Abstract
This study evaluated the suitability of five diatom species (Amphora sp., Cocconeis sp., Navicula ramosissima, Nitzschia sp., and Tryblionella sp.) as food to abalone Haliotis asinina early juveniles (5-mm shell length). Grazing periodicity, grazing rate, and feeding preference were measured; at the same time, abalone gut content was examined. Grazing incidence appeared to be continuous with significantly higher grazing intensity observed at nighttime from 6 p.m. to 10 p.m. (79%) than at daytime from 10 a.m. to 2 p.m. (40%) (p < 0.05). Grazing rates from 6 p.m. to 10 p.m. were significantly higher on N. ramosissima (1.6 × 105) among diatom species but was not statistically different from Cocconeis sp. (1.1 × 105) (p > 0.05). Broken cells of Amphora sp. (22%) were significantly higher in the gut of H. asinina compared to Cocconeis sp. (2.8%), N. ramosissima, (1.8%), and Tryblionella sp. (0.2%) although the abundance of Nitzschia sp. (6%) was not significantly different from Amphora sp. (p > 0.05). Early juveniles showed significant preference for Cocconeis sp. (18.6%), followed by Nitzschia sp. (16.2%), N. ramosissima (13.9%), and Amphora sp. (13.4%), with the least preference for Tryblionella sp. (7.8%). Survival of H. asinina was similar in 4 diatom species (46–71%) except in Tryblionella sp. (8–12%). These findings suggest that diatom species Cocconeis sp., Nitzschia sp., Amphora sp., and N. ramosissima are the suitable live food for H. asinina early juveniles. Knowledge from this study would contribute to the development of a feeding protocol that would maximize production of H. asinina early juveniles in the hatchery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abalones are important aquaculture species around the world (Najmudeen and Victor 2004; Siqueiros-Beltrones and Romero 2004; Kawamura et al. 2005; Onitsuka et al. 2007, 2010). Current global consumption continues to command high prices at US$80/kg with China as the biggest producer. In fact, abalone production in the global market grew by over 150,000 t in 2015 which is equivalent to an 11% increase of production from 2014. In 2016, the cost of whole live/fresh abalone species reached US$80 per kg (FAO 2017) . In 2019, abalone production worldwide increased to 203,374 t (198,945 t from Asia; 180,267 t from China) amounting to US$2,257,754. In the Philippines, there are four abalone species reported, namely, H. asinina, H. glabra, H. ovina, and H. varia (Fermin et al. 2008). Among these species, H. asinina has been commercially produced in the Philippines while the other species are caught in the wild (Tahil and Dy 2015). In 1996, the country recorded 448 t of H. asinina but declined to 199 t in 1998 to (FAO 2000). In 2019, Philippines recorded 190 t capture production of abalone (FAO 2021). Hatchery-produced abalones are an important source of stocks as their dwindling wild stocks are at risk due to fishing pressure and gleaning activity may also damage the reefs (Maliao et al. 2003; Salayo et al. 2020).
The Philippine donkey’s ear abalone H. asinina is listed as a major commercial gastropod species in the world. It is also among the greatest delicacies in Japanese cuisine (Kafuku and Ikenoue 1983). Locally known in the Philippines as lapas, kapinan, or sobra-sobra, which literally means huge or massive, its large visceral mass and foot overflow the shell. It could attain a very large size with a maximum shell length and body weight of 12 cm and 250 g, respectively, respectively (Tahil and Juinio-Menez 1999; FAO 2009; Encena and Bayona 2010). The aquaculture of H. asinina is considered profitable compared to other abalone species since a large percentage of its body is edible (Singhagraiwan and Doi 1993); it spawns year-round (Jarayabhand and Paphavasit 1996); and it has been considered as the fastest-growing abalone species in the world (Singhagraiwan and Sasaki 1991; Capinpin and Corre 1996; Yu et al. 2014). To boost the aquaculture production of abalone, SEAFDEC/AQD in the Philippines conducted studies to further improve the nursery and grow-out culture of abalone (Lebata-Ramos et al. 2021). Although extensive works have been done on the feeding, growth, and survival of adult abalone, few studies have focused on its early juvenile stage (Kruatrachue et al. 2004). In abalone, the larval settlement to early juvenile stage is a crucial phase in which high mortalities are observed (Ebert and Houk 1984). Early juveniles must reach the ideal size of 5–10 mm prior to stocking in commercial farms (Daume 2003; Dela Peña et al. 2010). Several studies have reported that benthic diatoms serve as chemical cues for settlement and are an important component in the diet of early juveniles (Kawamura et al. 1998; Siqueiros-Beltrones and Voltolina 2000; Carbajal-Miranda et al. 2005; Daume 2006). As such, it has become a hatchery practice to feed post-larvae abalone with natural benthic diatom populations that are highly diversified (Siqueiros-Beltrones et al. 2017); however, natural diatoms may have largely unpredictable species diversity and contain unwanted species (Correa-Reyes et al. 2001; Daume 2006).
Abalone juveniles are micro grazers dependent on the development and capacity of the radula (Roberts et al. 1999; Kawamura et al. 2001). At this stage, the juveniles are well adapted to grazing with benthic diatoms as food sources; as such, it is a common practice in the hatchery to provide live benthic diatoms (Gallardo and Buen 2003; Gordon et al. 2006; Chen 2007). However, the most suitable diatom species for H. asinina early juveniles is still unknown (Setyono 2005). Several reports on other species of Haliotis feeding on diatoms are presented in Table 1.
Most hatcheries in Thailand use thin films of benthic diatoms Nitzschia spp. and Navicula spp. for production of H. asinina and H. ovina (Jarayabhand and Paphavasit 1996). In the Philippines, the diatom N. ramosissima is currently used as feed to early juvenile abalones in SEAFDEC/AQD. Gallardo and Buen (2003) studied different biological films and found Navicula sp. or in combination with mucus (previously grazed by adult abalone) to be effective metamorphic and settlement inducers of larvae. Later, Gapasin and Polohan (2005) also found that Amphora sp. is effective in inducing larval settlement and, thus, this diatom species is considered as a potential candidate for mass propagation (De la Peña 2007; De la Peña et al. 2010). Meanwhile, Capinpin (2007) recommended Cocconeis sp. as the best food compared to other diatom species (Navicula mollis, Stauroneis sp., N. ramosissima, Pleurosigma sp.) when fed to early settling juveniles of H. asinina.
The present study used the five benthic diatoms (Cocconeis sp., Navicula ramosissima, Nitzschia sp., and Tryblionella sp.). Diatom sizes were within the recommended size of < 10 µm for abalone (Hahn 1989), except for Amphora sp. (mean size: 11–13 µm L; 3–3.5 W µm) (De la Peña and Franco 2013) which is slightly larger. All these diatoms form flat communities and are type B (adhesive prostate) except for N. ramosissima, which is a type A or gliding prostrate. There are three types of adhesiveness: + + + (strong degree of adhesiveness); + + (medium degree of adhesiveness); + (low degree of adhesiveness) (Kawamura 1996; Zhang et al. 2010). The most adhesive in these five benthic diatoms is Cocconeis sp. (+ + +), while Nitzschia sp. and Amphora sp. are of less adhesive types (+ +) (Table 2). The isolate Tryblionella sp. is a new collection in SEAFDEC/AQD and formerly identified as Diploneis sp. It has been observed that Tryblionella sp. is also a type B or slow-growing, prostrate form and less adhesive than Cocconeis sp. Martinez-Goss (1997) also described new taxa in the Philippines including two Nitzschia and one Tryblionella species, the latter of which was proposed to be named Tryblionella (Nitzschia) chutteri because of their common physical features. The complexity of the Nitzschia genus led to the transfer of Tryblionella species (formerly a section under genus Nitzschia) by Smith (1853) and eventually elevated to a genus level by Round et al. (1990) (Witknowski et al. 2004).
Understanding the feeding preference of any species is important in the development of appropriate and sustainable diets (Angell et al. 2012). Several studies pointed out that the use of high-quality diatom species is essential to improving the consistency of abalone production in hatcheries and reducing weaning mortality (Najmudeen and Victor 2004; Stott et al. 2004; Setyono 2005; Daume 2006; Xing et al. 2008; Onitsuka et al. 2010). Feeding selectivity based on examination of guts and grazing rates of given natural food were reported in several studies. Gut examination is often used to determine feeding selectivity in aquatic species (Infante 1978; Carter 1982; Madin and Cetta 1984; Eskinazi-Sant’Anna et al. 2002; del Próo et al. 2003). Grazing rate is another crucial parameter in understanding juvenile feeding behavior quantitatively (Searcy-Bernal et al. 2001; Day et al. 2004; Viana et al. 2007).
