Abstract
Heterotrophic nitrification and aerobic denitrification (HN-AD) bacteria are essential for aquaculture wastewater treatment to ensure product quality and environmental sustainability. Klebsiella aerogenes strain B23 was isolated from shrimp farm wastewater and identified based on phenotypic and phylogenetic characteristics. The strain demonstrated high removal efficiency and the maximum removal rates for ammonia (96.48%; 6.18 mg/L/h), nitrate (93.48%; 4.45 mg/L/h), and nitrite (90.92%; 2.38 mg/L/h). In wastewater from a shrimp farm, B23 reached a nitrate removal efficiency of 92.18% after 36 h. The expression of denitrifying genes (norV, norW, narJ, and narR) confirmed the HN-AD ability of strain B23. Single-factor experiments revealed that the optimal conditions for nitrate removal were a nitrate concentration of 5 mg/L, sucrose as a carbon source, temperature of 30 °C, pH of 7.5, rotation rate of 120 rpm, and salinity of 5‰. These findings demonstrate that strain B23 is a candidate for nitrogen removal in aquaculture wastewater treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Excess nitrogen is a significant consideration in aquaculture, especially within a limited water-exchanging system. In a small ecosystem, like a shrimp farm, nitrogen loading in wastewater is closely correlated with nitrogen input, including feed, fertilizer, and nitrogenous waste, such as animal carcasses, excrement, and unconsumed feed (Qiao et al. 2020; Zhang et al. 2022a). Under such circumstances, eutrophication can occur due to the discharge of untreated or inadequately treated nitrogenous wastewater into natural water sources, which leads to the deterioration of water quality, a lack of oxygen, and toxicity to aquatic organisms, which, in turn, threatens human food safety, particularly in the chain of production where contaminated aquatic organisms may be consumed by humans (Wang and Chu 2016; Qiang et al. 2020). Controlling and eliminating nitrogen pollution is conducive to healthy water systems and aquatic creatures (Thitanuwat and Wongsoonthornchai 2021; Xu et al. 2021).
Nitrogen biodegradation is a preferred method of wastewater management because it is economical and environmentally friendly (i.e., it does not produce secondary pollution) (Zhang et al. 2022a). Heterotrophic nitrification-aerobic denitrification (HN-AD) bacteria are being developed for aquaculture treatment due to their high removal rate, low space requirements, and cost-effective nitrogen removal in a single reactor. Traditional nitrogen-removing bacteria, such as anammox bacteria, aerobic denitrification bacteria, and short-cut nitrification-denitrification bacteria, typically require separate spaces and different oxygen conditions during the nitrification and denitrification processes (Lei et al. 2019; Chen et al. 2019; Yang et al. 2019b). Since the first HN-AD bacterial species, Paracoccus pantotrophus, was discovered by Robertson and Kuenen (1990), diverse HN-AD species from varying habitats were subsequently identified. These include Pseudomonas stutzeri TR2 and K50 (Takaya et al. 2003), Alcaligenes faecalis No. 4 (Joo et al. 2005), Bacillus methylotrophicus L7 (Zhang et al. 2012), Klebsiella pneumoniae CF-S9 (Padhi et al. 2013), Enterobacter cloacae HNR (Guo et al. 2016), Serratia marcescens W5 (Wang et al. 2016), Arthrobacter arilaitensis Y-10 (He et al. 2017), Halomonas alkaliphile HRL-9 (Ren et al. 2019), Sporidiobolus pararoseus Y1 (Zeng et al. 2020a), Barnettozyma californica K1 (Fang et al. 2021), and Pseudomonas sp. Y-5 (Zhang et al. 2022b). Many of these bacterial strains efficiently remove nitrogen and exhibit fast growth rates under sterile laboratory conditions or non-sterile open water systems.
In this study, we identified a HN-AD bacterium, Klebsiella aerogenes strain B23, that was isolated from the wastewater of a shrimp farm. The species was identified as K. aerogenes based on phenotypic and phylogenetic characteristics. Previous studies have shown that K. aerogenes is capable of biodegrading various substances, such as malachite green (Shang et al. 2019), spent engine oil (Ismail et al. 2014), soapy detergents (Amengialue et al. 2013), and crude oil (Akpe et al. 2013). However, few studies have evaluated the HN-AD ability of K. aerogenes. To our knowledge, this is the first report of the nitrogen removal characteristics of this species. We first evaluated the nitrogen and organic matter removal performance of K. aerogenes B23 using ammonium, nitrate, and nitrite as the sole nitrogen source both individually and in pairwise combinations. K. aerogenes B23 was also tested in samples of aquaculture wastewater to determine its practical nitrogen degradation ability. In addition, real-time quantitative PCR (qRT-PCR) was carried out to measure the expression of denitrifying genes under different nitration concentrations. Finally, we investigated the impact of the nitrate concentration, carbon sources, temperature, pH, rotation rate, and salinity on the nitrogen-removing capacity of K. aerogenes B23.
Material and methods
Medium
The heterotrophic nitrification medium (HNM) and denitrification media, DM-1 and DM-2, were composed of 5 g sucrose, 1 g of K2HPO4, 1 g of KH2PO4, 5 g of NaCl, and 2 mL of trace elements in 1 L of distilled water with either 0.38 g of NH4Cl, 0.72 g of KNO3, or 0.25 g of NaNO2 as the sole nitrogen source, respectively. The simultaneous nitrification and denitrification media (SND-1 and SND-2) contained 5 g of sucrose, 0.19 g of NH4Cl, 1 g of K2HPO4, 1 g of KH2PO4, 5 g of NaCl, 2 mL of trace elements, and 0.36 g of KNO3 or 0.25 g of NaNO2 in 1 L of distilled water. The components of the bromothymol blue (BTB) solid medium were the same as those in DM-1 with the addition of BTB 1 mL/L and agar powder 20 g/L. Luria–Bertani (LB) medium contained the following: 10 g of peptone, 5 g of yeast extract, and 5 g of NaCl in 1 L of distilled water. 1 × phosphate-buffered saline (PBS) was purchased from Biosharp Biotech Company (Beijing, China). Culture media were autoclaved at 121 °C for 20 min before use.
