Abstract
Fish trade and aquaculture activities are considered the major causes of fish and shellfish disease spread and transfer. An outbreak of infectious nature in captive stock of Java rabbitfish (Siganus javus) in brackish water tanks was investigated. Microscopic examination revealed different stages of the parasitic dinoflagellate Amyloodinium in the gill filaments of moribund fish. Histopathological studies confirmed severe infestation with gill erosion and lamellar fusion. The geographical lineage of the isolate was determined through partial sequencing of the 18S ribosomal RNA gene. BLAST analysis of the 18SrRNA gene sequence determined the 100% identity to Amyloodinium ocellatum and clustered with other isolates reported from Italy, Israel, the United States, Portugal, and Japan in the phylogenetic tree. The salinity requirement of the isolate was assessed by incubating tomont stages at salinities ranging from 0 to 30 ‰. Salinities below 5 ‰ were shown to inactivate and degrade 90% of the tomonts within 14 days of incubation, whereas salinities above 10 ‰ supported the parasite life cycle and its development. Further, the susceptibility of Asian seabass (Lates calcarifer) to A. ocellatum was elucidated in a challenge study. The current study demonstrated the potential threat of parasitic translocation with fish movement, the salinity regimes for their development, molecular detection including its impact on other cultivable fish species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish are farmed in a variety of aquatic habitats. They contract viruses, bacteria, and parasites spread undetected during fish transit, trade, and aquaculture activities. Parasitic dinoflagellates are a serious threat, particularly, Amyloodinium in the Oodiniaceae family cause heavy economic losses to fish culture in tropical and temperate environments (Lieke et al. 2019; Thomas et al. 2020; Ananda raja 2022; Moreira et al. 2023). The life cycle of parasitic dinoflagellates is direct and rapid, infection is apparent during mass mortality events. With the intensification of marine and brackish water finfish farming, parasitic dinoflagellates have turned out to be a serious risk factor and concern for aquaculture operations in India (Kumar et al. 2015; Kizhakudan et al. 2015; Thomas et al. 2020; Dhayanithi et al. 2022).
Amyloodinium has a wide host range, affecting the skin and gills of fish species, including larval and adult fish, in warmer climatic conditions (Kuperman and Matey 1999; Cruz-Lacierda et al. 2004; Thomas et al. 2020). At present, fish kills due to A. ocellatum are reported in captive brood stocks of marine fish and specific information about its salinity regimes, infectivity, and their host range is remaining unavailable (Kumar et al. 2015; Kizhakudan et al. 2015; Thomas et al. 2020; Dhayanithi et al. 2022).
Fish have varied susceptibility to Amyloodinium infection eliciting inflammatory and local immune responses with the production of Toll-like receptor (TLR-22), chemokines, interleukins (IL-1), and tumor necrosis factor α (TNF-α) in yellow tail (Seriola lalandi), European seabass (Dicentrarchus labrax), and silver pomfret (Pampus argenteus) and developed specific immunity in the hybrid striped bass and tomato clownfish (Amphiprion frenatus) that survived Amyloodiniosis (Smith et al. 1994; Cobb et al. 1998; Reyes-Becerril et al. 2015; Byadgi et al. 2021; Zhang et al. 2021).
Disease management strategies in aquaculture require access to vital information regarding the pathogens, the susceptibility of fishes and methods to limit the spread when introduced into new environment (Eissa et al. 2016). It is also important to have an understanding of environmental patterns and water quality indicators during disease outbreaks (Alosairi et al. 2021). The development of A. ocellatum is primarily affected by temperature and salinity conditions (18–30 °C). However, significant variations in salinity tolerance have been reported in different geographical isolates, indicating the variability in environmental adaptations (Paperna 1984; Alvarez-Pellitero 2008).
The present study investigated Amyloodiniosis in brood stock of Java rabbitfish, a prospective species for diversification (Rajaprabhu et al. 2021), the optimum salinity for A. ocellatum, including its detection by PCR and an infectivity experiment in Asian seabass was undertaken. The study signifies the translocation of parasites with their fish host to new niches and its transmissibility to susceptible hosts in the new environment.
