Abstract
This study was undertaken to assess the effects of dietary sodium propionate (SP) supplementation on the growth, haemato-immunological parameters, gut microbiota, and intestinal histology of European seabass (Dicentrarchus labrax) fry. In total, four diets were evaluated during a 90-day growth trial: a control (CTRL) diet without SP supplementation and three diets including 0.1%, 0.2%, or 0.3% SP, which were called SP1, SP2, and SP3, respectively. Diets were assigned to seabass (initial body weight: 0.45 ± 0.23 g), and the results revealed a 34%, 19%, and 15.6% increase in weight gain in SP3-, SP2-, and SP1-fed fish, respectively, concomitant with a lower feed conversion ratio in these fish than in fish not receiving the supplement (CTRL), which exhibited the lowest performance among all fish groups. Administration of 0.3% or 0.2% SP elevated the carcass protein content, major blood constituents, and immune function indicators compared to the CTRL. In addition, dietary inclusion of SP positively modified the bacterial community composition in the distal intestine (DI) of fish, causing an approximately 95% and 87% reduction in total microbial count in SP3- and SP2-fed fish, respectively, compared to CTRL fish. Vibrio spp. were non-existent (zero or negligible) in fish fed the SP2 and SP3 diets, and the number of faecal coliform bacteria gradually decreased to zero in SP3-fed fish. Additionally, an improvement in fish intestinal features, particularly in the DI, was evident from measurements of histological sections from the proximal, mid, and distal intestine. The greater villus area (height and width) and goblet cell count in fish fed the SP3 or SP2 diet than in fish not receiving the supplement suggested stimulation of digestive secretions with enhanced mucus production. In conclusion, enrichment of the diet with either 0.3% or 0.2% SP is capable of improving seabass fry growth performance, general health status, immune response efficiency, and intestinal function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
To enhance the general health and disease resistance of cultured fish, particularly during the fry stage, feeds may be supplemented with health-promoting additives, reducing the need to use chemotherapeutics. A wide variety of concepts and additives are being investigated for their ability to improve digestion and feed utilisation in the field of fish nutrition. Short-chain fatty acids (SCFAs)/organic acids (C1–C7) and/or their respective salts, commonly known as acidifiers, are potential substitutes for antibiotic growth promoters in aquafeeds that are receiving increasing attention (Ng and Koh, 2016; Lückstädt, 2008a,b). Acidifiers are a type of feed supplement that acts as conserving agents in the animal gut by reducing the pH of feed and inhibiting microbial growth (Lückstädt, 2008a). Many organic acids or SCFAs are also available in sodium, potassium, and calcium salts, with the advantages of being odourless, less corrosive, and easy to manipulate as a feed additive because of their solidity and lack of volatility (Partanen and Mroz, 1999). More recently, the use of dietary organic acids/salts as supplements in the culture of aquatic animals to improve health and limit the use of antibiotics has been the focus of much research on aquafeeds. According to several studies, some dietary acidifiers can significantly enhance the growth, feed utilisation, and immunity of fish (Hoseinifar et al., 2017; Ng and Koh, 2016). Whilst the potential for these acidifiers to act as growth promotors, immuno-stimulators, and health-status enhancers is known, fewer studies have assessed their effects on the intestinal microbiome (Ringø et al., 2015) or intestinal histology (Abdel-Mohsen et al., 2018). The intestinal microbiota has been linked to a wide range of biological processes that benefit the host including nutritional conditions and the immune system; therefore, it is undoubtedly an important factor determining the health status of fish.
European seabass (Dicentrarchus labrax, hereafter referred to as seabass) is regarded as a highly valuable marine fish and has a significant production volume in Mediterranean countries. Consequently, novel programmes to control disease outbreaks, reinforce immune competence, and enhance the viability of early fish stages are needed to achieve aquaculture sustainability and increase profitability. Nevertheless, there is currently an increasing interest in evaluating the potential of adding several feed supplements, including acidifiers, to seabass diets to enhance the growth, immunity, and efficiency of digestive and metabolic processes in fish. Among the more recent studies, the advantageous effects of sodium butyrate and sodium diformate on the growth, nutrient utilisation and basal health status of seabass fry, juveniles, and adults have been emphasised (Abdel-Mohsen et al., 2018; Wassef et al., 2017; Rimoldi et al., 2016). The organic acid sodium propionate (SP) has also been shown proven to promote growth naturally and effectively and support hygiene and feeding in aquatic organisms (da silva et al., 2016; Hosseini et al., 2011). The results of a few prior studies have revealed the beneficial effects of propionic acid/salts on immune response in Arctic charr, Salvelinus alpinus (Ringø, 1991); Nile tilapia, Oreochromis niloticus (Hassaan et al., 2014); zebra fish, Danio rerio (Safari et al., 2016), and Caspian white fish, Rutilus frisii kutum (Hoseinifar et al., 2017). However, the effects of dietary SP on seabass remain to be determined. Accordingly, the present study aims to evaluate the response of seabass fry to dietary supplementation with graded levels of SP in terms of growth performance, nutrient utilisation, carcass composition, health and immunity indicators, the gut microbiome, and intestinal histomorphology. These indicators will provide essential information on the optimal SP dosage, mainly as a biocontrol feed additive, to enforce disease resistance in seabass fry.
