1 (generally involved in the testis-determining pathway) and foxl2, cyp19a1a, wt4, rspo1 (implicated in the ovary-determining pathway) have been found to be sex-biased expressed and thermo-sensitive in fish (Fernandino et al. 2012; Heule et al. 2014; Shen et al. 2018; Shen and Wang 2018). In addition, the germ cells are important in mediating the direction of gonadal development in some species, and the degeneration or inhibition of germ cells by thermal stress are related to masculinization in many species (Shen and Wang 2018). Vasa have been reported to play pivotal roles in germ cell development and differentiation both in vertebrates (Tanaka et al. 2000; Hartung et al. 2014) and invertebrates (Hay et al. 1990; Yang et al. 2023). It is expressed only in germline cells and is a molecular marker gene in several fish species, such as medaka Oryzias latipes (Shinomiya et al. 2000), orange-spotted grouper Epinephelus coioides (Qu et al. 2020) and tambaqui Colossoma macropomum (Vasconcelos et al. 2019).
The tambaqui Colossoma macropomum is the second largest scaled fish from the Amazon basin, can reach up to 30 kg and up to 1 m in length (Araújo-Lima and Goulding 1988). It represents the most prominent native species in fish farming and consumption frequency in Brazil (IBGE 2021; Hilsdorf et al. 2022; St. Louis et al. 2022) and exhibits a sex-linked growth dimorphism (Almeida et al. 2016). Tambaqui is a gonochoristic species with a female homogametic system (XX/XY) and no sex-sensitivity to different pHs, in spite of originally living in basic and acidic waters (Morais et al. 2020; Varela et al. 2021). The gonad differentiation occurs in 40 mm total length juveniles and is characterised by a sex biased gonad gene expression in which the Wnt/β-catenin pathway directs the ovarian differentiation process, without the involvement of cyp19a1a (Lobo et al. 2020; Paixão et al. 2022). Among other genes, foxl2 and wt1, a classic antagonist of the Wnt/β-catenin pathway, are exclusively expressed in differentiating females and males respectively. And surprisingly the “male-bias” amh is differentially over-expressed by the females, being the first teleost to have higher expression of this gene in females during sexual differentiation. Among the classic thermo-sensitive sex-linked genes in teleost, tambaqui express ar and wt1 in developing testis and amh and foxl2 in developing ovaries (Lobo et al. 2020). However, it is still not known if the water temperature can affect sex ratio, which could facilitate sex manipulation in tambaqui farming and help in identifying possible threatness for the wild populations in global warming scenarios. Understanding the basis of tambaqui sexual development is therefore important to support the development of new farming technologies and for conservation biology. Thus, this work aimed to investigate the influence of water temperature on the sex determination and on the expression of genes involved in the sex differentiation of tambaqui.
Materials and methods
Ethics statement
All procedures of this work comply with the ethical of animal experimentation, adopted by the Brazilian College of Animal Experimentation (COBEA), and were approved by the Local Ethics Committee on Animal Use (CEUA) – of the Brazilian Institute for Research of the Amazon – INPA (n◦ 014/2017). The project has an Authorization of Access to Genetic Heritage under register A5784B5.
Animals, experimental conditions and sampling
A newly hatched full-sib progeny was obtained from a commercial hatchery at Presidente Figueiredo, Amazonas, Brazil. They were transported to the Laboratory of Ecophysiology and Molecular Evolution (LEEM), of the National Institute of Research in the Amazon (INPA/MCTI), Manaus, Brazil. During the first five days of acclimatization outdoors, a quick temperature trial was performed to establish the maximum and minimum temperatures tolerated by tambaqui fries. Briefly, we tested temperatures ranging from 31 to 33 °C and from 24 to 26 °C for 24 h in groups of 20 fries. Based on the mortality registered in each group, the lowest and highest water temperatures tolerated were 26 and 31 °C, respectively. Tambaqui larvae did not survive at the other temperatures tested.
On the 5th day, we gathered nine groups containing 60 larvae each, which were installed into 250 L tanks with aeration and water flow. Based on the pilot test of thermotolerance, the treatments consisted of three temperatures: 26 ºC, 28 ºC and 31 ºC, carried out in triplicates. To ensure the temperature stability of the experiment, a cooling system was used for the lowest temperature and thermostats heaters applied for the highest. For the third group (average 28 oC), there was no manipulation of the temperature, and the temperature followed the natural fluctuations of the water source of LEEM. The water temperature was monitored three times daily. To assure that treatment would cover the differentiation period of tambaqui, the treatments were applied during three months. Dissolved oxygen and pH were recorded twice and once a day, respectively. Every two weeks water samples were collected from each tank for analyses of ammonia, nitrite, alkalinity and hardness.
During the experiment, a total of 72 juveniles were sampled (on the days 20, 35, 65 and 95 post hatching; n = 6/treatment). For this, the fish were deeply anesthetized in a solution of buffered benzocaine (300 mg L− 1) (pH 7) followed by sectioning of the spinal cord. The juvenile trunks (after the removal of the head and region posterior to the urogenital papilla) were rapidly immersed in RNA later for total RNA isolation.