Materials and methods
Sample collection and microscopic examination of Java rabbit fish (Siganus javus)
Java rabbit fish (N = 360, wt ∼ 10–30 g) were collected during April to June, 2020 from the brackish water creeks of Vennangupattu (12°14’30.1"N 79°58’56.4"E), Tamil Nadu, India and were reared in brackish water tanks for captive maturation. Sudden mortalities occurred in fish during November, 2020 (temp-27 ± 2 ºC, salinity-15 ‰) and moribund fish samples were collected for investigations. Briefly, wet mount of the skin mucus and gill lamellae were prepared and observed under microscope (Nikon eclipse Ni, Japan). For ultrastructural studies, gills (1–2 cm) were preserved in 2.5% glutaraldehyde solution at 4 oC overnight and dehydrated with graded alcohol and chemically dried with hexamethyldisilazane and examined under the scanning electron microscope (JEOL Ltd, Japan) (Kumar et al. 2022). For histological analysis, gills were fixed in 10% formalin for 48 h, washed under running water, dehydrated using graded alcohol (50%, 70%, 90%, and 100%), cleared in xylene and embedded in liquid paraffin. Tissues were then sectioned (Leica RM2245, USA) and stained with haematoxylin and eosin and mounted on DPX according to standard procedure, and observed under high magnifications (Nikon eclipse Ni, Japan).
Molecular identification by polymerase chain reaction
DNA extraction
Genomic DNA was extracted using the lysis buffer-phenol-chloroform method with minor modifications (Sambrook et al. 1989). Briefly, the infested gill tissues (∼ 20 mg) were homogenized using sterile homogenising sticks in 200 µL of lysis buffer (50 mM Tris, 1 mM ethylene diamine tetra-acetic acid (EDTA), 500 mM NaCl, 1% SDS) with 2 µL of 20 mg/ml of proteinase K and the homogenate was incubated at 56 °C for 30 min and followed by 95 °C for 10 min. To the tissue digest, 200 µL of phenol-chloroform-isoamyl alcohol was added and centrifuged at 4 °C at 12,000 rpm for 10 min. The aqueous phase was then collected in sterile 1.5 ml microcentrifuge tubes and centrifuged with a mixture of an equal volume of chloroform and isoamyl alcohol (24:1). The supernatant was collected, and the DNA was precipitated in 1.5 volume of 100% alcohol at -20 °C for 20 min. The DNA precipitated was collected by centrifuging and washed in 70% alcohol, and air-dried under sterile conditions. The DNA pellet was dissolved in sterile nuclease-free water (50 µL) and stored at -20 °C for further use.
18SrRNA gene polymerase chain reaction
The 18SrRNA gene was amplified using published primers from Levy et al. (2007a); Marques et al. (2019) (AmyF1-5’ TAGATGTTCTGGGCTGCACG-3’, AmyR1-5’ CCTACGGAAACCTTTACGAC 3’; AmyF2-5’GACCTTGCCCGAGAGGG3’, AmyR2-5’CCGCCACAGTTTTCAGAAGC3’) in a 25 µL reaction volume consisting of 12.5 µL of 2× taq DNA polymerase PCR master mix RED (Ampliqon, Denmark), 0.5 µL each of forward and reverse primers (Eurofins, India) and 2 µL of 50 ng/ µL template genomic DNA. The cycle conditions for PCR were carried out as per published protocols (Levy et al. 2007a; Marques et al. 2019). Briefly, initial denaturation of 3 min at 94 °C, following 30 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 30 s, with a final extension of 10 min at 72 °C. First-step PCR products were diluted (1:10) and subjected to second-step PCR using AmyF2 and AmyR2 primers under the same reaction and cycling conditions. PCR products and 100 bp DNA ladder (Takara, Japan) were resolved on a 1.2% agarose- tris-acetate EDTA gel stained with 0.5 µg/mL ethidium bromide at 100 V for 20 min, visualized and photographed under a gel documentation system (Bio-Rad, USA). The PCR products were purified and sequenced at Genomics laboratory (Eurofins, India).