Isolation, screening, and identification
Ten liters of wastewater was collected from the comprehensive sewage outlet of Zhanjiang Yuehai Aquatic Seeding Company Limited, Donghai Island, Zhanjiang, China. The wastewater was inoculated with 10% (v/v) inoculum in a 250-mL triangular flask containing 200 mL of HNM and cultured on a rotary shaker at 120 rpm for 48 h at 30 °C to enrich the heterotrophic nitrifying bacteria. After enrichment, the sample was serially diluted onto BTB plates for separation and purification. During denitrification, nitrate was depleted by denitrifying bacteria, causing the pH value of the medium to increase and the color of the BTB indicator to change from yellow-green to blue. Colonies with bigger blue halos and obvious morphological differences were selected with an inoculating loop and streaked on a fresh DM agar plate for purification. After repeating the streaking procedure 4–5 times, some depurated colonies were harvested and their nitrogen removal capabilities were tested by measuring nitrate removal efficiency in DM-1 medium after 48 h. Finally, the strain with the greatest nitrogen removal efficiency was selected as the experimental strain and named as B23 for subsequent studies. Strain B23 was stored in 25% (v/v) sterile glycerol at −80 °C.
The 16S rRNA sequence of K. aerogenes B23 was determined using sequencing polymerase chain reaction (PCR). The genomic DNA of the isolate was extracted and used as a template for amplification. 16S rRNA genes were amplified using universal primers for bacteria (forward primer 27F: 5′-AGAGTTTGATCMTGGCTCAG-3′; reverse primer 1492R: 5′-GGTTACCTTGTTACGACTT-3′), which were designed by Sangon Biocompany (Shanghai, China). The PCR system (50 μL) was as follows: 25 μL of 2× EasyTaq® PCR SuperMix, 1 μL of template DNA, 1 μL of primers 27F, 1 μL of primers 1492R, and 22 μL of ddH2O. PCR was carried out as follows: pre-denaturation at 94 °C for 5 min, 35 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 1 min, with a final extension at 72 °C for 10 min. The PCR product was sent to Sangon Biocompany for sequencing.
For the sequence analysis and homology searches based on the 16S rDNA gene sequences, the BLAST program of NCBI was used. A phylogenetic tree was generated using MEGA7 with 1000 bootstrap replicates and the maximum composite likelihood method.
Assessment of nitrogen removal ability using single and mixed nitrogen sources
To test the HN-AD ability of K. aerogenes B23, shake flask experiments were conducted. The preserved, purified strains were first activated and inoculated into 100 mL of LB medium. After reaching the logarithmic growth phase (OD600 = 1.0), the bacterial specimens were washed with PBS three times and resuspended, before being inoculated into HNM, DM-1, DM-2, SND-1, and SND-2 with 5% (v/v) each. The flasks were sealed with breathable sealing films and shaken at 30 °C for 36 h. Samples were collected at 0, 2, 6, 12, 24, and 36 h and centrifuged at 10,000 rpm for 10 min. The supernatant was then tested for the removal capacity of ammonium, nitrate, nitrite, total nitrogen (TN), orthophosphate, and chemical oxygen demand (COD) during nitrification and denitrification. The non-inoculated medium was used as the control. All assays were performed in triplicate.
Aquaculture wastewater experiment
Ten liters of wastewater was also collected from the same shrimp farm (see the “Isolation, screening, and identification” section). K. aerogenes B23 was identified and chosen for the investigation of HN-AD ability in aquaculture wastewater. The initial concentrations of ammonium, nitrate, nitrite, TN, orthophosphate, and COD were 0.01, 68.75, 0.36, 116.87, 0.37, and 1357 mg/L, respectively. Following activation, the strain was inoculated into 100 mL of LB medium and cultured until it reached the logarithmic growth phase (OD600 = 1.0). The bacterial suspension was washed with sterile PBS three times and was resuspended. This bacterial suspension was inoculated into aquaculture wastewater at 5% (v/v), which was then sealed with a breathable sealing film. The culture conditions, sample collection, and measurement methods were as described in the “Assessment of nitrogen removal ability using single and mixed nitrogen sources” section. Wastewater with 5% (v/v) sterile PBS was used as the control. All assays were performed in triplicate.
Denitrifying gene expression analysis
Following incubation in DM-1 for 12 h (OD600 = 0.6), the total RNA of K. aerogenes B23 was isolated using a RaPure™ Bacteria RNA Isolation Kit (Yubo Biotech, Shanghai, China). Then, the purified total RNA was reverse-transcribed to cDNA using a First Strand cDNA Synthesis Kit (Yubo Biotech, Shanghai, China). Primers used for RT-qPCR were designed by Primer Premier 6.0 (Table 1) according to the genomic sequence of K. aerogenes B23. RT-qPCR was carried out on a CFX Connect Real-Time System (Bio-Rad, Hercules, CA, USA) with SYBR Premix Ex TaqTM GC (TaKaRa, Osaka, Japan). The reactions were performed under the following conditions: pre-denaturation at 95 °C for 30 s, 40 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 30 s, and extension at 72 °C for 15 s. The 16S rRNA gene was used as an internal control. Relative quantification was carried out using the 2−△△Ct method. K. aerogenes B23 cultured in DM-1 without nitrate served as the control. Analyses were performed in triplicate for each sample.
Impact of single-factor variables on nitrogen removal performance
All single-factor experiments were carried out aerobically at 30 °C in DM-1. For the nitrate concentration experiment, concentrations of 0, 5, 50, 500, and 1000 mg/L were used. In the carbon source experiment, glucose, sucrose, sodium citrate, sodium acetate, and disodium succinate were assessed. To assess the role of temperature, experiments were performed at 20 °C, 25 °C, 30 °C, 37 °C, and 40 °C at pH 7. In the pH experiment, the initial pH was adjusted to 6.0, 6.5, 7.0, 7.5, and 8.0 with rotation at 120 rpm. In the rotation rate experiment, the rotation rates were set at 10, 40, 80, 120, and 160 rpm at pH 7. In the salinity experiment, the salinity was set at 1, 5, 15, 30, and 40‰ (w/v), at a rotation rate of 120 rpm, pH 7, and 30 °C. The flasks were continuously cultured for 36 h. The OD600 values and nitrate concentration were measured periodically. All assays were performed in triplicate.