At the end of the treatment, the temperature of all tanks was stabilized at 28 °C and the fish were transported to the facilities of EMBRAPA Amazônia Ocidental, Manaus. Each group was allocated in a net-cage, all placed in a common earthen pond. The fish were maintained there for 8 months when the final sampling took place. At this sampling, the gonads were dissected, immediately fixed in Bouin’s solution for 24 h and included in paraffin or methacrylate for histology, according to routine procedures. Microscopic analyzes were performed using the Zeiss Axio Imager 2 integrated optical microscopy system.
RNA extraction and cDNA preparation
Total mRNA from trunks (n = 6/treatment) was isolated using Trizol reagent (Ambion RNA by life technologies), according to the manufacturer’s protocol. RNA integrity was confirmed by electrophoresis. The RNA quantity and purity were assessed by spectrophotometry on a nanodrop ND-1000 instrument (ThermoFisher Scientifics). After quantification the samples were treated with DNAse using the RQ1 RNase-free DNAse kit (Promega). After DNase treatment, mRNA samples were again quantified by fluorimetry with the aid of Qubit 3.0 (Thermo Fisher). Complementary DNA was prepared by reverse transcription of 6 µg of total RNA using the GoScript™ Reverse Transcriptase System Kit (Promega) according to the manufacturer’s recommendations.
Quantitative PCR
We chose two genes involved in the female pathway (foxl2 and amh) and two from the male pathway (wt1b and ar), known to play roles during early tambaqui differentiation (Lobo et al. 2020). In addition, we evaluated the transcription of vasa, a key gene for germline survival and development.
The qPCR primers were designed from mRNA sequences retrieved from the NCBI database (No. GSE130895), avoiding regions of conserved domains, and looking for intron-exon junctions to avoid amplification of genomic DNA (Table 1). The Primer-BLAST (NCBI National Center for Biotechnology Information) and OligoAnalyzer Tool and PrimerQuest Tool (Integrated DNA Technologies) were used for primer design and analysis, respectively.
Quantitative PCR was performed in duplicates in 96-well optical plates, each well containing 10 µL of Fast Sybr Green Master Mix (Applied Biosystems), 100 ng of cDNA and 200 nM of each forward and reverse primer to a final volume of 20 µL. Thermocycling was conducted in a 7500 Fast Real-Time PCR System v2.3 (Applied Biosystems). The following thermal profile for qPCR was used: initial denaturation step of 95 °C for 10 min, followed by 3-step cycling for a total of 40 cycles of denaturation for 15 s at 95 °C, annealing for 1 min at 60 °C and extension for 30 s at 72 °C. No template controls for each gene were run in all qPCR plates. To validate the assays, the amplification efficiency was first estimated using serial dilutions of a pool of ovary and testes cDNA (1:4, 1:16, 1:64, 1:256) and the determination coefficient (R2) were all above 0.98. The melt curve was analysed at the end of each assay. The relative gene expression was calculated using the 2 − ΔΔCt method. All values were normalized to β-actin (Nascimento et al. 2016) and calibrated to the average ΔCt of the group maintained at 28 °C.
Statistical analysis
All results were checked for homogeneity of variances by the Shapiro-Wilk test, and are presented as mean ± sd. For the analysis of the physical-chemical parameters of the water and the mortality rate, one-way ANOVA was used, followed by Tukey test for multiple comparisons of means when necessary. For expression analysis, the non-parametric Kruskal-Wallis test was used, followed by Dunn’s multiple comparison, since the assumption of homogeneity of variances was not met. Differences in sex ratio between the groups and deviation from 1:1 sex ratio were analysed using the chi-square test. All statistical analyses were performed in GraphPad Prism 9.4.1.
Results
Experimental conditions
The water temperature of the treatments was stable during the experiment. The highest variation was − 2.7 degrees in the temperature 28 °C, and the standard deviation was 0.30, 0.94 and 0.29 for the treatments of 26, 28 and 31 °C, respectively (Table 2). Dissolved oxygen decreased along with the increase of water temperature but did not reach the lower limit of tolerance for tambaqui (3 mg L− 1). The other physical-chemical parameters analysed did not differ during the experiment (p < 0.05; Table 2).
Fish growth and gene expression in early gonadal development
At the first sampling (20 dph), the fish had a mean standard length of 21 ± 1.0, 22 ± 2.1 and 34 ± 2.35 mm in the groups of 26 °C, 28 and 31 °C, respectively. At 95 days post hatching (dph), standard length was 55 ± 3.0, 55 ± 1.9, 79 ± 4.5 mm at 26 °C, 28 and 31 °C, respectively, indicating that the treatment started before and finished after the period of sex differentiation (Lobo et al. 2020) (Fig. 1a). vasa transcripts were detected in all samples. In each sampling, there was no difference in the mean vasa expression between the groups. However, the transcription increased with fish growth, most likely owing to their gonad growth and constant multiplication of germ cells (Fig. 1b). Interestingly, in all samplings there was a group of fish that presented low vasa expression, irrespective of the water temperature. On the contrary, there were other fish with higher expression of this gene, mainly around the differentiation period (40 mm; at approximately 40 dph; Fig. 1).