Material and methods
Experimental facilities
This experiment was conducted in the Fish Nutrition Laboratory, National Institute of Oceanography and Fisheries (NIOF), Alexandria. Hatchery-bred seabass fry (initial body weight (IBW) of approximately 0.5 g) was maintained in a reservoir tank and fed a control (CTRL) diet for a 2-week acclimatisation period. Thereafter, 8 groups of 150 fish were distributed among 0.5-m3 fibreglass tanks filled with filtered and UV-treated fresh seawater with a flow-through (120 l/h) system in duplicate for each treatment. The water temperature was adjusted to 22 ± 1 °C and monitored by a thermometer, and a dissolved oxygen concentration of 7–8 mg/l was maintained at a saturation level of 90–100% (YSI Professional Series Instrument, USA). The salinity was approximately 35 g/l (Portable Refractometer, Operating Instructions, GG-201/211), the pH was 8.0 ± 0.5 (pH/temperature branch metre, Italy), and the nitrogen concentrations were N2 = 0.54 ± 0.03 mg/l, NH3 = 0.73 ± 0.05 mg/l, and NH4 = 0.77 ± 0.04 mg/l (YSI EcoSense® 9300 Photometer, England). Water quality parameters were periodically checked throughout the whole experimental period to ensure that experimental fish are fulfilling the requirements of ARRIVE guidelines and in accordance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
All analysed water quality parameters remained within the acceptable standards for the cultivation of seabass (Vázquez and Muñoz-Cueto, 2014). The trial was conducted under a natural photoperiod regime of 14L:10D and lasted for 90 days.
Experimental diets
Four diets were formulated on an isonitrogenous (~ 52% CP), isolipidic (~ 16% L), and isoenergetic (~ 52 kJ/kg, gross energy) basis and supplemented with 1 of 4 graded levels of SP: 0.0% (CTRL diet), 0.1% (SP1 diet), 0.2% (SP2 diet), or 0.3% (SP3 diet). The ingredient composition of all diets was the same, except for SP, and fulfilled the nutritional requirements of seabass fry (NRC (National Research Council), 2011). Diets were produced in the Fish Nutrition Laboratory, NIOF, Alexandria, as crumbles (0.2-mm diameter), and the tested doses of SP (AGROCEL® Agranco Corp., USA) were selected based upon previous research with other organic salts for the same species (Wassef et al., 2017, Abdel-Mohsen et al., 2018; Rimoldi et al., 2016). Details of the diet processing method were previously described for a similar sodium butyrate experiment (Abdel-Mohsen et al., 2018). Diets were dispensed into duplicate tanks for each dietary treatment 4 times a day (8.00, 11.00, 14.00, and 17.00 h), 7 days a week, to apparent visual satiety and carefully controlled through feed intake to avoid feed wastage. The formulation and proximate composition of the experimental diets are given in Table 1.
Sample collection
Haemato-immunological assays
At the end of the feeding period (day 91), 24 h after the last meal, 5 fish per tank were lightly anaesthetised (50 mg clove oil/l), and blood samples were immediately collected from the caudal vein by heparinised syringes and pooled for each treatment. Haematological analyses were performed following standard techniques presented in Dacie and Lewis (2006). Another set of blood samples was collected and left to coagulate at − 4 °C and then centrifuged at 3500g for 10 min. Sera were stored at − 80 °C for later use in immunological assays.
Intestinal microbiota assessments
Immediately after blood sampling, fish were euthanised, and the distal intestine (DI) was carefully aseptically excised from each fish specimen (using sterile tools) and homogenised in 10 ml of 3% sterile sodium chloride solution. These were defined as the “stock” samples. Briefly, a series of ten-time dilutions of the stock sample were followed to obtain five consecutive diluted samples labelled from 10−1 to 10−5. Microbiota community composition was determined in 1 ml from the last dilution by assays of growth on plated selective agar media. For the total count of viable bacteria (TBC), seawater agar was used (Zobell, 1946); for Vibrio spp., thiosulfate-citrate-bile salt-sucrose (TCBS) agar was used (Kobayashi et al., 1963); and for Escherichia coli, mFC agar was used (ISO (International Organization for Standardization) No. 9308/1, 1990). All plates were incubated at 30 °C for 24–48 h for enumeration, except for the mFC medium, which was incubated at 44 °C for 24 h. For cultivation of acid-fermentative bacteria, de Man, Rogosa and Sharpe (MRS) medium was used and incubated at 37 °C for 48 h under anaerobic conditions (Amiza et al., 2006). All instruments were cleaned and disinfected between individual fish specimens, and all samples were analysed in triplicate.
Intestinal histology
At termination of the trial, 3 fish from each tank were randomly selected, and their intestines were carefully excised and visually divided into 3 segments: the proximal intestine (PI), immediately following the stomach; the DI, the widened part internally from the anus; and the mid intestine (MI), the section between the PI and DI. Samples of intestinal tissues from the three portions were preserved in 4% phosphate-buffered formalin (pH of 7.4), transverse sectioned to a thickness of 5 μm (SHANDON HYPERCUT, USA), stained with haematoxylin and eosin (H&E) and mounted on slides according to standard histological techniques. The sections were processed and examined for overall features of intestinal morphology and composition by using a light microscope (Nikon Phase Contrast Dry, 0.90, Japan).