Studies on abalone diets have focused mostly on adults while those of small juveniles are sparse (del Próo et al. 2003). The present study investigates the grazing periodicity, grazing rate, diatom-specific consumption, and feeding preference of early juvenile abalone H. asinina (5 mm) on five benthic diatom species (Amphora sp., Cocconeis sp., Navicula ramosissima, Nitzschia sp., and Tryblionella sp.) in order to determine the most suitable diatom diet to improve the survival of H. asinina settling to early juvenile stage in the hatchery.
Materials and methods
Preparation of diatom feed and conditioning of abalone juveniles
Five non-axenic benthic diatom species, Amphora sp., Cocconeis sp., N. ramosissima, Nitzschia sp., and Tryblionella sp., from the culture collection of the Larval Food Laboratory of SEAFDEC/AQD in Tigbauan, Iloilo, were used. Table 2 shows the descriptions and other information on the diatom species used in this study. The diatom starters were grown in F/2 medium of Guillard and Ryther (1962) in unaerated 500-mL Erlenmeyer flasks (N = 6) with medium renewed every 7–10 days and maintained at 20 °C (SD = 2 °C, N = 10) and 12L:12D photoperiod using one cool-white daylight (Philips, 40 W) fluorescent tube (De la Peña and Franco 2013). The diatoms were harvested in the log phase which served as inocula for diatom cultures in 3-L jars grown in the same conditions which were harvested on the fourth day of culture used as feed for succeeding abalone experiments.
Two-month-old early juvenile abalones with a mean shell length of 5.43 mm (SE = 0.38 mm, N = 150) produced from the natural and spontaneous spawning at the SEAFDEC/AQD abalone hatchery were obtained. These were maintained in 40 cm × 24 cm × 12 cm hapa nets suspended horizontally in 250-L fiberglass tanks prior to use in experiments. Before the feeding experiments, the abalone juveniles were supplied daily with a mixture of the five diatom species in equal densities (1–2 × 105 cells ml−1 of each species) for 1 week to condition them on these diatoms. The holding net cage was provided with continuous aeration in a flow-through system at 2 L min−1 exchange rate. The water salinity and temperature recorded in the juvenile abalone holding tank was 31‰ (SD = 2‰, N = 7) and 28 °C (SD = 1 °C, N = 7), respectively.
Experiment 1: determination of feeding periodicity
Diatom slurry of each strain was collected from 3-L starter jars using a “stiff bristles” Chinese paint brush. Equal densities of each of the 5 diatoms were inoculated into a 3-L jar to obtain an initial density of 1 × 106 cells mL−1 of mixed strain. The inoculum was seeded into 30-mL Petri dishes (d = 90 mm) to have approximately 1.0 × 105 cells cm−2 cover. Capinpin (2007) used initial densities of 4.6 × 105 cells cm−2 whereas Searcy-Bernal et al. (2001) reported maximum grazing rate levels off at high initial densities of 2.0 × 105 cells cm−2. The diatom film was allowed to develop for 5 days by maintaining the seeded petri dishes at light intensity of 53.8 µmol s−1 m−2 using one cool-white daylight fluorescent tube (40 W, Philips), salinity of 27‰ (SD = 1‰, N = 15), and temperature of 25 °C (SD = 2 °C, N = 15).
By the 4th day of diatom film culture, twenty-four 5-mm healthy juveniles were selected from the 250-L holding tank and transferred to a 3-L container with filtered ozonated seawater. These juveniles were provided with continuous aeration while being starved for 24 h before use in the experiment. The mean shell length and body weight of juveniles used in the experiment were 5.4 mm (SD = 0.003 mm, N = 24) and 0.03 g (SD = 0.0 g, N = 24), respectively. Juvenile shell length was measured under a stereomicroscope (Motic Plus Image 2.0, USA) while for weight measurements, a digital analytical balance (Sartorius Genius, Germany) was used. After size measurements were completed, the 30 juveniles were individually transferred to each Petri dish previously seeded with 5-day-old mixed diatoms.
The grazing activity in the next 72 h was observed and recorded. Since abalone is nocturnal (Tahil and Juinio-Menez 1999), the first observation period began at 6 p.m. and every 4 h thereafter. That is at a 4-h interval: 6 p.m.–10 p.m., 10 p.m.–2 a.m., 2 a.m.–6 a.m., 6 a.m.–10 a.m., 10 a.m.–2 p.m., and 2 p.m.–6 p.m. The Petri dishes containing the abalone were set up in an area with transparent roofing where natural light can penetrate with no artificial lighting. The periods of observation were 100% dark for the periods 10 p.m.–2 a.m., 75% dark, and 25% light for 6 p.m.–10 p.m. and 2 a.m.–6 a.m., whereas they were 25% dark and 75% light for periods 2 p.m.–6 p.m., and 100% light for periods 6 a.m.–10 a.m. and 10 a.m.–2 p.m. The light intensity measurements in photosynthetic photon flux density (PPFD) in micromoles per square meter per second were done by placing the probe of the hand-held light meter (Li-Cor Biosciences 250, USA) on the cover of three representative Petri dishes at the start and every 4 h thereafter. Ambient temperatures were recorded at 27 °C (SD = 3 °C, N = 21). Water change was done daily using pre-filtered ozonated seawater and freshwater to ascertain good water quality and to maintain salinity at 29‰ (SD = 3‰, N = 21). Grazing was based on the extent of clearing of diatom cover in the petri dish per time interval. The area grazed was marked with a marker on the underside of the Petri dish to track grazing between 4-h intervals. The percent incidence of juveniles grazing was computed using Eq. 1:
Experiment 2: determination of diatom-specific grazing rate
An experiment following a completely randomized design with 5 treatments with 14 replicates each was conducted for a period of 4 h (6 p.m.–10 p.m.) based on results of experiment 1. This is to determine the diatom-specific grazing rate of H. asinina early juveniles with shell length 5.39 mm (SE = 0.037 mm, N = 70). Three to 5 mL of diatom slurry obtained from the 3-L jar cultures containing the monospecific diatoms were inoculated in 70 Petri dishes (v = 30 mL; d = 90 mm), with each diatom strain of 14 replicates. After the diatom film has settled for 4 days, single abalone juvenile was transferred to each of the diatom-seeded Petri dishes. Additional 15 Petri dishes were seeded with each diatom strain (3 per strain) but without abalone to serve as blanks for diatom density in the absence of grazing. No artificial light was provided during the entire period to simulate dark conditions (6–10 p.m.). Daily water change was also done using pre-filtered ozonated seawater and freshwater. Temperature and salinity were recorded per hour in Petri dish blanks at 25 °C (SD = 1 °C, N = 15) and 27‰ (SD = 1‰, N = 15), respectively.
Three replicated samples of 1 cm2 were collected hourly in the Petri dishes of each diatom without abalone using the new “still bristles” Chinese paintbrush while viewed under an inverted microscope (Olympus CK53, Japan). To ensure collection of samples of exact amount, Petri dishes were marked with lines using a pointed colored pen at 1 cm2 per area using a ruler. Similarly, the blanks were allowed to settle for 4 days simultaneously with those inoculated with abalone prior to collection. Exactly 0.1 mL of brushed diatom cells was collected using a pre-calibrated single-channel pipette (Eppendorf 20–200 µL, Germany), and transferred to 1.5-mL calibrated tubes (Eppendorf) pre-filled with 0.1 mL seawater. Then, 1–2 drops of bleach solution containing 5.25% sodium hypochlorite were added for less than 4 h (Carr et al. 1986) on the samples to prevent the aggregation of benthic diatoms until complete oxidation (disappearance of brownish coloration and presence of white turbid solution) was achieved. Sterile distilled water was added to complete volume of 1 mL then set aside for enumeration at a later time. Diatom counts were done through a direct hemacytometer (Improved Neubauer, Bright Line, BOECO, Germany) method. Diatom density (in cells cm−2) per diatom strain was calculated using Eq. 2:
wherein Cell count is the count taken from a hemacytometer.
The factor of dilution for the 0.1-mL sample is × 10, from 1 mL/0.1 mL = 10.