Body standard length (a) and relative expression of vasa (b) of tambaqui Colossoma macropomum reared in different water temperatures during the phase of early gonad development. Dotted line points the beginning of sex differentiation in tambaqui. Results are shown as mean ± SD (n = 6/group). Different letters indicate different mean values of the same group at different ages
At 15 days of treatment, when undifferentiated larvae were 20 dph, only the female-biased genes showed some regulation in transcription (Fig. 2). At this age, amh expression showed two groups at 31 oC, one with higher transcription than the other, while foxl2 expression decreased along with elevating temperatures. With further growth of the fish, and entering differentiation, amh maintained the expression pattern of two clusters in all the temperatures, with exception that after differentiation (95 dph) the expression was very low in the fish (already immature juveniles) reared at 31 oC, with exception of one sample (Fig. 2). In contrast, foxl2 did not display temperature or age regulation from 35 to 95 dph, except for one fish, in which it was upregulated at 28 oC at 95 dph. The same individual also displayed high amh expression and low wt1b and ar transcriptions, i.e., mostly likely it was a developing female.
The expression of the genes involved in the male development displayed a later regulation. wt1b and ar mRNA were detected at baseline levels in all larvae with no changes related to temperature up to 35 dph, when at 28 °C two out of six fish showed upregulation of wt1b and at 31 oC half of the group presented ar upregulated. Soon after the morphological gonad differentiation (65 dph), the expression of wt1b and ar were divided into two groups at 31 °C, probably biased in favour of the males. wt1b and ar mRNAs were regulated in immature juveniles (95 dph) as different individuals presented different relative expression values, without any temperature effect on these transcriptional levels (Fig. 2).
Survival, sex ratio and gonad morphology
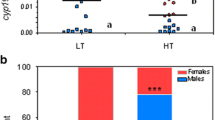
The survival ratio at the end of the study was similar in all groups and ranged from 75.7 to 81.6%. The sex of 271 (86 to 93/treatment) juveniles was histologically identified at 11 months old, and the sex ratio was similar in all groups. Table 3 shows the number and percentage of males and females in each group.
No relevant difference was observed in the gonadal parenchyma of fish at 11 months of age in all groups. The females presented immature ovaries (Fig. 3a and b). Some ovaries were characterized by the presence of nests of oogonia surrounded by pre-follicular cells in the germinal epithelium; the majority (74%) were characterized by the onset of meiosis with the individualization of pre-vitellogenic oocytes surrounded by follicular cells. Males had testis in two stages, immature or at the beginning of maturation (Fig. 3c and d); a small number of males had testes with germinal epithelium containing cysts of undifferentiated and differentiated type A spermatogonia. Most males (72%) had maturing testis, characterized by the presence of cysts of spermatocytes, spermatids and some free spermatozoa in the lumen of the tubules.
Section (5 μm) of Colossoma macropomum gonads. a and b) immature ovary; c) immature testis; d) testis in initial maturation; Black asterisk shows nest of oogonia. White asterisk shows spermatozoa. CT: connective tissue; GE: germinal epithelium; PFC: prefollicular cell; Oo: ovogonia; PvO: pre-vitellogenic oocytes; FC: follicular cell; Aund: type A undifferentiated spermatogonia; Adiff: type A differentiated spermatogonia; SC: Sertoli cell; Sc: spermatocyte; St: spermatid. The scale bar represents 25 μm
Discussion
Tambaqui is traditionally the main source of animal protein in the north of Brazil and northern-neighbouring countries. Additionally, it is farmed as pure or in the formation of interspecific hybrids, being important for the native aquaculture industry (Hilsdorf et al. 2022). A possible sex ratio susceptibility of this species to different water temperatures represents a risk in the face of global climate changes. Besides, knowing the forces that drive sex differentiation in a farmed species is always fundamental for the development of new technologies based on sex manipulation to increase production at harvest. The sensitivity of tambaqui to thermal variations, particularly in its reproduction, growth, metabolism, and physiology was reviewed by Amanajás and Val (2022), and shows that the species grows and seems to regulate its metabolism more effectively between 25 and 32 °C. In an effort to fully characterize the system of sex determination of tambaqui, in this study we raised undifferentiated larvae at three different temperatures, two of them being the extremes tolerated by the species at larval stage, during sex differentiation, i.e., up to 3 months of age. As Colossoma macropomum forms a single large panmictic population over the broad range in the Amazon basin of Brazil, Bolivia, and Peru (Farias et al. 2010) and the breeding stocks and wild populations display similar genetic diversity (Aguiar et al. 2013), we performed our study using a single population, assuming that there are no subpopulations nor family differences on the sex determination or sex sensitivity to temperature in tambaqui, as seen in other worldwide distributed species such as tilapia (Baroiller et al. 2009) and zebrafish (Valdivieso et al. 2022). The results show that water temperature does not influence tambaqui sex ratio, which exhibited the proportion of 1:1 at all temperatures analysed, which is also similar to that found in wild populations (Villacorta-Correa and Saint-Paul 1999). Hence a possible gene dose effect for environmentally induced sex reversal (Quinn et al. 2007) can be ruled out since the larvae would not survive at higher temperatures. Furthermore, they are within the temperature range of thermal comfort and the natural environment of the species. Similarly, tambaqui sex ratio is not changed by water pH (Morais et al. 2020). A possible environmental susceptibility could lead to changes in the gene pathway culminating in switches on the phenotypic sex of the fish (Fernandino et al. 2013). Instead, our results reinforce the monofactorial sex determination system hypothesis in tambaqui, where the single or strongly (inter)linked genetic factors located on a single chromosome overlap the environmental factor temperature in gonadal differentiation and consequently indicates a genetic sex termination (GSD; Devlin and Nagahama 2002). Although chromosomal dimorphism between tambaqui males and females has not been identified by karyotype (Nakayama et al. 2012), there is still the possibility of the presence of homomorphic sex chromosomes similar to autosomes.
