Abstract
Light quality is important for microalgal biomass and bio-component accumulation. In this study, 8 kinds of combined monochromatic red (R) and blue light (B) were employed to grow Phaeodactylum tricornutum using white light (W) as a control. The results indicated that P. tricornutum had the highest specific growth rate under white light, reaching 0.151 (day−1), and the highest biomass (dry weight), reaching 302.77 mg L−1. The red light and 2R5B were the best for P. tricornutum producing carotenoids and protein, respectively. Carbohydrate was not significantly affected by light quality. The lipid content and lipid production under 5R2B were the highest, reaching 27.85% (per dry weight) and 75.56 mg/L (per culture), respectively. The best production of palmitic acid (C16:0), palmitoleic acid (C16:1 (n7)), and eicosapentaenoic acid (EPA, C20:5 (n3)) was observed at 4R3B, red light, and 1R6B respectively. The highest proportions per total fatty acids of C16:0, C16:1 (n7), and EPA were determined as 26.57%, 60.59%, and 13.87%, which were 15.71%, 11.37%, and 29.49% higher than those under white light, respectively. Compared with white light, cells that were grown under blue light, red light, and 1R6B revealed the improved lipid nutrition quality, reduced AI and TI values, increased HI (h/H) values, and increased n3:n6 ratios (under 1R6B only). Combined monochromatic light (except 1R6B) produced biodiesel with higher CN values, lower DU and IV values, and higher oxidation stability, but slightly reduced fluidity at low temperatures. The manipulated spectrum or light quality is a promising strategy to regulate the product property of P. tricornutum cultures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are single-celled organisms capable of photosynthesis in aquatic ecosystems, active lipid producers, and ideal biofuel feedstocks because they can produce biofuels while consuming carbon dioxide, which is one of the major contributors to global warming (Salam et al. 2016; Bhattacharya and Goswami 2020; Jin et al. 2021). To make the production of biofuels from microalgae more economically viable, the concept of microalgal “biorefinery” was proposed and emphasized the simultaneous extraction of high-value by-products including valuable pigments, proteins, polysaccharides, and polyunsaturated fatty acids during the production of biofuels (Gao et al. 2017; Bhattacharya and Goswami 2020).
At the same time, microalgae are the basis of the marine food web. Different species of marine microalgae can synthesize a number of nutrients that are important for the health of higher organisms (Nalder et al. 2015). Among these nutrients, microalgae lipids, especially their fatty acid composition and content, are of particular concern (Aysu et al. 2018). The most typical components of these nutrient lipids are the long-chain n3 polyunsaturated fatty acids (PUFAs). For example, eicosapentaenoic acid (EPA, 20:5(n3)) and docosahexaenoic acid (DHA, 22:6(n3)) are considered essential for survival, growth, and development in many marine animals, especially in the larval stage (Aysu et al. 2018; Li et al. 2019). This is largely due to the limited ability of higher organisms to synthesize these compounds from scratch (Mühlroth et al. 2013; Li et al. 2019). In addition to promoting optimal growth and health in animals that feed directly on microalgae, it is of profound significance that animals accumulate these compounds from feeding on microalgae and provide nutrients for subsequent human consumption (Nalder et al. 2015; Li et al. 2019). Microalgae are likewise being used in aquaculture feed formulation which ultimately improves the nutritional value of aquatic products for human health (El-Baky et al. 2004; Gharajeh et al. 2020). As a monounsaturated fatty acid (MUFA), palmitoleic acid (C16:1(n7)) is released from adipose tissue and it can regulate the metabolism of distant organs. Therefore, palmitoleic acid has recently been identified as a lipidic hormone factor that can regulate lipid metabolism and affect the formation of obesity (Bergman et al. 2013; Hu et al. 2019). Its medical and health value has attracted increasing attention. The commercial production of palmitoleic acid is limited and difficult to meet the market demand. Therefore, it is necessary to find new sources of raw materials, such as microalgae, for its production (Bergman et al. 2013; Wang et al. 2016; Hu et al. 2019).
Phaeodactylum tricornutum is a unicellular marine diatom and has been extensively used as a food source in marine aquaculture and as a model diatom for biochemistry and genomics study. Under optimal conditions, P. tricornutum is easily to be cultivated with fast growth to achieve high biomass (Remmers et al. 2017; Duarte et al. 2021). This alga produces a large amount of valuable fatty acids and can produce high content of triacylglycerol (TAG) of up to 25% of dried cell weight in a photobioreactor under optimal conditions (Remmers et al. 2017). Its EPA content is likewise higher than those in Chlorella vulgaris, Dunaliella salina, and many other commercially utilized algae species (Remmers et al. 2017). As a potential source of biofuels and valuable fatty acids, P. tricornutum is becoming one of the most popular microalgae in the research on algal lipid production (Gao et al. 2017; Duarte et al. 2021).
The growth, lipid content, and fatty acid composition of P. tricornutum are affected by culture conditions, such as temperature, light, and nutrients (Gao et al. 2017; Costa et al. 2013; Duarte et al. 2021). Among them, the influence of light, which is the energy source of algal photosynthesis, is particularly notable (Jungandreas et al. 2014; Maltsev et al. 2021). Microalgal pigments like chlorophyll and carotenoids absorb the photons with the peak wavelengths at the red and blue regions on the visible spectrum. It was reported that blue light increased the content of saturated fatty acids (SFAs), and red light increased the content of EPA in P. tricornutum cells by the comparison of the treatments with red, blue, and white light (Duarte et al. 2021). With the illumination of a combined red and blue light with a fixed proportion of 1:1, the biomass and lipid content of P. tricornutum was reported higher than that cultured with white light (Sirisuk et al. 2018). These studies initiated the optimizing illumination spectrum for P. tricornutum, and preliminarily proved that light quality regulates lipid composition. Our previous work on green algae, namely D. salina and Nannochloropsis oculata, has demonstrated that the properly combined monochromatic red and blue light has a strong potential in promoting algal photosynthetic metabolism (Jin et al. 2021; Yu et al. 2022). Therefore, further optimization of light quality for P. tricornutum to produce high-value fatty acids still needs detailed experiments (Maltsev et al. 2021).
In this study, the effects of different light qualities with different proportions of red and blue monochromatic light on the growth, lipid content, and fatty acid composition of P. tricornutum were investigated. In addition, lipid nutrition quality and biodiesel quality of P. tricornutum under different light quality were also evaluated. Analysis of lipid changes under different light qualities will provide a deeper understanding of lipid metabolism in this species and allows more efficient use in biodiesel production, aquaculture, and human nutritional supplements.
Materials and methods
Algal strain and culture conditions
The experimental axenic alga Phaeodactylum tricornutum was provided with the number of MEL106 by the Center for the Collection of Marine Bacteria and Phytoplankton (CCMBP) of the State Key Laboratory of Marine Environmental Science at Xiamen University, China.