The grazing rate of a diatom species by the juvenile abalone was estimated based on the area of the diatom that had been cleared every hour during the peak hours as previously identified in experiment 1. The area of diatoms grazed by each abalone juvenile was determined by marking the cleared area with a compact disc labeling pen on the underside of the Petri dish. Observations of grazing were done hourly with each hour color coded until the end of the experiment after a period of 4 h. The area grazed by the juvenile per hour was measured using the Motic Image 2.0 program stereomicroscope (Viana et al. 2007). The camera attached to the stereomicroscope captured the grazed area in each Petri dish, and the contour of the cleared area in the image was traced using an irregularly shaped measuring tool, which automatically calculated the area in square centimeters. The hourly grazing rate of each diatom species by the juvenile abalone was then calculated and expressed as area cleared per hour. The diatom strain-specific grazing rate of the abalone juvenile was computed based on the hourly mean. Grazing rate (cells juvenile−1 h−1) is determined using the diatom cover of blank Petri dish replicates per strain as control density (cells cm−2) multiplied by the area (cm−2) grazed by the abalone in the Petri dish with abalones (Eq. 3):
Experiment 3: feeding preference of 5-mm H. asinina early juveniles
An experiment to determine the feeding preference of H. asinina early juveniles using a randomized block design (RBD) with 5 treatments and 5 replicates was conducted for 48 h. The diatom patch of corrugated polyvinyl material is known to provide an excellent substrate since it offers refuge for abalone juveniles (Dela Peña et al. 2010). Fifty pieces of rectangular corrugated polyvinyl patches each with dimension of 50 mm × 75 mm were roughened with sandpaper, cleaned with water, and placed in 3-L containers containing 104 cells mL−1 monospecific diatom cultures of the 5 diatom species. The patches were allowed to develop sufficient diatom cover for 5 days by providing strong aeration and 1 cool-white daylight fluorescent tube (40 W, Philips; 24D: 0D photoperiod) with a light intensity of 53.8 µmol s−1 m−2 (SD = 0.1 µmol s−1 m−2, N = 15). Light intensities were measured using a hand-held light meter (Li-Cor Biosciences 250, USA). After 5 days, the corrugated polyvinyl patches, 1 per diatom type, were randomly distributed equally into five 7-L circular, wide-mouth basins (upper r = 17.1 cm; lower r = 12.1 cm) at 5 patches per basin. These patches were arranged randomly in a pentagon shape in the basins where 1 patch per diatom species is contained in each basin. Each patch has a 45-mm distance from the center. Once done, 2 L of filtered (5 μm) seawater was added slowly so as not to disturb the diatom film in the patches. Then, 10 juveniles, SL 5.3 mm (SE = 0.01 mm, N = 50) and BW 0.02 g (SE = 0.0 g, N = 50), were positioned at the center of each basin. No lighting was provided throughout the duration of the experiment and was in total darkness with 0 µmol s−1 m−2 PPFD. The percentage incidence of juvenile grazing in the diatom patches was plotted by the hour for 48 h to show the pattern of feeding. Mild aeration was provided, and daily cleaning and water change were done. Cleaning included removal of feces and detached diatoms using a siphon. Temperature was determined at 25 °C (SD = 1 °C, N = 20). Daily water change involved replacing 20% of the total volume of seawater in the basin with fresh filtered seawater or freshwater to maintain salinity at 33‰ (SD = 2‰, N = 20). The relative incidence of juveniles grazing was tracked over time following the formula as described in Experiment 1.
Experiment 4: diatom-specific consumption of H. asinina early juveniles
Twenty-four juveniles from experiment 1, 50 juveniles from experiment 2, and 50 juveniles from experiment 3 of the previous experiments were immediately subjected to gut content analysis as described by Norman-Boudreau et al. (1986) and Zhang et al. (2010) to determine the relative abundance of broken diatom cells. Each of the juveniles from experiments 1 and 3 was transferred into a clean petri dish, allowed to settle, and re-attached to the petri dish. From experiment 2, 10 representative juveniles per diatom were singly placed into a clean petri dish. Debris and loose diatoms adhering to the surface of the abalone juveniles were removed by passing 5–10 rinses of 10-µm filtered ozone-treated seawater over the animals. Cleaned juveniles were then immersed in a saturated ethylenediaminetetraacetic acid disodium salt solution (EDTA) and left to stand at room temperature for 4–20 h. Abalone juveniles were then examined to ensure that the shell decalcified and the diatoms adhering to the shell surface sloughed off. All decalcified abalone were transferred to calibrated Eppendorf tubes, washed with distilled water to remove EDTA solution, and replaced by 2–3 drops of commercial bleach (5.2% sodium hypochlorite). Soaking of decalcified abalone tissues in bleach was up to a maximum of 4 h to completely dissolve the tissues and all other organic matter, except the siliceous frustules of the diatoms. Distilled water was added to a calibrated volume of 1 mL. Treated samples were kept in distilled water until examination (Carr et al. 1986; Zhang et al. 2010) using a light compound microscope (Zeiss-Axiolab, Germany).
The percent relative abundance of each diatom strain in the abalone gut was computed according to Eq. 4 (Zhang et al. 2010):
where \(N\) is the number of broken cells of a diatom species per mL.
and \({N}_{o}\) is the total number of cells per mL.
Determination of survival rates of H. asinina early juveniles
In a separate study, a large-scale feeding experiment (MR de la Peña; unpublished data) was done in 5-unit 1-t fiberglass tanks to determine the growth and survival of 3-mm and 5-mm H. asinina early juveniles fed the five diatom species. Thirty pieces of corrugated polyvinyl plates (23 cm × 35 cm) previously colonized with 20–30% crustose coralline algae for 3–6 months in the SEAFDEC/AQD hatchery were hung horizontally with flow-through sand-filtered seawater (SEAFDEC/AQD abalone hatchery protocol). The tanks were assigned randomly to the five monospecific algal treatments: N. ramosissima, Amphora sp., Tryblionella sp., Cocconeis sp., and Nitzschia sp. Diatom slurry from the laboratory were harvested and used as starter, counted prior to addition to the tanks. The desired feeding rate was based on the dry weight of each species to provide a 10-µg ml−1 amount of food to develop the initial diatom and 10% of the initial volume on succeeding days (Dela Peña et al. 2010). A total of 1500 healthy juveniles averaged 5.29 mm (SE = 0.13) were harvested from SEAFDEC/AQD’s abalone hatchery and distributed randomly in five tanks. In each tank, two sizes of abalone juveniles (3 mm and 5 mm SL) were stocked in hapa nets (40 cm × 24 cm × 12 cm) with 3 pseudo-replicates per size at a stocking density of 50 ind. hapa net−1. The feeding trial was done for 60 days with 300% water change to ascertain provision of clean, oxygenated seawater and maintain normal good water quality (pH range 8.2–8.3; ammonia < 0.01 mg L−1; DO range > 7 mg DO L−1) (SEAFDEC/AQD abalone hatchery protocol). The percent survival was computed as the percentage of postlarvae surviving every 15 days whereas the final percent survival was determined by counting all postlarvae that survived at day 60. Specific growth rates were also determined following the formula of Ricker 1979 (Pereira and Rasse 2007).
Statistical analysis
The values for percent incidence and grazing rate (experiments 1, 2, and 3) were arcsine-transformed whereas the relative abundances of broken cells (experiment 4) were square root transformed before analysis. Tests for normality using the Shapiro–Wilk test and homogeneity of variances using Levene’s test were run on all transformed data. For homogeneous data sets (% incidence or grazing rate), significant differences among treatments (diatom strain or time of day) were analyzed using one-way analysis of variance (ANOVA) in a completely randomized design (CRD) for experiments 2 and 4. Values for experiment 1 were analyzed using repeated measure analysis of variance, tested for Mauchly’s test for sphericity and post hoc test with a Bonferroni adjustment. Values for experiment 3 were analyzed using univariate analysis of variance in a randomized block design (RBD). A Tukey HSD post hoc test was run to determine statistically significant treatments at α = 0.05. Linear trend lines were plotted, and the slopes were compared across time periods per diatom strains that could indicate temporal differences in diatom-strain specific grazing intensity. Statistical procedures were run using the IBM SPSS Statistics 23.0 program.