Analytical methods
The concentrations of ammonium, nitrate, nitrite, TN, orthophosphate, COD, and dissolved oxygen (DO) were separately determined using standard methods (State Environmental Protection Administration of China 2002). Ammonium was measured by Nessler’s reagent spectrophotometry. Nitrate was analyzed by UV spectrophotometry. Nitrite was determined by the N-(1-naphthalene)-diaminoethane photometry method. TN was surveyed by UV spectrophotometry. Orthophosphate was measured by Mo-Sb anti-spectrophotometry. COD was determined by the potassium dichromate method. DO was surveyed by iodometry. The OD600 value was measured using an ultraviolet spectrophotometer. The nitrogen removal rates were calculated using the following equations: Re (%) = (C0 − C1)/C0 × 100, where Re represents the removal efficiency and C0 and C1 represent the initial and final nitrogen concentrations; Rr (mg/L/h) = (C2 − C3)/T1 − T2, where Rr represents the removal rate, C2 and C3 represent the nitrogen concentrations of T1 and T2, and T1 and T2 represent the time points. One-way analysis variance (ANOVA) and Student’s t-tests were used for comparisons (p < 0.05) using SPSS Statistics 21.0. Each experiment was performed in triplicate and the results are presented as means ± standard deviation of means. Values followed by different letters are significantly different at p < 0.05.
Results and discussion
Strain isolation and identification
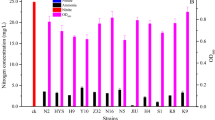
After four to five rounds of selection and purification on BTB and DM-1 plates, 16 purified strains were prepared for the initial screening. The secondary screening process assessed the nitrogen removal ability of the strains. K. aerogenes B23 showed the highest nitrogen removal efficiency (94.68%), as shown in Fig. 1. Therefore, K. aerogenes B23 was selected for subsequent experiments.
The colonies of K. aerogenes B23 on LB plates were characterized by a whitish color, shiny and translucent appearance, moist, and slightly convex with neat edges. The average colony diameter was 2.0–3.0 mm. The 16S rRNA gene of the strain was obtained, consisting of 1434 bp. By a search against the NCBI nucleic acid database, 16S rRNA of the strain had 99% similarity with 16S rRNA of K. aerogenes strain MH100730.1. A phylogenetic tree based on partial 16S rRNA sequences was constructed to classify K. aerogenes B23, as shown in Fig. 2. By combining physiological and biochemical characteristics, the strain was identified as K. aerogenes (accession number OQ913507.1 in GenBank). Previous studies have reported the HN-AD capacity of Klebsiella pneumoniae in the same genus (Padhi et al. 2013).
Nitrogen removal capacity with varying nitrogen sources
Ammonium, nitrate, and nitrite were used as the sole nitrogen source during the aerobic culture of K. aerogenes B23 to study its nitrogen removal characteristics. The initial concentrations of ammonium, nitrate, and nitrite were 100, 100, and 50 mg/L, respectively.
During the culture of K. aerogenes B23 with ammonium as the sole nitrogen source, the ammonium concentration displayed decreased with the increasing cell population. The concentration remained stable during the stationary phase and increased slightly towards the end of the experiment (Fig. 3a). After 36 h of incubation, the ammonium removal efficiency was 96.48% with an average removal rate of 2.71 mg/L/h, which was higher than those reported for Pseudomonas sp. ADN-42 (1.38 mg/L/h) (Jin et al. 2015), Pseudomonas putida Y-12 (2.14 mg/L/h) (Ye et al. 2017), and Chryseobacterium sp. R31 (1.11 mg/L/h) (Kundu et al. 2014). The high ammonium degradation during the logarithmic phase was due to both the biosynthetic processes associated with the proliferation of the microorganisms and nitrification to form nitrate, nitrite, and other nitrification by-products (Lang et al. 2020). The difference in the TN removal efficiency when the nitrogen source was either ammonium (91.05%) or nitrate (92.77%) was not significant. However, a minor reduction was observed in the TN with nitrite (83.28%) as the sole nitrogen source in comparison, which was similar to the results for Pseudomonas mendocina TJPU04, with intermediate product formation observed in the process of ammonium removal (He et al. 2019). COD exhibited a similar trend to that of ammonium. COD removal mainly occurred during the logarithmic phase of bacterial growth with a maximum removal rate of 83.75 mg/L/h at 2–6 h. This result showed that inorganic substances, as well as ammonium, dissolved in the medium were fully utilized in the nitrification process for the proliferation of bacteria. The excellent nitrogen and COD removal abilities exhibited by K. aerogenes B23 suggest that it is an outstanding heterotrophic nitrifier.
After 6 h, the growth rate of K. aerogenes B23 increased rapidly, despite the slightly slower initial growth observed when using ammonium as the sole nitrogen source, as depicted in Fig. 3b. During the logarithmic phase (6–24 h), K. aerogenes B23 showed an outstanding ability to remove nitrate (3.86 mg/L/h), which was highly correlated with the growth rate of the strain within 24 h. At 36 h, the majority of nitrate was removed and the removal efficiency was 93.48%. The average removal rate of nitrate was 2.58 mg/L/h, which was higher than the estimates for some denitrifying bacteria, such as K. pneumonia CF-S9 (2.2 mg/L/h) (Padhi et al. 2013) and Rhodococcus sp. CPZ24 (0.93 mg/L/h) (Chen et al. 2012). Nitrite concentrations remained low and fluctuated within 2–24 h, with a maximum concentration of 3.07 mg/L at 12 h. Nitrite was undetectable by 36 h. It has been reported that nitrite accumulates when ammonium or nitrate is used as the nitrogen source (Huang et al. 2013; Padhi et al. 2013; He et al. 2019). A similar study speculated that nitrite may act as an electron acceptor by taking the place of nitrate during the denitrification of nitrate (Sun et al. 2016). A different phenomenon was observed in an experiment involving Acinetobacter sp. ND7, where denitrification was carried out in medium with nitrate as the sole nitrogen source, with barely detectable levels of nitrite (Xia et al. 2020). By 36 h, K. aerogenes B23 reached the maximum OD600 value (OD600 = 1.55). The strain showed a relatively slower growth rate when the nitrogen source was nitrate than with ammonium, which may be due to the accumulation of nitrite during the denitrification process (Ruan et al. 2020). Ammonium levels were not measurable within 36 h.
