Abstract
Two series of experiments were conducted to determine suitable growth factors for the mass propagation of the local algal isolate Amphora sp. A higher growth rate of 0.2 doubling (μ) day−1 was attained at a lower photosynthetic photon flux density (PPFD; 11.4 μmol photon m−2s−1) compared to cultures exposed to higher levels of PPFD (16.1 μmol photon m−2s−1, −0.1 μ day −1; 31.3 μmol photon m−2s−1, 0.0 μ day−1). Cultures located inside the laboratory had a significantly higher cell density (133 × 104 cells cm−2) and growth rate (0.3 μ day−1) compared to those located outdoors (100 × 104 cells cm−2, 0.2 μ day−1). A comparison of nutrient medium across two locations showed that lipid content was significantly higher in cultures enriched with F/2MTM (macronutrients + trace metals) and F/2MV (macronutrients + vitamins). Saturated fatty acids were also present in high concentrations in cultures enriched with F/2M (macronutrients only). Significantly higher amounts of saturated fatty acids were observed in cultures located outdoors (33.1%) compared to those located indoors (26.6%). The protein, carbohydrates and n-6 fatty acid content of Amphora sp. were influenced by the location and enrichment of the cultures. This study has identified growth conditions for mass culture of Amphora sp. and determined biochemical composition under those culture conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Benthic diatoms in abalone culture act as inductors for larval settlement and as food for the early juvenile stages (Ebert and Houk 1984; Uki and Kikuchi 1979; Kawamura et al. 1995). They excrete extracellular polymeric substances that play an important role in abalone larval settlement (Hoagland et al. 1993; Kawamura and Takami 1995). The current juvenile seed production of the tropical abalone Haliotis asinina in the Southeast Asian Fisheries Development Center Aquaculture Department (SEAFDEC/AQD) relies on the natural populations of mixed diatoms in the rearing tanks. This culture method does not provide the continuous supply of suitable diatom species needed by the abalone larvae (Ebert and Houk 1984; Norman-Boudreau et al. 1986). The provision of preferred diatom species for the growth and survival of juvenile abalones is required (Uki and Kikuchi 1979). Furthermore, the biomass produced from mixed diatom species is inadequate for growing larvae. A high production of microalgal biomass is one of the critical requirements of successful shellfish hatchery aquaculture (Persoone and Claus 1980; De Pauw et al. 1983). In the Philippines, Navicula sp. improved larval settlement in the tropical abalone H. asinina (Gallardo and Buen 2003). Culture conditions favoring maximum cell growth of Amphora sp.—an endemic species—must be identified for cost-effective production. It is important to determine changes in the chemical composition of algae, since this could affect its nutritive value when it is used as larval feed. The biochemical composition of microalgae varies as a function of inorganic nutrient enrichment, temperature and light (Harrison et al. 1977; Sakshaug and Holm-Hansen 1977; Fabregas et al. 1985, 1986; Thompson et al. 1992; Wifkors 1986; Wang et al. 1997; Simental-Trinidad et al. 2001). An all nutrient-enriched medium such as F/2 (Guillard and Ryther 1962) is expensive and difficult to procure. The use of agricultural-grade fertilizer in the production of microalgae has been shown to produce sufficient algal biomass (Renaud et al. 1991; Gonzales-Rodriguez and Maestrini 1984; Fabregas et al. 1987; Simental-Trinidad et al. 2001). In this study, Amphora sp., a new isolate from SEAFDEC/AQD, was evaluated for its capacity to grow in outdoor conditions, since the use of a temperature-controlled culture room entails additional hatchery cost. Consideration of the biochemical composition of microalgae as food for abalone postlarvae could lead to the production of diatoms with better nutritional value, promoting higher growth and survival of abalone in the hatchery. Amphora sp. has not yet been identified to the species level because of its small size.
The aim of this study was to evaluate the growth rate and nutritive value of the newly isolated benthic diatom, Amphora sp., by manipulating the enrichment medium, photosynthetic photon flux density (PPFD), and culture location.