The LED illuminator used for the algal culture was composed of 7 light-emitting units with a working power of 1 W each, that is, each illuminator had the working power of 7 W. The illuminators assembled with 7 units emitting white light (W) were used as control, while those with m units emitting red light plus n units emitting blue light (m = 0, 1, 2, 3, 4, 5, 6, or 7, m + n = 7, mRnB) were used as treatments. Therefore, gradually varied proportions of monochromatic red and blue light were formed as R (7R0B), 1R6B, 2R5B, 3R4B, 4R3B, 5R2B, 6R1B, and B (0R7B) (Jin et al. 2021). The exact spectral quality of each illuminator was determined by a plant light analyzer (PLA-30, EVERFINE Corporation, China), and the actual relative photon flux densities of each illuminator are shown in Fig. 1. Three parallels are established for each treatment.
P. tricornutum in the logarithmic growth phase with OD690nm of 0.6 were aseptically inoculated into 150 mL autoclaved silicon-containing seawater f/2 medium in a 250-mL Erlenmeyer flask with a 0.45-µm filter film cover. The alga was cultivated under illuminators with a unified light intensity of 65 ± 2 µmol·m2·s−1, with a temperature of 22 ± 2 ℃, with a light–dark cycle of 16 h:8 h, and without aeration. The culture period is 14 days, shaking once each morning and evening (Jin et al. 2021; Yu et al. 2022; Haro et al. 2017).
Determination of algal growth and dry weight of biomass
Algae culture with a volume of 200 µL from each treatment was taken into a 96-well plate every day. The OD690nm was measured with a microplate reader (Sanco, Shanghai, China) to draw a growth curve. The cell density was calculated according to the standard curve for OD690nm vs. cell density of P. tricornutum, to calculate the specific growth rate µ (day−1) as Formula (1):
where Ct1 and Ct2 are the cell density (106 cells mL−1) when the culture time is t1 and t2, respectively (Kong et al. 2010). According to the specific growth rate µ, the doubling rate K (day−1) [K = µ/ln2] and the generation time T (day) [T = 1/K] were determined (Garali et al. 2016).
At the end of the experiment, 100 mL of algae culture of each treatment was centrifuged (3700 r/min) for 10 min (Hitachi, Kyoto, Japan). The supernatant was removed, and the pellet was re-suspended and washed with 10 mL of distilled H2O before being centrifuged again. The precipitate was transferred to a clean glass bottle and lyophilized in a freeze dryer (Labconco, Kansas, USA) for 24 h, before the biomass dry weight measurement (Chen et al. 2015).
Determination of pigment, protein, carbohydrate, and lipid content
The pigment content of the sample was examined using an absorbance method (Sujitha, et al. 2016). The algal sample (5 mL) was subjected to centrifugation (4000 rpm, 10 min, 4 ℃) to collect the pellets. Five milliliters of acetone:water (90%, v/v) was added to the collected pellets, and kept standing overnight in dark at 4 ℃. Then, the cells were lysed by an ultrasonic cell disruptor on ice for 10 min (5-s working after 3-s interval, 700 w). After centrifugation (4000 rpm, 10 min, 4 ℃), the supernatant was subjected to the optical density measurement at the wavelength of 470, 644, and 661 nm. The concentration (mg L−1) for each pigment was calculated according to Formulae (2) and (3) by Sujitha et al. (2016).
where Awavelength is the optical density measured at the wavelength of 470, 644, or 661 nm, respectively, and Chl-a in Formula (3) is the concentration of chlorophyll-a (mg L−1) obtained in Formula (2) (Sujitha, et al. 2016).
Using D-glucose and serum albumin (BSA) as standards, the carbohydrates and protein contents were examined by a phenol sulfuric acid method and a Kaunas blue method, respectively (Dubois et al. 1956; Bradford 1976). The algal sample (30 mL) was subjected to centrifugation (4000 rpm, 10 min, 4 ℃) to collect the pellets, and then was re-suspended and lysed by an ultrasonic cell disruptor (Scientz, Ningbo, China) on ice with 30 mL distilled water. After centrifugation (4000 rpm, 10 min, 4 ℃), the supernatant was subjected to the protein and carbohydrate content measurements.
For lipid content determination, 20 mg of lyophilized algal powder was transferred into a centrifuge tube with the addition of 3.8 mL of a mixed solution (pure water:chloroform:methanol = 4:5:10). The mixture was extracted in an ice bath by 8-min ultrasonication (3-s working after 2-s interval, 200 w). With the addition of 1 mL water and 1 mL chloroform, the sample was vibrated and centrifuged. The water phase and cell debris were discarded. The chloroform phase was washed with distilled water, centrifuged for stratification, and transferred to a dried test tube. After the evaporation of the solvent under nitrogen flow, the total lipid weight was measured (Liu, 2014; Yu et al. 2022).
Fatty acid composition analysis
Fatty acid methyl esterification
n-Hexane was added to extracted lipid in the proportion of 1 mL n-hexane for every 1 mg lipid. After fully dissolving and 10-min ultrasonic treatments, 0.8 mL of 11% KOH methanol solution was added. After sonication for 10 min and the addition of 0.125 g anhydrous sodium acetate, the sample was vibrated and then stood still for stratification. The supernatant with a volume of 0.6 mL was filtered through a 0.22-µm membrane and injected into a sample bottle for GC–MS analysis (Cha et al. 2011).
GC–MS analysis
Fatty acid composition was analyzed by ITQ900 GC–MS (Thermo Fisher Scientific, Waltham, USA) with a TR-5MS (30 m × 0.25 mm × 0.25 µm). The carrier gas was helium (99.999%) with a constant flow of 0.8 mL min−1. The sample (1 µL) was injected into the injection port (230 ℃) in a splitless mode. One quality control (QC) sample was inserted after every 9 samples. The temperature program of GC was set as 120 ℃ for 1 min, then increased to 240 ℃ at 3 ℃/min, and kept for 10 min. The temperature of the EI ion source of MS was 220 ℃. The solvent delay was set as 4 min, mass spectra were acquired in full-scan mode, and the mass range was from 50 to 800 amu. The peaks with percentages of peak areas were recorded and subjected to peak identification. Based on NIST Mass Spectral Library (2008) and the GC–MS profiles of the standard of Fatty Acid Methyl Esters (FAMEs, Supelco CRM47885) and the samples prepared from fatty acids (including C16:1(n7)) after methyl esterification, each FAME in the sample was identified (as shown in supplementary Fig. 1). The relative content of each fatty acid was calculated using the peak area normalization method (Qari & Oves 2020; Yu et al. 2022).
Lipid nutrition quality analysis
Through fatty acid profile analysis, the lipid nutritional quality in terms of the n3:n6 ratio was calculated. Indices for atherosclerosis (AI), thrombosis (TI), hypocholesterolemic (HI), and hypocholesterolemic/hypercholesterolemic (h/H) were also obtained for each sample. The calculation of each index is as Formulae (4)–(7), respectively (Gharajeh et al. 2020).