In addition to ammonium and nitrate, it was observed that K. aerogenes B23 could also utilize and remove nitrite as the sole nitrogen source (Fig. 3c). The increase in OD600 values confirmed that nitrogen was transformed into biomass. However, the concentration of nitrite was about 50 mg/L, which is toxic and can inhibit respiration and cell proliferation in microbial populations (Van Hulle et al. 2007). Previous reports have also shown that high concentrations of nitrite can harm the ability of microorganisms to remove nitrogen, as seen in Paracoccus versutus LYM (Zhang et al. 2015) and Pseudomonas stutzeri D6 (Yang et al. 2012). During the first 6 h, K. aerogenes B23 exhibited slow growth with the OD600 value increasing from 0.06 to 0.21, which was slightly slower than its growth in sole ammonium or nitrate medium. However, K. aerogenes B23 then underwent rapid proliferation in the logarithmic growth phase, with an average growth rate of 0.063/h calculated using the OD600 value during 6–24 h, which was lower than the average growth rate of 0.069/h when ammonium was the sole nitrogen source. Towards the end of the experiment, the nitrite concentration decreased rapidly to 6.88 mg/L. The maximal removal rate of the strain was 2.38 mg/L/h at 6–12 h. The nitrite removal efficiency at 24 h was 81.09% and reached 90.92% at 36 h. In addition, nitrate accumulation was observed when nitrite became the sole nitrogen source, reaching its maximum concentration of 8.24 mg/L at 12 h followed by a decrease to 2.89 mg/L at 36 h. A similar study proposed that this trend may account for the oxidation of nitrite and denitrification of the strain (He et al. 2019). Compared with the orthophosphate removal efficiency in medium containing only ammonium (58.43%) or nitrate (55.31%), the removal efficiency was relatively lower (44.76%) in medium containing nitrite as the sole source, even though the concentration of nitrite decreased from 2.48 to 1.37 mg/L. The changes in COD revealed that carbon sources were utilized with lower efficiency compared with that observed in sole ammonium and nitrate experiments. This may be due to the fact that the best proliferation was achieved with ammonium (OD600 = 1.61) as the sole nitrogen source, followed by nitrate (OD600 = 1.55) and nitrite (OD600 = 1.41). Based on the removal performance of nitrate and nitrite, it can be concluded that K. aerogenes B23 is an efficient aerobic denitrifier that can broadly utilize inorganic nitrogen.
Nitrogen removal capacity of K. aerogenes B23 with mixed nitrogen sources
To study the simultaneous nitrification and denitrification capacity of K. aerogenes B23, mixed nitrogen sources consisting of ammonium and either nitrate or nitrite (an intermediate product of nitrification) were used. The results showed that, compared to media with a single nitrogen source, K. aerogenes B23 exhibited significantly better growth and nitrogen removal efficiency in the SND-1 medium (Fig. 4a), while its growth rate and nitrogen removal efficiency were lower in the SND-2 medium (Fig. 4b). This phenomenon may result from the toxicity of ammonium and nitrite, both of which served as nitrogen sources in the SND-2 medium. The TN removal efficiency of the strain reached 94.8% in SND-1 medium and 89% in SND-2 medium. In both mixed media, ammonium was utilized first and was not detected by the end of experiment. A similar study suggested that the preferential removal of ammonium and nitrite by bacterial strains could be beneficial for aquaculture, as high levels of ammonium and nitrite can be toxic to aquatic animals (Zhang et al. 2022a). Within 24 h, K. aerogenes B23 showed a higher nitrate removal efficiency of 87.45% with an average denitrification rate of 1.83 mg/L/h in the SND-1 medium than those for nitrite in SND-2 medium, with a removal efficiency of 62.99% and a denitrification rate of 1.30 mg/L/h. These results suggest that K. aerogenes B23 has a better denitrification performance with nitrate as the nitrogen source rather than nitrite. During the experiment, no more than 4.78 mg/L nitrate was detected in the SND-2 medium, suggesting that nitrate was converted from ammonium and nitrite. This is supported by our previous observations that nitrate is produced when ammonium or nitrite served as the sole nitrogen source. In contrast, a small amount of accumulated nitrite was detected in the SND-1 medium, with less than 3.16 mg/L nitrite measured within 48 h. This is a common phenomenon observed in previous studies where ammonium and nitrate were used as nitrogen sources (Zhao et al. 2010; Zou et al. 2014). At the end of the experiment, 19.03% of nitrite remained in the SND-2 medium, whereas in SND-1 and SND-2 media, the removal efficiencies of orthophosphate were 51.75% and 39.48%, respectively. Moreover, 61.67% and 47.92% removal efficiencies were calculated for COD. These results provide a theoretical basis for the application of HN-AD bacteria in aquaculture and wastewater treatment.
Nitrogen removal capacity of K. aerogenes B23 in shrimp farm aquaculture wastewater
The successful application of HN-AD bacteria, such as Bacillus amyloliquefaciens H4, in the removal of nitrite and ammonium in industrial aquaculture water management has been reported (Zhang et al. 2022a). To demonstrate the practical applicability of the newly isolated K. aerogenes B23, its nitrogen removal efficiency was evaluated in a non-sterile environment. The strain was inoculated into wastewater samples from a shrimp farm. In the control group, which consisted of sterile PBS, TN, ammonium, nitrate, nitrite, orthophosphate, and COD remained unchanged within the 36-h incubation period (shown in Fig. 5a), indicating that indigenous microorganisms did not contribute to nitrogen removal. However, nitrate, nitrite, and TN concentrations (shown in Fig. 5b) differed significantly between the wastewater supplemented K. aerogenes B23 and the control group. Nitrate utilization in wastewater resembled that in the sole nitrate experiment discussed above, despite the uncontrolled components of the wastewater. The concentration of nitrate was reduced from 68.75 to 5.37 mg/L with a removal efficiency reaching 92.18% at 36 h. The results demonstrated that the nitrate removal performance of K. aerogenes B23 in non-sterile wastewater was equivalent to that in a sterile medium. The application of K. aerogenes B23 in wastewater also efficiently decreased the concentration of nitrite from 0.36 to 0.15 mg/L and TN from 116.87 to 43.22 mg/L. A similar study by He et al. (2019) found that nitrate and nitrite removal efficiencies under non-sterile conditions were significantly higher than those under sterile conditions. Since the initial concentration of ammonium was no more than 0.02 mg/L, K. aerogenes B23 did not demonstrate the ability to remove ammonium but maintained its concentration at a low level with slight fluctuations. On the other hand, the concentration of orthophosphate decreased significantly from 0.36 to 0.05 mg/L after 36 h. The removal efficiency of COD (47.01%) in wastewater was as good as that in the sterile conditions in the laboratory, indicating that the application of K. aerogenes B23 in wastewater has the potential to become a practical solution, although it is still limited to laboratory settings at this time.