Sequencing and phylogenetic analysis
The forward and reverse FASTA sequences of 18SrRNA gene retrieved from the chromatogram were edited and aligned using the BioEdit 7.2 tool (Hall 1999). The aligned FASTA sequences were used for homology search using Basic Local Alignment Search Tool (BLAST) in the NCBI database (http://www.ncbi.nih.gov/BLAST). The closest genetic identity of the current isolates was found. Further, the 18SrRNA gene sequences of eight Amyloodinium isolates of India (OP889363.1-present study); the North Adriatic Sea (KY474336.1-Italy); Gulf of Mexico (JX905204.1); common clownfish (AF080096.1-USA); seabass (MG768977.1-Portugal); Red Sea (DQ490257.1-Israel); Tiger pufferfish (LC475458.1-Japan); Mediterranean sea (DQ490256.1), two isolates of Piscinoodinium (EF016921.1 and EF016917.1-USA and six other representative dinoflagellates of O. Gonyaulacales (KX097020.1-Alexandrium fragae and EU130569.1-Dinophysis acuminata); O.Gymnodinales (EF492504.1-Karenia brevis); O. Noctilucales (KR527303.1-Noctiluca scintillans); O.Syndiniales (KX097020.1-Amoebophrya); F. Symbiodiniaceae (AB016581.1-Symbiodinium) along with Ichthyophthirius multifiliis (KJ690572.1) of Phylum Ciliophora as an outgroup were used for phylogenetic analysis using the Molecular Evolutionary Genetics Analysis (MEGA-X) (10.1.8). The best fit model was evaluated after multiple sequence alignment and a phylogenetic tree was generated with 1000 bootstrap replicates and using Maximum likelihood method by employing Kimura 2-parameter with gamma distribution rates (K2 + G) model (Felsenstein 1985; Saitou and Nei 1987; Tamura et al. 2004; Kumar et al. 2018).
Salinity test for A. ocellatum
Collection of tomonts
Tomont stages were collected as described in a previous study (Masson et al. 2013). Infested gills were excised and placed in sterile seawater in a petriplate. Tomonts adhered on the gill lamellae were gently flushed with sterile phosphate buffered saline (PBS) using a pipette, free tomonts in seawater were collected, and rinsed twice and suspended in PBS for further use.
Salinity test
Tomonts (N = 100) were dispersed into 15 ml of 0, 5, 10, 15, 20, and 30 ‰ of saline water in triplicates and incubated at 30 °C under natural conditions. Aliquotes were collected from each treatment every 2 days intervals and were observed under higher magnification until 14 days. The number of tomonts were counted at the end of 14th day and analysed statistically using One-way ANOVA in SPSS statistics 29.0.0.0.
Experimental challenge on Asian seabass (L. Calcarifer) using A. ocellatum
An experimental challenge was carried out to check the infectivity A. ocellatum on Asian seabass. Briefly, Asian seabass (N = 60; wt-100 g) were distributed at the rate of 10 fish/tank into 6 tanks of 500 L capacity (salinity-30 ‰, temperature-28 oC) with continuous aeration and were fed commercial seabass feed twice daily at 2% of their body weight. Tomonts (500 no ≈ 256 × 500 dinospores) were added to three fish tanks (Roberts-Thomson et al. 2006). The remaining fish (N = 30) in three tanks served as the control group. Fish exposed to parasites were routinely checked for the presence of the parasite, and the mortalities. The number of mortalities was recorded per day and the cumulative mortalities were calculated. Wet mounts of gills from dead fish were examined for parasites.
Results
Gross signs and microscopic examination
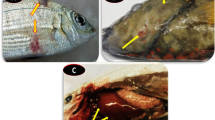
Dead fish appeared asphyxiated with opened mouth and excess mucus covering over the body. Opercular examination revealed the presence of coarse sand and stone particles in the gill chamber. Wet mount preparation of gills of the fish revealed round or ovoid developmental stages of the marine dinoflagellate Amyloodinium. The gills were heavily infested with the round to oval trophont stages (Fig. 1a&b). Microscopic examination revealed round to ovoid trophonts (20–50 µM) adhered to gill lamellae (Fig. 2a&b), tomonts (20–100 µM) were appeared in single or in clusters at different stages of their development (Fig. 2c, SF1), and biflagellated dinospores (< 10 µM) (Fig. 2d). Tomonts appeared round with granular cell contents under the ultrastructural examination of the gills with scanning electron microscopy (∼ 20 µM- Fig. 3).