Materials and methods
Algal culture
In order to select species that can adapt to Philippine environmental conditions, the dominant microalga identified as Amphora sp. was isolated from SEAFDEC/AQD abalone hatchery tanks. Amphora sp. is a raphid pennate diatom characterized by a smooth, arched valve on the dorsal side with a weakly convex ventral margin. The ends are slightly protracted and barely rostrate. Approximately 22–26 parallel and evenly distributed striae are present per 10 μm body length. The alga measures 10.8–12.87 μm in length and 2.97–3.44 μm in width—a size suitable for the early juvenile stage of abalone (Norman-Boudreau et al. 1986). It is classified as a small periphitic diatom that is acceptable for sea urchin larvae (Ito and Kitamura 1997). This species can form flat diatom communities and is strongly adhesive—two characteristics considered favorable for abalone larvae by Kawamura and Kikuchi (1992), Kawamura (1994) and Kawamura et al. (1998). Amphora sp. belongs to family Naviculaceae, which is identified as an acceptable food for abalone (Uki and Kikuchi 1979; Grant 1981; Ohgai et al. 1991). Amphora sp. was isolated from a mixed diatom slurry using the microcapillary pipette method (Hoshaw and Rosowski 1973). The diatom slurry was scraped from polyvinyl corrugated plates with attached abalone juveniles. This diatom was chosen since it was the dominant population at the time of isolation. It was precultivated for several generations under laboratory conditions of light (10.0 μmol photon m−2s−1) and temperature (22–23°C) before the start of the experiment.
The alga was cultured in 8 L rectangular acrylic glass aquaria containing 5 L culture medium. Ten pieces of 12 × 12 × 12 cm2 area acrylic glass plates were installed in each aquarium to serve as diatom settlement plates. The plates were provided with orange polyvinyl stand pipes to keep them in an upright and slanting position. A two-point mild aeration was provided by glass-wool filtered air to provide an effective gas exchange and to allow the alga to settle onto the acrylic glass plates.
Seawater (32 g L−1) obtained by directly pumping water 30 m from the shore, was passed through a sand filter before stocking to the concrete reservoir. The seawater was re-filtered in a smaller sand filter facility in our algal laboratory. Before being used for medium preparation, it was re-filtered through a 5 μm-rated filter bag and sterilized following the method of de la Peña and Villegas (2005). A standard inoculum (non-axenic) density of 50,000 cells mL−1 was added to each aquarium. Cell counts were determined with a Neubauer hemocytometer (AO Brightline; American Optical, Buffalo, NY) and a compound microscope (Martinez et al. 1975). The light intensity was measured at the center of the culture vessel using a LI-COR light meter (model LI-250; LI-COR, Lincoln, NE).
Effects of light intensity and culture medium
Two essential factors in the culture of Amphora sp. were tested in the first experiment: culture medium and light intensity. The basal culture medium was the F/2 medium (Guillard and Ryther 1962) composed of macronutrients (NaNO3, 42.08 mg L−1; NaH2PO4·H2O, 5.0 mg L−1; FeCl3·6H2O, 1.5 mg L−1; Na2EDTA, 5.0 mg L−1), trace metals (CuSO4·5H2O, 0.01 mg L−1; ZnSO4·7H20, 0.02 mg L−1; CoCl2·6H20, 0.20 mg L−1; MnCl2·4H2O, 3.60 mg L−1; NaMoO4·2H20, 0.13 mg L−1) and vitamins (B1, 0.20 mg L−1; B12, 0.001 mg L−1; Biotin, 0.001 mg L−1). This basal medium was modified into three treatments (T1–T3), as F/2MTM (macronutrients + trace metals; T1), F/2MV (macronutrients + vitamins; T2) and F/2M (macronutrients; T3) and tested for the growth of the diatom for an 8-day culture period. The next factor tested was the response of the diatom to PPFD, i.e., 11.4 μmol photons m−2s−1 (one bulb), 16.1 μmol photons m−2s−1 (two bulbs) and 31.3 μmol photons m−2s−1 (three bulbs). The 40 W cool-white fluorescent lamps (General Electric) were installed 34 cm above the culture vessel. Diatom samples were collected daily by scraping the total surface area of the plate using a soft paintbrush and diluting the concentrated slurry with sterile seawater to make up a total volume of 50 mL. The final algal count was adjusted based on the dilution factor. Growth rates [cell doubling (μ) day−1] were calculated based on successive counts over 4 days as:
where N 1 = cell density at time 1 (t 1) and N 0 = cell density at time 0 (t 0) (Wood et al. 2005).