In tambaqui foxl2 and amh are upregulated in pre-differentiating females, while wt1b and ar are more expressed by the future males (Lobo et al. 2020). Hence, we analysed the expression of these genes in the present study to investigate a possible transcription regulation by water temperature. foxl2, amh, ar and wt1b were detected from undifferentiated larvae to immature juvenile tambaqui (from 20 to 95 dph). Surprisingly, only foxl2 showed a thermo-sensitive expression before sex differentiation (20 dph), when transcription decreased from the lowest to the highest temperature before the onset of tambaqui morphological sex differentiation. The foxl2 gene belongs to the female pathway and promotes ovarian development by upregulating cyp19a1a expression, the key enzyme involved in the synthesis of estrogens, increasing germ cell number, and repressing male pathway gene expression (Zhang et al. 2017). In teleosts, foxl2 transcription can be thermosensitive and downregulated at high temperatures, such as in olive sole GSD/TE Paralichthys olivaceus, whose expression of foxl2 is repressed during masculinization at high temperature (Fan et al. 2019) and bluegill Lepomis macrochirus that prior to sexual differentiation the expression of foxl2 was also repressed at higher temperature, however there was no significant difference in sex ratio (Shen et al. 2018). Recently, it was discovered that high water temperature also decreases foxl2 expression in Yesso scallop Patinopecten yessoensis (Liu et al. 2022). However, the effects on sex change are controversial and species-specific (Yamaguchi et al. 2010; Yamaguchi and Kitano 2012; Shen et al. 2018). In this study, the effect of temperature on foxl2 expression follows that observed in other teleosts, i.e., downregulation at higher temperatures. However, this effect was not sufficient to affect ovary differentiation, as the sex ratio was not altered. A similar response was recently observed in juvenile tambaqui treated with anti-estradiol compounds; the treatments influenced gene expression (including cyp19a1a) and reduced E2 plasma levels without changing the sex ratio (Paixão et al. 2022). This result strengthens the hypothesis of a strict genetic sex determination of the species. On the other hand, it must be further investigated if the role of foxl2 does not involve TSD in tambaqui as it does in other species. Furthermore, in contrast to the most species studied to date, foxl2 transcription profile seems not to be positively correlated with cyp19a1a in tambaqui, as its transcription was identified by real-time quantitative PCR (qPCR) during the early stages of sex development that increased in fishes with 2 to 4 cm with an important inter-individual variability (Paixão et al. 2022). The unexpected expression of genes related to ovarian differentiation have been reported, especially in characids, such as Astyanax mexicanus in which none of the ovarian differentiation genes, i.e., foxl2a, cyp19a1a, and wnt4b displayed an early sexually dimorphic expression (Imarazene et al. 2021). Controversial findings have also been reported in cyprinids, such as in Acrossocheilus fasciatus where the level of foxl2 expression was significantly lower in females during the sex determination period, which in this species is very long and late from 4 to 12 months (Ren et al. 2023).
All four genes analysed here presented a bimodal expression either before (mostly the female-bias amh and foxl2), during, or after sex differentiation (only male-bias genes wt1b and ar). At 20 dph the expression of amh was high and displayed into two clusters. This was more pronounced in the group with the longest body length (mean of 23 mm) reared at 31 oC). On the other hand, at 65 dph when mean fish length was from 45 to 48 mm, both wt1b and ar were highly expressed in average, but with some individuals showing high other low transcription, which can be interpreted as sex-biased expression favouring the males. This is in agreement with the length of sex differentiation of the species, as ovaries differentiate earlier at approximately 40 mm of total length, while testis remains as undifferentiated gonads until later on (Lobo et al. 2020).
In three months, old immature juveniles, only wt1b and ar showed bimodal expression profiles. amh and foxl2 transcriptions were almost null, except for one fish that presented very high relative expression of both genes. The fact that only testis-supporting genes remained upregulated in these juveniles suggests that their role is not restricted during male differentiation, but also crucial for testis maintenance. Similarly, during sex differentiation of the Amur sturgeon Acipenser schrenckii ar expression was relatively late (Zhang et al. 2022). In mammals wt1 is essential for the development of the urogenital system (Gao et al. 2014). In teleost fish two paralogs have been identified, namely wt1a and wt1b, as being involved in the maintenance of primordial germ cells, regulatory mechanisms at differentiation and maintenance of the gonads, but not in sex determination (Perner et al. 2007; Jiang et al. 2017). The expression of wt1b in tambaqui juvenile trunks was marked by high values in fish of 45 to 48 mm standard length, i.e., just after the beginning of sex differentiation. Similar results were found for trunks of juveniles and adult males of the African cichlid Astatotilapia burtoni (Böhne et al. 2013; Heule et al. 2014) and in males of yellow croaker Larimichthys crocea (Xiao et al. 2019), suggesting that wt1b is an important gene not only for the testis development, but also maintenance.