Biodiesel quality analysis
Biodiesel properties in terms of saponification value (SV), iodine value (IV), cetane number (CN), degree of unsaturation (DU), long-chain saturation factor (LCSF), and cold filter plug point (CFPP) were determined by Formulae (8)–(13), respectively (Francisco et al. 2010; Wu & Miao 2014).
where M is the molecular weight of the fatty acid, D represents the number of double bonds, N is the proportion of each fatty acid component, MUFA is the proportion of monounsaturated fatty acids by weight (wt %), PUFA is the proportion of polyunsaturated fatty acids by weight (wt %), and C16 and C18 are the proportion of saturated fatty acids C16:0 and C18:0 by weight (wt %).
Data analysis
IBM SPSS22 software was used for one-way analysis of variance (one-way ANOVA) with normality and homogeneity test (P ≥ 0.05). Multiple comparisons were performed by least significant difference (LSD) or Dunnett’s T3 test. Uppercase letters represent extremely significant differences (P ≤ 0.01), and lowercase letters represent significant differences (P ≤ 0.05) in all figures (Wang et al. 2016).
Results
Effect of light quality on the growth of P. tricornutum
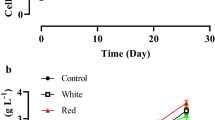
The effect of different light qualities on the growth of P. tricornutum is shown in Fig. 2a. P. tricornutum could grow under all light quality tested, and grow rapidly in the first 6 days; however, the growth rate slowed down after the 6th day. Growth rates of P. tricornutum under the control of white light (W) and the two monochromatic light, red light (R) and blue light (B), were superior to those under the combined monochromatic light. The treatment of 1R6B achieved the best growth among the treatments with combined monochromatic light treatments. At the end of the experiment, the best growth rate of P. tricornutum was observed under white light (W), and the poorest one was 6R1B. In the final stage of the experiment corresponding to the days12 to 14, the algae under white light (W) were still growing, while the algae under other light treatments entered the stationary or decline phase.
Effects of the light source on the growth of Phaeodactylum tricornutum (means ± SD, n = 3). a Growth curve of P. tricornutum at different incubation times under different treatments. b Cell density of P. tricornutum at harvest under different treatments. c Growth rate (µ), doubling rate (K), and generation time (T) of P. tricornutum under different treatments. d Dry weight of P. tricornutum at harvest under different treatments. Different letters indicate a statistical difference as determined by LSD test or Dunnett’s T3 test, uppercase letters indicated P < 0.01, and lowercase letters indicated P < 0.05
The final cell densities of P. tricornutum under different light qualities were extremely varied (Fig. 2b). The cell density under white light (W) was the highest one corresponding to 5.15 × 106 cells mL−1, while the one under 6R1B was the lowest one with 3.91 × 106 cells mL−1, The ones under the two monochromatic lights (R and B) were moderate. The highest cell density under combined monochromatic light was observed in 4R3B with 4.85 × 106 cells mL−1, followed by 5R2B with 4.78 × 106 cells mL−1.
From the results of the specific growth rates (Fig. 2c), white light (W) had the highest µ of 0.151 day−1, followed by 4R3B and 5R2B, and the lowest was 6R1B, with only 0.132 day−1. The longest generation time (T) of 6R1B was 5.26 days, while white light only took 4.58 days. The generation time under monochromatic light (R and B) and the other combined monochromatic light ranged between those under 6R1B and W.
The difference in the dry weight of the biomass obtained in the final harvest was extremely significant (Fig. 2d). The dry weight of algal cells under white light (W) was the highest one at 302.77 mg L−1 and showed no significant difference with those under R, B, 1R6B, and 5R2B (about 270 mg L−1). The dry weight of treatments of 2R5B, 3R4B, and 4R3B was only about 230 mg L−1, which was significantly lower than those under W, R, B, 1R6B, and 5R2B. The lowest dry weight of the biomass was obtained under 6R1B (189.47 mg L−1), which was 62.6% of that under W and was significantly lower than all other groups.
Effect of light quality on the protein, carbohydrate, and pigment accumulation of P. tricornutum
Light quality significantly affected protein accumulation in P. tricornutum (Fig. 3a). The protein production of white light (W) was 16.47 mg L−1, and similar values were observed in both two monochromatic lights (R and B). Protein production under all combined monochromatic light tended to be higher than that of white light (W), especially 2R5B, which was up to 74.11 mg L−1, 4.5 times that of white light (W). Light quality had no significant effect on carbohydrate accumulation in P. tricornutum (Fig. 3b). Carbohydrate production of white light (W) was 153.44 mg L−1. The carbohydrate content under the combined monochromatic light with high proportions of blue light (1R6B) was 203.44 mg L−1 and higher than the other treatments without significant difference. The effect of light quality on chlorophyll-a content in P. tricornutum was significant (Fig. 3c). Although there was no significant difference in chlorophyll-a content in cells grown under R light when compared to control, cells grown under the other light treatments (B and combined monochromatic light) achieved lower chlorophyll-a contents than that under white light (W). Especially under combined monochromatic light from 3R4B to 6R1B, the contents of chlorophyll-a were only about 60% of that under white light (W). Similar to chlorophyll-a, the carotenoid content of P. tricornutum was also the highest under red light (R) (1.12 mg L−1), which was significantly higher than that of white light (W) (Fig. 3d). All other combined monochromatic light and monochrome blue light (B) have significantly lower levels of carotenoids than white light (W), The lowest carotenoid content was 3R4B (0.66 mg L−1), which was 66% of that of white light (W) and 59% of that of red light (R), respectively.
Effect of the light source on protein, carbohydrate, and pigment accumulation of Phaeodactylum tricornutum (means ± SD, n = 3). a Protein production of P. tricornutum under different treatments. b Carbohydrate production of P. tricornutum under different treatments. c Chlorophyll-a concentration of P. tricornutum under different treatments. d Carotenoid concentration of P. tricornutum under different treatments. Different letters indicate a statistical difference as determined by LSD test or Dunnett’s T3 test, uppercase letters indicated P < 0.01, and lowercase letters indicated P < 0.05
Effect of light quality on the lipid accumulation of P. tricornutum
After 14 days of cultivation, different light qualities had extremely significant effects on the lipid contents (DW %) of P. tricornutum (Fig. 4a). The lipid content of cells under white light (W) was the lowest, only 16.15%. The lipid contents under the treatments of blue light (B), red light (R), or combined monochromatic light were higher than that of white light. Among them, the lipid content under red light (R) was 26.41%, and that under blue light (B) was 22.26%. The cells grown under combined monochromatic light presented increased lipid content. With an increasing proportion of red light in combined monochromatic light, the lipid content also increased, from 20.09% at 1R6B to the highest value of 27.85% at 5R2B, which is 1.7 times that in the control white light (W).
