Abstract
Nocardiosis is a chronic infectious disease that afflicts multiple fish species and poses a growing threat to the aquaculture. In this study, Nocardia sp. infection was identified in jade perch, Scortum barcoo, from a pond culture in Guangzhou, China. The infected fish were characterized by dark body color, occasional skin ulcerations, and nodular lesions in the kidney, liver, heart, and other organs with typical granuloma structure under light microscopy. Ziehl–Neelsen staining revealed a large quantity of bacterial aggregates within lesions. Histological examination of the kidney, liver, and spleen demonstrated typical granuloma accompanied by histopathological changes. A bacterial strain with consistent morphology was isolated from multiple tissues. Symptoms of nocardiosis were also observed in healthy fish after artificial infection with the isolated strain. Physiological and biochemical experiments suggested that the isolated strain shared the basic characteristics of Nocardia, and the causative pathogen was subsequently confirmed as Nocardia seriolae by sequencing of 16S ribosomal RNA and the gyrB gene. To our knowledge, this is the first report of nocardiosis in jade perch; it may pose a serious risk to the aquaculture of jade perch due to prolonged incubation prior to symptom emergence and the low efficacy of current treatments in china.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nocardiosis is a chronic wasting disease caused by Nocardia spp. infection. As important pathogen in aquaculture, Nocardia are aerobic gram-positive branching filamentous Actinomycetes and belong specifically to the family Nocardiae (Eshraghi 2015). The early stages of nocardiosis in fish are largely asymptomatic. Granuloma is the primary histological sign, which progressively invades into multiple organs and tissues, such as the kidney, liver, spleen, intestine, and muscle, and generates evident lesions. Due to this gradual emergence of symptoms, nocardiosis is usually not identified until moderate to advanced stages. Furthermore, treatment is labor-intensive and expensive with low efficacy and high recurrence. Thus, nocardiosis is widely prevalent in South America, North America, South Asia, and Australia, and has resulted in substantial economic losses to the local aquaculture industries (Kudo et al. 1988; Cornwell et al. 2011; Elkesh et al. 2013; Vu-Khac et al. 2016). Indeed, N. seriolae is considered the main pathogenic bacteria in Japanese aquaculture and is now posing an ever-increasing danger to the aquaculture industry in China (Chen et al. 2000; Wang et al. 2005, 2007; Wang et al. 2009), particularly for species cultured at high density, such as Micropterus salmoides, Channa argus, Larimichthys crocea, Lateolabrax japonicus, Terapon jarbua, and Trachinotus ovatus.
Jade perch, Scortum barcoo, originally native to Australia, has become a pivotal commercial freshwater fish species due to its fresh taste, no intermuscular bone, strong adaptability to a variety of environments, and high resistance to diseases. Jade perch is also rich in ω-3 fatty acids beneficial to human health. This fish can be cultured at high density and so is a particularly suitable species for aquaculture. Since the successful introduction and cultivation of jade perch from Australia in 2001, it has proven successful among Chinese consumers, resulting in a parallel increase in Chinese aquaculture production. In fact, jade perch has become one of the major cultured fish species in China owing to breakthroughs in artificial propagation. To meet this new demand, there has been a gradual expansion of cultivation areas to include fish ponds, net cages, and factorized cultivation modes. Only in Guangdong province of China, the annual production of jade perch has reached 8000 tons in recent years (Chen et al. 2007; Zhao et al. 2011). Currently, the disease burden on cultured jade perch is not that severe, and is mainly due to infection by Trichodina and other parasites during fry culture. Since 2013, we have studied a large-scale infection in a jade perch aquaculture population manifested by surface and organ signs of nocardiasis. We isolated N. seriolae from the diseased fish and observed identical symptoms after inoculation in healthy fish, confirming the causative species.
Materials and methods
Origin and symptoms of diseased fish
Ten diseased fish, weighing approximately 300 g, were obtained from an aquaculture farm in Nansha District, Guangzhou, China. Signs and symptoms of disease were observed and the internal signs analyzed after dissection. The tissues surrounding nodules were spread on glass microscope slides, and pressed under cover slips for observation of the granuloma. The slides were stained with Ziehl–Neelsen (ZN) and bacterial filament morphology was observed under light microscopy.