Histopathological examination of the A. ocellatum infested gill lamellae showed ovoid dividing tomonts located at the interfilamental sulcus. Secondary gill lamellae were curled and fused with adjacent filament. Severe congestion, generalized mild to moderate hypertrophy and hypercellularity of lamellar epithelial cells were observed with synechiae formation by fusion along the lengths of some lamellae and/or at the lamellar tips (Fig. 4).
Histopathological examination of primary lamella of Java rabbitfish, S. javus infested with A. ocellatum. Note ovoid dividing tomonts (arrow head) located at the interfilamental sulcus. Secondary gill lamellae are curled (black arrows) and fused with adjacent filament. Severe congestion, generalized mild to moderate hypertrophy and hypercellularity of lamellar epithelial cells are observed with synechiae formation by fusion along the lengths of some lamellae and/or at the lamellar tips (white arrows). Note the formation of pseudocystic spaces by synechiae. H&E. Scale bar: 50 μm
A. ocellatum has a direct life cycle with a single host (Fig. 5). The illustration depicts the triphasic transmission cycle of A. ocellatum in Java rabbitfish where the free-living dinospore (infective stage) finds the fish host and transforms into a feeding stage called trophont. Trophonts are the obligatory parasites of fish where they feed on the epithelial layers of skin and gills develop into tomont stages. Tomonts are the dividing phase of the parasite where it undergoes multiple divisions and produces dinospores. Duration of the life cycle varies with different isolates and geographical locations under the influence of salinity and temperature.
Illustration of life cycle of A. ocellatum in Java rabbitfish (S. javus). The life cycle depicted a direct transmission cycle of A. ocellatum with a single fish host, where the free-living dinopore finds the suitable fish host and develops into a trophont feeding on the epithelial layers of skin and gills of host. Trophont develops into tomonts which undergoes multiple divisions and transforms into dinospores which are available for new infections
Molecular detection using 18SrRNA gene based PCR
PCR amplification of the DNA extracted from A. ocellatum infested gill tissue using 18SrRNA gene primers yielded a 336 base pairs (bp) amplicon in the first-step and a 225 bp amplicon in the second step (Fig. 6). The PCR product was sequenced and sequence generated were submitted to the NCBI Genbank® (accession number OP889363).
BLAST analysis and phylogenetic tree
The BLASTN analysis revealed that the 18SrRNA gene sequences from the present study were 100% identical to an Italian isolate of A. ocellatum from the North Adriatic Sea (KY474336.1). The phylogenetic tree built using 18SrRNA sequences of eight A. ocellatum and two Piscinoodinium, and other 8 representative dinoflagellates revealed that the Indian isolate clustered with Amyloodinium isolates in a separate clade along with other A. ocellatum isolates from Italy, USA, Mediterranean Sea, Israel, Portugal, and Japan, whereas Ichthyophthirius multifiliis of Phylum Ciliophora formed an outgroup in the phylogenetic tree (Fig. 7).
Phylogenetic tree of 18SrRNA gene of A. ocellatum isolates based on neighbor joining method. 18SrRNA gene of eight Amyloodinium isolates of India (OP889363.1-present study); the North Adriatic Sea (KY474336.1-Italy); Gulf of Mexico (JX905204.1); common clownfish (AF080096.1-USA); seabass (MG768977.1-Portugal); Red Sea (DQ490257.1-Israel); Tiger pufferfish (LC475458.1-Japan); Mediterranean sea (DQ490256.1), two isolates of Piscinoodinium (EF016921.1 and EF016917.1-USA and six other representative dinoflagellates of O. Gonyaulacales (KX097020.1-Alexandrium fragae and EU130569.1-Dinophysis acuminata); O.Gymnodinales (EF492504.1-Karenia brevis); O. Noctilucales (KR527303.1-Noctiluca scintillans); O.Syndiniales (KX097020.1-Amoebophrya); F. Symbiodiniaceae (AB016581.1-Symbiodinium) along with Ichthyophthirius multifiliis (KJ690572.1) of Phylum Ciliophora as an outgroup were used for phylogenetic analysis using the Molecular Evolutionary Genetics Analysis (MEGA-X) (10.1.8)
Effect of salinity on tomonts
Tomonts incubated at different salinities (0, 5, 10, 15, 20, 25, 30 ‰) were counted at the end of 14th day and expressed as percentage of the initial number graphically in Fig. 8. The initial number of tomonts (100 no) were significantly reduced to < 10%, (p value < 0.001) at 0 and 5 ‰ salinity and the microscopic examination revealed that the content of the tomonts were shrunken and with no divisional activities (Fig. 9-a &b, SF2, SF3). No tomonts were observed at 10 ‰ and 15 ‰ salinities (SF4, SF5) and the number of tomonts incubated at higher salinities of 20 ‰ and 30 ‰ were reduced to less than 30% and the tomonts appeared intact and were found to be at different stages of multiplication (SF5, Fig. 9-c&d).