The present study revealed a peculiar expression profile of vasa in trunks of juveniles before and after the window of gonadal differentiation. In all four samplings, there were always individuals with low vasa expression while others presented high relative transcription values. From 20 to 35 dph, coinciding with the period of gonadal differentiation in the species, that starts at 40 mm standard length (Lobo et al. 2020), the vasa expression increased up to three fold in some fish, while it remained low in others. After this period, the expression persisted increasing in some and low in others, even when differentiation was completed (95 dph). In teleost, the female differentiation occurs first than that in males, and ovarian differentiation is generally marked by a higher number of germ cells (Kobayashi et al. 2000; Xu et al. 2005; Imarazene et al. 2021). Hence, the clear variability on vasa expression among individuals can be associated with the sex dimorphic number of germ cells in tambaqui. Vasa is essential for the development of the germline in vertebrates and invertebrates (Begum et al. 2022), and vasa expression is restricted to gonads (Vasconcelos et al. 2019). Although temperature did not produce any significant effect on vasa transcript abundance, the increase from 20 to 95 dph was more accentuated in fish reared at 31 °C compared to 26 and 28 °C. This result disagrees from those obtained for tilapia Oreochromis niloticus and pufferfish Takifugu rubripes in which higher temperatures induced germ cell degeneration and masculinization of ovarian somatic cells (Lee et al. 2009; Pandit et al. 2015). However, in turtle Mauremys mutica the level of vasa mRNA in female-producing temperature (FPT) embryos at 33 °C was significantly higher than that of male-producing temperature (MPT) embryos at 25 °C (Liu et al. 2021). In the bluegill Lepomis macrochirus, thermal stress delayed proliferation and reduced numbers of germ cells in the low-temperature treatment (17 °C) and morphological sex differentiation had not been detected up to 97 dph (Shen et al. 2018). In this study, the expression of vasa at the lowest temperature treatment (26 °C) exhibited a similar pattern to 28 and 31 °C from 20 to 65 dph, but with a decrease at 95 dph.
In conclusion, our results showed that tambaqui sexual development was not sensitive to temperature, in spite of an early disturbance in foxl2 transcription (downregulated by higher temperature). Although some regulation can be observed in the expression of amh, wt1, foxl2 and ar in all treated groups, this seems to be more related to the genetic sex of each individual than to temperature. While the female-bias genes are expressed earlier (before morphological sex differentiation), the male-bias genes are upregulated later and seems to be required for testis maintenance. As TSD species can be threatened by scenarios of climate change, our results strongly reinforce the idea that tambaqui present a strict genetic sex determination system, which, on the other hand, can facilitate the development of efficient techniques to produce monosex population of the species.
Data Availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Aguiar J, Schneider H, Gomes F et al (2013) Genetic variation in native and farmed populations of Tambaqui (Colossoma macropomum) in the brazilian Amazon: regional discrepancies in farming systems. An Acad Bras Ciênc 85(04):1439–1447. https://doi.org/10.1590/0001-376520130007
Almeida FL, Lopes JS, Crescencio R et al (2016) Early puberty of farmed tambaqui (Colossoma macropomum): possible influence of male sexual maturation on harvest weight. Aquaculture 452:224–232. https://doi.org/10.1016/j.aquaculture.2015.10.031
Amanajás RD, Val AL (2022) Thermal biology of tambaqui (Colossoma macropomum): General insights for aquaculture in a changing world. Rev Aquac 15:380–390. https://doi.org/10.1111/raq.12732
Araújo-Lima C, GOULDING M (1988) Os frutos do tambaqui: ecologia, conservação e cultivo na Amazônia. CNPq /Instituto de Desenvolvimento Sustentável Mamirauá, Brasilia/Tefe
Baroiller JF, D’Cotta H, Bezault E, Wessels S, Hoerstgen-Schwark G (2009) Tilapia sex determination: where temperature and genetics meet. Comp Biochem Physiol Part A 153:30–38. https://doi.org/10.1016/j.cbpa.2008.11.018
Begum S, Gnanasree SM, Anusha N, Senthilkumaran B (2022) Germ cell markers in fishes - a review. Aquac Fish 7:540–552. https://doi.org/10.1016/j.aaf.2022.03.015
Böhne A, Heule C, Boileau N, Salzburger W (2013) Expression and sequence evolution of aromatase cyp19a1 and other sexual development genes in east african cichlid fishes. Mol Biol Evol 30:2268–2285. https://doi.org/10.1093/molbev/mst124