Bacterial isolation
Diseased fish with typical signs and symptoms were washed in tap water, and 75% ethanol to sterilize the surface. Swabs taken from the liver, kidney, spleen, and subcutaneous tissues were streaked onto solid agar of various compositions, such as sheep blood agar or brain heart infusion (BHI) agar, and cultured at 28 °C for 3–7 days. The growth and morphology of the bacterial colonies were observed daily.
Experimental infection
Colonies of Nocardia sp. showing waxy and dry, in appearance with a rough surface on agar plate. Due to the bacteria within the colonies closely adhere together, so single bacterium could not be isolated after directly dissolving in water. Initially, colonies with characteristics consistent with Nocardia sp. were collected using an inoculating loop, crushed in a sterilized mortar bowl to isolate the bacteria, dissolved in physiological saline, centrifuged, washed 2–3 times, and diluted to 1.0 × 108 and 1.0 × 109 colony-forming units (CFU)/ml. Healthy jade perch, weighing approximately 100 g, were provided by the Lab of Fish Genetics and Fish Breeding of our Institute, and were kept for temporary culture in the aquarium for 2 weeks. Experimental fish were intraperitoneally injected with either concentration of bacterial suspension (10 fish/dose group) at 0.2 ml/fish. Control fish (n = 10) were injected with an equivalent volume of physiological saline. Injected fish were then cultured at 27 ± 2 °C with aeration throughout the day, and daily wastewater exchange. Animal conditions were observed and recorded. The organs of dying fish were sampled and prepared for bacterial isolation on sheep blood agar plates.
Identification of pathogenic bacteria
Isolated strains were identified according to Bergey’s Manual of Systematic Bacteriology and the methods proposed for Rapid Identification and Systematics of Actinobacteria by Ruan and Huang (2011). The identification procedures included enzyme production, hydrolysis activity, carbon source utilization, and growth/survival dependence on temperature, salinity, and pH that according to Chen et al. (2000) and Wang et al. (2005).
Pathological examination
The spleen, kidney, and other tissues were sampled from fish with evident signs of infection, fixed in Bouin’s solution for 24 h, dehydrated in an ethanol gradient, made transparent with xylene, embedded in paraffin, and sectioned at 3 μm. Section were subjected to conventional H&E (Hematoxylin And Eosin) staining, and observed under a light microscope.
Molecular identification and analysis of pathogenic bacteria
DNA was extracted from isolated strain of jade perch using a bacterial genomic DNA extract kit (Tiangen) strictly according to the manufacturers’ instructions and stored at − 20 °C. The bacterial 16S ribosomal RNA (rRNA) was amplified by PCR using the primers forward AGAGTTTGATCCTGGTCAGAACGAACGCT and reverse TACGGCTACCTTGTTACGACTTCACCCC in a 50 μl reaction volume. The reaction conditions were denaturation at 94 °C for 4 min, followed by 30 cycles of 94 °C for 30 s, annealing at 55 °C for 1 min, extension at 72 °C for 2 min, and a final extension at 72 °C for 8 min. The PCR product was detected by 1% agarose gel electrophoresis, isolated from the gel, purified by gel extraction kit, inserted into the pMD18-T vector, and then sequenced by Guangzhou IGE Biotechnology Ltd., China. Similar sequences were identified using BLAST. ClustalW 1.8 software was adopted to compare multiple sequences of Nocardia spp. 16S rRNA from NCBI Genbank with the query sequence. A phylogenetic tree was constructed using the MEGA4.0 software package.
The gyrB gene of isolated strain was subject to cloning, sequencing, comparison, and analysis as described for 16S rRNA using the primer set forward GTCGGTCGTACCAGTAGGAT and reverse AAGGCGGAAGCGAAGTCTCA.
Results
Symptoms of diseased fish
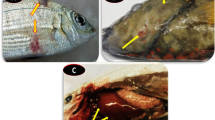
The diseased fish had dark bodies, floated on the surface, and exhibited relatively slow responses to environmental disturbances. Skin ulceration was observed at multiple sites but especially at the fin base. Skin blisters were occasionally observed with rupture and erythema surrounding the margins, and faint yellow pus was observed after cutting off the blister (Fig. 1). Nodular masses, approximately 1–5 mm in diameter, were observed on the surface of the liver, kidney, heart, spleen, and other organs (Fig. 2a, b). Multiple organs exhibited varying degrees of enlargement, especially the kidney and head-kidney.