Data analyses
Growth calculations
At the start of the experiment, the fish in each tank were bulk weighed (IBW, g/fish) and counted before monitoring the growth and survival of the fish in each dietary treatment. Three fish from each tank (6 per treatment) were randomly sampled at the start and end of the trial and homogenised into a composite sample for initial and final carcass composition analyses. At the end of the trial and after 1 day of feed deprivation, all fish from each tank were lightly anaesthetised and bulk weighed, and their final body weight (FBW) was then recorded. Growth and feed utilisation indices were calculated using the following indicators: weight gain (WG, g) = FBW − IBW, specific growth rate (SGR, %/day) = 100 (ln FBW − ln IBW) / t, average daily gain (ADG, g/fish/day) = WG/t, where FBW and IBW were measured in g and “t” is the time of the experiment (days). The feeding rate (FR; %BW/day) was calculated as 100 × {crude feed intake / (IBW + FBW)/2/days}, the feed conversion ratio (FCR) was calculated as dry feed consumed (g)/fish WG (g), the protein efficiency ratio (PER) was calculated as 100 (fish WG, g) / (protein intake, g), and the protein productive value (PPV) was calculated as 100 (protein gain, g) / protein fed (g). The percentage survival of fish (S, %) was the ratio between the numbers of fish at the end to the total number of fish at the start of the experiment.
Analytical procedures
The proximate composition of diets and fish was assessed according to the standard methodology of AOAC (2015). Dry matter was determined by oven drying at 105 °C; crude protein (total N × 6.25), by using a semi-automatic Kjeldahl procedure (VELP Scientifica, UDK 126, Italy) after acid digestion; and crude lipid, gravimetrically after extraction by a chloroform/methanol (2:1 v/v) mixture (40–60 °C). The ash content was measured by burning the samples in a muffle furnace at 550 °C for 6 h. Crude fibre was analysed by the acid hydrolysis method, whereas the nitrogen-free extract was calculated as the difference. Gross energy was obtained based upon 23.6, 39.5, and 17.2 kJ/g for proteins, lipids, and carbohydrates, respectively.
Haemato-immunological analyses
Haemoglobin was determined according to Van-Kampen and Zijlstra (1961) procedure; whilst haematocrit, total number of red blood cells (RBCs, erythrocytes), and white blood cells (WBCs, leukocytes), differential leukocyte counting, and blood indices: mean cell volume (MCV), mean cell haemoglobin (MCH), and MCH concentration (MCHC) were all measured according to Dacie and Lewis (2006) techniques. All blood samples were analysed in triplicate.
The respiratory burst activity of leukocytes was assessed by Secombes (1990) method, phagocytic activity by the method in Kawahara et al. (1991), and serum lysozyme activity (LA) in Ellis (1990) with some modifications. Total myeloperoxidase content (MPO) in serum was measured according to the technique in Sahoo et al. (2005) and total immunoglobulin M (IgM) in Siwicki and Anderson (1993). All samples were analysed in triplicate.
Intestinal measurements
The previously reported criteria of Wang et al. (2017) were chosen in the present study to measure and evaluate the morphological characteristics of seabass intestine. Six parameters were measured with transverse sections for each of the 3 intestinal portions: the muscular layer (ML), submucosa layer (SML), lamina propria (LP), villus height (VH), width (VW), and goblet cell (GC) count. Ten measurements were recorded for each parameter to obtain the average value per treatment (6 parameters × 10 measurements × 3 intestinal portions × 4 dietary treatments).
Statistical analysis
Data are presented as means ± standard error (SE) (n = 3, unless otherwise stated). All data were subjected to a Shapiro-Wilk test (SAS Institute Inc., Cary, NC), and the results indicated a normal distribution (W = 0.90). To determine significant differences among dietary treatments, mean values were analysed by using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test, with a 0.05 probability level for significance. Before ANOVA, all data expressed as percentages were subjected to arcsine square root transformation.
Results
Growth and feeding performance
The acidifying effect of dietary SP had caused no negative effect on seabass appetite, and fish equally consumed the experimental diets without any noticeable behavioural or vigour change among dietary treatments. Feeding rate (%BW) was not significantly influenced by SP presence or level (P > 0.05) (Table 2). However, after treatment for 90 days, some growth performance criteria were found to be affected by SP presence and/or level (P < 0.05), with the SP3 diet resulting in the highest/best numerical values overall. A beneficial effect of the SP3 diet was observed for the FBW, WG, and daily WG (DWG, g/d), which showed significantly higher values at all tested SP concentrations than in the CTRL. A 34%, 19%, and 15.6% increase in WG was noted in SP3-, SP2-, and SP1-fed fish compared to CTRL-fed fish, which exhibited the lowest growth values among all fish groups. The SGR remained unaltered (P > 0.05) by the dietary treatments. Fish fed the SP3 diet exhibited significantly higher survival (97.5%) than those in all the other treatments, whilst the survival of fish fed the CTRL diet was the lowest (94%) but not significantly different from that of fish fed the other SP-treated diets.
The feed utilisation indices indicated significant positive effects (P < 0.05) of dietary SP on the FCR, the PER, and energy utilisation (EU, %), whereas the FR (% BW/d) remained unaffected (P > 0.05) among dietary groups. However, the FCR progressively improved/decreased with an increase in SP level, reaching the most significant value in the SP3-fed fish (1.20), followed by the SP2-fed fish (1.45), whilst the highest value was observed in the CTRL (1.82) fish group. Similarly, a gradual increase in the PER was observed with an increase in the dietary SP level, and the highest value was recorded in fish fed the SP3 diet, followed by those fed the SP2 diet, whereas those fed the CTRL or SP1 diet exhibited the lowest values. In addition, increasing levels of SP did not significantly influence (P > 0.05) the PPV, energy gain or energy retention, whereas the EU was significantly higher in both the SP3 and SP2 fish groups than in the CTRL and SP1 fish groups (Table 2).