Graphical representation of number of tomonts present at different salinities on 14th day of salinity test. Tomonts (100 nos) were incubated at 0, 5, 10, 15, 20 & 30 ‰ and were counted and expressed as percentage at the end of 14th day. Tomonts were reduced to below 10% at 0 and 5 ‰ salinity and less than 30% at 20 and 30 ‰ salinity. Tomonts were completely absent at 10 and 15 ‰ salinity
Experimental infection on Asian seabass (L. Calcarifer)
The first mortality in Asian seabass exposed to A. ocellatum was occurred on the fourteenth day, cumulative mortality reached 25% (16th day post challenge), 50% (18th day post challenge), and 100% (28th day of post challenge) within 4th, 6th, and 10th day post initial mortality (Fig. 10-a) Infested fish appeared dark coloured, with a thin white layer of substrate covering the dorsal body surface, and excess mucus on gills and skin (Fig. 10- b). Microscopic examination confirmed the presence of several stages of the parasites varying from 20 to 80 nos per square millimeter of gill lamellae (Fig. 10-c).
Discussion
Heavy infestations of parasites pose a significant threat to marine and brackishwater aquaculture, resulting in massive fish mortality and economic losses. Warm-water fishes are particularly vulnerable to parasitic dinoflagellates and ciliates notably including Amyloodinium, Piscinoodinium, Ichthyodinium, Ichthyophthirius, and Cryptocaryon. These organisms present a substantial risk to aquaculture systems due to their rapid generation time, low host specificity and often leading to high mortality rates ranging from 80 to 100% during outbreaks (Noga and Levy 2006; Lieke et al. 2019; Ananda raja 2022; Sudhagar et al. 2022; Moreira et al. 2023). Among parasitic dinoflagellates, Amyloodinium and Piscinoodinium excerts considerable impact in aquaculture systems. Though A.ocellatum is closely similar to Piscinoodinium they are genetically quite distinct, belonging to monospecific genus (Levy et al. 2007a). Moreover, their remarkable tolerance to varying salinity levels and their expected expansion to higher altitudes under global warming effects is a predicted threat to non-parasitized fishes (Moreira et al. 2023).
Amyloodinium has a broad host range affecting various cultured warm-water fish species such as silver moony (Monodactylus argenteus), milkfish (Chanos chanos), mangrove red snapper (Lutjanus argentimaculatus), European seabass (Dicentrarchus labrax), silver pompano (Trachinotus blochii), Indian halibut (Psettodes erumei), striped grey mullet (Mugil cephalus), and seabream (Sparus aurata) (Baticados and Quinition 1984; Cruz-Lacierda et al. 2004; Kumar et al. 2015; Kizhakudan et al. 2015; Moreira et al. 2017; Bessat and Fadel 2018; Thomas et al. 2020). Amyloodinium has been detected in captive fish populations including silver pompano, Indian halibut, silver moony, and percula clown fish, causing concerns in fish hatcheries and brood stock development facilities across India (Kumar et al. 2015; Kizhakudan et al. 2015; Thomas et al. 2020; Dhayanithi et al. 2022). Aquaculture operations have been found to transmit pathogens with their fish hosts potentially harming native fauna (Fishar 2006). Meanwhile, wild-caught fish frequently contain parasites unique to their region, adapt to the new territory and can infiltrate the adjoining fish facilities (Menconi et al. 2019). Some parasitic stages are microscopic and highly resilient to disinfection, evade detection and release with fishes into new environment (Menconi et al. 2019). The present isolate of A. ocellatum was detected in a captive stock of Java rabbit fish and the first documented case of Amyloodiniosis in Java rabbit fish demonstrating severe pathogenicity in affected fish (Cruz-Lacierda et al. 2004). The detection of Amyloodinium at molecular level may aid in developing diagnostics to detect early infections in fish and environmental samples for faster disease prediction and treatment (Levy et al. 2007a). Furthermore, in contrast to other parasitic dinoflagellates like Icthybodo and Piscinoodinium, the phylogenetic analysis based on the SSU gene revealed the presence of a single species of A. ocellatum across various geographical locations with more than 99% sequence similarity (Levy et al. 2007a; Francis-Floyd and Floyd 2011). Despite similar mode of attachment to host, molecular data suggest that the convergent evolution towards ectoparasitism occurred independently in Amyloodinium and Piscinoodinium, placing the later along with free living and endosymbiotic dinoflagellate of O. Sussiales (Levy et al., 2007b).