Effects of enrichment medium and culture site
The second experiment tested the effect of five enrichment media and two culture sites on the growth of Amphora sp. The same basal medium as in the first series of experiments was used, plus two additional complete media (F/2MVTM, complete F/2; Commercial II, CII). The CII medium (Renaud et al. 1991) was composed of technical and agricultural grade reagents (ammonium sulfate 21-0-0, 150.0 mg L−1, urea 46-0-0, 7.5 mg L−1, super phosphate 16-20-0, 25 mg L−1, FeCl3·6H2O, 5.0 mg L−1, Na2EDTA, 5.0 mg L−1). The next factor tested was the response of the diatom to culture site, i.e., inside the laboratory (indoor, 25 ± 1°C) and outside the laboratory (outdoor, 26–31°C). Based on the results of the first series, one 40 W cool-white fluorescent tube was provided for this experiment. Continuous lighting was provided in cultures located inside, while cultures located outside were provided with light during nighttime only, since sunlight provided the required irradiance during daytime. The culture medium was replaced every 4 days for a culture period of 32 days to allow further diatom matting on the plate. Diatom samples were collected every 4 days by scraping an area of 16 cm2 prior to changing the culture medium. The plates were subdivided into nine small squares (16 cm2) to avoid sampling the same area. Collection of samples and computation of growth rate was the same as in the first experiment.
Chemical composition and fatty acid analysis
Samples for analysis of protein, carbohydrates, chlorophyll a and fatty acids were scraped from diatom plates on the 12th day of culture in the second experimental series. Samples for fatty acids were collected on pre-combusted GF/F filters placed in screw-capped test tubes. Lipids were extracted immediately following the method of Folch et al. (1957). Fatty acid methyl esters were prepared by direct esterification (Lepage and Roy 1984) of lipid extracts. The component fatty acids were separated and identified using a Shimadzu GC-17A gas-liquid chromatograph with capillary column (Omegawax-320, Supelco, Bellefonte, PA; 30 m length, 0.32 mm i.d., 0.25 um film thickness). Fatty acids were identified using known standards and well-defined cod liver oil preparations. Samples for protein were determined using a modified Lowry protein assay (Bensadoun and Weinstein 1976) using bovine serum albumin (99%) as standard. Carbohydrates were measured using the phenol-sulphuric method of Dubois et al. (1956) and Kochert (1978) using glucose (99%) as standard. Chlorophyll a was determined using the method of Brown (1991).
Statistical analysis
In the first experiment, statistical analysis was carried out using a 3 × 3 factorial design to test the effects of light intensity and nutrient medium on cell growth of Amphora sp. In the second experiment, a 5 × 2 factorial design was used to compare cell growth of Amphora sp. cultured in five different culture media and at two culture sites. Both experiments employed five replicates per treatment group. Cell count data were transformed to their logarithms before two-way analysis of variance (ANOVA), in which statistical significance of the main effects and interaction was tested. Differences between means of replicate analysis were tested for statistical significance (P > 0.05) with the Duncan’s multiple range test (SAS 1991).
The chemical and fatty acid composition of Amphora sp. was subjected to similar statistical analysis in the second experiment.
Results
Effects of light intensity and culture medium
The results of 3 × 3 factor factorial ANOVA showed that the two factors, light intensity and culture medium, had no significant effect on cell growth of Amphora sp. The variables tested did not affect each other, as no significant interaction effects were found between the two factor combinations (light intensity × culture medium). Regardless of PPFD, the cell density of cultures enriched with F/2MTM, F/2MV and F/2M were not significantly different (P > 0.05, Table 1). No cell doubling was recorded in cultures enriched with F/2MTM and F/2MV. A slow growth rate of 0.1 μ day−1 was observed in cultures enriched with F/2M. Comparison of levels of PPFD across nutrient medium showed that increasing levels of PPFD did not significantly affect the cell density of Amphora sp. (P > 0.05, Table 2). However, higher growth rate was observed in cultures exposed to 11.4 μmol photon m−2s−1 PPFD (0.2 μ day−1) compared to cultures exposed to higher PPFD (16.1 μmol photon m−2s−1, −0.1 μ day−1; 31.3 μmol photon m−2s−1, 0.0 μ day−1).