Results
Experiment 1: feeding periodicity of early juveniles
The grazing activity of abalone juveniles on mixed-species diatom food was significantly more intense or peaked in the period of sunset to nighttime at 6 p.m.–10 p.m. (79%) than at daytime at 10 a.m.–2 p.m. (40%) but not significantly higher in other feeding periods at 10 p.m.–2 a.m. (68%), 2 p.m.–6 pm (64%), and 2 a.m.–6 a.m. (60%), F (1, 23) = 1662.66, p = 0.000 (Fig. 1a). This observation is related to the highest photosynthetic photon flux density (PPFD) of 215.1 µmol m2 s−1 (SD = 3 µmol m2 s−1, N = 15) recorded at 10 a.m.–2 p.m. which resulted to lowest feeding activity. The peak feeding activity that was observed at 6 p.m.–10 p.m. was recorded with the lowest PPFD of 43.7 µmol m2 s−1 (SD = 11.9 µmol m2 s−1, N = 15). This period represented sunset to nighttime. The incidence of grazing of early juveniles and light intensity were not significantly correlated (Fig. 1b, R2 = 0.5442; p = 0.094).
a Feeding periodicity of 5 mm Haliotis asinina early juveniles every 4 h for 3 days. Highest photosynthetic photon flux density (PPFD) of 215.1 ± 3 µmol m2 s−1 at 10 a.m.–2 p.m., which represents the peak of daytime and low feeding activity. Peak feeding activity at 6 p.m.–10 p.m. with the lowest PPFD 43.7 ± 11.9 µmol m2 s−1 that constitutes sunset to night time. Dark bars represent dark times, gray bars as mixed dark and light times and white bars as light times. The different letters on the bars indicate significant differences. Vertical bars indicate standard error. F (1,23) = 1662.66, p = 0.000). b A correlation relationship of light intensity and grazing activity of 5-mm Haliotis asinina early juveniles showing a decreasing incidence of grazing as the photosynthetic flux density (PPFD) increases (slope of − 0.1215, R.2 = 0.5442. F (1,4) = 4.776, p = 0.094
Experiment 2: grazing rate of abalone juvenile per diatom strain
The grazing rate activities of 5-mm abalone juveniles observed during peak hours (6 p.m.–10 p.m.) as determined in the feeding periodicity experiment are expressed as the highest mean grazing rate. The early juveniles that recorded the highest grazing rate were juveniles on N. ramosissima (1.6 × 105 cells juvenile−1 h−1, SE = 2.4 × 104 cells juvenile−1 h−1) but not higher on Cocconeis sp. (1.1 × 105 cells juvenile−1 h−1, SE = 1.4 × 104 cells juvenile−1 h−1), but higher than on Tryblionella sp. (6.3 × 104 cells juvenile−1 h−1, SE = 1.6 × 104 cells juvenile−1 h−1), Nitzschia sp. (5.3 × 104 cells juvenile−1 h−1, SE = 1.3 × 104 cells juvenile−1 h−1), and Amphora sp. (3.4 × 104 cells juvenile−1 h−1, SE = 4.2 × 103 cells juvenile−1 h−1), with no significant difference between the former two and latter three, F (4, 15) = 9.876, p = 0.000 (Fig. 2).
Experiment 3: feeding preference of early abalone juvenile
The juveniles at the onset of feeding explored all the diatom patches. After a feeding spree, activities stabilized, and regardless of diatom strain, it was observed that 100% of the juveniles tend to remain under the patch rather than on top of the patch after a period of exploration. The highest peak of feeding activity was attained at the 15th hour with those abalone feeding on Cocconeis sp. patches (28%, SE = 2%) and the lowest with Tryblionella sp. (10.0%, SE = 0.5%). Comparing the five means of incidence of grazing for the entire period, juveniles frequented patches of Cocconeis sp. (19%, SE = 0.8%), and significantly higher than on Nitzschia sp. patches (16.6%, SE = 0.69%), and significantly higher on N. ramosissima (14.3%, SE = 0.6%) and on Amphora sp. (13.9%, SE = 0.5%), and significantly higher on Tryblionella sp. (8.3%, SE = 0.4%) patches, F (4,192) = 65.817 (p < 0.05) (Fig. 3a).
a Feeding preference of 5-mm Haliotis asinina early juveniles in monospecific diatom patches of Cocconeis sp. (18.6%, SD = 6.2%)a, Nitzschia sp. (16.2%, SD = 5.4%)ab, Navicula ramosissima (13.9%, SD = 4.7%)b, Amphora sp. (13.4%, SD = 3.8%)b, and Tryblionella sp. (7.8%, SD = 3.4%)c monitored hourly for a period of 48 h. F (4,192) = 65.817, p = 0.000. b Linear trend lines of incidence of 5-mm Haliotis asinina early juveniles in monospecific diatom patches monitored hourly for 2 days. Trendlines show positive slope for Cocconeis sp. (0.28, R2 = 0.43); F (1,47) = 36.68, p = 0.000, Nitzschia sp. (0.33, R2 = 0.79); F (1,47) = 144.37, p = 0.000, Navicula ramosissima (0.27, R2 = 0.67); F (1,47) = 98.65, p = 0.000, and Amphora sp. (0.08, R2 = 0.1); F (1,47) = 4.33, p = 0.043. Negative slope for Tryblionella sp. (− 0.11, R.2 = 0.22); F (1,47) = 12.41
Also, using the linear trend line to get the overall incidence pattern from start to finish, a positive slope was obtained for Nitzschia sp. (0.33; r2 = 0.754; p = 0.000), Cocconeis sp. (0.28; r2 = 0.43; p = 0.000), N. ramosissima (0.27; r2 = 0.67; p = 0.000), and Amphora sp. (0.08; r2 = 0.1; p = 0.043), whereas, in contrast a negative slope was obtained for Tryblionella sp. (− 0.11; r2 = 0.22; p = 0.101). The low p values (< 0.05) obtained showed a significant relationship of the diatoms Cocconeis sp., N. ramosissima, and Amphora towards time period except Tryblionella sp. (Fig. 3b).
Experiment 4: diatom-specific consumption of abalone juveniles
The differences in the quantity of diatom cells consumed per juvenile with different experimental units (Petri dish or patch) are shown as relative abundance of broken cells per juvenile per milliliter (Fig. 4a). The mean diatom gut content of abalone juveniles with mixed monospecific diatoms was 24,531 cells juvenile−1 mL−1 (n = 30), the highest of which is Amphora (6302 cells juvenile−1 mL−1), then Nitzschia (1354 cells juvenile−1 mL−1), Cocconeis (313 cells juvenile−1 mL−1), Tryblionella (52 cells juvenile−1 mL−1), and N. ramosissima (0 cells juvenile−1 mL−1). When offered as a single diatom, all the juvenile guts contain all the diatoms species except Cocconeis sp., with N. ramosissima (176,375 cells juvenile−1 mL−1) as the highest, followed by Nitzschia sp. (87,750 cells juvenile−1 mL−1) then Amphora sp. (87,750 cells juvenile−1 mL−1), and least when fed Tryblionella sp. (20,750 cells juvenile−1 mL−1). When diatom food was provided in polyvinyl corrugated patches, all (N = 50) abalone juveniles ingested Amphora sp. (203,495 cells juvenile−1 mL−1). Other diatom species were fed on by a smaller number of abalone juvenile and in less quantity, e.g., only 35 juveniles ingested Cocconeis sp. (18,402 cells juvenile−1 mL−1), 29 juveniles on Nitzschia sp. (31,650 cells juvenile−1 mL−1), 13 juveniles on N. ramosissima (13,700 cells juvenile−1 mL−1), and only 2 juveniles fed on Tryblionella sp. (1500 cells juvenile−1 mL−1).
a Relative abundance of (%) broken cells in guts of 5-mm Haliotis asinina early juveniles per diatom strain in experiments 1 (mixed diatoms), 2 (monospecific diatoms), and 3 (monospecific-preference). b Relative abundance of (%) broken cells in guts of 5 mm Haliotis asinina early juveniles per diatom strain. The different letters on the bars indicate significant differences. F (4,10) = 7.057, p = 0.006. Vertical bars indicate standard error
Overall, in terms of percent relative broken cells, based on combined results of the three gut examination experiments, the highest relative abundance of broken cells found was of Amphora sp. (21.8%, SE = 4.7) and Nitzschia sp. (5.9%, SE = 3.6) than Cocconeis sp. (2.8%, SE = 2.3), N. ramosissima (1.8%, SE = 1.8), and Tryblionella sp. (0.2%, SE = 0.19), with no significant difference between the former two and the latter three (F (4,10) = 7.057, p = 0.006) (Fig. 4b).