Proximate composition of fish
None of the provided diets affected seabass whole-body composition, except for protein content, which exhibited a significant (P < 0.05) increase (16.13% or 15.0%, respectively) in SP3- or SP2-fed fish compared to CTRL fish (14.76%) (Table 3).
Haematology and blood biochemistry
The SP-containing diets positively (P < 0.05) affected major blood constituents of seabass fry (Table 4). The haemoglobin content progressively increased with each increase in dietary SP level, reaching its highest value in SP3-fed fish, which was comparable (P > 0.05) to that of SP2- and SP1-fed fish but significantly lower than that of CTRL fish. A similar trend was also observed for haematocrit values. The total erythrocyte (RBC) count was highest in the SP1 and SP2 fish groups, followed by the SP3 group, whereas CTRL fish exhibited a significantly (P < 0.05) lower RBC count than fish in all the other dietary groups. Regarding erythrocyte characteristics, the MCV increased significantly in only SP2 fish, and the MCHC significantly increased in SP3 fish, whilst MCH was significantly higher in all SP-fed fish, particularly the SP3 and SP2 fish groups, than in CTRL-fed fish. The total WBC/leukocyte count was significantly higher with SP dietary inclusion, particularly in SP2- and SP3-fed fish, than in the CTRL and SP1 dietary groups. Both the lymphocyte and monocyte percentages were significantly higher in fish fed either the SP2 or SP3 diet than in those fed the CTRL or SP1 diet (Table 4). These data suggest that both SP2 and SP3 diets led to enhanced blood profiles in seabass fry over 90 days of feeding with SP-supplemented diets.
Immunological assays
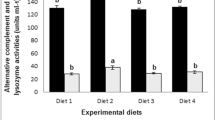
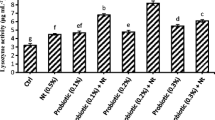
Immunity indicators in fish serum, namely, the respiratory burst activity of leukocytes (NBT), phagocytic (PA), lysozyme (LA), myeloperoxidase (MPO) enzyme and bactericidal activities, and total immunoglobulin M (IgM), were all estimated at the end of the trial and are represented in Table 4 (bottom section). Fish fed the SP3 or SP2 diet displayed a significantly higher respiratory burst activity, phagocytic activity of leukocytes index, myeloperoxidase enzyme activity, and bactericidal activity than CTRL fish, which showed values relatively lower than but comparable (P > 0.05) to those of SP1-fish. However, the LA and IgM did not respond (P > 0.05) to dietary SP presence and level. In summary, both 0.2% and 0.3% dietary SP supplementation significantly increased the main measured immunity indicators compared to the CTRL (untreated) diet.
Intestinal microbiota
The microbial populations from the DI of seabass fry fed SP-containing diets were identical among the replicate samples of each treatment. Supplementation with SP significantly modified the bacterial community of the DI, causing an approximately 95%, 87%, and 44% reduction in the total bacterial count at the 0.3%, 0.2%, and 0.1% levels, respectively, compared with the CTRL diet, which showed the highest bacterial count among all treatments (Table 5). Vibrio spp. were non-existent (zero or negligible) in the DI of fish fed either the SP2 or SP3 diet but were significantly lower in SP1-fed fish than in CTRL-fed fish. Similarly, estimates of faecal coliform bacteria gradually decreased with each increase in SP level, reaching significance in SP2-fed fish and zero (an approximately 52% reduction) in SP3-fed fish compared to CTRL-fed fish. However, the beneficial acid-fermentative bacteria were significantly affected by neither SP presence nor SP level.
Intestinal histology
Six parameters were measured in intestinal sections, namely, the thickness of the ML, SML, VH, and VW in the epithelium brush border of the lumen, GC count, and LP. The means of each parameter from each of the three segments of the seabass intestine (PI, MI, and DI) are given in Table 6. For most measurements, the majority of significant variations were detected in the DI portion. Both the ML and SML were significantly different among dietary treatments in only the DI. Fish fed the SP3 diet exhibited the greatest thickness (P < 0.05) of the ML, followed by SP2-fed fish, whereas SP1-fed fish showed a value comparable (P > 0.05) to that of CTRL-fed fish (Fig. 1a–d). The thickness of the SML was significantly higher in SP3-fed fish than in fish in all the other treatments, but diet had no appreciable effect on the SML in either the SP1 or SP2 fish group relative to the CTRL fish group (Fig. 2). Additionally, dietary SP significantly influenced the intestinal VH in the epithelium brush border of both the PI and DI. In the PI, the VH significantly and gradually increased in all SP-fed fish relative to that in CTRL-fed fish and peaked in the SP3-fed fish group. In the DI, only SP3-fed fish displayed a significant elevation in VH relative to fish in the other treatments or the CTRL. However, the VW gradually and progressively increased with each increase in dietary SP level in both the MI and DI compared to that of CTRL-fed fish. In the MI, the most significant VW values were observed in SP3- and SP2-fed fish, followed by SP1-fed fish. Similarly, in the DI, the VW was remarkably higher in SP3-fed fish than in CTRL-fed fish and other SP-fed fish groups. Therefore, the SP3 diet significantly improved the villus area (VH and VW) in the epithelium brush border of the DI compared to the CTRL diet (Fig. 2a–d).