Effects of enrichment medium and culture site
Results of 5 × 2 factor factorial ANOVA showed that culture site, but not nutrient enrichment, had a significant influence on cell growth of Amphora sp. The variables tested did not affect each other, as no interaction effects were found between the two factor combinations (enrichment medium × culture site). Comparison of levels of nutrient medium across location of cultures showed that the cell density of Amphora sp. enriched with F/2MTM, F/2MV, F/2M and CII medium were not significantly different compared to cultures enriched with complete F/2 (F/2MTMV) (P > 0.05, Table 3). The growth rate was similar (0.2 μ day−1) in cultures enriched with the five nutrient media (Table 3). Regardless of nutrient medium, cultures located inside the laboratory (B1) had a significantly higher cell density(133 × 104 cells cm−2) and growth rate (0.3 μ day−1) compared to cultures located outdoor (B2) (cell density,100.4 × 104 cells cm−2; growth rate, 0.2 μ day−1) (P < 0.05, Table 4). Cultures enriched with Commercial II could sustain algal growth up to 32 days in both indoor and outdoor culture conditions compared to other fertilization schemes (Fig. 1).
Chemical and fatty acid composition
The results of 5 × 2 factor factorial ANOVA showed that culture site and enrichment medium had no significant effects on the lipid, saturated fatty acid, unsaturated fatty acid, n-3 fatty acid, and n-3 highly unsaturated fatty acids (HUFA) content or the ratios of n-3 to n-6 polyunsaturated fatty acids (PUFA) of Amphora sp. Regardless of culture site, results of one-way ANOVA showed a significantly higher lipid (P < 0.05) content in cultures enriched with F/2MTM (61.3%) and F/2MV (60.2%) compared to cultures enriched with F/2MTMV (32.4%) and CII (38.6%), but no significant difference from cultures enriched with F/2M (45.1%). The saturated fatty acid content was significantly higher (P< 0.05) in cultures enriched with F/2M (37.1%) compared to other treatments (Table 5). No significant differences were noted in the unsaturated fatty acids, n-3 fatty acids, and n-3 HUFA or the ratios of n-3 to n-6 polyunsaturated fatty acids (PUFA) among the treatments. Regardless of nutrient enrichment, the lipids, unsaturated fatty acids, n-3 fatty acids, n-6 fatty acids, n-3 HUFA and the ratios of n-3 to n-6 PUFA of cultures exposed to two locations were not significantly different (P > 0.05, Table 6). However, significantly higher saturated fatty acids (P < 0.05) were recorded in cultures located outdoors (33.1%) compared to cultures located inside the laboratory (26.6%).
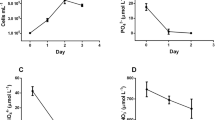
The n-6 fatty acids, protein, carbohydrate and chlorophyll a content of Amphora sp. were influenced by culture location and culture medium, as significant interaction effects were found between the two factors (enrichment medium × culture site) tested. The n-6 fatty acids content of Amphora sp. enriched with CII and cultured outside (11.3%) was comparable to that of cultures enriched with complete F/2 (11.1%) grown inside the laboratory (Fig. 2a). A higher protein and carbohydrate content of Amphora sp. was noted in cultures located inside the laboratory compared to cultures grown outside (Fig. 2b,c). In contrast, the chlorophyll a content was lower in cultures located indoors compared to cultures located outdoors (Fig. 2d). The proximate chemical composition (protein, carbohydrates, chlorophyll a) of Amphora sp. was highly dependent on the type of enrichment used.
Discussion
The low cell density and zero growth rate of Amphora sp. in the first experiment could be due to culture age (lag phase) at the time of sampling. The cells were still adjusting to the new culture environment (incomplete medium and slow aeration).