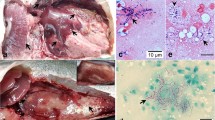
Histopathological manifestations of Nocardia sp. infection. Nodular lesions were identified in the liver, kidney, heart, and other organs (a, b). Evident nodular structure was seen in wet-mount preparations of kidney (arrow, c). Ziehl–Neelsen staining revealed bacterial filament masses stained purple red (d). Scale bars, c = 100 μm; d = 10 μm
Nodular masses of varying sizes enveloped by membrane-like structures were observed in wet-mount preparations of spleen, kidney, and subcutaneous tissues (Fig. 2c). After ZN staining, bacteria on the slides stained purple red, showed filamentous, branching, and beaded structure (Fig. 2d).
Bacterial isolation
Bacteria with uniform shape were isolated from the liver, kidney, spleen, and subcutaneous tissues. These bacteria grew slowly in vitro. Colonies could be observed after 3–5 days of culture on sheep blood agar at 28 °C, while 5–7 days of culture was required on BHI agar. Bacterial colonies gradually enlarged, thickened, and formed irregular margins with prominent elevation which appeared wrinkled, waxy, and granular surface. Colonies were opaque, chalky white, or yellow-gray in color.
Experimental infection
Jade perch presented with typical manifestations of early-stage infection following inoculation, including slowed responses and skin darkening, which gradually progressed to erythema of the fin base and death. Dissection of the dying fish revealed numerous nodular lesions within the abdominal cavity. In addition to liver, spleen, kidney, and other vital tissues, white nodes were observed on the mesentery. Thus, inoculation with cultured bacteria from the original aquaculture colony reproduced the typical lesions and symptoms of nocardiosis. Bacterial colonies consisting of uniformly shaped lesions were isolated from different diseased tissues and confirmed as N. seriolae by molecular identification. At 17 days after infection, all fish in both dose groups died, suggesting that the isolated stain was the original pathogenic bacteria.
Physiological and biochemical identification of the pathogenic bacteria
The biochemical characteristics, temperature dependence of growth, salt tolerance, hydrolysis activity, and carbon source utilization of the isolated strain are illustrated in Table 1. Based upon the methods in Rapid Identification and Systematic of Actinobacteria, and the findings by Kudo et al. (1988), Chen et al. (2000) and Wang et al. (2005), the physiological and biochemical characteristics of the isolated strain were consistent with Nocardia sp.
Histopathological changes
Histological examination demonstrated granulomas in the kidney, liver, spleen, and other organs, accompanied by various histopathological changes (Fig. 3). Most granulomas were round/ovoid in shape. Necrotic lesions surrounded by layers of macrophages were observed at the center and an epithelial cell-derived membrane was observed surrounding these structures, consistent with the nodular structures observed in wet-mount preparations from the infected jade perch. In the liver, nodes of varying sizes were noted, and cell swelling, necrosis, and even vacuolization lesions were observed in the surrounding tissues (Fig. 3a). A large quantity of nodes could be seen within the kidney, accompanied by glomerulus telangiectasis, renal tubular epithelial cell edema, degeneration, and even necrosis (Fig. 3b). The number of lymphocytes was increased substantially in the head-kidney and spleen of infected fish (Fig. 3c, d), and granulomas enveloped by fibrocytes were loosely distributed throughout the tissues, which mainly consisted of fibrous connective tissues and large-body cells. In the capsular membrane structures of the spleen and kidney, numerous necrotic lesions formed by dead cellular masses were identified, distributed outside the capsule and occasionally surrounded by epithelial cells. Bacterial filaments could be identified within necrotic lesions as well as within small punctate nodes on the organ surface of artificially infected fish and the original population (Fig. 3e).