a–d Photo micrographs of TS in distal intestine of seabass fry fed sodium propionate (SP)–containing diets, showing details of villus (V) structure: a, b fish fed CTRL and SP1 diets, showing enterocytes (E); goblet cells (GC, stars) some leucocytes infiltration (head arrows) from lamina propria (LP), c fish fed SP2 diet showing normal enterocytes (E) with basal nucleus; normal lamina propira (LP) with the lowest leucocytes infiltration, goblet cells (GC, stars); d fish fed SP3 diet with goblet cells (GC, stars); lamina propira (LP) and few leucocytes infiltration (head arrows). [H&E, × 100]
The secreting GC count was unaffected (P > 0.05) by diet in both the PI and MI, whereas in the DI, a remarkable increase in GC number (P < 0.05) was observed in all SP-fed fish, particularly SP2- and SP3-fed fish, relative to that in CTRL-fed fish. Moreover, a significant (P < 0.05) effect of SP level on the LP measure was observed in both the proximal and distal portions of the intestine. In the PI, the LP was increased in both SP2- and SP3-fed fish relative to that in CTRL-fed fish, whilst for SP1-fed fish, it remained unchanged (P > 0.05). In the MI, the dietary treatments resulted in similar LP measures. In contrast, in the DI, the LP was reduced in both SP2- and SP3-fed fish compared to that in either the CTRL fish or SP1-fed fish (Table 6).
Discussion
SP and calcium propionate have been found to have positive effects on the performance and health of some species of fish and shrimp (Hoseinifar et al., 2017; Ng and Koh, 2016). Overall, the present study supports the use of SP in seabass fry diets to enhance overall performance, nutrient utilisation, and general health status. Variation in the response of seabass to dietary SP was evident among the 3 tested doses and was more pronounced at the relatively high levels (0.3% and 0.2%). Whilst the medium (0.2%) and high (0.3%) doses of SP significantly enhanced the growth and survival of seabass fry and affected the gastrointestinal microbiota in this study, the lowest dose (0.1%) had a relatively weak or sometimes no effect. The highest SP dose (0.3%) had no negative effects on the FR (%BW/d) but enhanced both growth and feed efficiency (Table 2). Our findings confirm that of Hoseinifar et al. (2016), in which 0.25% dietary SP had beneficial effects on the growth performance of Caspian white fish (R. frisii kutum) fry.
The higher WG in seabass fed SP diets in the present research may be linked to SP is utilised in the gastrointestinal tract of seabass as an additional energy source (Lückstädt, 2008a, b), leading to an enhanced growth rate and better protein utilisation than those observed in fish not receiving the supplement. Moreover, the increased PER indicated that more dietary energy/protein was released by the use of SP. Our experiment yielded good growth similar to or greater than that previously reported for seabass of a similar size (Kousoulaki et al., 2015), reflecting the appropriateness of the diet formulation, rearing conditions and fish welfare.
The most important modes of action of acidifiers are their antimicrobial effect and stimulation and activation of digestive enzymes (Lückstädt, 2008a, b). Organic acids/salts might alter the availability and enhance the absorption of certain nutrients, such as amino acids, lipids, and phosphorus (Sarker et al., 2012; Morken et al., 2011). Our results indicated that SP has beneficial effects on seabass fry, leading to enhanced survival, growth, and disease resistance and a controlling effect on the gastrointestinal microbiota composition. These findings are consistent with those of prior studies that demonstrated improvement in these criteria upon dietary supplementation with propionic acid/salts for other fish species (Ringø, 1991; Hassaan et al., 2014; Safari et al., 2016; Reda et al., 2016; Hoseinifar et al., 2016) and shrimps (da Silva et al., 2016). However, the advantageous effect of SP on the FCR of seabass in the present study is in contrast to the lack of a significant effect on the FCR of Caspian white fish (Hoseinifar et al., 2016).
Fish fry relies on only their innate immune system whilst their adaptive immune system is still developing, making them susceptible to infectious diseases (Magnadottír, 2006). Again, the antimicrobial effect of SP was evident in the current study, which led to higher survival (97.5%) of 0.3% SP-fed seabass fry than of fry not receiving the supplement (94%). This effect may be attributed to the fact that dietary acidification by SP is an effective strategy for reducing the impacts of pathogens and improving disease resistance in seabass fry, suggesting an immunity-boosting effect. These findings are consistent with those of previous reports, which indicated that diet acidification is commonly practised to enhance disease resistance in aquaculture production (Menanteau-Ledouble et al., 2017).
The effects of dietary supplementation with organic acids/salts on the haemato-immunological parameters of aquatic animals are poorly studied, but the antimicrobial activity of these substances against a broad spectrum of pathogens such as bacteria has been demonstrated (Ringo et al., 2015), and SP is no exception. Acidifiers reduce the gut pH and provide nutrients to the host, changes that partly explain the reported beneficial effects of acidifiers in fish, such as improved growth, disease resistance, and immunity (Hoseinifar et al., 2017). In line with our findings, feeding 1% or 2% propionic acid to Nile tilapia (O. niloticus) and 0.25% SP to Caspian white fish (R. frisii kutum) significantly improved certain immunological indicators, including the serum bactericidal index, LA, and nitric oxide (Reda et al., 2016; Hoseinifar et al., 2016).