Effect of nitrate concentration on the expression of denitrifying genes
qRT-PCR was performed to measure changes in the expression of denitrifying genes in K. aerogenes B23 in different nitrate concentrations (Fig. 6), using the same strain cultured in a control medium without nitrate as a control. The levels denitrifying genes differed to varying degrees between DM-1 and the control. The norV and norW genes are responsible for coding nitrite oxidoreductase (NOR) (which plays a key role in the oxidation of nitrite to nitrate) (Hutchings et al. 2002); their expressions were significantly upregulated (p < 0.01) after exposure to 100 mg/L nitrate. In addition, a slight up-regulation in gene expression of narJ (p > 0.05) was detected in the treatment group. The narJ gene encodes nitrate reductase (NAR), which is essential for the reduction of nitrate to nitrite. NarJ is a specific chaperone protein required for the assembly of the membrane nitrate reductase complex. After assembly of the NarGH complex on the membrane, the NarJ protein remains in the cytoplasm (Liu and Demoss 1997). NirB nitrite reductase reduces nitrite in the cytoplasm using NADH as an electron donor (Wang et al. 2000). In this study, nirB gene expression was slightly lower in the treatment group than in the control group (p > 0.05). The observed changes in the expression of denitrifying genes in K. aerogenes B23 provide strong evidence that the strain is capable of both heterotrophic nitrification and aerobic denitrification.
Factors affecting the nitrate removal capacity: single-factor experiments
The growth and nitrate utilization of bacterial strains are significantly affected by various factors, such as the nitrate concentration, carbon sources, temperature, pH, rotation rate, and salinity. A series of single-factor experiments was conducted to investigate the nitrogen removal ability of K. aerogenes B23 under different culture conditions (Table 2).
Effect of nitrate concentration
In aquaculture wastewater management, it is important for HN-AD bacteria to adapt to the various nitrogen concentrations in their environment and reduce nitrogen efficiently in the event of nitrogen pollution. Therefore, a bacterial strain with superior nitrogen-removing abilities able to survive in harsh environments would be of significant value (Zhang et al. 2012). In this experiment, isolated K. aerogenes B23 was cultured in media where nitrate at various concentrations served as the sole nitrogen source. As shown in Table 2, K. aerogenes B23 had the greatest growth performance (OD600 = 1.613) at a nitrate concentration of 50 mg/L, with a nitrogen removal efficiency of 96.79%. The same strain grown in media containing 5 mg/L nitrate exhibited poorer growth (OD600 = 1.126); however, the nitrogen removal efficiency was as high as 99.35%. It is possible that the less concentrated medium lacked sufficient nitrogen to support maximal bacterial growth. Similar results have been reported for Pseudomonas mendocina TJPU04 (He et al. 2019) cultured with ammonium as the nitrogen source. The growth and nitrate removal efficiency of K. aerogenes B23 could not be observed and tested without nitrate in the medium (0 mg/L).
When the nitrate concentration was increased to 500 mg/L and 1000 mg/L, K. aerogenes B23 did not achieve higher nitrogen removal efficiencies than that at 50 mg/L; however, removal efficiencies were still 83.44% and 64.39%, respectively. These results showed that K. aerogenes B23 could conduct aerobic nitrification at high nitrate concentrations.
Effect of the carbon source
As carbon is a major energy provider and electron donor, carbon sources play important roles not only in the growth and proliferation of microorganisms but also in denitrification during the logarithmic phase of growth (Lang et al. 2019; Su et al. 2020). Bacteria have the inherent ability to use diverse carbon sources, which is essential for their survival in complex heterogeneous and dynamic environments. Nitrate was used as the sole nitrogen source and electron acceptor in this experiment. The utilization efficiency of nitrate by K. aerogenes B23 differed depending on the carbon source (Table 2). To investigate the effect of different carbon sources, glucose, sucrose, sodium citrate, sodium acetate, and disodium succinate were used individually as the sole carbon source for the growth assays. The use of sucrose elicited the highest OD600 value (OD600 = 1.548) and nitrogen removal efficiency (95.18%), followed by glucose and sodium citrate. Poor growth characteristics were observed when sodium acetate and disodium succinate were used as the sole carbon source. This differed from results of similar experiments with other HN-AD strains; the optimal carbon source for nitrogen removal by Bacillus amyloliquefaciens H4 (Zhang et al. 2022a) and Alcaligenes faecalis WT14 (Chen et al. 2021) was sodium citrate and that for Acinetobacter indicus ZJB20129 (Ke et al. 2022) was succinate. Our results strongly suggest that K. aerogenes B23 preferentially uses sugars (glucose and sucrose) rather than organic acid salts (sodium citrate, sodium acetate, and disodium succinate) as a carbon source. Thus, sucrose was employed as the carbon source for K. aerogenes B23 for subsequent experiments.
Effect of temperature
As exhibited in Table 2, the growth and nitrate removal efficiency of K. aerogenes B23 was temperature-dependent. Optimal denitrification activity was observed at temperatures ranging from 30 to 35 °C, and the highest removal rate of nitrate was 96.72% at 30 °C. It has been suggested that certain enzymes associated with nitrification and denitrification are triggered by elevated temperatures (Yang et al. 2019a). However, a slightly lower nitrate removal efficiency (95.37%) was detected at 35 °C than at 30 °C. The strain productivity was greatly inhibited by high temperatures; the nitrate removal efficiency was only 64.26% when cultured at 40 °C. Overall, the optimal culture temperature for K. aerogenes B23 was 30 °C. These data indicate that K. aerogenes B23 is capable of adapting to a high-temperature environment (30–40 °C), typical of aquaculture wastewater treatment systems.
Effect of pH
Table 2 demonstrates that the initial pH value had a significant effect on the growth and nitrogen removal efficiencies of K. aerogenes B23. No significant growth or nitrate removal was observed when the strain was cultured at an initial pH of 6, suggesting that acidic environments can limit bacterial growth and metabolism as well as their ability to remove nitrogen (Zeng et al. 2020b; Ke et al. 2022). Neutral and weakly alkaline environments were more conducive for K. aerogenes B23 growth and nitrate removal. K. aerogenes B23 maintained a nitrate removal efficiency of greater than 90% when the culture pH range was 7–8, consistent with previous findings showing that some HN-AD bacteria could produce alkali substances during the denitrification process, which can alter the pH environment (Zhu et al. 2012; Ma et al. 2022). Therefore, the outstanding nitrogen removal performance of K. aerogenes B23 in neutral and weak alkaline environments would contribute to its practical use in aquaculture wastewater treatment (Duan et al. 2019).