Large-scale culture of early juveniles for 60 days
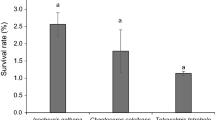
The large-scale feeding experiment conducted by de la Pena (SEAFDEC/AQD Annual Report 2015) showed that the highest percent survival rate after 60 days was attained by 3-mm early juveniles fed Nitzschia sp. (71.3%, SE = 0.7%) but not significantly higher than those fed N. ramosissima (66.0%, SE = 5.0%), Amphora sp. (55.33%, SE = 6.67%), and Cocconeis sp. (51.3%, SE = 7.4%) but significantly higher than those fed Tryblionella sp. (12.0%, SE = 2.3%), F (4,10) = 23.411, p = 0.000). Similarly, the percent survival of 5-mm juveniles was highest in those fed N. ramosissima (65.3%, SE = 3.3%), Amphora sp. (60.7%, SE = 2.4%), Nitzschia sp. (59.3%¸ SE = 4.1%), and Cocconeis sp. (46.0%, SE = 7.2%), but significantly higher than those fed Tryblionella sp. (8.0%, SE = 4.2%), F (4,10) = 25.42, p = 0.000) (Fig. 5).
The % survival of 3-mm and 5-mm Haliotis asinina early juveniles in 1-t FGT for 60 days fed the five diatom strains. The different letters on the bars indicate significant differences. Vertical bars indicate standard error. For 3 mm: F (4,10) = 23.41, p = 0.000, and for 5 mm F (4,10) = 25.42), p = 0.000
Discussion
Diatom characteristics such as size, growth form, adhesiveness, and movement influence the grazing periodicity, grazing rate, feeding preference, and ultimately survival of H. asinina early juveniles. In this study, the grazing rate of abalone was higher in N. ramosissima and Cocconeis sp. while their feeding preference was highest in Cocconeis sp. The gliding movement exhibited by N. ramosissima could have contributed to efficient capture by early juveniles, and although Cocconeis sp. is highly adhesive, the radula of abalone at this stage can now efficiently capture the small cells of Cocconeis sp. Furthermore, Onitsuka et al. (2007) reported that Cocconeis sp. can be easily ruptured during ingestion and passage through the gut of 0.6–0.8 mm SL abalone which explains the low abundance in the gut of early juveniles. Amphora sp. was the most abundant in the gut of abalone early juveniles followed by Nitzschia sp. The high abundance of these 2 diatom species in the gut of abalone can be attributed to their size. It is important to note that among the 5 diatoms, Amphora sp. is the largest, thus explaining its longer retention time in the gut of abalone. Amphora sp. are larger compared to other diatom species but can still fit in the 30-µm mouth size of abalone (2-day post metamorphosis) and be ingested (Kawamura et al. 2005). Amphora is a prostrate and not as adhesive as others, and thus can be easily detached by the juvenile radula and found to be suitable for high larval growth when mixed with Navicula in another abalone species (H. rubra) (Daume et al. 2000). Survival of abalone was similar among 4 diatom species except when fed with Tryblionella sp. indicating the suitability of N. ramosissima, Amphora sp., Nitzschia sp., and Cocconeis sp. as food items to H. asinina early juveniles.
In the study, abalone early juveniles (5 mm) exhibited cryptic behavior as indicated by their tendency to stay underneath diatom patches after exploration above the patches. Additionally, the cryptic tendency of abalone juveniles was demonstrated by an increase in their feeding activity during low light levels (i.e., during dusk and night times) which is similar to the observations of Tahil and Juinio-Menez (1999). This is also in agreement with the study of Hahn (1989) that grazing activity of H. discus hannai seems heightened when light levels are low. Likewise, Searcy-Bernal et al. (2003) reported higher growth rates for H. fulgens at lower light intensity. Searcy-Bernal and Gorrostieta-Hurtado (2007) also reported a higher grazing rate of 37/cell/hour during darkness than in light at 27/cell/hour which then resulted to a higher growth rate in the dark (growth rate = 38 µm/day) than in 10–13 µmol quanta m−2 s−1 lighted conditions (growth rate = 34 µm/day).
The present study revealed that at the population level, grazing is an asynchronous activity. At daytime, a number of abalone individuals are grazing but the peak of grazing activity occurred during nighttime for most members of the population. Grazing behavior offers a selective advantage to juvenile abalone since at this stage they are vulnerable to predation (Takami et al. 1995). The lowest mean grazing rate was achieved in Amphora sp. and could be attributed to its bigger size (11–13 µm L; 3.0–3.5 µm W) as compared to the other diatoms used in this study. As such, bigger size diatom corresponds to a lesser number of cells grazed by abalone per area. The high grazing rate for Cocconeis sp. can be attributed to its small size such that a higher number of Cocconeis sp. cells were grazed per area by the abalone juvenile as confirmed in the study by Matthews and Cook (1995). They reported a higher number of Cocconeis sp. in the gut of 14-day-old H. midae postlarvae as compared to Amphora proteoides, Nitzschia sp., and Nitzschia closterium. On the other hand, in the present study, early juveniles fed N. ramosissima showed higher grazing activity to compensate for its hard cells that are difficult to break as such gut fullness was not readily achieved. Capinpin (2007) likewise reported that N. ramosissima cells could pass intact in 1-mm H. asinina postlarvae. Zhang et al. (2010) also observed that the high ingestion rate of Navicula spp. was due to its low adhesive strength and ability to form clumps. Navicula spp. accounted for 43.2–91.5% of the total cell density as the dominant species in guts of 12- to 24-day old postlarvae after settlement (Zhang et al. 2010). Also, Navicula sp. is the most studied benthic diatom due to its cellular mucus (Kawamura and Takami 1995) and could thrive in cheaper commercial fertilizers (Simental-Trinidad et al. 2001). Cocconeis is strongly adherent but found to stimulate rapid growth in abalone Haliotis diversicolor (Onitsuka et al. 2007). Cocconeis sp. is reported to have inductive potential for settlement of Haliotis discus veliger larvae (Kafuku and Ikenoue 1983; Takami et al. 2002; Capinpin 2007; Iba 2008) and found to be the only diatom found in the gut of H. corrugata (del Próo et al. 2003). Similarly, Cocconeis sp. were highly abundant in the digestive tracts of newly metamorphosed abalone Haliotis kamtschatkana × Haliotis rufenscens (Norman-Boudreau et al. 1986), Haliotis midae (Matthews and Cook 1995), and H. asinina (Capinpin 2007). In this study, H. asinina early juveniles have higher grazing rates than those of 60-day-old H. fulgens juveniles (Searcy-Bernal et al. 2001).
There was an unceasing feeding activity in the first 16 h but lagged in the next succeeding hours. After this latent period, an increasing trend was observed in the feeding activity of abalone juveniles in diatom patches except in Tryblionella sp. Furthermore, juveniles moved from one diatom strain patch to another indicating selection and preference behavior. It was also observed that early juveniles showed the least preference for Tryblionella sp. when given a choice as compared to other benthic diatoms Cocconeis sp., Nitzschia sp., N. ramosissima, and Amphora sp. For instance, it was observed that a juvenile abalone originally settled in Cocconeis sp. had transferred to Tryblionella sp. patch but returned to Cocconeis sp. patch after hours of inactivity in the Tryblionella patch. In another instance, a juvenile was noted to transfer from the Nitzschia sp. patch to the Amphora sp. patch and remained in the Amphora sp. patch until the end of the experiment. This behavior depicts the preference of early juveniles for a particular diatom species. Matthews and Cook (1995) also reported preference of H. midae for related prostrate diatoms Cocconeis sublittoralis Hendey and Amphora proteoides Hustedt. Still, they would consume the more loosely packed diatoms like Diploneis placida and Nitzschia palea if their preferred diatom strain was not available. Navicula sp. are type A gliding prostate diatoms (Kawamura 1996). They are also widely used as settlement cues for postlarval abalone because they improve the growth and survival of abalone whether in combination with other inducers, diatoms, green filamentous algae, and artificial diets (Uki and Kikuchi 1979; Kawamura and Takami 1995; Brown et al. 1997; Kawamura et al. 1998; Searcy-Bernal and Anguiano‐Beltrán 1998; Daume et al. 1999; Gomez-Montes et al. 2003; Day et al. 2004; Viana et al. 2007; Xing et al. 2008; dela Peña et al. 2010; Courtois de Vicose et al. 2010; Tung and Alfaro 2011).