de Morais IS, Reis VR, de Almeida FL (2020) The influence of the water pH on the sex ratio of tambaqui Colossoma macropomum (CUVIER, 1818). Aquac Rep 17:100334. https://doi.org/10.1016/j.aqrep.2020.100334
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental inf luences. Aquaculture 208:191–364. https://doi.org/10.1016/S0044-8486(02)00057-1
Fan Z, Zou Y, Liang D et al (2019) Roles of forkhead box protein L2 (foxl2) during gonad differentiation and maintenance in a fish, the olive flounder (Paralichthys olivaceus). Reprod Fertil Dev 31:1742–1752. https://doi.org/10.1071/RD18233
Farias IP, Torrico JP, García-Dávila C et al (2010) Are rapids a barrier for floodplain fishes of the Amazon basin? A demographic study of the keystone floodplain species Colossoma macropomum (Teleostei: Characiformes). Mol Phylogenet Evol 56(3):1129–1135. https://doi.org/10.1016/j.ympev.2010.03.028
Fernandino JI, Hattori RS, Kishii A et al (2012) The cortisol and androgen pathways cross talk in high temperature-induced masculinization: the 11β-hydroxysteroid dehydrogenase as a key enzyme. Endocrinology 153:6003–6011. https://doi.org/10.1210/en.2012-1517
Fernandino JI, Hattori RS, Moreno Acosta OD et al (2013) Environmental stress-induced testis differentiation: androgen as a by-product of cortisol inactivation. Gen Comp Endocrinol 192:36–44. https://doi.org/10.1016/j.ygcen.2013.05.024
Gao F, Zhang J, Wang X et al (2014) Wt1 functions in ovarian follicle development by regulating granulosa cell differentiation. Hum Mol Genet 23:333–341. https://doi.org/10.1093/hmg/ddt423
Geffroy B, Besson M, Sánchez-Baizán N et al (2021) Unraveling the genotype by environment interaction in a thermosensitive fish with a polygenic sex determination system. Proc Natl Acad Sci 118(50):e2112660118. https://doi.org/10.1073/pnas.2112660118
Hartung O, Forbes MM, Marlow FL (2014) Zebrafish vasa is required for germ-cell differentiation and maintenance. Mol Reprod Dev 81:946–961. https://doi.org/10.1002/mrd.22414
Hay B, Yeh Jan L, Nung Jan Y (1990) Localization of vasa, a component of Drosophila polar granules, in maternal-effect mutants that alter embryonic anteroposterior polarity. Development 109(2):425–433. https://doi.org/10.1242/dev.109.2.425
Heule C, Göppert C, Salzburger W, Böhne A (2014) Genetics and timing of sex determination in the east african cichlid fish Astatotilapia burtoni BMC Genet 15(1):1–17. https://doi.org/10.1186/s12863-014-0140-5
Hilsdorf AWS, Hallerman E, Valladão GMR et al (2022) The farming and husbandry of Colossoma macropomum: from amazonian waters to sustainable production. Rev Aquac 14:993–1027. https://doi.org/10.1111/raq.12638
IBGE, Instituto Brasileiro de Geografia e Estatística (2021) Produção da Pecuária Municipal 2021. Rio de Janeiro
Imarazene B, Beille S, Jouanno E et al (2021) Primordial germ cell Migration and histological and molecular characterization of gonadal differentiation in Pachón Cavefish Astyanax mexicanus Sex Dev 14:80–98. https://doi.org/10.1159/000513378
Jiang D, Chen J, Fan Z et al (2017) CRISPR/Cas9-induced disruption of wt1a and wt1b reveals their different roles in kidney and gonad development in Nile tilapia. Dev Biol 428:63–73. https://doi.org/10.1016/j.ydbio.2017.05.017
Kobayashi T, Kajiura-Kobayashi H, Nagahama Y (2000) Differential expression of vasa homologue gene in the germ cells during oogenesis and spermatogenesis in a teleost fish, tilapia, Oreochromis niloticus Mech Dev 99:139–142. https://doi.org/10.1016/S0925-4773(00)00464-0
Kossack ME, Draper BW (2019) Genetic regulation of sex determination and maintenance in zebrafish (Danio rerio). In: Capel B (ed) Sex Determination in Vertebrates, pp 119–149
Lee KH, Yamaguchi A, Rashid H et al (2009) Germ cell degeneration in high-temperature treated pufferfish, Takifugu rubripes Sex Dev 3:225–232. https://doi.org/10.1159/000228723
Liu X, Zhu Y, Zhao Y et al (2021) Vasa expression is associated with sex differentiation in the asian yellow pond turtle, Mauremys mutica J Exp Zool B Mol Dev Evol 336:431–442. https://doi.org/10.1002/jez.b.23064
Liu T, Li R, Liu L et al (2022) The Effect of temperature on gonadal sex differentiation of Yesso Scallop Patinopecten yessoensis Front Cell Dev Biol 9:3850. https://doi.org/10.3389/fcell.2021.803046
Lobo IKC, do Nascimento ÁR, Yamagishi MEB et al (2020) Transcriptome of tambaqui Colossoma macropomum during gonad differentiation: different molecular signals leading to sex identity. Genomics 112:2478–2488. https://doi.org/10.1016/j.ygeno.2020.01.022