In the second experiment (using the same enrichment), cell density and growth rate increased with the adoption of a semi-continuous culture system. Renewal of culture medium allowed the cells to recover and form thicker diatom mats on the plate. Cultures exposed to low PPFD showed a higher cell density compared to cultures exposed to higher PPFD. The difference in growth could be attributed to a report that benthic diatoms are efficient in utilizing low irradiances for inorganic assimilation (Rivkin and Putt 1987). This result was similar to earlier works wherein better algal growth was noted under low irradiances (Admiraal 1977; Palmisano et al. 1985; Cahoon et al. 1993; Kronkamp et al. 1998). Benthic microalgae exposed to relatively lower irradiances have distinct photo adaptations to enhance their growth (Morris 1981; Rivkin and De Laca 1990). In the culture of benthic diatoms for abalone postlarvae, the level of light was reduced by providing black polyethylene plastic netting to control the type of algal community that grew in polyvinyl plates (Ito and Kitamura 1997). In contrast, the observations of Sanchez-Saavedra and Voltolina (1996) in planktonic diatoms found no significant difference in cell division rate when cells were exposed to increasing irradiances. Correa-Reyes et al. (2001) showed that some diatoms could grow in high light irradiances without photoinhibition. These contrasting results showed that the saturation light intensity may vary among diatom species. In this study, the low PPFD did not limit the growth of Amphora sp., and this was demonstrated by an erratic growth rate when Amphora sp. was subjected to higher PPFD.
Cultures enriched with F/2M, F/2MTM and F/2MV showed similar cell density to cultures enriched with complete F/2 and CII. This result agrees with earlier work in which microalgae were successfully grown using basic macronutrients and cheap agricultural fertilizers (Gonzales-Rodriguez and Maestrini 1984; Fabregas et al. 1987; Renaud et al. 1991; Okauchi and Kawamura 1997; Simental-Trinidad et al. 2001). In microalgal culture, the available nitrates and phosphates are the most important nutrients controlling growth (Wifkors 1986; Austin et al. 1990) Hence, removal of trace metals and vitamins did not decrease cell density. The use of the semi-continuous system (renewal of medium every 4 days) in this study was able to sustain the growth of Amphora sp. for 32 days in both laboratory and outdoor conditions. This result could be due to the fact that nutrient and light limitation was avoided in this system (Harrison et al. 1990). The observation that cultures located inside showed a higher cell density compared to cultures located outside could be due to the fact that cultures located outside are subjected to environmental fluctuations. In outdoor conditions, temperature and irradiance fluctuate. Lighting from one 40-W fluorescent tube was provided only during nighttime. During the daytime, PPFD was low, since the cultures were shaded with polyvinyl plastic roofing. In indoor conditions, the cultures were continuously lit with one 40-W fluorescent bulb, and the temperature was kept constant at 25°C. Temperature and light are two of the primary factors considered to regulate algal growth (Rivkin and Putt 1987). Wang et al. (1997) observed different growth rates in four benthic diatom species at varying levels of temperature and light intensity.
The chemical composition of Amphora sp. varied at different levels of nutrient enrichment. Regardless of culture location, a high lipid content was observed in cultures enriched with F/2MTM or F/2MV compared to cultures enriched with complete F/2 and CII fertilizers. This result suggests that propagation of high lipid Amphora sp. is possible with the use of basic F/2 macronutrients only. The lipid and fatty acid composition of Amphora sp. did not vary in the two locations except for the saturated fat content, which is high in cultures located outside. This result is beneficial to abalone larvae since higher levels of saturated fatty acids provide extra energy for growing larvae (Thompson et al. 1993; Brown et al. 1996). The n-6 fatty acid content of Amphora sp. varied at different enrichment and culture locations. High n-6 fatty acid was attained in cultures located inside (enriched with complete F/2) and in cultures located outside (enriched with CII). This result suggests that high n-6 fatty acid Amphora sp. can be grown in hatchery conditions using cheap fertilizers. The results of the ratios of n-3 to n-6 PUFA of Amphora sp. indicate that this is acceptable for abalone larvae based on the nutritional index of Watanabe et al. (1983).