During the gut examination of early juveniles, Amphora sp. was found to be the dominant diatom species. As shown in Fig. 4a, Amphora sp. consistently demonstrated the highest relative abundance of percentage broken cells for 3 experimental setups as compared with other diatoms, confirming the results of Kawamura (1996). Zhang et al. (2010) also found Amphora spp. as the most abundant diatom in the gut of early postlarvae of H. diversicolor supertexta. Sawatpeera et al. (1998) examined the guts of bigger H. asinina (68.3 mm SL) and 99.3% benthic diatoms were under the genera Nitzschia, Amphora, and Cocconeis which indicates the ingestion of these diatoms by abalone even until later adult stages. Creencia et al. (2016) reported that Navicula, Cocconeis, and Nitzschia were found in the stomach of 4- to 75-day-old H. diversicolor with relatively increasing size distribution as the abalone ages. Cocconeis spp. is a type B diatom (Kawamura 1996; Kawamura et al. 2005) and has highly adhesive strength, but several reports identified it as food during settlement to the early juvenile stage of abalone H. discus (Kafuku and Ikenoue 1983; Takami et al. 2002; Capinpin 2007) which resulted in a rapid growth rate (Onitsuka et al. 2007). Likewise, Cocconeis sp. is also detected as one of the dominant diatoms in the digestive tracts of newly metamorphosed abalone (Norman-Boudreau et al. 1986; Matthews and Cook 1995; Capinpin 2007). Furthermore, Onitsuka et al. (2007) found that Cocconeis sp. can easily be ruptured during ingestion and during passage through the gut in larger than 0.6–0.8-mm SL abalone H. diversicolor.
The grazing capability of abalones towards a particular diatom depends on the attachment, size, and frustule strength of the diatom (Kawamura et al. 1998). In this study, early juveniles showed a higher grazing rate in N. ramosissima followed by Cocconeis sp. However, Cocconeis sp. was less abundant in the gut and this may be due to several reports that cells of Cocconeis sp. can be easily ruptured (Daume et al. 1997; Roberts et al. 1999) and completely digested, thus not visible upon examination of the gut. Among the diatom species, Tryblionella sp. does not appear to be a suitable food item for early juvenile abalone (5 mm) because they are least preferred, and a lower grazing rate was observed in this diatom species. In addition, the low survival of early juvenile abalone fed Tryblionella sp. further supported the non-suitability of this diatom. The high mortality can be linked to starvation which then leads to inadequate nutrition for the maintenance, growth, and development of early juveniles. Indeed, the growth and survival of abalone are dependent on the digestibility and nutritional properties of diatom species as reported by Gordon et al. (2006), Zhang et al. (2010), and Ding et al. (2016). So far, there are no studies on Tryblionella sp. As live food for abalone juveniles, but this diatom species is commonly found in the stomach of limpet Fissurella volcano (León-Cisneros et al. 2017) and as an epiphytic diatom associated with red mangrove (Siqueiros-Beltrones and Lopez-Fuerte 2004).
Conclusion
This study showed that diatom species Cocconeis sp., Nitzschia sp., Amphora sp., and N. ramosissima are the most suitable live food for H. asinina early juveniles (5 mm). Feeding periodicity is not strictly nocturnal for early juveniles but reaches its peak during low light intensity periods. On the practical side, since peak feeding of abalone takes place during nighttime or in the absence of light, provision of artificial light is no longer necessary thus reducing the hatchery operation cost. In addition, the present findings can serve as the basis for developing a more efficient feeding regimen to improve abalone hatchery production and in designing the hatchery.
Data availability
The data generated from this study are available from the corresponding author upon request.
References
Angell A, Pirozzi I, de Nys R, Paul N (2012) Feeding preferences and the nutritional value of tropical algae for the abalone Haliotis asinina. PLoS ONE 7:e38857. https://doi.org/10.1371/journal.pone.0038857
Brown M, Jeffrey S, Volkman J, Dunstan G (1997) Nutritional properties of microalgae for mariculture. Aquaculture 151:315–331
Capinpin E, Corre K (1996) Growth rate of the Philippine abalone, Haliotis asinina fed an artificial diet and macroalgae. Aquaculture 144:81–89. https://doi.org/10.1016/S0044-8486(96)01332-4
Capinpin EC Jr (2007) Feeding, growth, and survival of postlarval abalone Haliotis asinina on different benthic diatoms. Sci Diliman 19:45–59
Carbajal-Miranda M, Sánchez-Saavedra M, Simental J (2005) Effect of monospecific and mixed benthic diatom cultures on the growth of red abalone postlarvae Haliotis rufescens (Swainson 1822). J Shellfish Res 24:401–405. https://doi.org/10.2983/0730-8000(2005)24[401:EOMAMB]2.0.CO;2
Carr J, Hergenrader G, Troelstrup Jr NH (1986) A simple inexpensive method for cleaning diatoms. Transactions of the American Microscopical Society. 152–157
Carter C (1982) A technique for direct microscopic observation of periphyton assemblages on aquatic macrophytes. J Aquat Plant Manage 20:53–56
Chen Y-C (2007) Immobilization of twelve benthic diatom species for long-term storage and as feed for postlarval abalone Haliotis diversicolor. Aquaculture 263:97–106. https://doi.org/10.1016/j.aquaculture.2006.12.008
Correa-Reyes J, Sánchez-Saavedra M, Siqueiros-Beltrones D, Norberto F (2001) Isolation and growth of eight strains of benthic diatoms, cultured under two light conditions. J Shellfish Res 20:603–610
Correa-Reyes J, Sánchez-Saavedra M, Viana M, Flores-Acevedo N, Peláez C (2009) Effect of eight benthic diatoms as feed on the growth of red abalone (Haliotis rufescens) postlarvae. J Appl Phycol 21:387–393. https://doi.org/10.1007/s10811-008-9381-x
Courtois de Vicose G, Vera M, Bilbao A, Izquierdo M (2010) Larval settlement of Haliotis tuberculata coccinea in response to different inductive cues and the effect of larval density on settlement, early growth, and survival. J Shellfish Res 29:587–591
Creencia L, Noro T, Fukumoto M (2016) Composition, size and relative density of diatoms in stomach of 4-to-75-day-old juvenile abalone (Reeve). Palawan Sci 8:1–12
Daume S. (2003). Early life history of abalone (Haliotis rubra, H. laevigata): settlement, survival, and early growth. Final report for FRDC Project 1998/306 (psu.edu)
Daume S (2006) The roles of bacteria and micro and macro algae in abalone aquaculture: a review. J Shellfish Res 25:151–157. https://doi.org/10.2983/0730-8000(2006)25[151:TROBAM]2.0.CO;2
Daume S, Brand S, Woelkerling J (1997) Effects of postlarval abalone (Haliotis rubra) grazing on the epiphytic diatom assemblage of coralline red algae. Molluscan Res 18:119–130. https://doi.org/10.1080/13235818.1997.10673686
Daume S, Brand-Gardner S, Woelkerling W (1999) Preferential settlement of abalone larvae: diatom films vs. non-geniculate coralline red algae. Aquaculture 174:243–254
Daume S, Krsinich A, Farrell S, Gervis M (2000) Settlement and early growth of Haliotis rubra in response to different algal species. J Appl Phycol 12:479–488. https://doi.org/10.1023/A:1008110828581
Day R, Gilmour P, Huchette S (2004) Effects of density and food supply on postlarval abalone: behaviour, growth and mortality. J Shellfish Res 23:1009–1018
De la Peña M (2007) Cell growth and nutritive value of the tropical benthic diatom, Amphora sp., at varying levels of nutrients and light intensity, and different culture locations. J Appl Phycol 19:647–655. https://doi.org/10.1007/s10811-007-9189-0
De la Peña M, Bautista J, Buen-Ursua S, Bayona N, Titular V (2010) Settlement, growth and survival of the donkey-s ear abalone Haliotis asinina (Linne) in response to diatom diets and attachment substrate. Philipp J Sci 139:27–33