Effect of the light source on lipid production of Phaeodactylum tricornutum (means ± SD, n = 3). a Lipid content of P. tricornutum under different treatments. b Lipid production of P. tricornutum under different treatments. Different letters indicate a statistical difference as determined by LSD test or Dunnett’s T3 test, uppercase letters indicated P < 0.01, and lowercase letters indicated P < 0.05
Lipid production is a function of both the dry weight of the biomass and lipid content (Han et al., 2016). The results in Fig. 4b showed that the lipid production in the treatments of blue light (B), red light (R), or combined monochromatic light tended to be higher than that of white light (W), which was the lowest one with only 48.90 mg L−1. Lipid production in the treatments of R and B was higher than that of control white light (W), reaching 72.33 mg L−1 and 59.71 mg L−1, respectively. The lipid production of the combined monochromatic light was not always higher due to the low dry weight of biomass, although their lipid contents were relatively high. However, due to the highest lipid content and relatively high dry weight of biomass, the 5R2B in the combined light achieved the highest lipid production among all the treatments and achieved 75.56 mg L−1, which was 1.5 times that in the control white light (W).
Effect of light quality on the fatty acid composition of P. tricornutum
The main fatty acids of P. tricornutum under different treatments mainly contained C14:0, C16:0, C16:1(n7), C16:3(n3), C18:0, C18:1(n9), C18:2(n6), C18:3(n6), and C20:5 (n3), as shown in Table 1. Except for C18:0, variation of light quality had significant effects on the composition of detected fatty acids (P < 0.01). Under all light quality, palmitoleic acid C16:1(n7) was the fatty acid with the highest proportion in the total lipid composition of P. tricornutum, with a minimum proportion of 52.67% (under 6R1B) and a maximum of 60.59% (under R). Fatty acids accounting for the low proportions were C16:3(n3), C18:0, C18:2(n6), and C18:3(n6), which accounted for only about 1% under various treatments. SFA, including C14:0, C16:0, and C18:0, accounted for about 30% of all fatty acids in P. tricornutum. The high SFA content was observed under 2R5B, 3R4B, and 4R3B, which was significantly higher than those under R, B, and 1R6B. Monounsaturated fatty acids (MUFA), including C16:1(n7) and C18:1(n9), accounted for about 60% of all fatty acids in P. tricornutum and showed little difference with the variation of light quality. PUFA, including C16:3(n3), C18:2(n6), C18:3(n6), and C20:5(n3), accounted for about 10% of all fatty acids in P. tricornutum. Control (W) and monochromatic red and blue light (R and B) achieved relatively high PUFA content but showed no significant difference with those of combined monochromatic light (P > 0.01). Interestingly, PUFA content under 1R6B reached 13.87%, which is significantly higher than other combined monochromatic treatments (P < 0.01).
Against each fatty acid content in P. tricornutum lipid under white light (W), the amplitude of variations for the 9 identified fatty acids caused by the changes in light quality was obtained and shown in Fig. 5. In the cases of SFA, the variations of fatty acid C14:0 under treatments of monochromatic light (R and B) and combined monochromatic light all followed the negative trend compared to control W. A similar result was also observed for C18:0, except for slight positive effects in the treatments of B and 5R2B. The SFA, C16:0, showed positive variations in samples grown under all combined monochromatic light except 1R6B. The maximum variation was observed under 4R3B, which is 15.71% higher than the control (W). Among all the treatments, R was the least suitable for P. tricornutum to accumulate SFA. In the cases of MUFA, palmitoleic acid (C16:1(n7)) had little change in the treatments other than R, which significantly increased the proportion of C16:1(n7) to be 11.37% higher than that of control (W). The increasing variations of C18:1(n9) were observed with the increase of the proportions of red light in combined monochromatic light, such as those in the treatments of 4R3B, 5R2B, and 6R1B. In the cases of PUFA, blue light (B) and the combinations with high proportions of monochromatic blue light (1R6B) were beneficial to increasing long-chain PUFAs, including C18:2(n6), C18:3(n6), and C20:5(n3), but the same did not occur for C16:3(n3). Both red light (R) and blue light (B) benefited to accumulate C18:2(n6) and C20:5(n3). Most contents of C20:5(n3) in the algae under the combined monochromatic light were lower than that in control (W), except for the treatment of R, B, and 1R6B. Especially in the treatments of B and 1R6B, the content of C20:5(n3) was 23.93% and 29.49% higher than that of control (W) (Fig. 5), and reached 10.32% and 10.79% of total fatty acids (Table 1), respectively. The C20:5(n3) in 1R6B was the highest value among all treatments. Therefore, monochromatic blue light, or combinations with high proportions of monochromatic blue light (1R6B), was beneficial to increase the content of EPA. In a summary, the red light was not conducive to the accumulation of SFA of P. tricornutum, but beneficial for the increase of UFAs, especially medium-chain ones including C16:1(n7), C16:3(n3), and C18:2(n6). Blue light, or combinations with high proportions of monochromatic blue light (1R6B), was not conducive to the accumulation of SFA and medium-chain UFAs, but beneficial to the increase of UFAs with long-chain of 18C and above, especially C18:2(n6), C18:3(n6), and C20:5(n3). The combined monochromatic light, except for 1R6B, mainly helped to accumulate the SFA of C16:0.
The amplitude of variation (%) for FAMEs of Phaeodactylum tricornutum under different monochromatic light sources against that under white light. The amplitude of variation (%) for each fatty acid content was calculated as the variation between the treatment and control (white) divided by the control
Effect of light quality on the lipid nutritional quality of P. tricornutum
The n3:n6 ratio in oily food has proven to be an applicable index for nutritional value. The World Health Organization (WHO) recommends that the n3:n6 ratio in the diet should be greater than 5:1 (Gharajeh et al. 2020). In the present study, the n3:n6 ratio in control (W) was 5.19, which was significantly transcended by the treatment of 1R6B. The values of most other treatments except for 5R2B were slightly lower than that of the white light (W) without a significant difference (Table 2). To comprehensively evaluate the effects of light quality on the nutritional value of P. tricornutum lipid composition, the ratio of atherosclerosis index (AI), thrombosis index (TI), hypocholesterolemic index (HI), and hypocholesterolemic to hypercholesterolemic indices (h/H) under each light quality was also calculated. Since C20:3 and C22:4 were not detected for all samples, h/H resulted in the same values as HI. The TI, AI, and HI of the lipid sample represent their anti-thrombogenic, anti-atherogenic, and hypercholesterolemic potential, respectively. A diet rich in low AI and TI food sources helps avoid cardiovascular disorders in humans while a diet rich in high HI and h/H ratio food sources benefits cholesterol level reduction (Gharajeh et al. 2020). As shown in Table 2, the AI value of lipid obtained in control (W) was 0.55. The lowest AI value was achieved in the treatment of monochromatic red light (R), which is only 0.43. AI under monochromatic blue light (B) and combined monochromatic light with high proportions of blue light (1R6B) were also lower than that of control (W). TI values under various light qualities did not exceed 0.60. TI followed a similar trend to AI, and the treatments of R, B, and 1R6B achieved lower values than 0.44 in control (W). HI (h/H) in control (W) was 0.57. The range of HI (h/H) in treatments was 0.47––0.65. HI (h/H) in the treatments of R, B, and 1R6B were higher than that in control (W).