The chemical contents of the diatom, such as protein, carbohydrates and chlorophyll a, were affected by both location and enrichment medium. The higher protein and carbohydrate contents in cultures located inside over outside cultures could be due to the more regulated cultural conditions (constant irradiance and temperature) compared to the outside (hatchery) conditions, which were fluctuating. The quality and quantity of light may affect biomass production and biochemical composition of microalgae (Simental-Trinidad et al. 2001; Wang et al. 1997). Microalgae exposed to changes in environmental factors vary in protein and carbohydrate content (Varum and Myklestad 1984). Modification of chemical composition could also be due to inorganic nutrient concentration of the culture medium (Harrison et al. 1977; Hitchcock 1980). Variability of protein may be caused by varying nitrate concentrations (Sakshaug and Holm-Hansen 1977; Fabregas et al. 1985; Harrison et al. 1990). The low chlorophyll a content of cultures located inside was similar to the observations of Renaud et al. (1991) in Isochrysis galbana.
In summary, this study was able to determine optimum growth conditions for the mass culture of the tropical Amphora sp., and identified its biochemical and fatty acid composition under these conditions. Furthermore, this study recommends the use of the semi-continuous culture system (renewal of medium every 4 days) to increase diatom biomass needed by abalone postlarvae.
References
Admiraal W (1977) Influence of light and temperature on the growth rate of estuarine bethic diatoms in culture. Mar Biol 39:1–9
Austin AP, Ridley-Thomas CI, Lucey WP, Austin DJD (1990) Effects of nutrient enrichment on marine periphyton: implications for abalone culture. Bot Mar 33:235–239
Bensadoun A, Weinstein D (1976) Assay of protein in the presence of interfering materials. Anal Biochem 70:241–250
Brown MR (1991) The amino-acid sugar composition of 16 species of microalgae used in mariculture. J Exp Mar Biol Ecol 145:79–99
Brown MR, Dunstan GA, Norwood SJ, Miller KA (1996) Effects of harvest stage and light on the biochemical composition of the diatom Thalassiosira pseudonana. J Phycol 32:64–73
Cahoon LB, Beretich GR, Thomas CJ, McDonald AM (1993) Benthic microalgal production at Stellwagen Bank, Massachusetts Bay. Mar Ecol Progr Ser 102:179–185
Correa-Reyes JB, Sanchez-Saavedra MP, Siqueiros-Beltrones DA, Flores-Acevedo N (2001) Isolation and growth of eight strains of benthic diatoms cultured under two light conditions. J Shellfish Res 20:603–610
De la Peña MR, Villegas CT (2005) Cell growth, effect of filtrate and nutritive value of the tropical Prasinophyte Tetraselmis tetrathele Butcher at different phases of culture. Aquac Res 36:1500–1508
De Pauw N, Verboven J, Claus C (1983) Large-scale microalgal production for nursery rearing of marine bivalves. Aquac Eng 2:27–47
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Ebert EE, Houk JL (1984) Elements and innovations in the cultivation of red abalone Haliotis rufescens. Aquaculture 39:375–392
Fabregas J, Herrero C, Cabezas B, Abalde J (1985) Mass culture and biochemical variability of the marine microalga Tetraselmis suecica (Kylin) Butch with high nutrient concentrations. Aquaculture 49:231–241
Fabregas J, Herrero C, Cabezas B, Abalde J (1986) Biomass production and biochemical composition in mass cultures of the marine microalga Isochrysis galbana Parke at varying nutrient concentrations. Aquaculture 53:101–113
Fabregas J, Toribio L, Abalde J, Cabezas B, Herrero C (1987) Approach to biomass production of the marine microalga Tetraselmis suecica (Kylin) Butch using common garden fertilizer and soil extract as cheap nutrient supply in batch cultures. Aquac Eng 6:141–150
Folch J, Lees M, Sloane-Stanley GH (1957) A simple method of the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–506