De la Peña M, Franco A (2013). Culture of marine phytoplankton for aquaculture seed production. Aquaculture extension manual 55. Southeast Asian Fisheries Development Center, Aquaculture Department. pp 33. http://repository.seafdec.org.ph/handle/10862/3045
del Próo S, Serviere-Zaragoza E, Beltrones D (2003) Natural diet of juvenile abalone Haliotis fulgens and H. corrugata (Mollusca: Gastropoda) in Bahia Tortugas. Mexico Pac Sci 57:319–324. https://doi.org/10.1353/psc.2003.0025
Ding J, Huang B, Hu Y, Wang X (2016) The effects of different monospecific benthic diatoms on larval settlement, metamorphosis, survival, and growth of Haliotis asinina Linnaeus in the South China Sea. Aquac Int 25:367–377. https://doi.org/10.1007/s10499-016-0035-8
Ebert E, Houk J (1984) Elements and innovations in the cultivation of red abalone Haliotis rufescens. Aquaculture 39:375–392. https://doi.org/10.1016/0044-8486(84)90279-5
Encena V, Bayona N. (2010). Farming of the tropical abalone. Aquaculture extension manual 49, Southeast Asian Fisheries Development Center. p 24. http://repository.seafdec.org.ph/handle/10862/2157
Eskinazi-Sant’Anna E, Maia-Barbosa P, Barbosa F (2002) On the natural diet of Daphnia laevis in the eutrophic Pampulha reservoir (Belo Horizonte, Minas Gerais). Braz J Biol 62:445–452. https://doi.org/10.1590/s1519-69842002000300007
FAO (2000) FAO Yearbook of Fishery Statistics 83, 1989–1998. Italy, Rome
FAO (2009) The state of food and agriculture. FAO, Rome, Italy
FAO (2017) Global fish market reports, FAO, Rome, Italy
FAO (2021) Global capture production, fishery statistical collection. FAO, Rome, Italy
Fermin A, dela Peña M, Gapasin R, Teruel M, Ursua SM, Encena II VC, Bayona NC (2008) Abalone hatchery. SEAFDEC Aquaculture Department, Tigbauan, Iloilo, Philippines
Gallardo W, Buen SM (2003) Evaluation of mucus, Navicula, and mixed diatoms as larval settlement inducers for the tropical abalone Haliotis asinina. Aquaculture 221:357–364. https://doi.org/10.1016/S0044-8486(03)00121-2
Gapasin R, Polohan B (2005) Response of the Tropical abalone, Haliotis asinina, larvae on combinations of attachment cues. Hydrobiologia 548:301–306. https://doi.org/10.1007/s10750-005-0754-8
Gomez-Montez L, Garcia-Esquivel Z, D’ Abramo L, Shimada A, Vasquez-Pelaez C, Viana M (2003) Effect of dietary protein: energy ratio on intake, growth and metabolism of juvenile green abalone Haliotis fulgens. Aquaculture 220:769-780
Gordon N, Neori A, Shpigel M, Lee J, Harpaz S (2006) Effect of diatom diets on growth and survival of the abalone Haliotis discus hannai postlarvae. Aquaculture 252:225–233. https://doi.org/10.1016/j.aquaculture.2005.06.034
Guillard R, Ryther J (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol 8:229–239. https://doi.org/10.1139/m62-029
Hahn KO (1989) Handbook of culture of abalone and other marine gastropods. CRC Press, Boca Raton
Iba W (2008) Nutrition requirement of cultured abalone post larvae and juveniles a review. Indones Aquac J 3:45–57. https://doi.org/10.15578/iaj.3.1.2008.45-57
Infante A (1978) Natural food of herbivorous zooplankton of Lake Valencia (Venezuela). Arch Hydrobiol 82:347–358
Jarayabhand P, Paphavasit N (1996) A review of the culture of tropical abalone with special reference to Thailand. Aquaculture 140:159–168. https://doi.org/10.1016/0044-8486(95)01194-3
Kafuku T, Ikenoue H (1983) Modern methods of aquaculture in Japan. Development in Aquaculture and Fisheries Science. Elsevier Scientific Pub., Amsterdam, NL
Kawamura T (1996) The role of benthic diatoms in the early life stages of the Japanese abalone (Haliotis discus hannai). In: Watanabe Y, Yamashita Y, Oozeki Y (eds) Survival strategies in early life stages of marine resources. A A Balkema, Rotterdam, NL, pp 355–367
Kawamura T, Roberts R, Nicholson CM (1998) Factors affecting the food value of diatom strains for post-larval abalone Haliotis iris. Aquaculture 160:81–88. https://doi.org/10.1016/S0044-8486(97)00223-8
Kawamura T, Roberts R, Takami H (2005) Importance of periphyton in abalone culture. In: Azim ME, Verdegem MCJ, van Dam AA, Beveridge MCM (eds) Periphyton: ecology, exploitation and management. CAB International Publishing, London, pp 269–284
Kawamura T, Takami H, Roberts R, Yamashita Y (2001) Radula development in abalone Haliotis discus hannai from larva to adult in relation to feeding transitions. Fish Sci 67:596–605. https://doi.org/10.1046/j.1444-2906.2001.00295.xKawamuraT,RobertsRD,YamashitaY(2001)RaduladevelopmentinabaloneHaliotisdiscushannaifromlarvatoadultinrelationtofeedingtransitions.FishSci67:596-605.10.1046/j.1444-2906.2001.00295.x
Kawamura T, Takami H (1995) Analysis of feeding and growth rate of newly metamorphosed abalone Haliotis discus hannai fed on four species of benthic diatom. Fish Sci 61:357–358. https://doi.org/10.2331/fishsci.61.357
Kruatrachue M, Sawatpeera S, Chitramvong Y, Sonchaeng P, Upatham ES, Sangpradub S. (2004). Comparative growth performance of early juvenile fed various artificial diets. Journal of Shellfish Research 23 (1). https://link.gale.com/apps/doc/A118543933/AONE?
Lebata-Ramos J, Mediavilla J, Solis E, Sibonga R, Alicante F, Dionela C (2021) Nursery and grow-out culture of the abalone Haliotis asinina on a reef flat: a comparison of growth and survival using different culture containers. Aquaculture 541:736786. https://doi.org/10.1016/j.aquaculture.2021.736786
León-Cisneros K, Mazariegos-Villarreal A, Miranda-Saucedo C, Argumedo-Hernández U, Siqueiros-Beltrones D, Serviere-Zaragoza E (2017) Diet of the volcano keyhole limpet fissurella volcano (Gastropoda: Fissurellidae) in subtropical rocky reefs of the Baja California Peninsula. Pac Sci 71:57–66. https://doi.org/10.2984/71.1.5
Madin L, Cetta C (1984) The use of gut fluorescence to estimate grazing by oceanic salps. J Plankton Res 6:475–492. https://doi.org/10.1093/plankt/6.3.475
Maliao R, Webb E, Jensen K (2003) A survey of stock of the donkey’s ear abalone, Haliotis asinina L. in the Sagay Marine Reserve, Philippines: evaluating the effectiveness of marine protected area enforcement. Fish Res 66:343–353. https://doi.org/10.1016/S0165-7836(03)00181-4
Martinez-Goss, (1997) New Nitzschia and Tryblionella from Laguna de Bay, Philippines. Proc Acad Natl Sci Phila 147:119–123
Matthews I, Cook P (1995) Diatom diet of abalone post-larvae (Haliotis midae) and the effect of pre-grazing the diatom overstorey. Mar Freshw Res 46:545–548. https://doi.org/10.1071/MF9950545
Najmudeen T, Victor A (2004) Seed production and juvenile rearing of the tropical abalone Haliotis varia Linnaeus 1758. Aquaculture 234:277–292. https://doi.org/10.1016/j.aquaculture.2003.12.013
Norman-Boudreau K, Burns D, Cooke C, Austin A (1986) A simple technique for detection of feeding in newly metamorphosed abalone. Aquaculture 51:313–317. https://doi.org/10.1016/0044-8486(86)90322-4
Onitsuka T, Kawamura T, Ohashi S, Horii T, Watanabe Y (2007) Dietary value of benthic diatoms for post-larval abalone Haliotis diversicolor associated with feeding transitions. Fish Sci 73:295–302. https://doi.org/10.1111/j.1444-2906.2007.01335.x
Onitsuka T, Kawamura T, Ohashi S, Iwanaga S, Horii T, Watanabe Y (2010) Effects of delayed metamorphosis and delayed post-settlement feeding on post-larval survival and growth of the abalone Haliotis diversicolor. Aquaculture 298:239–244. https://doi.org/10.1016/j.aquaculture.2009.11.009
Paul A, Paul J, Hood D, Neve R (1977) Observations on food preferences daily ration requirements and growth of Haliotis kamtschatkana in captivity. Veliger 19:303–309
Pereira L, Rasse S (2007) Evaluation of growth and survival of juveniles of the Japanese abalone Haliotis discus hannai in two culture systems suspended in tanks. J Shellfish Res 26:769–776. https://doi.org/10.2983/0730-8000(2007)26[769:EOGASO]2.0.CO;2
Roberts R, Kawamura T, Nicholson C (1999) Growth and survival of postlarval abalone (Haliotis iris) in relation to development and diatom diet. J Shellfish Res 18:243–250
Round FE, Crawford, RM, Mann DG (1990) Diatoms: biology and morphology of the genera. Cambridge University Press, Cambridge