Investigating the effect of dietary components on the gut microbiota is important as the gastrointestinal tract (GIT) is thought to be one of the major routes of infection in fish. Very few studies have reported changes in the bacterial community in the GIT of seabass (Guerreiro et al., 2018; Sun et al., 2013; Carda-Dieguez et al., 2013) or a reduction in Vibrio in the intestine of other marine fish with the use of propionic acid or salts (Hoseinifar et al., 2017). However, the effects of organic acids/salts go beyond the modification of gut microflora, and other benefits, such as improved digestive enzyme activity, increased pancreatic secretion, enhanced development of the intestinal epithelium and intestinal barrier integrity, and better anti-inflammatory properties, have often been observed and identified as likely causes of enhanced performance when supplementing diets with some of these organic salts (Piñeiro et al., 2013). The positive changes in gut microbiota composition observed in our study indicated that the overall microbial community was positively modified to a large extent by dietary SP and contributed to the enhanced growth performance, immune status, and protein efficiency in seabass fed a 0.3% or 0.2% propionate diet. The observed modification in the intestinal microbial community likely developed due to propionate presence and/or level causing a decrease in the intestinal pH, which inhibits the growth of certain pathogenic bacteria (Ringo et al., 2015). Our results confirmed that SP possesses inhibitory potential against Vibrio spp., as suggested by Defoirdt et al. (2011), but the beneficial acid-fermentative bacteria did not respond positively to SP presence nor level (Cotter and Hill, 2003). The present research confirms previous reports that dietary sodium/calcium propionate can effectively enhance the gut health of seabass fry and disease resistance of tilapia (Hassaan et al., 2014), Caspian white fish (Hoseinifar et al., 2016) and shrimps (da silva et al., 2016).
Studies on the effects of dietary acidifiers on fish intestinal histomorphology are very limited. The results of the present research clearly show that SP has an impact on the morphological characteristics of three regions (PI, MI, and DI) of the seabass intestine, especially the distal portion. In general, the intestinal structure of seabass is in agreement with the features of intestinal layers previously reported by Bonaldo et al. (2008), Cerezuela et al. (2012), Rimoldi et al. (2016), and Abdel Mohsen et al. (2018). The present investigation revealed an increase in the thickness of both the ML and SML in the DI of fish fed the 0.3% SP diet relative to that in fish fed the non-supplemented diet, in contrast to the results of Rimoldi et al. (2016) for seabass (540 g) administered an acidified high soy protein diet with 0.2% sodium butyrate. These differences are probably due to the use of different acidifiers, fish sizes, and diet compositions. The epithelium brush border layer is the area where much of the absorption of nutrients occurs (Cerezuela et al., 2012). Therefore, the increase in LP and GC number observed in the present study may have been associated with increased nutrient absorption in seabass fry fed propionate diets. In aquatic animals, intestinal VH is regarded as a proxy for absorption ability (Cerezuela et al., 2012). Similarly, our findings showed that diets containing propionate caused an increase in VH in the PI and DI and VW in the DI, which revealed the trophic effects of propionate on the intestinal epithelium of seabass through an increase in the VH and crypt depth. This increase in villus area could be related to the number of absorptive cells, which would make these villi appear more or less bulky. Furthermore, it was recently reported that sodium butyrate improved the intestinal morphology of seabass fry by increasing the gut absorptive area in the lumen, microvillus density and height, and villus structural complexity (Abdel-Mohsen et al., 2018). In contrast, shortening of the primary and secondary intestinal mucosal folds is suggested to be a typical sign of intestinal inflammation (Rimoldi et al., 2016). Our findings are consistent with that of a previous report in which acidifiers (such as SP) stimulated gut secretions, which increased nutrient utilisation and protein synthesis and, ultimately, fish protein content (Freccia et al., 2014). Fish intestinal mucosa consists of a simple epithelium with abundant GCs and an LP containing infiltrated leucocytes and a continuous mucus layer. In our research, a comparison of GC numbers along the entire intestine of seabass fry revealed a higher count in the posterior portion (DI) than in the anterior portion (PI). GCs secrete high-molecular-weight glycoproteins called mucins (Forstner, 1978). The mucus layer keeps the epithelium moist, acts as a lubricant, traps microbes, and aids in the expulsion of microorganisms (Basset et al., 2003). Consequently, higher GC numbers also relate to increased protection and lubrication for faecal exclusion (Dai et al., 2007) when seabass are fed a 0.3% or 0.2% SP diet. To summarise, SP can stimulate digestive secretions, increase VH and villus density, and increase mucus production through an increase in the number of GCs.
Conclusions
Overall, several beneficial effects of including 0.3% (or sometimes 0.2%) SP in the diets of seabass fry were observed, such as an improvement in growth, the FCR, the PER, and EU, thereby improving survival and yield. Therefore, SP positively affected seabass fry performance and protein content and was very efficient for further improving health and immunity. Moreover, SP positively modified the gut microbiota as well as intestinal morphological characteristics, thereby promoting fish gut health and well-being. These results show for the first time the potential for SP to act as an antibiotic-free growth promoter for seabass fry, possibly leading to heightened protection against pathogens; hence, it can be considered a potential biocontrol agent in small-fish culture, being additionally safe for consumers and the environment.