Effect of light quality on the biodiesel quality of P. tricornutum
To comprehensively evaluate the effects of light quality on the biodiesel quality of P. tricornutum lipid, relative indicators were calculated (Fig. 6). The CN value of biodiesel under white light was 45.5, much higher than the CN values under the two monochromatic light (R) and (B) and combined monochromatic light of 1R6B. All the CN values under other combined monochromatic light were higher than that of white light, especially 4R3B and 5R2B, as 47.5 and 47.3. The DU value of white light was 82.8 wt%, and the DU values of all the combined monochromatic light except 1R6B were lower than that of white light. The lowest one was 4R3B with the DU value as only 78.4 wt%. IV values of biodiesel obtained in this study were all lower than 120 g I2 100 g−1, and that under white light was 102 g I2 100 g−1. Like DU, IV values of combined monochromatic light except 1R6B were all lower than that of white light, and the lowest one was 4R3B, whose IV value was 94 g I2 100 g−1. The CFPP of all biodiesel obtained under different light qualities was below 0 ℃, and the fluidity was good at low temperatures. The CFPP of white light was − 6.7 ℃. CFPP values of the combined monochromatic light except 1R6B were higher than that of white light. Two monochromatic lights (R and B) made the CFPP values lower than that of white light. In general, the biodiesel quality of P. tricornutum cultured under monochromatic light (R and B) was lower than that of white light. The biodiesel produced by combined monochromatic light culture (except 1R6B) had higher CN value, lower DU and IV values, and higher oxidation stability, and was more suitable for long-term storage, but its fluidity was slightly reduced at low temperatures.
Discussion
Light quality is an important factor for the biomass accumulation of microalgae, which grow through autotrophism and photosynthesis (Maltsev et al. 2021). P. tricornutum grew fastest under white light, and the final dry weight of biomass was also the highest in this study. This is in line with Costa’s results that P. tricornutum grew faster under white light than under red and blue light and achieved the highest photosynthesis efficiency (Costa et al. 2013). In this study, the growth rates under monochromatic red light and blue light were slightly lower than that under control white light, and that under the monochromatic red light is relatively higher than that under monochromatic blue light. Meanwhile, P. tricornutum had been grown under a red light until the end of the exponential period and then transferred to white light in Sharma’s study, and their results tended to that red light might stimulate the formation of P. tricornutum biomass (Sharma et al. 2020). A red-shifted antenna complex of photosynthetic apparatus and distinct cell morphologies had been identified for chromatic acclimation of P. tricornutum during red-enhanced ambient light (Herbstová et al. 2017). However, it was also reported that the biomass formation of a blue-green alga Arthrospira platensis under red light was the highest and that under blue light was the lowest (Wang et al. 2007). In our previous study of green algae N. oculata cultivated under monochromatic light and combined monochromatic light, it was found that N. oculata grew worst under red light and grew better under combined monochromatic light (Yu et al. 2022). Okumura et al. cultured the green algae Botryococcus braunii under the monochromatic blue light and the mixed red–green–blue monochromatic light and found they enhanced biomass and growth rate (Okumura et al. 2015). In this study, except for 1R6B and 5R2B, the growth rate and the dry weight of diatom P. tricornutum under combined light treatment were slower and lower than those under white light treatment, respectively. Although the culture conditions were various in those studies, their results agreed that the optimal illumination spectra to improve the biomass yield for microalgae are species-specific due to the different pigment compositions of microalgae (Maltsev et al. 2021).
Not only microalgae growth but also the accumulation of metabolites in the cells, including lipids, proteins, and pigments, is influenced by light quality. The results of this study showed that light quality significantly affected protein and pigment content. The pigment content of P. tricornutum under the combined light was generally low, but the same condition could increase the protein production of P. tricornutum, especially the combined light 2R5B with a significant effect. Monochromatic red light can significantly increase the pigment content of P. tricornutum, especially carotenoid content. Although white light was helpful for the growth of P. tricornutum in this study, it was not beneficial to the accumulation of lipids. White light and 1R6B achieved the high dry weight of biomass at the cost of the lowest lipid content. Differently, combined monochromatic red and blue light, monochromatic red light (R), and monochromatic blue light (B) were more conducive to the accumulation of lipids than white light, at the expense of the slight reduction in the growth of P. tricornutum. The monochromatic red light (R) or combinations with high proportions of red light led to higher lipid contents, indicating that red light is conducive to the accumulation of lipids in P. tricornutum. This is the same as Sharma’s study, in which the lipid content of P. tricornutum under red light was 27.8% higher than that under white light after 10 days of culture (Sharma et al. 2020). The simple variation on the luminous flux of special spectral components regulating the metabolic process of microalgae cells towards accumulating proteins, carbohydrates, or lipids is very important. It was reported for P. tricornutum that the transformation from red light to blue light increased the lipid content, while the reverse transformation from blue light to red light promoted carbohydrates (Jungandreas et al. 2014). It is reported that after red light acclimation, the cellular carbon/nitrogen ratio, lipid, and carbohydrate of P. tricornutum increased, which led to changes in lipid content (Severes et al. 2017). The red light was conducive to lipid accumulation for P. tricornutum in this study, which is consistent with the above literature. Lipid production under the treatment of 5R2B was the highest, which is 1.5 times that in white light (W). For all other treatments with monochromatic light, the lipid production resulted in a level that was at least no less than that in W. These data proved that the algal lipid synthesis was affected not only by nutrient supply which was widely reported but also by illuminating light quality like in this study (Cha et al. 2011; Wu & Miao 2014). Another aspect, the white light led to high biomass growth, and the combined monochromatic led to high lipid accumulation. This result may also help the algal two-stage cultivation, in which the alga was subjected to the first-stage conditions to achieve high biomass and then transferred to the second-stage conditions to accumulate lipids (Ali et al., 2021). The switching of illuminators in large-scale cultivations or bioreactors should be more feasible (Karpagam et al., 2022).