Martínez P, Viñas AM, Sánchez L et al (2014) Genetic architecture of sex determination in fish: applications to sex ratio control in aquaculture. Front Genet 5:340. https://doi.org/10.3389/fgene.2014.00340
Miyoshi K, Hattori RS, Strüssmann CA et al (2020) Phenotypic/genotypic sex mismatches and temperature-dependent sex determination in a wild population of an Old World atherinid, the cobaltcap silverside Hypoatherina tsurugae Mol Ecol 29:2349–2358. https://doi.org/10.1111/mec.15490
Nagahama Y, Chakraborty T, Paul-Prasanth B et al (2021) Sex determination, gonadal sex differentiation, and plasticity in vertebrate species. Physiol Rev 101:1237–1308. https://doi.org/10.1152/physrev.00044.2019
Nakayama CM, Feldberg E, Antonio L, Bertollo C (2012) Karyotype differentiation and cytotaxonomic considerations in species of Serrasalmidae (Characiformes) from the Amazon basin. Neotropical Ichthyol 10:53–58. https://doi.org/10.1590/S1679-62252012000100005
Nascimento AR, Silva GF, Gualberto GF, Almeida FL (2016) Validation of reference genes for real-time quantitative PCR in tambaqui (Colossoma macropomum). Genet Mol Res 15(4):gmr15049228. https://doi.org/10.4238/gmr15049228
Ospina-Álvarez N, Piferrer F (2008) Temperature-dependent sex determination in fish revisited: Prevalence, a single sex ratio response pattern, and possible effects of climate change. PLoS ONE 3(7):e2837. https://doi.org/10.1371/journal.pone.0002837
Paixão RV, Silva GF, Caetano AR et al (2022) Phylogenomic and expression analysis of Colossoma macropomum cyp19a1a and cyp19a1b and their non-classical role in tambaqui sex differentiation. Gene 843:146795. https://doi.org/10.1016/j.gene.2022.146795
Pandit NP, Bhandari RK, Kobayashi Y, Nakamura M (2015) High temperature-induced sterility in the female Nile tilapia, Oreochromis niloticus Gen Comp Endocrinol 213:110–117. https://doi.org/10.1016/j.ygcen.2015.01.028
Perner B, Englert C, Bollig F (2007) The Wilms tumor genes wt1a and wt1b control different steps during formation of the zebrafish pronephros. Dev Biol 309:87–96. https://doi.org/10.1016/j.ydbio.2007.06.022
Qu L, Wu X, Liu M et al (2020) Identification and characterization of germ cell genes vasa and dazl in a protogynous hermaphrodite fish, orange-spotted grouper (Epinephelus coioides). Gene Expr Patterns 35:119095. https://doi.org/10.1016/j.gep.2020.119095
Quinn AE, Georges A, Sarre SD et al (2007) Temperature sex reversal implies sex gene dosage in a reptile. Science 316(5823):411–411. https://doi.org/10.1126/science.1135925
Ren Y, Mu Y, Zhao B et al (2023) dmrt3, nom1, abce1, and pkmyt1 play key roles in gonadal sex determination in Acrossocheilus fasciatus. Aquaculture International 31:317–332. https://doi.org/10.1007/s10499-022-00976-7
Sarre SD, Georges A, Quinn A (2004) The ends of a continuum: genetic and temperature-dependent sex determination in reptiles. BioEssays 26:639–645. https://doi.org/10.1002/bies.20050
Shao C, Li Q, Chen S et al (2014) Epigenetic modification and inheritance in sexual reversal of fish. Genome Res 24:604–615. https://doi.org/10.1101/gr.162172.113
Shen ZG, Wang HP (2014) Molecular players involved in temperature-dependent sex determination and sex differentiation in Teleost fish. Genet Selection Evol 46(1):26. https://doi.org/10.1186/1297-9686-46-26
Shen Z-G, Wang H-P (2018) Environmental sex determination and sex differentiation in Teleosts - How sex is established. In: Sex control in aquaculture, pp 85–115
Shen ZG, Eissa N, Yao H et al (2018) Effects of temperature on the expression of two ovarian differentiation-related genes foxl2 and cyp19a1a. Front Physiol 9:1208. https://doi.org/10.3389/fphys.2018.01208
Shinomiya A, Tanaka M, Kobayashi T et al (2000) The vasa-like gene, olvas, identifies the migration path of primordial germ cells during embryonic body formation stage in the medaka, Oryzias latipes Dev Growth Differ 42:317–326. https://doi.org/10.1046/j.1440-169X.2000.00521.x
St. Louis TJ, Pedroza Filho MX, Flores RMV (2022) Consumption frequencies, determinants, and habits of aquaculture species in Brazil. Aquacult Int 30:919–936. https://doi.org/10.1007/s10499-022-00838-2
Tanaka SS, Toyooka Y, Akasu R et al (2000) The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev 14:841–853. https://doi.org/10.1101/gad.14.7.841
Taranger GL, Carrillo M, Schulz RW et al (2010) Control of puberty in farmed fish. Gen Comp Endocrinol 165:483–515. https://doi.org/10.1016/j.ygcen.2009.05.004
Valdivieso A, Wilson CA, Amores A et al (2022) Environmentally-induced sex reversal in fish with chromosomal vs. polygenic sex determination. Environ Res 213:113549. https://doi.org/10.1016/j.envres.2022.113549