References
Abdel-Mohsen HH, Wassef EA, El-Bermawy NM, Abdel-Meguid NE, Saleh NE, Barakat Kh M, Shaltout OE (2018) Advantageous effects of dietary butyrate on growth, immunity competence, intestinal microbiota and histomorphology of European Seabass (Dicentrarchus labrax) fry. Egyptian Journal of Aquatic Biology and Fisheries 22(4):93–110
Amiza MA, Zakiah J, Khim L, Lay KW (2006) Fermentation of tempoyak using isolated tempoyak culture. Res J Microbiol 1(3):243–254
AOAC (2015) Official methods of analysis of the Association of official Analytical Chemists. 20th Ed. 1, Benjamin Franklin Station, Washington DC
Basset C, Holton J, O’Mahony R, Roitt R (2003) Innate immunity and pathogen-host interaction. Vaccine 21:12–23
Bonaldo A, Roem AJ, Fagioli P, Pecchini A, Cipollini I, Gatta PP (2008) Influence of dietary levels of soybean meal on the performance and gut histology of gilthead seabream (Sparus aurata L.) and European seabass (Dicentrarchus labrax L.). Aquac Res 39:970–978
Carda-Dieguez M, Mira A, Fouz B (2013) Pyrosequencing survey of intestinal microbiota diversity in cultured seabass (Dicentrarchus labrax) fed functional diets. FEMS Microbiol Ecol 87:451–459
Cerezuela R, Fumanal M, Tapia-Paniagua ST, Meseguer M, Morinigo MA, Esteban MA (2012) Histological alterations and microbial ecology of the intestine in gilthead seabream (Sparus aurata L.) fed dietary probiotics and microalgae. Cell Tissue Res 350:477–489
Cotter PD, Hill C (2003) Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol Mol Biol Rev 67:429–453
da Silva BC, Vieira FN, Mouriño JLP, Bolivar N, Seiffert WQ (2016) Butyrate and propionate improve the growth performance of Litopenaeus vannamei. Aquac Res 47:612–623. https://doi.org/10.1111/are.12520
Dacie J, Lewis S (2006) Practical hematology. Churchill Livingstone, London, pp 26–47
Dai X, Shu M, Fang W (2007) Histological and ultra-structural study of the digestive tract of rice field eel, Monopoterus albus. J Applied Ichthyology 23:177–183
Defoirdt T, Sorgeloos P, Bossier P (2011) Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol 14:251–258
Ellis AE (1990) Serum antiproteases in fish. In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS, van Muiswinkel WB (eds) Techniques in fish immunology. SOS Publications, Fair Haven, pp 95–99
Forstner JF (1978) Intestinal mucins in health and disease. Digestion 17: 234–263. Publishing Policy and Support Branch, Office of Knowledge Exchange, Research and Extension, Rome
Freccia A, Sousa SM, Meurer F, Butzge AJ, Mewes JK, Bombardelli RA (2014) Essential oils in the initial phase of broodstock diets of Nile tilapia. R Bras Zootec 43:1–7
Guerreiro I, Serra CR, Oliva-Teles A, Enes P (2018) Gut microbiota of European sea bass (Dicentrarchus labrax) is modulated by short-chain fructooligo-saccharides and xylooligo-saccharides. Short communication. Aquacult Int 26:279–288. https://doi.org/10.1007/s10499-017-0220-4
Hassaan M, Wafa M, Soltan M, Goda A, Mogheth N (2014) Effect of dietary organic salts on growth, nutrient digestibility, mineral absorption and some biochemical indices of Nile tilapia; Oreochromis niloticus L. fingerlings. World Appl Sci J 29:47–55
Hoseinifar SH, Zoheiri F, Caipang CM (2016) Dietary sodium propionate improved performance, mucosal and humoral immune responses in Caspian white fish (Rutilus frisii kutum) fry. Fish and Shellfish Immunology 55:523–528
Hoseinifar SH, Sun YZ, Caipang CM (2017) Short chain fatty acids as feed supplements for sustainable aquaculture: an updated review. Review Article Aquaculture Research 48(4):1380–1391
Hosseini E, Grootaert C, Verstraete W, Van de Wiele T (2011) Propionate as a health-promoting microbial metabolite in the human gut. Nutr Rev 69:245–258
ISO, International Standard ISO 9308-1 (1990) Water Quality Detection and Enumeration of Coliform Organisms, Thermotolerant Coliform Organisms and Presumptive Escherichia coli. Part 1: Membrane Filtration Method. International Organization for Standardization. Geneva, Switzerland. https://www.iso.org/standard/16966.html
Kawahara E, Ueda T, Nomura S (1991) In vitro phagocytic activity of white spotted shark cells after injection with Aeromonas salmonicida extracellular products. Gyobyo Kenkyu/Fish Pathol, Japan 26(4):213–214
Kobayashi T, Enomato S, Sakazaki R, Kuwahara S (1963) A new selective medium for pathogenic Vibrios TCBS agar (modified Nakanishi’s agar). Japanese J Bacteriology 18:387–391
Kousoulaki K, Sæther BS, Albrektsen S, Noble C (2015) Review on European seabass (Dicentrarchus labrax, Linnaeus, 1758) nutrition and feed management: a practical guide for optimizing feed formulation and farming protocols. Aquac Nutr 21:129–151
Lückstädt C (2008a) The use of acidifiers in fish nutrition. CAB Reviews: Perspectives in Agriculture. Veterinary Science, Nutrition and Natural Resources. 3 (044): 1–8. https://www.cababstractsplus.org/cabreviews
Lückstädt C (2008b) Effect of organic acid containing additives in worldwide aquaculture–sustainable production the non-antibiotic way: 71-77. In: Lückstüadt C (ed) Acidifiers in animal nutrition. A guide for feed preservation and acidification to promote animal performance. Nottingham University Press, Nottingham
Magnadottír B (2006) Innate immunity of fish (overview). Fish and Shell fish Immunology 20:137–151
Menanteau-Ledouble S, Krauss I, Gonçalves RA, Weber B, Santos GA, El-Matbouli M (2017) Antimicrobial effect of Biotronic® Top3 supplement and efficacy in protecting rainbow trout (Oncorhynchus mykiss) from infection by Aeromonas salmonicida subsp. salmonicida. Res Vet Sci, 114: 95-100. https://doi.org/10.1016/j.rvsc.2017.03.010.