Previous studies had shown that red light stimulated the formation of PUFAs in P. tricornutum and reduced the amount of MUFAs (Sharma et al. 2020; Duarte et al. 2021). The use of red light increased the content of EPA in the green algae Tetraselmis suecica and the contents of EPA and docosahexaenoic acid (DHA, C22:6(n3)) in green algae C. vulgaris (Abiusi et al. 2020), while yellow and green LEDs were also reported to increase EPA and DHA contents in the green algae C. vulgaris. A blue light was reported with promotion for the formation of PUFAs and alanine (ALA) in green algae C. vulgaris, C. pyrenoidosa, and Scenedesmus quadricauda (Zhong et al. 2018). In this study, it was found that combined monochromatic light can only promote the accumulation of SFAs, especially C16:0. The increase of UFAs was observed under single monochromatic light (R or B) and the combined monochromatic light with a high proportion of blue light. A red light could not increase the content of SFAs in P. tricornutum, but increased the content of UFAs, especially C16:1(n7), which was identified as a lipidic hormone factor regulating lipid metabolism and affecting the formation of obesity (Bergman et al. 2013; Hu et al. 2019). Treatments of B and 1R6B, which are monochromatic light with a high proportion of blue light, were particularly beneficial for the accumulation of UFAs with long-chain fatty acids, especially C18:2(n6) and C20:5(n3). It should be mentioned that C20:5(n3) (EPA) contents in glycolipid and phospholipid of P. tricornutum are much higher than that in TAG (Remmers et al. 2017). Variance in total lipid extraction would lead to a large difference in EPA content. Furthermore, many factors other than light quality may also affect the composition of fatty acids, including growth phase, light intensity, nutrition supply, temperature, and aeration (Ramos et al. 2009; Remmers et al. 2017; Haro et al. 2017; Duarte et al., 2021). In this study, all those factors were unified and simplified to avoid possible variance in those factors. Therefore, the changes in the composition of fatty acids are reliable and will undoubtedly affect the nutritional value and biodiesel quality of lipids produced by P. tricornutum (Hu et al. 2019; Gharajeh et al. 2020).
Eating oily food with appropriate composition of fatty acids can promote human health. The n3:n6 ratio has proved to be an effective indicator of the nutritional value of oily food. The inappropriate intake of n3 and n6 fatty acids potentially causes chronic diseases, cancer, diabetes, cardiovascular diseases, or inflammatory diseases (Mühlroth et al. 2013; Anitha et al. 2018). Therefore, it is of great importance for humans to consume high n3:n6 ratio-rich food. However, modern people’s main diet and increased fried/fast food often lead to insufficient intake of n-3 fatty acids (Mühlroth et al. 2013). Ratios of n3:n6 in P. tricornutum ranged from 4.61 under 5R2B to the highest ratio of 5.73 under 1R6B. The comparable n3:n6 ratio of 5.08 has previously been recorded for Dunaliella salina (El-Baky et al. 2004). The high n3:n6 ratio of P. tricornutum in this study shows that the lipid of P. tricornutum possesses a strong potential in compensation for the human insufficient intake of n3 fatty acids, and the combined monochromatic light of 1R6B and 6R1B, in particular, could make the value of n3:n6 ratio even higher. Nutritional sources with low atherosclerosis index (AI) and thrombosis index (TI) help to reduce the risk of cardiovascular disease. The lower AI and TI, and the higher HI (h/H), will make the food source healthier (Lopes et al. 2014). Analysis of the lipid nutritional index of P. tricornutum under each light quality showed that R, B, and 1R6B led to not only the lower AI and TI but also the higher HI, in comparison with white light (W). So these treatments were helpful in further improving the lipid nutritional quality of P. tricornutum. Especially, the performance of the treatment of 1R6B is the most prominent in the promotion of the lipid nutritional quality of P. tricornutum. The lipid products of P. tricornutum cultivated under light quality like 1R6B with high nutritional value and high content of EPA are suitable for not only aquaculture feed but also human health food supplements and medical biotechnology.
CN is the important index for biodiesel quality, which is significantly correlated to the ignition delay time and combustion quality. Generally, the longer the carbon chain of fatty acids and the higher the saturation of fatty acids, the higher the cetane number is (Ramos et al. 2009). The reference standard criteria for biodiesel application by European standard EN 14,214 and ASTM international standard D6751 were CN > 51 and CN > 47, respectively (Gharajeh et al. 2020). Qualitative biodiesel properties based on the fatty acid profiles for the lipids obtained from all treatments were estimated and compared with each other in this study. The CN values became higher than that of white light under combined monochromatic light, especially 4R3B and 5R2B. These results indicated that combined monochromatic light could improve the biodiesel quality, and P. tricornutum cultured under combined monochromatic light of 4R3B and 5R2B could be used as direct raw materials for biodiesel. DU is measured according to the proportions of monounsaturated and polyunsaturated fatty acids (Francisco et al. 2010; Wu & Miao 2014). The unsaturation of fatty acids affects the feasibility of long-term storage of biodiesel. Polyunsaturated fatty acids are more easily oxidized than monounsaturated fatty acids and tend to produce biodiesel with less oxidation stability (Ramos et al. 2009; Wu et al. 2014). The DU values under all the combined monochromatic light except 1R6B were lower than that under white light. With low PUFA and DU, biodiesel produced by P. tricornutum under the combined monochromatic light was more suitable for long-term storage. IV is a rough measurement of the total unsaturation of biodiesel, which is usually employed to reflect the oxidation stability of biodiesel. The oxidation stability of high IV biodiesel is lower than that of low IV biodiesel (Wu et al. 2014; Gharajeh et al. 2020). The IV value proposed by the European standard is less than 120 g I2 100 g−1 (Gharajeh et al. 2020). Like DU, IV values of combined monochromatic light except 1R6B were all lower than that of white light. Low IV and DU of the biodiesel extracted from P. tricornutum cultured under the combined monochromatic light indicate higher oxidation stability and are more suitable for long-term storage. CFPP is another important parameter for biodiesel and is related to the flow performance at low temperatures (Wu et al. 2014). Low-temperature performance of biodiesel mainly depends on the content of saturated fatty acids, while the influence of unsaturated fatty acid composition can be ignored (Ramos et al. 2009). That is, CFPP is correlated with saturated fatty acid content. The higher contents of stearic acid (C18:0) and palmitic acid (C16:0) usually result in a higher CFPP (Ramos et al. 2009). The CFPP values of the biodiesel obtained from P. tricornutum cultured under all light quality were far lower than 0 ℃, indicating that the biodiesel from P. tricornutum had very good liquidity at low temperatures, although the CFPP increased slightly due to the increase of C16:0 ratio under combined monochromatic light (Wu and Miao, 2014; Gharajeh et al. 2020). In general, the biodiesel quality of P. tricornutum cultured under two monochromatic lights (R and B) was inferior to white light. The biodiesel produced by combined monochromatic light culture (except 1R6B) had higher CN, lower DU and IV values, and higher oxidation stability, and was more suitable for long-term storage, but its fluidity was slightly restricted at low temperatures. Compared with three reported Dunaliella sp. strains of ABRRNW-B1, ABRRNW-G2/1, and ABRRNW-I1, biodiesel produced by P. tricornutum (white light) had much higher CN than their CN values, which was 17.3, 13.8, and 16.6, respectively (Gharajeh et al. 2020), and much lower IV, DU, and CFPP. The result indicates that P. tricornutum is a very suitable raw material for biodiesel production, and its biodiesel quality can be further improved by a combined monochromatic light strategy.