Gallardo WG, Buen SMA (2003) Evaluation of mucus, Navicula and mixed diatoms as larval settlement inducer for the tropical abalone Haliotis asinina. Aquaculture 221:357–364
Gonzalez-Rodriguez E, Maestrini SY (1984) The use of some agricultural fertilizers for the mass production of marine algae. Aquaculture 36:245–256
Grant JF (1981) Abalone culture in Japan: Development and current commercial practice. Tasmanian Fisheries Research. Tasmanian Fisheries Development Authority, Hobart, Tasmania
Guillard RRL, Ryther JH (1962) Studies on marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonela confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Harrison PJ, Conwy HL, Holmes RW, Davis CO (1977) Marine diatoms grown in silicate or ammonium limitation III. Cellular chemical composition and morphology of Chaetoceros edulis, Skeletonema costatum and Thalassiosira gravida. Mar Biol 43:19–31
Harrison PJ, Thompson PA, Calderwood GS (1990) Effects of nutrient and light limitation on the biochemical composition of phytoplankton. J Appl Phycol 2:45–56
Hitchcock GL (1980) Diel variation in chlorophyll a, carbohydrate and protein content of the marine diatom Skeletonema costatum. Mar Biol 57:271–278
Hoagland KD, Rowsowski JR, Gretz MR, Roemer SC (1993) Diatom extracellular polymeric substances: function, fine structure, chemistry and physiology. J Phycol 29:537–566
Hoshaw RW, Rosowski JR (1973) Methods for microscopic algae. In: Stein JR (ed.) Handbook of Phycological Methods, Culture Methods and Growth Measurements. Cambridge University Press, London, pp 53–68
Ito S, Kitamura H (1997) Induction of larval metamorphosis in the sea cucumber Stichopus japonicus by periphitic diatoms. Hydrobiologia 358:281–284
Kawamura T (1994) The role of benthic diatom in the early life stages of the Japanese abalone (Haliotis discus hannai). In: Watanabe Y, Yamashita Y, and Oozeki Y (eds) Survival strategies in early life stages of marine resources. Balkema, Rotterdam, pp 355–367
Kawamura T, Kikuchi T (1992) Effects of benthic diatoms on settlement and metamorphosis of abalone larvae (in Japanese with English abstract). Suisanzoshoku 40:403–409
Kawamura T, Takami H (1995) Analysis of feeding and growth rate of newly metamorphosed abalone Haliotis discus Hannai fed on four species of benthic diatoms. Fish Sci 61:357–358
Kawamura T, Saido T, Takami H, Yamashita Y (1995) Dietary value of benthic diatoms for the growth of post-larval abalone Haliotis discus Hannai. J Exp Mar Biol Ecol 194:189–199
Kawamura T, Roberts RD, Takami H (1998) A review of the feeding and growth of postlarval abalone. J Shellfish Res 17:615–625
Kochert G (1978) Carbohydrate determination by the phenol-sulfuric acid method. In: Hellebust JA, Craigie JS (eds) Handbook of phycological methods, physiological and biochemical methods. Cambridge University Press, London, pp 95–97
Kronkamp J, Barranguet C, Peene J (1998) Determination of microphytobenthos PSII quantum efficiency and photosynthetic activity by means of variable chlorophyll fluorescence. Mar Ecol Prog Ser 162:45–55
Lepage G, Roy CC (1984) Improved recovery of fatty acid through direct transesterification without prior extraction and purification. J Lipid Res 25:1391–1396
Martinez MR, Chakroff RP, Pantastico JB (1975) Direct phytoplankton counting techniques using a haemacytometer. Philipp Agric 59:43–50
Morris I (1981) Photosynthesis products, physiological state and phytoplankton growth. Can Bull Fish Aquati Sci 210:83–102
Norman-Boudreau K, Burns D, Coke CA, Austin A (1986) A simple technique for detection of feeding in newly metamorphosed abalone. Aquaculture 51:313–317
Ohgai M, Wakano M, Nagai S (1991) Effect of attached microalgae in the settlement of larvae and growth of juveniles in abalone, Haliotis discus hannai Ino (in Japanese with English abstract). Suisanzoshoku 39:263–266
Okauchi M, Kawamura K (1997) Optimum medium for large-scale culture of Tetraselmis tetrathele. Hydrobiologia 358:217–222