Histopathological changes in jade perch infected with Nocardia sp. Round/ovoid granuloma lesions of varying sizes were observed in the liver (a), kidney (b), spleen (c), and head-kidney (d). Necrotic tissues (N) were identified within the lesions and epithelial cells were noted at the periphery. Necrotic lesions consisting of a large quantity of dead cellular masses were identified within the capsular membrane structures of spleen and kidney (e). Scale bars, a, b, c = 50 μm; d, e = 20 μm
Molecular identification and systematic evolution analysis of pathogenic bacteria
DNA fragments of the expected size were obtained by PCR amplification using primers targeting 16S rRNA and the gyrB gene. The obtained 16S rRNA sequence was 1492 bp in size. The sequence has been submitted to GenBank with accession number KY 029036, and completely identical to the 16S rRNA gene of N. seriolae isolated from Seriola dumerili (NCBI: AB255698). The obtained gyrB gene was 2296 bp in size, and the sequence has been submitted to GenBank with accession number KY 368575. BLAST analysis demonstrated that six bases differed from the N. seriolae sequence isolated from Anguila japonica (NCBI: CP017839.1), with 99.74% homology and no insertions or deletions. Based on pathophysiological responses, growth characteristics, and sequencing, N. seriolae was confirmed as the pathogenic bacteria in this infected population of jade perch.
The 16S rRNA and gyrB sequences of different Nocardia species were downloaded from Genbank, and the MEGA 4.0 software package used to construct phylogenetic trees of Nocardia spp. (Figs. 4 and 5). According to these phylogenetic trees, many Nocardia species could be discriminated based on either 16S rRNA or gyrB gene sequences. These genes could thus serve as useful markers to identify Nocardia species and their phylogenetic relationships.
Discussion and conclusion
Jade perch retrieved from an aquaculture farm in Nansha District, Guangzhou, China, exhibited surface ulcerations and nodular masses in vital organs suggestive of nocardiosis. Wet-mount preparations revealed granuloma lesions containing ZN-stained filamentous, branching bacteria, consistent with nocardial infection. Moreover, the bacteria isolated from the diseased fish reproduced these signs and symptom in healthy jade perch. Finally, we identified the pathogenic species as N. seriolae, confirming nocardiosis. N. seriolae can cause long-term occult infection within the fish body, but it seldom causes mass death (Wang et al. 2007; Cornwell et al. 2011; Elkesh et al. 2013). Thus, fish at different stages of infection co-existed within the fish pond, again typical of nocardial infection.
Like many Actinomyces, Nocardia sp. is widely distributed in both terrestrial and aquatic environments, including water, soil, bottom mud, and rotting animals and plants (Beaman and Beaman 1994; Saubolle and Sussland 2003). Nocardia sp. is a typical opportunistic pathogen and infection may be facilitated by the aquaculture environment. Although the bacterial concentration in the water is not that high, intensified culture and high animal density are likely to compromise fish immunity and increase the risk of bacterial infection (Wang et al. 2005). Jade perch are particularly amenable to aquaculture due to their mild disposition and low oxygen demand. Consequently, the culture density of jade perch is high, especially during the winter, which significantly deteriorates the water quality and decreases immune function, thereby increasing the incidence of disease.
These results indicate that N. seriolae is able to form granulomas within jade perch resembling those caused by Mycobacterium marinum infection (Swaim et al. 2006). Granuloma formation is a pivotal host strategy to control parasitic bacteria. However, granuloma formation does not eliminate the bacteria and paradoxically can actually sustain infection by acting as a habitat for the bacteria, thereby contributing to the refractory nature of this disease (Davis and Ramakrishnan 2009; Gauthier and Rhodes 2009; Yang et al. 2012). In nocardiasis fish, the granuloma consists mainly of macrophages and Nocardia sp., possibly due to the long-term interaction between pathogen and host. The host accumulates a large quantity of immune cells to resist pathogen invasion and utilizes epithelial cells to surround the lesions and prevent systemic spread of bacteria, eventually leading to the formation of granuloma tissues. The formation of granulomas is probably the primary cause of the enduring disease course (Itano et al. 2006; Wang et al. 2014). Simultaneously, the tangible barrier provided by granuloma may impede antibiotic penetration, to some extent causes the pathogen within the granuloma to escape the effect of the drug, which affects the antibiotic treatment with low efficacy (Dartois 2014). At present, research on N. seriolae infection of fish mainly stresses etiology, while bacterial proliferation, diffusion, and reaction in the host are poorly understood, hampering effective disease prevention and control. Systematic investigations of infection, progression, and tissue pathogenesis will provide clues to effective prevention and treatment strategies against nocardiosis .