Effect of the rotation rate
Sufficient DO in the surrounding environment is vital for nitrogen transformation. Rotating the culture flask can facilitate the transfer of oxygen from the atmosphere to the liquid and increase DO levels (Liu et al. 2021). We found that the nitrate removal efficiency and growth were closely related to the rotation speed. As shown in Table 2, the OD600 value and the nitrate removal efficiency increased from 0.982 to 1.654 and 80.61% to 97.04%, respectively, with a rise in the rotation rate from 10 to 120 rpm (DO value increased from 0.47 to 5.13 mg/L). However, further increasing the rotation rate to 160 (DO value = 6.24 mg/L) resulted in an evident decline in the nitrate removal efficiency, indicating that an excessively high rotation rate could have a negative impact on nitrate removal by K. aerogenes B23. An analogous phenomenon was observed in Acinetobacter sp. ND7, where the nitrate removal efficiency was over 90% when the rotation speed was 40–120 rpm and then decreased sharply to 60.31% as the rotation rate was increased to 150 rpm (Xia et al. 2020). The decrease observed in the OD600 value and the nitrogen removal efficiency at the rotation rate over 150 rpm was also observed in another similar study, Ma et al. (2022) assumed that violent shaking could harm bacterial growth and the nitrogen removal ability of Rhodococcus erythropolis Y10. This may be because bacteria may enter the decline phase early because of the fast consumption of carbon sources at a high rotational speed. In this study, a rotation rate of 120 rpm was optimal for K. aerogenes B23 to degrade nitrate and was chosen for subsequent experiments.
Effect of salinity
The salinity level of aquaculture wastewater can vary widely depending on the cultivated environment, such as seawater, freshwater, and brackish water. Thus, it is vital for bacterial strains to survive under different salinity levels for their widespread application in nitrogen biodegradation. The results shown in Table 2 demonstrate that K. aerogenes B23 exhibited high tolerance to a salinity range of 1 to 40‰ (w/v). The strain grew well in a salinity range of 1 to 30‰, with high OD600 values that were no less than 1.487. Moreover, K. aerogenes B23 achieved high nitrate removal efficiencies of 95.88%, 93.64%, and 92.45% within 36 h when the salinity was 5 ‰, 15‰, and 30‰, respectively. These results suggest the remarkable adaptability of K. aerogenes B23 for efficient aerobic denitrification in a wide range of salinity environments. However, a decrease in the nitrogen removal efficiency was observed when the salinity reached 40‰; it is possible that the high salinity caused cell plasmolysis and decreased microbial activity, as reported in previous studies (Duan et al. 2015). Other salt-resistant bacteria, such as Bacillus strain N31 (Huang et al. 2017) and Marinobacter sp. F6 (Zheng et al. 2012), exhibit efficient nitrogen degradation, highlighting the potential of K. aerogenes B23 to survive and adapt in a wide salinity range (1–40‰) for practical applications.
Conclusion
In this study, we isolated and identified K. aerogenes B23 as a highly efficient HN-AD bacterial strain. Our findings indicate that K. aerogenes B23 is capable of removing inorganic nitrogen, orthophosphate, and COD and performs as well in aquaculture wastewater samples as in the laboratory medium. Moreover, K. aerogenes B23 demonstrated exceptional nitrogen removal ability across a wide range of nitrate concentrations, temperatures, and salinity levels. These results highlight the practical value of K. aerogenes B23 in wastewater treatment and water quality remediation, making it a promising candidate for further exploration.
References
Akpe AR, Ekundayo AO, Esumeh FI (2013) Degradation of crude oil by bacteria: a role for plasmid-borne genes. Glob J Sci Front Res 13:20–26
Amengialue OO, Ibeh IN, Egharevba AP et al (2013) Studies on the bacterial flora of synthetic-detergent effluent and their biodegradation potentials. Glob J Biol Agric Health Sci 2:14–19
Chen J, Xu J, Zhang S et al (2021) Nitrogen removal characteristics of a novel heterotrophic nitrification and aerobic denitrification bacteria, Alcaligenes faecalis strain WT14. J Environ Manag 282:111961. https://doi.org/10.1016/j.jenvman.2021.111961
Chen P, Li J, Li QX et al (2012) Simultaneous heterotrophic nitrification and aerobic denitrification by bacterium Rhodococcus sp. CPZ24. Bioresour Technol 116:266–270. https://doi.org/10.1016/j.biortech.2012.02.050
Chen S, He S, Wu C, Du D (2019) Characteristics of heterotrophic nitrification and aerobic denitrification bacterium Acinetobacter sp. T1 and its application for pig farm wastewater treatment - ScienceDirect. J Biosci Bioeng 127:201–205. https://doi.org/10.1016/j.jbiosc.2018.07.025
Duan J, Fang H, Su B et al (2015) Characterization of a halophilic heterotrophic nitrification-aerobic denitrification bacterium and its application on treatment of saline wastewater. Bioresour Technol 179:421–428. https://doi.org/10.1016/j.biortech.2014.12.057
Duan Y, Wang Y, Liu Q et al (2019) Changes in the intestine barrier function of Litopenaeus vannamei in response to pH stress. Fish Shellfish Immunol 88:142–149. https://doi.org/10.1016/j.fsi.2019.02.047
Fang J, Liao S, Zhang S et al (2021) Characteristics of a novel heterotrophic nitrification-aerobic denitrification yeast, Barnettozyma californica K1. Bioresour Technol 339:125665. https://doi.org/10.1016/j.biortech.2021.125665
Guo LJ, Zhao B, An Q, Tian M (2016) Characteristics of a novel aerobic denitrifying bacterium, Enterobacter cloacae strain HNR. Appl Biochem Biotechnol 178:947–959. https://doi.org/10.1007/s12010-015-1920-8
He T, Xie D, Li Z et al (2017) Ammonium stimulates nitrate reduction during simultaneous nitrification and denitrification process by Arthrobacter arilaitensis Y-10. Bioresour Technol 239:66–73. https://doi.org/10.1016/j.biortech.2017.04.125