Conclusion
White light is better than monochromatic light for the cell growth and accumulation of biomass of P. tricornutum. Combined light, especially 2R5B, is the best illuminating spectrum for P. tricornutum producing protein. Monochrome red light is the best for P. tricornutum producing pigments, especially carotenoids. Monochromatic blue and red light and their combinations are superior to white light in lipid accumulation by P. tricornutum. Among them, 5R2B is the best one for lipid production with the highest lipid content and acceptable dry weight of biomass. All combined monochromatic light except 1R6B enhances P. tricornutum accumulating SFA of C16:0, red light helps it accumulate C16:1(n7), and blue light and combined monochromatic light with a high proportion of blue light like 1R6B are conducive for P. tricornutum to accumulate EPA. Red light, blue light, and 1R6B help to further improve the lipid nutritional quality of P. tricornutum. Especially, combined monochromatic light with a high proportion of blue light like 1R6B leads to the highest lipid nutrition quality of P. tricornutum. Combined monochromatic light helps to further improve the biodiesel quality of P. tricornutum, with higher CN values and lower DU and IV values.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abiusi F, Wijffels RH, Janssen M (2020) Doubling of microalgae productivity by oxygen balanced mixotrophy[J]. ACS Sustain Chem Eng 8(15):6065–6074. https://doi.org/10.1021/acssuschemeng.0c00990
Ali HEA, El-fayoumy EA, Rasmy WE et al (2021) Two-stage cultivation of Chlorella vulgaris using light and salt stress conditions for simultaneous production of lipid, carotenoids, and antioxidants. J Appl Phycol 33:227–239. https://doi.org/10.1007/s10811-020-02308-9
Anitha S, Shah A, Ali B (2018) Modulation of lipid productivity under nitrogen, salinity and temperature stress in microalgae Dunaliella sp [J]. J Environ Biol 39(5):625–632. https://doi.org/10.22438/jeb/39/5/mrn-761
Aysu T, Fermoso J, Sanna A (2018) Ceria on alumina support for catalytic pyrolysis of Pavlova sp microalgae to high-quality bio-oils[J]. J Energy Chem 27(3):874–882. https://doi.org/10.1016/j.jechem.2017.06.014
Bergman BC, Howard D, Schauer IE et al (2013) The importance of palmitoleic acid to adipocyte insulin resistance and whole-body insulin sensitivity in type 1 diabetes [J]. J Clin Endocr Metab 98(1):E40–E50. https://doi.org/10.1210/jc.2012-2892
Bhattacharya M, Goswami S (2020) Microalgae - A green multi-product biorefinery for future industrial prospects[J]. Biocatal Agr Biotechnol 25:101580. https://doi.org/10.1016/j.bcab.2020.101580
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding[J]. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cha TS, Chen JW, Goh EG et al (2011) Differential regulation of fatty acid biosynthesis in two Chlorella species in response to nitrate treatments and the potential of binary blending microalgae oils for biodiesel application[J]. Bioresource Technol 102(22):10633–10640. https://doi.org/10.1016/j.biortech.2011.09.042
Chen CY, Chen YC, Huang HC et al (2015) Enhancing the production of eicosapentaenoic acid (EPA) from Nannochloropsis oceanica CY2 using innovative photobioreactors with optimal light source arrangements[J]. Bioresource Technol 191:407–413. https://doi.org/10.1016/j.biortech.2015.03.001
Costa BS, Jungandreas A, Jakob T et al (2013) Blue light is essential for high light acclimation and photoprotection in the diatom Phaeodactylum tricornutum[J]. J Exp Bot 64(2):483–493. https://doi.org/10.1093/jxb/ers340
Duarte B, Feijão E, Goessling JW et al (2021) Pigment and fatty acid production under different light qualities in the diatom Phaeodactylum tricornutum[J]. Appl Sci 11(6):2550. https://doi.org/10.3390/app11062550
Dubois M, Gilles KA, Ton JKH et al (1956) Colorimetric method for determination of sugars and related substances[J]. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
El-Baky HHA, El-Baz FK, El-Baroty GS (2004) Production of lipids rich in omega 3 fatty acids from the halotolerant alga Dunaliella salina[J]. Biotechnol 3(1):102–108. https://doi.org/10.3923/biotech.2004.102.108
Francisco EC, Neves DB, Lopes EJ, Franco et al (2010) Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality[J]. J Chem Technol Biot 85(3):395–403. https://doi.org/10.1002/jctb.2338
Gao BY, Chen AL, Zhang WY et al (2017) Co-production of lipids, eicosapentaenoic acid, fucoxanthin, and chrysolaminarin by Phaeodactylum tricornutum cultured in a flat-plate photobioreactor under varying nitrogen conditions[J]. J Ocean U China 16(5):916–924. https://doi.org/10.1007/s11802-017-3174-2
Garali SMB, Sahraoui I, Iglesia PDL et al (2016) Effects of nitrogen supply on Pseudo-nitzschia calliantha, and Pseudo-nitzschia, cf seriata: field and laboratory experiments[J]. Ecotoxicology 25(6):1211–1225. https://doi.org/10.1007/s10646-016-1675-1
Gharajeh NH, Valizadeh M, Dorani E et al (2020) Biochemical profiling of three indigenous Dunaliella isolates with main focus on fatty acid composition towards potential biotechnological application[J]. Biotechnol Rep 26:e00479. https://doi.org/10.1016/j.btre.2020.e00479
Han F, Pei H, Hu W et al (2016) Beneficial changes in biomass and lipid of microalgae Anabaena variabilis facing the ultrasonic stress environment[J]. Bioresource Technol 209: 16–22. Bioresource Technol 253:175–181. https://doi.org/10.1016/j.biortech.2018.01.020
Haro P, Sáez K, Gómez PI (2017) Physiological plasticity of a Chilean strain of the diatom Phaeodactylum tricornutum: the effect of culture conditions on the quantity and quality of lipid production[J]. J Appl Phycol 29:2771–2782. https://doi.org/10.1007/s10811-017-1212-5
Herbstová M, Bína D, Kaňa R et al (2017) Red-light phenotype in a marine diatom involves a specialized oligomeric red-shifted antenna and altered cell morphology. Sci Rep 7:11976. https://doi.org/10.1038/s41598-017-12247-0
Hu W, Fitzgerald M, Topp B et al (2019) A review of biological functions, health benefits, and possible de novo biosynthetic pathway of palmitoleic acid in macadamia nuts[J]. J Funct Foods 62:103520. https://doi.org/10.1016/j.jff.2019.103520
Jin CL, Yu BQ, Qian SY et al (2021) Impact of combined monochromatic light on the biocomponent productivity of Dunaliella salina[J]. J Renew Sustain Ener 13(2):023101. https://doi.org/10.1063/5.0041330