The 16S rRNA gene is a vital molecular biomarker for phylogenetic systematics and so is frequently utilized for phylogenetic analyses and determination of classification level. The 16S rRNA sequence of Nocardia is highly conserved, and so can be used to discriminate species within the genus. A growing number of genes are now applied to analyze prokaryotic phylogenetic relationships. Indeed, Takeda et al. (2010) distinguished 56 strains of Nocardia by comparative analysis of the gyrB gene. In contrast, Yang et al. (2007) reported that the gyrB gene is superior to the 16S rRNA gene for phylogenetic classification of the Nocardiopsis genus. In this research, the gyrB gene sequence of N. seriolae exhibits a slightly higher degree of variation than 16S rRNA, which contains more genetic evolutionary information and so is a more suitable molecular marker for analytical study of the phylogenetic relationships within this bacterial species.
Gene sequences contain abundant evolutionary clock information from which biological relationships among species can be ascertained (Christen 2008). As the impact of genomic and molecular data grows in epidemiology, studies about bacterial systematic evolution and genetic epidemiology have increased. Renibacterium salmoninarum is the pathogen of bacterial kidney disease and forms granulomas that show symptoms consistent with the nocardial infection. Bacterial kidney disease usually exhibits hidden infection in fish which transmits the pathogen conveniently, present a similar epidemiology to nocardiosis (Murray et al. 2011; Hall et al. 2015). R. salmoninarum is more suitable for low temperatures below 18 °C, mainly infected the coldwater fish, salmonids, in both cultured and wild worldwide (Brynildsrud et al. 2014; Purcell et al. 2016); Nocardiosis has a wider temperature range, occurs not only in coldwater fish but also tropical fish. Nocardial infection had been identified in so many fish species including salmonids, showing a broader host range (Chen et al. 2000; Wang et al. 2007; Cornwell et al. 2011; Elkesh et al. 2013; Vu-Khac et al. 2016). Herefore, the genetic relationship of Nocardia from different sources can be more complicated. Brynildsrud et al. (2014) exploited 68 diverse isolates of R. salmoninarum representing broad geographical and different host using genome sequencing technology, and the phylogenetic results indicated all isolates can delineated into two lineages that might help us to understand the process of bacterial evolution and epidemiology in salmonids. As intracellular pathogens, N. seriolae, like R. salmoninarum, have complex pathogen-host interactions to phagocytes in fish, presenting chronic infection with a broad host range, so the genetic epidemiology of N. seriolae deserve further exploration.
Nocardiosis is a serious threat to the seawater aquaculture industry in East Asia, especially N. seriolae. Seriolae dumerili, Lateolabrax japonicus, Mugil cephalus, Lutianus erythroptrus, Morone saxatilis, Larimichthys crocea, and even freshwater fish species have been infected with N. seriolae (Chen et al. 2000; Wang et al. 2005; Itano et al. 2006; Shimahara et al. 2009; Cornwell et al. 2011). In recent years, the infection rate of nocardiosis in M. salmoides, C. argus, and other freshwater cultured species has increased, causing widespread alarm among aquaculture practitioners. In aquaculture, infectious diseases with wide host ranges, such as iridovirus and betanodavirus, is reported from seawater to freshwater, share many prevalent characteristics (Furusawa et al. 2007; Dong et al. 2008; Jeong et al. 2008; Whittington et al. 2010; Crane and Hyatt 2011; Shetty et al. 2012; Liu et al. 2015; Rimmer et al. 2016). These phenomena covering infection, host-pathogen interaction and synergistic evolution deserve extensive investigation. The frozen marine wild trash fish has been used as an important feed for Micropterus salmoides, Channa spp., and other freshwater fish species in the Zhujiang Delta of Guangdong Province, China, and represents a potential pathway for N. seriolae transmission (Gomez et al. 2010). As a pathogenic bacterium with a wide host range, N. seriolae seldom causes acute death of the host and so is prone to trans-species transmission. Therefore, comparing the different origins of N. seriolae between seawater and freshwater fish will enhance our understanding of the mechanism underlying infection and transmission of nocardiosis in fish.