He X, Sun Q, Xu T et al (2019) Removal of nitrogen by heterotrophic nitrification–aerobic denitrification of a novel halotolerant bacterium Pseudomonas mendocina TJPU04. Bioprocess Biosyst Eng 42:853–866. https://doi.org/10.1007/s00449-019-02088-8
Huang F, Pan L, Lv N, Tang X (2017) Characterization of novel Bacillus strain N31 from mariculture water capable of halophilic heterotrophic nitrification–aerobic denitrification. J Biosci Bioeng 124:564–571. https://doi.org/10.1016/j.jbiosc.2017.06.008
Huang X, Li W, Zhang D, Qin W (2013) Ammonium removal by a novel oligotrophic Acinetobacter sp. Y16 capable of heterotrophic nitrification–aerobic denitrification at low temperature. Bioresour Technol 146:44–50. https://doi.org/10.1016/j.biortech.2013.07.046
Hutchings MI, Mandhana N, Spiro S (2002) The NorR protein of Escherichia coli activates expression of the flavorubredoxin gene norV in response to reactive nitrogen species. J Bacteriol 184:4640–4643. https://doi.org/10.1128/jb.184.16.4640-4643.2002
Ismail HY, Ijah U, Riskuwa ML, Allamin II (2014) Biodegradation of spent engine oil by bacteria isolated from the rhizosphere of legumes grown in contaminated soil. Int J Environ 3:63–75. https://doi.org/10.3126/ije.v3i2.10515
Jin R, Liu T, Liu G et al (2015) Simultaneous heterotrophic nitrification and aerobic denitrification by the marine origin bacterium Pseudomonas sp. ADN-42. Appl Biochem Biotechnol 175:2000–2011. https://doi.org/10.1007/s12010-014-1406-0
Joo HS, Mitsuyo H, Makoto S (2005) Characteristics of ammonium removal by heterotrophic nitrification-aerobic denitrification by Alcaligenes faecalis No. 4. J Biosci Bioeng 100:184–191. https://doi.org/10.1263/jbb.100.184
Ke X, Liu C, Tang SQ et al (2022) Characterization of Acinetobacter indicus ZJB20129 for heterotrophic nitrification and aerobic denitrification isolated from an urban sewage treatment plant. Bioresour Technol 347:126423. https://doi.org/10.1016/j.biortech.2021.126423
Kundu P, Pramanik A, Dasgupta A et al (2014) Simultaneous heterotrophic nitrification and aerobic denitrification by Chryseobacterium sp. R31 isolated from abattoir wastewater. BioMed Res Int 2014:436056. https://doi.org/10.1155/2014/436056
Lang X, Li Q, Ji M et al (2020) Isolation and niche characteristics in simultaneous nitrification and denitrification application of an aerobic denitrifier, Acinetobacter sp. YS2. Bioresour Technol 302:122799. https://doi.org/10.1016/j.biortech.2020.122799
Lang X, Li Q, Xu Y et al (2019) Aerobic denitrifiers with petroleum metabolizing ability isolated from caprolactam sewage treatment pool. Bioresour Technol 290:121719
Lei X, Jia Y, Chen Y, Hu Y (2019) Simultaneous nitrification and denitrification without nitrite accumulation by a novel isolated Ochrobactrum anthropic LJ81. Bioresour Technol 272:442–450. https://doi.org/10.1016/j.biortech.2018.10.060
Liu X, Demoss JA (1997) Characterization of NarJ, a system-specific chaperone required for nitrate reductase biogenesis in Escherichia coli. J Biol Chem 272:24266–24271. https://doi.org/10.1074/jbc.272.39.24266
Liu X, Hu S, Sun R et al (2021) Dissolved oxygen disturbs nitrate transformation by modifying microbial community, co-occurrence networks, and functional genes during aerobic-anoxic transition. Sci Total Environ 790:148245. https://doi.org/10.1016/j.scitotenv.2021.148245
Ma S, Huang S, Tian Y, Lu X (2022) Heterotrophic ammonium assimilation: an important driving force for aerobic denitrification of Rhodococcus erythropolis strain Y10. Chemosphere 291:132910. https://doi.org/10.1016/j.chemosphere.2021.132910
Padhi SK, Tripathy S, Sen R et al (2013) Characterisation of heterotrophic nitrifying and aerobic denitrifying Klebsiella pneumoniae CF-S9 strain for bioremediation of wastewater. Int Biodeterior Biodegrad 78:67–73. https://doi.org/10.1016/j.ibiod.2013.01.001
Qiang J, Zhou Z, Wang K et al (2020) Coupling ammonia nitrogen adsorption and regeneration unit with a high-load anoxic/aerobic process to achieve rapid and efficient pollutants removal for wastewater treatment. Water Res 170:115280. https://doi.org/10.1016/j.watres.2019.115280
Qiao Z, Sun R, Wu Y et al (2020) Characteristics and metabolic pathway of sediment biomass for heterotrophic nitrification and aerobic denitrification in aquatic ecosystems. Environ Res 191:110069. https://doi.org/10.1016/j.envres.2020.110069
Ren J, Wei C, Ma H et al (2019) The nitrogen-removal efficiency of a novel high-efficiency salt-tolerant aerobic denitrifier, Halomonas alkaliphile HRL-9, isolated from a seawater biofilter. Int J Environ Res Public Health 16:4451. https://doi.org/10.3390/ijerph16224451
Robertson LA, Kuenen JG (1990) Combined heterotrophic nitrification and aerobic denitrification in Thiosphaera pantotropha and other bacteria. Antonie Van Leeuwenhoek 57:139–152. https://doi.org/10.1007/BF00403948
Ruan Y, Taherzadeh MJ, Kong D et al (2020) Nitrogen removal performance and metabolic pathways analysis of a novel aerobic denitrifying halotolerant Pseudomonas balearica strain RAD-17. Microorganisms 8:72. https://doi.org/10.3390/microorganisms8010072
Shang N, Ding M, Dai M et al (2019) Biodegradation of malachite green by an endophytic bacterium Klebsiella aerogenes S27 involving a novel oxidoreductase. Appl Microbiol Biotechnol 103:2141–2153. https://doi.org/10.1007/s00253-018-09583-0
State Environmental Protection Administration of China (2002) Water and wastewater analysis methods. China Environmental Science Press, Beijing
Su C, Zhang M, Lin L et al (2020) Reduction of iron oxides and microbial community composition in iron-rich soils with different organic carbon as electron donors. Int Biodeterior Biodegrad 148:104881. https://doi.org/10.1016/j.ibiod.2019.104881
Sun Z, Lv Y, Liu Y, Ren R (2016) Removal of nitrogen by heterotrophic nitrification-aerobic denitrification of a novel metal resistant bacterium Cupriavidus sp. S1. Bioresour Technol 220:142–150. https://doi.org/10.1016/j.biortech.2016.07.110