Jungandreas A, Costa BS, Jakob T et al (2014) The acclimation of Phaeodactylum tricornutum to blue and red light does not influence the photosynthetic light reaction but strongly disturbs the carbon allocation pattern[J]. PLoS ONE 9(8):e99727. https://doi.org/10.1371/journal.pone.0099727
Karpagam R, Jawaharraj K, Ashokkumar B et al (2022) A cheap two-step cultivation of Phaeodactylum tricornutum for increased TAG production and differential expression of TAG biosynthesis associated genes[J]. J Biotech 354:53–62. https://doi.org/10.1016/j.jbiotec.2022.06.002
Kong QX, Zhu LZ, Shen XY (2010) The toxicity of naphthalene to marine Chlorella vulgaris under different nutrient conditions[J]. J Hazard Mater 178(1–3):282–286. https://doi.org/10.1016/j.jhazmat.2010.01.074
Li XP, Liu JP, Chen GY et al (2019) Extraction and purification of eicosapentaenoic acid and docosahexaenoic acid from microalgae: a critical review[J]. Algal Res 43:101619. https://doi.org/10.1016/j.algal.2019.101619
Liu JY (2014) Optimisation of biomass and lipid production by adjusting the interspecific competition mode of Dunaliella salina and Nannochloropsis gaditana in mixed culture[J]. J Appl Phycol 26(1):163–171. https://doi.org/10.1007/s10811-013-0099-z
Lopes LD, Bger BR, Cavalli KF et al (2014) Fatty acid profile, quality lipid index and bioactive compounds of flour from grape residues[J]. Cienc Investig Agrar 41(2):225–234. https://doi.org/10.4067/S0718-16202014000200009
Maltsev Y, Maltseva K, Kulikovskiy M et al (2021) Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition[J]. Biol 10(10):1060–1084. https://doi.org/10.3390/biology10101060
Mühlroth K, Li G, Røkke P et al (2013) Pathways of lipid metabolism in marine algae, co-expression network, bottlenecks and candidate genes for enhanced production of EPA and DHA in species of Chromista[J]. Mar Drugs 11(11):4662–4697. https://doi.org/10.3390/md11114662
Nalder TD, Miller MR, Packer MA (2015) Changes in lipid class content and composition of Isochrysis sp (T-Iso) grown in batch culture[J]. Aquacult Int 23(5):1293–1312. https://doi.org/10.1007/s10499-015-9884-9
Okumura C, Saffreena N, Rahman MA et al (2015) Economic efficiency of different light wavelengths and intensities using LEDs for the cultivation of green microalga Botryococcus braunii (NIES-836) for biofuel production[J]. Environ Prog Sustain 34(1):269–275. https://doi.org/10.1002/ep.11951
Qari HA, Oves M (2020) Fatty acid synthesis by Chlamydomonas reinhardtii in phosphorus limitation[J]. J Bioenerg Biomembr 52(1):27–38. https://doi.org/10.1007/s10863-019-09813-8
Ramos MJ, Fernandez CM, Casas A et al (2009) Influence of fatty acid composition of raw materials on biodiesel properties[J]. Prep Biochem Biotech 100(1):261–268. https://doi.org/10.1016/j.biortech.2008.06.039
Remmers IM, Martens DE, Wijffels RH et al (2017) Dynamics of triacylglycerol and EPA production in Phaeodactylum tricornutum under nitrogen starvation at different light intensities[J]. PLoS ONE 12(4):e0175630. https://doi.org/10.1371/journal.pone.0175630
Salam KA, Velasquez-Orta SB, Harvey AP (2016) A sustainable integrated in situ, transesterification of microalgae for biodiesel production and associated co˗products ˗ a review[J]. Renew Sust Energ Rev 65:1179–1198. https://doi.org/10.1016/j.rser.2016.07.068
Severes A, Hegde S, D’Souza L et al (2017) Use of light emitting diodes (LEDs) for enhanced lipid production in micro-algae based biofuels[J]. J Photoch Photobio B 170:235–240. https://doi.org/10.1016/j.jphotobiol.2017.04.023
Sharma N, Fleurent G, Awwad F et al (2020) Red light variation an effective alternative to regulate biomass and lipid profiles in Phaeodactylum tricornutum[J]. Appl Sci 10(7):2531. https://doi.org/10.3390/app10072531
Sirisuk P, Ra CH, Jeong GT et al (2018) Effects of wavelength mixing ratio and photoperiod on microalgal biomass and lipid production in a two-phase culture system using LED illumination[J]. Bioresource Technol 253:175–181. https://doi.org/10.1016/j.biortech.2018.01.020
Sujitha BS, Muthu A (2016) Influence of abscisic acid on growth, biomass and lipid yield of Scenedesmus quadricauda under nitrogen starved condition[J]. Bioresource Technol 213:198–203. https://doi.org/10.1016/j.biortech.2016.02.078
Wang CY, Fu CC, Liu YC (2007) Effects of using light-emitting diodes on the cultivation of Spirulina platensis[J]. Biochem Eng J 37(1):21–25. https://doi.org/10.1016/j.bej.2007.03.004
Wang H, Gao LL, Zhou WJ et al (2016) Growth and palmitoleic acid accumulation of filamentous oleaginous microalgae Tribonema minus at varying temperatures and light regimes[J]. Bioproc Biosyst Eng 39(10):1589–1595. https://doi.org/10.1007/s00449-016-1633-6
Wu HQ, Miao XL (2014) Biodiesel quality and biochemical changes of microalgae Chlorella pyrenoidosa and Scenedesmus obliquus in response to nitrate levels[J]. Bioresource Technol 170:421–427. https://doi.org/10.1016/j.biortech.2014.08.017
Yu BQ, Qian SY, Liu Q et al (2022) The response of bio-component production of Nannochloris oculata to the combinations of monochromatic lights[J]. J Ocean U China 21(1):243–251. https://doi.org/10.1007/s11802-022-4896-3
Zhong Y, Jin P, Cheng JJ (2018) A comprehensive comparable study of the physiological properties of four microalgal species under different light wavelength conditions[J]. Planta 248(2):489–498. https://doi.org/10.1007/s00425-018-2899-5
Funding
This work was supported by the National Natural Science Foundation of China (nos. 42177459, 41776156, and 41271521).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Miaoping Dong, Yi Zhang, Qiuyan Yu, Qing Liu, Xiaojian Zhou, and Cuili Jin. The first draft of the manuscript was written by Xiaojian Zhou and Cuili Jin. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Human and animal ethics
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Ronan Sulpice
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, M., Zhang, Y., Yu, Q. et al. Regulation of light quality on lipid production, biodiesel quality, and nutritional quality of Phaeodactylum tricornutum. Aquacult Int 31, 1231–1251 (2023). https://doi.org/10.1007/s10499-022-01024-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-01024-0