Abbreviations
- N.:
-
Nocardia
- 16S rRNA:
-
16S ribosomal RNA gene
- gyrB:
-
Gyrase B
- BHI:
-
Brain heart infusion
- ZN:
-
Ziehl–Neelsen staining
- H&E:
-
Hematoxylin and eosin staining
References
Beaman BL, Beaman LV (1994) Nocardia species: host-parasite relationships. Clin Microbiol Rev 7:213–264
Brynildsrud O, Feil EJ, Bohlin J, Castillo-Ramirez S, Colquhoun D, McCarthy U, Verner-Jeffreys DW (2014) Microevolution of Renibacterium salmoninarum: evidence for intercontinental dissemination associated with fish movements. ISME J 8:746–756
Chen SC, Lee JL, Lai JC, Gu YW, Wang CT, Chang HY, Tsai KH (2000) Nocardiosis in sea bass, Lateolabrax japonicus, in Taiwan. J Fish Dis 23:299–307
Chen KC, Zhu XP, Du HJ, Xie G, Liu YH, Zheng GM, Chen YL (2007) Effects of temperature and salinity on the embryonic development of jade perch Scortum barcoo. J Fishery Sci China 14:1032–1037
Christen R (2008) Global sequencing: a review of current molecular data and new methods available to assess microbial diversity. Microbes Environ 23:253–268
Cornwell ER, Cinelli MJ, Mcintosh DM, Blank GS, Wooster GA, Groocock GH, Getchell RG, Bowser PR (2011) Epizootic Nocardia infection in cultured weakfish, Crynoscion regalis (Bloch and Schneider). J Fish Dis 34:567–571
Crane M, Hyatt A (2011) Viruses of fish: an overview of significant pathogens. Viruses 3:2025–2046
Dartois V (2014) The path of anti-tuberculosis drugs: from blood to lesions to mycobacterial cells. Nat Rev Microbiol 12:159–167
Davis JM, Ramakrishnan L (2009) The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136:37–49
Dong C, Weng S, Shi X, Xu X, Shi N, He J (2008) Development of a mandarin fish Siniperca chuatsi fry cell line suitable for the study of infectious spleen and kidney necrosis virus (ISKNV). Virus Res 135:273–281
Elkesh A, Kantham KL, Shinn AP, Crumlish M, Richards RH (2013) Systemic nocardiosis in a Mediterranean population of cultured meagre, Argyrosomus regius Asso (Perciformes: Sciaenidae). J Fish Dis 36:141–149
Eshraghi S (2015) Molecular typing of Nocardia species. J Med Bacteriol 1:38–45
Furusawa R, Okinaka Y, Uematsu K, Nakai T (2007) Screening of freshwater fish species for their susceptibility to a betanodavirus. Dis Aquat Org 77:119–125
Gauthier DT, Rhodes MW (2009) Mycobacteriosis in fishes: a review. Vet J 180:33–47
Gomez DK, Mori KI, Okinaka Y, Nakai T, Park SC (2010) Trash fish can be a source of betanodaviruses for cultured marine fish. Aquaculture 302:158–163
Hall LM, Duguid S, Wallace IS, Murray AG (2015) Estimating the prevalence of Renibacterium salmoninarum-infected salmonid production sites. J Fish Dis 38:231–235
Itano T, Kawakami H, Kono T, Sakai M (2006) Experimental induction of nocardiosis in yellowtail, Seriola quinqueradiata Temminck & Schlegel by artificial challenge. J Fish Dis 29:529–534
Jeong JB, Kim HY, Jun LJ, Lyu JH, Park NG, Kim JK, Do Jeong H (2008) Outbreaks and risks of infectious spleen and kidney necrosis virus disease in freshwater ornamental fishes. Dis Aquat Org 78:209–215
Kudo T, Hatai K, Seino A (1988) Nocardia seriolae sp. nov. causing nocardiosis of cultured fish. Int J Syst Evol Microbiol 38:173–178
Liu XD, Huang JN, Weng SP, Hu XQ, Chen WJ, Qin ZD, Wang WM (2015) Infections of nervous necrosis virus in wild and cage-reared marine fish from South China Sea with unexpected wide host ranges. J Fish Dis 38:533–540