Takaya N, Catalan-Sakairi MA, Sakaguchi Y et al (2003) Aerobic denitrifying bacteria that produce low levels of nitrous oxide. Appl Environ Microbiol 69:3152–3157. https://doi.org/10.1128/aem.69.6.3152-3157.2003
Thitanuwat B, Wongsoonthornchai M (2021) Estimation of nitrogen loading to surface water from agriculture based area and its application for water pollution mitigation. Environ Nat Resour J 19:230–238. https://doi.org/10.32526/ennrj/19/2020239
Van Hulle SW, Volcke EI, Teruel JL et al (2007) Influence of temperature and pH on the kinetics of the Sharon nitritation process. J Chem Technol Biotechnol 82:471–480. https://doi.org/10.1002/jctb.1692
Wang H, Gunsalus RP, Wang H, Gunsalus RP (2000) The nrfA and nirB nitrite reductase operons in Escherichia coli are expressed differently in response to nitrate than to nitrite. J Bacteriol 182:5813–5822. https://doi.org/10.1128/JB.182.20.5813-5822.2000
Wang J, Chu L (2016) Biological nitrate removal from water and wastewater by solid-phase denitrification process. Biotechnol Adv 34:1103–1112. https://doi.org/10.1016/j.biotechadv.2016.07.001
Wang T, Dang Q, Liu C et al (2016) Heterotrophic nitrogen removal by a newly-isolated alkalitolerant microorganism, Serratia marcescens W5. Bioresour Technol 211:618–627. https://doi.org/10.1016/j.biortech.2016.03.142
Xia L, Li X, Fan W, Wang J (2020) Heterotrophic nitrification and aerobic denitrification by a novel Acinetobacter sp. ND7 isolated from municipal activated sludge. Bioresour Technol 301:122749. https://doi.org/10.1016/j.biortech.2020.122749
Xu W, Xu Y, Su H et al (2021) Production performance, inorganic nitrogen control and bacterial community characteristics in a controlled biofloc-based system for indoor and outdoor super-intensive culture of Litopenaeus vannamei. Aquac 531:735–749. https://doi.org/10.1016/j.aquaculture.2020.735749
Yang L, Wang XH, Cui S et al (2019a) Simultaneous removal of nitrogen and phosphorous by heterotrophic nitrification-aerobic denitrification of a metal resistant bacterium Pseudomonas putida strain NP5. Bioresour Technol 285:121360. https://doi.org/10.1016/j.biortech.2019.121360
Yang M, Lu D, Yang J et al (2019b) Carbon and nitrogen metabolic pathways and interaction of cold-resistant heterotrophic nitrifying bacteria under aerobic and anaerobic conditions. Chemosphere 234:162–170. https://doi.org/10.1016/j.chemosphere.2019.06.052
Yang X, Wang S, Zhou L (2012) Effect of carbon source, C/N ratio, nitrate and dissolved oxygen concentration on nitrite and ammonium production from denitrification process by Pseudomonas stutzeri D6. Bioresour Technol 104:65–72. https://doi.org/10.1016/j.biortech.2011.10.026
Ye Q, Li K, Li Z et al (2017) Heterotrophic nitrification-aerobic denitrification performance of strain Y-12 under low temperature and high concentration of inorganic nitrogen conditions. Water 9:835. https://doi.org/10.3390/w9110835
Zeng J, Liao S, Qiu M et al (2020a) Effects of carbon sources on the removal of ammonium, nitrite and nitrate nitrogen by the red yeast Sporidiobolus pararoseus Y1. Bioresour Technol 312:123593. https://doi.org/10.1016/j.biortech.2020.123593
Zeng X, Huang JJ, Hua B, Champagne P (2020b) Nitrogen removal bacterial strains, MSNA-1 and MSD4, with wide ranges of salinity and pH resistances. Bioresour Technol 310:123309. https://doi.org/10.1016/j.biortech.2020.123309
Zhang F, Xie F, Zhou K et al (2022a) Nitrogen removal performance of novel isolated Bacillus sp. capable of simultaneous heterotrophic nitrification and aerobic denitrification. Appl Biochem Biotechnol 194:3196–3211. https://doi.org/10.1007/s12010-022-03877-w
Zhang QL, Liu Y, Ai GM et al (2012) The characteristics of a novel heterotrophic nitrification–aerobic denitrification bacterium, Bacillus methylotrophicus strain L7. Bioresour Technol 108:35–44. https://doi.org/10.1016/j.biortech.2011.12.139
Zhang X, Xia Y, Zeng Y et al (2022b) Simultaneous nitrification and denitrification by Pseudomonas. sp Y-5 in a high nitrogen environment. Environ Sci Pollut Res Int 29:69491–69501. https://doi.org/10.1007/s11356-022-20708-x
Zhang Y, Shi Z, Chen M et al (2015) Evaluation of simultaneous nitrification and denitrification under controlled conditions by an aerobic denitrifier culture. Bioresour Technol 175:602–605. https://doi.org/10.1016/j.biortech.2014.10.016
Zhao B, He YL, Zhang XF (2010) Nitrogen removal capability through simultaneous heterotrophic nitrification and aerobic denitrification by Bacillus. sp. Environ Technol 31:409–416. https://doi.org/10.1080/09593330903508922
Zheng H, Liu Y, Gao X et al (2012) Characterization of a marine origin aerobic nitrifying-denitrifying bacterium. J Biosci Bioeng 114:33–37
Zhu L, Ding W, Feng LJ et al (2012) Isolation of aerobic denitrifiers and characterization for their potential application in the bioremediation of oligotrophic ecosystem. Bioresour Technol 108:1–7. https://doi.org/10.1016/j.biortech.2011.12.033
Zou S, Yao S, Ni J (2014) High-efficient nitrogen removal by coupling enriched autotrophic-nitrification and aerobic-denitrification consortiums at cold temperature. Bioresour Technol 161:288–296. https://doi.org/10.1016/j.biortech.2014.03.066
Funding
The research was financially supported by Lingnan Normal University 2020 Campus Level Project (ZL2033).
Author information
Authors and Affiliations
Contributions
Yanyan Chen: investigation, formal analysis, writing—original draft. Juanjuan Zhong: investigation. Bingqi Li: investigation. Wenjing Dai: methodology, resources. Zhu Yang: writing—review and editing. Cuiming Huang: data curation, conceptualization. Jiahua Zeng: project administration, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling editor: Brian Austin
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Zhong, J., Li, B. et al. Exploring the nitrogen removal capacity of Klebsiella aerogenes B23 isolated from shrimp farm wastewater: heterotrophic nitrification and aerobic denitrification. Aquacult Int 32, 1453–1471 (2024). https://doi.org/10.1007/s10499-023-01224-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01224-2