Salayo N, Azuma T, Castel R, Barrido RT, Tormon-West DHM, Shibuno T (2020) Stock enhancement of abalone Haliotis asinina in multi-use buffer zone of Sagay Marine Reserve in the Philippines. Aquaculture 523:735138
Sawatpeera S, Upatham E, Kruatrachue M, Ingsrisawang V, Singhagraiwan T, Chitramvong Y, Parkpoomkamol K (1998) BIOLOGY-determination of gut contents of Thai abalone Haliotis asinina Linnaeus. J Shellfish Res 17:765–770
SEAFDEC/AQD Annual Report 2012, Southeast Asian Fisheries Development Center, Tigbauan Iloilo
Searcy-Bernal R, Anguiano‐Beltrán C (1998) Optimizing the concentration of gamma‐aminobutyric acid (GABA) for inducing larval metamorphosis in the red abalone Haliotis rufescens (Mollusca: Gastropoda). J World Aquac Soc 29(4):463–470. https://doi.org/10.1111/j.1749-7345.1998.tb00670.x
Searcy-Bernal R, Gorrostieta-Hurtado E (2007) Effect of darkness and water flow rate on survival, grazing and growth rates of abalone Haliotis rufescens postlarvae. J Shellfish Res 26:789–794. https://doi.org/10.2983/0730-8000(2007)26[789:EODAWF]2.0.CO;2
Searcy-Bernal R, Casandra A, Esparza-Hernández A (2003) The effect of irradiance on the survival and growth of abalone postlarvae Haliotis fulgens fed Navicula incerta. Aquaculture 228:237–248. https://doi.org/10.1016/S0044-8486(03)00269-2
Searcy-Bernal R, Velez-Espino LA, Anguiano-Beltran C (2001) Effect of biofilm density on grazing and growth rates of Haliotis fulgens postlarvae. J Shellfish Res 20:587–592
Setyono DED (2005) Abalone (Haliotis asinina L) 5 Early juvenile rearing and ongrowing culture. Oceana 30:1–10. https://doi.org/10.14203/mri.v30i0.420
Simental-Trinidad J, Sanchez—Saavedra M, Correa-Reyes J (2001) Biochemical composition of benthic marine diatoms using as culture medium a common agricultural fertilizer. Journal of Shellfish Research 20:611–617
Singhagraiwan T, Doi M (1993) Seed production and culture of a tropical abalone, Haliotis asinine Linne. Research Project of Fishery Resource Development in the Kingdom of Thailand, Bangkok, Thailand
Singhagraiwan T, Sasaki M (1991) Breeding and early development of donkey’s ear abalone, Haliotis asinine Linne. Thail Mar Fish, Res Bull 2:15–20
Siqueiros-Beltrones D, Lopez-Fuerte F (2004) Benthic diatoms associated to red mangrove (Rhizophora mangle L.) prop roots in Baha Magdalena, BCS Mexico, Biologia Troical 54
Siqueiros-Beltrones D, Romero G (2004) Benthic diatom assemblages in an abalone (Haliotis spp.) habitat in the Baja California Peninsula. Pac Sci 58:435–446. https://doi.org/10.1353/psc.2004.0027
Siqueiros-Beltrones D, Voltolina D (2000) Grazing selectivity of red abalone (Haliotis rufescens) post-larvae on benthic diatom films under culture conditions. J World Aquac Soc 31:239–246. https://doi.org/10.1111/j.1749-7345.2000.tb00359.x
Siqueiros-Beltrones D, Argumedo-Hernández U, López-Fuerte F (2017) Diversity of benthic diatoms in the Guerrero Negro Lagoon (El Vizcaíno Biosfere Reserve), Baja California Peninsula, Mexico. Rev Mex Biodiversidadz 88:21–35. https://doi.org/10.1016/j.rmb.2017.01.026
Smith W (1853) A synopsis of the British Diatomaceae, vol 1. John Voorst, London, pp 89, 31
Stott A, Takeuchi T, Koike Y (2004) An alternative culture system for the hatchery production of abalone without using livefood. Aquaculture 236:341–360. https://doi.org/10.1016/j.aquaculture.2004.03.008
Tahil A, Dy D (2015) Effects of reduced pH on the growth and survival of postlarvae of the donkey’s ear abalone, Haliotis asinina (L.). Aqua Int: Environ Sci 23:141–153. https://doi.org/10.1007/s10499-014-9804-4
Tahil A, Juinio-Menez M (1999) Natural diet, feeding periodicity and functional response to food density of the abalone, Haliotis asinina L., (Gastropoda). Aquac Res 30:95–107. https://doi.org/10.1046/j.1365-2109.1999.00294.x
Takami H, Kawamura T, Yamashita Y (2002) Effects of delayed metamorphosis on larval competence, and postlarval survival and growth of abalone Haliotis discus hannai. Aquaculture 213:311–322. https://doi.org/10.1016/S0044-8486(02)00338-1
Takami H, Yamakawa H, Nakano H (1995) Survival and physiological stress of juvenile disk abalone Haliotis discus discus during long-term starvation. Fish Sci 61:111–115. https://doi.org/10.2331/fishsci.61.111
Uki N, Kikuchi S (1979) Food value of six benthic micro-algae on growth of juvenile abalone, Haliotis discus hannai. Bull Tohoku Reg Fish Res Lab 40:47–52
Viana M, Reyes J, Lazo J, Frías-Díaz R, Durazo E, Peláez C (2007) Digestive physiology and metabolism of green abalone Haliotis fulgens from postlarvae to juvenile, fed three different diatoms. Aquaculture 271:449–460. https://doi.org/10.1016/j.aquaculture.2007.04.072
Watson D, Daume S, Prince J, Beazley L, Knott B (2004) The influence of light intensity on the density of different diatoms as feed for juvenile greenlip abalone (Haliotis laevigata). Aquaculture 235:345–359. https://doi.org/10.1016/j.aquaculture.2004.01.039
Witkowski A, Lange-Bertalot H, Kocliokek P, Ruppel M, Wawrzyniak-Wydrowska B, Bak M, Brzezinska A (2004) Four new species of Nitzschia ssect. Tryblionella (Bacillariophyceae) resembling N. parvula. Phycologia 43:579–595
Xing RI, Wang C, Xb C, Chang Y (2008) Settlement, growth and survival of abalone, Haliotis discus hannai, in response to eight monospecific benthic diatoms. J Appl Phycol 20:47–53. https://doi.org/10.1007/s10811-007-9179-2
Yu SN, Duan ZL, Huang B, Wang YL, Wang XB, Li Y, Zhang SF, Zhang YD, Wang T, Qiu Y (2014) Effects of different diet combinations on the growth of juvenile abalone (Haliotis asinina Linnaeus). Anim Feed Sci Technol 194:106–112. https://doi.org/10.1016/j.anifeedsci.2014.04.018
Zhang Y, Gao Y, Liang J, Chen C, Zhao D, Li X, Li Y, Wu W (2010) Diatom diet selectivity by early post-larval abalone Haliotis diversicolor supertexta under hatchery conditions. Chin J Ocean Limnol 28:1187–1194. https://doi.org/10.1007/s00343-010-0019-x
Acknowledgements
We thank Mr. Vicente Balinas for the review of the statistical analysis of our data; Dr. Josefa Tan-Fermin, Dr. Evelyn Grace Ayson, and the anonymous reviewers for their helpful comments; and Mary Anne Mandario for the review and editing of our final manuscript. Special thanks are given to the phycology laboratory staff (Jilla Alcalde-Tornalejo, Ellen Grace Ledesma, Loina Henzel Delgado-Maquilan), the abalone hatchery staffs (Nestor Bayona and Rafael Barrido), and my trainee PJ Sajol for their invaluable assistance.
Funding
The Department of Science and Technology—Philippine Council for Agriculture and the Natural Resources Research and Development funded this study under the National Abalone Program of the Philippines, project on refinement of hatchery technology for the donkey’s ear abalone (Study code: NR04-M2009T), with the Aquaculture Department of the Southeast Asian Fisheries Development (SEAFDEC/AQD) as implementing agency.
Author information
Authors and Affiliations
Contributions
All authors (AVF, MRDP, and MFJN) contributed to the study conception, design, and writing of the draft and final manuscript. MRDP is the overall project leader and funding manager. AVF prepared the materials, performed the experiments and data collection, and analyzed the data. All the authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study complied with the institutional, national, and international animal ethics guidelines and policy, and was approved by the Aquaculture Department, Southeast Asian Fisheries Development Center (SEAFDEC/AQD) ethics committee.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling editor: Gavin Burnell.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Villa-Franco, A.U., dela Peña, M.R. & Nievales, M.F.J. Grazing periodicity, grazing rate, feeding preference, and gut examination of early juveniles of abalone Haliotis asinina–fed five benthic diatom species. Aquacult Int 30, 2343–2364 (2022). https://doi.org/10.1007/s10499-022-00906-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-00906-7