Palmisano AC, Soohoo JB, White DC, Smith GA, Stanton GR, Burcle LH (1985) Shade adapted benthic diatoms beneath Antarctic sea ice. J Phycol 21:664–667
Persoone G, Claus C (1980) Mass culture of algae: a bottleneck in the nursery culturing of mollusks. In: Shelef G, Soeder CJ (eds) Algae biomass: production and use. Elsevier/North Holland Biomedical, Amsterdam, pp 265–285
Renaud SM, Parry DL, Luong-van T, Kuo C, Padovan A, Sammy N (1991) Effect of light intensity in the proximate biochemical and fatty acid composition of Isochrysis sp. and Nannochloropsis oculata for use in tropical aquaculture. J Appl Phycol 3:43–53
Rivkin RB, De Laca TE (1990) Trophic dynamics in Antarctic benthic communities. I. In situ ingestion of microalgae by foraminefera and metazoan meiofauna. Mar Ecol Prog Ser 64:129–136
Rivkin RB, Putt M (1987) Photosynthesis and cell division by the Antarctic microalgae: comparison of benthic, planktonic and ice algae. J Phycol 23:223–229
Sakshaug E, Holm-Hansen O (1977) Chemical composition of Skeletonema costatum (Grev.) Cleve and Pavlova (Monochrysis) lutheri (Droop) Green as a function of nitrate, phosphate and iron limited growth. J Exp Mar Biol Ecol 29:1–34
Sanchez-Saavedra MP, Voltolina D (1996) Effect of blue-green light on growth rate and chemical composition of three diatoms. J Appl Phycol 8:131–137
Simental-Trinidad JA, Sanchez-Saavedra MP, Correa-Reyes JG (2001) Biochemical composition of benthic marine diatoms using a common agricultural fertilizer as culture medium. J Shellfish Res 20:611–617
SAS Institute (1991) SAS systems for linear models. 3rd edn, Cary, NC
Thompson PA, Guo M, Harrison PJ, White JNC (1992) Effects of variation of temperature. I. On the biochemical composition of eight species of marine phytoplankton. J Phycol 28:481–488
Thompson PA, Guo M, Harrison PJ (1993) The influence of irradiance on the biochemical composition of three phytoplankton species and their nutritional value for larvae of the Pacific oyster (Crassostrea gigas). Mar Biol 117:259–268
Uki N, Kikuchi S (1979) Food value of six benthic micro-algae in growth of juvenile abalone, Haliotis discus hannai (in Japanese with English abstract) Bull. Tohoku Reg Fish Res Lab 40:47–52
Wang QH, Wang SH, Ding MJ, Li M, Shi RF, Cheng AH (1997) Studies on the culture conditions of benthic diatoms for feeding abalone. I. Effects of temperature and light intensity on growth rate. Chin J Limnol 15:296–302
Watanabe T, Kitajima C, Fujita S (1983) Nutritional values of live organisms used in Japan for mass propagation of fish: a review. Aquaculture 34:115–143
Wifkors GH (1986) Altering growth and gross chemical composition of two microalgal molluscan food species by varying nitrate and phosphate. Aquaculture 59:1–14
Wood EJ (1961) Studies on Australia and New Zealand diatoms. IV. Description of further sedentary species. In: Salmon JT (ed) Transactions of the Royal Society of New Zealand, vol 88. Royal Society of New Zealand, Wellington
Wood AM, Everroad RC, Wingard LM (2005) Measuring growth rates in microalgal cultures. In: Andersen RA (ed) Algal culturing techniques, Elsevier, New York, pp 269–286
Varum JK, Myklestad S (1984) Effects of light, salinity and nutrient limitation on the products of β1-3-d glucan and exo-d-glucanase activity in Skeletonema costatum (Grev.) Cleve. J Exp Mar Biol Ecol 83:13–25
Acknowledgments
I wish to thank Mr. Daniel Lojera for his assistance in diatom culture, Dr. Soichiro Suda for the identification of the diatom sample, Ms. Marget Arnaiz for the identification of fatty acid composition, and Prof. Roman Sañares for statistical analysis. This study was supported by SEAFDEC/AQD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de la Peña, M.R. Cell growth and nutritive value of the tropical benthic diatom, Amphora sp., at varying levels of nutrients and light intensity, and different culture locations. J Appl Phycol 19, 647–655 (2007). https://doi.org/10.1007/s10811-007-9189-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-007-9189-0