Morken T, Kraugerud OF, Barrows FT, Sørensen M, Storebakken T, Øverland M (2011) Sodium diformate and extrusion temperature affect nutrient digestibility and physical quality of diets with fish meal and barley protein concentrate for rainbow trout (Oncorhynchus mykiss). Aquaculture 317:138–145
Ng WK, Koh CB (2016) The utilization and mode of action of organic acids in the feeds of cultured aquatic animals. Rev Aquac 0:1–27
NRC (National Research Council) (2011) Nutrient requirements of fish and shrimp. National Council of the National Academies, Washington DC, p 376
Partanen KH, Mroz Z (1999) Organic acids for performance enhancement in pig diets. Nutr Res Rev 12:117–145
Piñeiro PS, Ferreiro UV, Calvar NE, Leal JM (Eds) (2013) New additives and ingredients in the formulation of aquafeeds. Centro Tecnológico del Mar Fundación CETMAR, Special Report, 81 pp
Reda RM, Mahmoud R, Selim KM, El-Araby IE (2016) Effects of dietary acidifiers on growth, hematology, immune response and disease resistance of Nile tilapia, Oreochromis niloticus. Fish and Shellfish Immunology 50:255–262
Rimoldi S, Finzi G, Ceccotti C, Girardello R, Grimaldi A, Ascione C, Terova G (2016) Butyrate and taurine exert a mitigating effect on the inflamed distal intestine of European sea bass fed with a high percentage of soybean meal. Fisheries and Aquatic Sciences 19:19–40. https://doi.org/10.1186/s41240-016-0041-9
Ringø E (1991) Effects of dietary lactate and propionate on growth and digesta in Arctic charr, Salvelinus alpinus (L.). Aquaculture 96:321–333
Ringo E, Zhou Z, Vecino JLG, Wadsworth S, Romero J, Krogdahl Å, Olsen RE, Dimitroglou A, Foey A, Daveis S, Owen M, Lauzon HL, Martinsen LL, De Schryver P, Bossier P, Sperstad S, Merrifield DL (2015) Effect of dietary components on the gut microbiota of aquatic animals. A never-endeng story? Aquac Nutr 22:1–42. https://doi.org/10.1111/anu.12346
Safari R, Hoseinifar SH, Kavandi M (2016) Modulation of antioxidant defense and immune response in zebra fish (Danio rerio) using dietary sodium propionate. Fish Physiol Biochem 42:1733–1739
Sahoo P, Kumari J, Mishra B (2005) Non-specific immune responses in juveniles of Indian major carps. J. App. Ichthyology 21(2):151–155
Sarker MSA, Satoh S, Kamata K, Haga Y, Yamamoto Y (2012) Supplementation effect(s) of organic acids and/or lipid to plant protein-based diets on juvenile yellowtail, Seriola quinqueradiata Temminck et Schlegel 1845, growth and, nitrogen and phosphorus excretion. Aquac Res 43:538–545
Secombes CJ (1990) Isolation of salmonid macrophages and analysis of their killing activity. In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS, Van Muiswinkel WB (eds) Techniques of fish immunology. SOS Publications, Fair Haven, pp 137–154 137e152
Siwicki AK, Anderson DP (1993) Nonspecific defense mechanisms assay in fish: II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs and total immunoglobulin (Ig) level in serum. In: Fish Disease Diagnosis and Prevention Methods. (Eds: Siwicki AK, Anderson DP and Waluga J). (Publ.,Wydawnictwo Instytutu Rybactwa Strodladowego) Olsztyn, Poland: 105-111
Sun H, Jami E, Harpaz S, Mizrahi I (2013) Involvement of dietary salt in shaping bacterial communities in European seabass (Dicentrarchus labrax). Sci Rep 3:1558
Van-Kampen EJ, Zijlstra WG (1961) Standardization of hemoglobin-metery. Clin.Chem.Acta 6:438–544
Vázquez F, Muñoz-Cueto J (2014) Biology of European seabass. CRC Press, Boca Raton
Wang J, Qingyan T, Wang Z, Mai K, Xu W, Zhang Y, Ai Q (2017) Effects of fish meal replacement by soybean meal with supplementation of functional compound additives on intestinal morphology and microbiome of Japanese seabass (Lateolabrax japonicus). Aquacul Res 48:2186–2197
Wassef EA, Abdel-Momen SA, Saleh NE, Al-Zayat AM, Ashry AM (2017) Is sodium diformate a beneficial feed supplement for European seabass (Dicentrarchus labrax)? Effect on growth performance and health status. Egypt J Aquat Res 43:229–234
Zobell CE (1946) Marine Microbiology. Chronica Botanica, Waltham, p 240
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wassef, E.A., Saleh, N.E., Abdel-Meguid, N.E. et al. Sodium propionate as a dietary acidifier for European seabass (Dicentrarchus labrax) fry: immune competence, gut microbiome, and intestinal histology benefits. Aquacult Int 28, 95–111 (2020). https://doi.org/10.1007/s10499-019-00446-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-019-00446-7