Murray AG, Hall M, Munro LA, Wallace IS (2011) Modelling management strategies for a disease including undetected sub-clinical infection: bacterial kidney disease in Scottish salmon and trout farms. Epidemics 3:171–182
Purcell MK, McKibben CL, Pearman-Gillman S, Elliott DG, Winton JR (2016) Effects of temperature on Renibacterium salmoninarum infection and transmission potential in Chinook salmon, Oncorhynchus tshawytscha (Walbaum). J Fish Dis 39:787–798
Rimmer AE, Whittington RJ, Tweedie A, Becker JA (2016) Susceptibility of a number of Australian freshwater fishes to dwarf gourami iridovirus (infectious spleen and kidney necrosis virus). J Fish Dis 39:1–18
Ruan JS, Huang Y (2011) Rapid identification and systematics of Actinobacteria. Science Press, Beijing, pp 69–78
Saubolle MA, Sussland D (2003) Nocardiosis review of clinical and laboratory experience. J Clin Microbial 41:4497–4501
Shetty M, Maiti B, Santhosh KS, Venugopal MN, Karunasagar I (2012) Betanodavirus of marine and freshwater fish: distribution, genomic organization, diagnosis and control measures. Indian J Virol 23:14–123
Shimahara Y, Huang YF, Tsai MA, Wang PC, Yoshida T, Lee JL, Chen SC (2009) Genotypic and phenotypic analysis of fish pathogen, Nocardia seriolae, isolated in Taiwan. Aquaculture 294:165–171
Swaim LE, Connolly LE, Volkman HE, Humbert O, Born DE Ramakrishnan L (2006) Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infect Immun 74:6108–6117
Takeda K, Kang Y, Yazawa K, Gonoi T, Mikami Y (2010) Phylogenetic studies of Nocardia species based on gyrB gene analyses. J Med Micro 59:165–171
Vu-Khac H, Chen SC, Pham TH, Nguyen TG, Trinh TH (2016) Isolation and genetic characterization of Nocardia seriolae from snubnose pompano Trachinotus blochii in Vietnam. Dis Aquat Org 120:173–177
Wang GL, Yuan SP, Jin S (2005) Nocardiosis in large yellow croaker, Larimichthys crocea (Richardson). J Fish Dis 28:339–345
Wang GL, Xu YJ, Jin S, Zhu JL, Yuan SP (2007) Nocardiosis in snakehead, Ophiocephalus argus cantor. Aquaculture 271:54–60
Wang PC, Chen SD, Tsai MA, Weng YJ, Chu SY, Chern RS, Chen SC (2009) Nocardia seriolae infection in the three striped tigerfish, Terapon jarbua (Forsskål). J Fish Dis 32:301–310
Wang PC, Tsai MA, Liang YC, Chan Y, Chen SC (2014) Nocardia seriolae, a causative agent of systematic granuloma in spotted butterfish, Scatophagus argus, Linn. Afr J Microbiol Res 8:3441–3452
Whittington RJ, Becker JA, Dennis MM (2010) Iridovirus infections in finfish–critical review with emphasis on ranaviruses. J Fish Dis 33:95–122
Yang LL, Zhi XY, Li WJ (2007) Phylogenetic analysis of Nocardiopsis species based on 16S rRNA, gyrB, sod and rpoB gene sequences. Acta Microbiol Sin 47:951–955
Yang CT, Cambier CJ, Davis JM, Hall CJ, Crosier PS, Ramakrishnan L (2012) Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe 12:301–312
Zhao J, Chen KC, Zhu XP, Zheng GM, Chen YL, Pan DB (2011) Analysis and evaluation of muscle nutrients in jade perch Scortum barcoo. J Dalian Ocean Univ 18:93–96
Acknowledgements
This study was supported by Special Scientific Research Funds for Central Non-profit Institutes, Chinese Academy of Fishery Sciences (No. 2013A0609), and the National Science Foundation in China (No. 31302212).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, F., Wang, X., Liu, C. et al. Nocardia seriolae infection in cultured jade perch, Scortum barcoo . Aquacult Int 25, 2201–2212 (2017). https://doi.org/10.1007/s10499-017-0184-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-017-0184-4