Valenzuela N (2008) Sexual development and the evolution of sex determination. Sex Dev 2:64–72. https://doi.org/10.1159/000129691
Valenzuela N, Adams DC, Janzen FJ (2003) Pattern does not equal process: exactly when is sex environmentally determined? Am Nat 161:676–683. https://doi.org/10.1086/368292
Varela ES, Bekaert M, Ganeco-Kirschnik LN et al (2021) A high-density linkage map and sex-linked markers for the Amazon Tambaqui Colossoma macropomum BMC Genomics 22(1):1–10. https://doi.org/10.1186/s12864-021-08037-8
Vasconcelos ACN, Streit DP, Octavera A et al (2019) Isolation and characterization of a germ cell marker in teleost fish Colossoma macropomum Gene 683:54–60. https://doi.org/10.1016/j.gene.2018.10.027
Villacorta-Correa MA, Saint-Paul U (1999) Structural indexes and sexual maturity of tambaqui Colossoma macropomum (Cuvier, 1818) (Characiformes: Characidae) in central Amazon, Brazil. Rev Bras Biol 59:637–652. https://doi.org/10.1590/S0034-71081999000400013
Xiao J, Cao K, Zou Y et al (2019) Sex-biased gene discovery from the gonadal transcriptomes of the large yellow croaker (Larimichthys crocea). Aquac Fish 4:9–16. https://doi.org/10.1016/j.aaf.2019.01.001
Xu H, Gui J, Hong Y (2005) Differential expression of vasa RNA and protein during spermatogenesis and oogenesis in the gibel carp (Carassius auratus gibelio), a bisexually and gynogenetically reproducing vertebrate. Developmental dynamics: an official publication of the American Association of Anatomists 233(3):872–882. https://doi.org/10.1002/dvdy.20410
Yamaguchi T, Kitano T (2012) High temperature induces cyp26b1 mRNA expression and delays meiotic initiation of germ cells by increasing cortisol levels during gonadal sex differentiation in japanese flounder. Biochem Biophys Res Commun 419:287–292. https://doi.org/10.1016/j.bbrc.2012.02.012
Yamaguchi T, Yoshinaga N, Yazawa T et al (2010) Cortisol is involved in temperature-dependent sex determination in the japanese flounder. Endocrinology 151:3900–3908. https://doi.org/10.1210/en.2010-0228
Yang X, Chen D, Zheng S et al (2023) The Prmt5-Vasa module is essential for spermatogenesis in Bombyx mori PLoS Genet 19:e1010600. https://doi.org/10.1371/journal.pgen.1010600
Zhang X, Li M, Ma H et al (2017) Mutation of foxl2 or cyp19a1a results in female to male sex reversal in XX nile tilapia. Endocrinology 158:2634–2647. https://doi.org/10.1210/en.2017-00127
Zhang X, Wu W, Zhou J et al (2022) MiR-34b/c play a role in early sex differentiation of Amur sturgeon, Acipenser schrenckii. Front Zool 19(1):23. https://doi.org/10.1186/s12983-022-00469-6
Funding
This work was financially supported by the Brazilian Agricultural Research Corporation (Embrapa) (SEG 12.16.05.018.00.01.00), INCT ADAPTA (465540/2014-7) / FAPEAM (062.1187/2017) / CAPES (finance code 001) and supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) with a scholarship for Vanessa Ribeiro Reis.
Author information
Authors and Affiliations
Contributions
Vanessa Ribeiro Reis performed the investigation, analysed, and interpreted the data, and wrote the article. Rômulo Veiga Paixão analysed the data and wrote the article. Iraní da Silva de Morais and Izabel Correa Bandeira were responsible for data collection and technical support in the lab. Adalberto Luís Val provided resources, infrastructure, and technical support to carry out the experiment, and review and comment the manuscript. Gilvan Ferreira da Silva conceived and supervised the study, was responsible for conceptual advice, review and editing of the article and Fernanda Loureiro de Almeida O’Sullivan was responsible for conceptualization, supervision, funding acquisition, project administration, resources, data curation, writing - review and editing.
Corresponding author
Ethics declarations
Ethics approval
All procedures of this work comply with the ethical of animal experimentation, adopted by the Brazilian College of Animal Experimentation (COBEA), and were approved by the Local Ethics Committee on Animal Use (CEUA) – of the Brazilian Institute for Research of the Amazon – INPA (n◦ 014/2017). The project has an Authorization of Access to Genetic Heritage under register A5784B5.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Pierre Boudry.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Reis, V.R., Paixão, R.V., de Morais, I.d. et al. Effect of temperature on the early sexual development of tambaqui Colossoma macropomum. Aquacult Int 32, 1719–1733 (2024). https://doi.org/10.1007/s10499-023-01238-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01238-w