The temperature and salinity adaptations of A. ocellatum are varied across different geographical isolates, the optimum temperature ranges from 18 to 30 °C and the salinity tolerance across 50 ‰ to 12 ‰ (Paperna 1984; Noga and Levy 1995). Noga and Levy (1995) have suggested that reducing salinities may help to alleviate the burden of A. ocellatum in marine aquarium fish during disease outbreaks, although A. ocellatum outbreaks have been reported in estuarine waters with salinities as low as 2 to 3‰. Therefore, understanding the salinity regimes of the current isolate is highly essential for developing effective preventive strategies against the parasite in tropical aquaculture systems. Our findings revealed that A. ocellatum isolated from Java rabbitfish can be controlled by lowering salinities < 5 ‰, and a 14-day exposure of tomont stages to freshwater inhibits the division and aids their destruction. Lower salinities may be a potential option for recently translocated and infected fish stocks in order to control the infection and prevent parasite spread to local fauna. Our findings on the present parasitic isolate have shown that salinities ranging from 10 to 20 ‰ are conducive for parasite propagation and a potential threat to fishes in tropical brackishwater systems.
Amyloodinium infestation has been the cause of fish mortalities in marine fish hatcheries, cages and farms (Thomas et al. 2020; Bessat and Fadel 2018; Kumar et al. 2015; Kizhakudan et al. 2015; Moreira et al. 2017, 2023) due to their high rate of proliferation and quick spread via free-living dinospores (Noga and Levy 1995). The current study demonstrated the development and transmission of A. ocellatum via tomont stages to native fish fauna. Though devastating, bio-securing the hatcheries with proper quarantine, disinfection, and adequate treatment, parasite infections in marine brood stocks can be effectively prevented (Kumar et al. 2015; Paperna 1984). However, due to the restricted use of medications and pesticides in water bodies, these parasite diseases continue to be a problem in cages, pens, and similar open aquaculture systems. Successful preventive approaches to sensitive marine and brackish water larviculture systems have yet to be investigated. Zooplankton predator, Artemia have been successfully used to reduce the parasite burden in red drums (Oestmann et al. 1995) and similar kind of environmentally safe natural predators such as rotifers and copepods can be a useful aid to reduce the parasitic stages in sensitive marine larviculture systems. Vaccination against parasitic flagellates has yet to be explored; however, given reports of antibody-mediated immune responses to recurring infections (Smith et al. 1994; Cobb et al. 1998), the potential for vaccine development could be investigated further.
Conclusions
The current investigation reveals the presence of Amyloodinium ocellatum, an obligatory fish parasite in the rabbit fish stocks for captive maturation and breeding programme. A PCR based detection and identification of the parasite was undertaken in the current study which aids in faster implementation of preventive and therapeutic strategies to contain the disease spread. Additionally, the invasive nature of A. ocellatum in Asian seabass and its salinity tolerance were investigated. The early infection by Amyloodinium can be treated using freshwater dip treatments in closed aquaculture systems, however such treatment is impractical in extensive fish culture facilities and scope of vaccination of fishes against such ectoparasites should be explored. Conclusively, a biosecurity plan based on continuous monitoring for newly recruited fish stocks for aquaculture programs must be effectively applied using adequate quarantine and disinfection strategies to stop the introduction and spread of disease agents to native fish species.
Data availability
The molecular sequence data generated in this study were deposited in the NCBI database under the accession number OP889363.1.
References
Alosairi Y, Al-Ragum A, Al-Houti D (2021) Environmental mechanisms associated with fish kill in a semi-enclosed water body: an integrated numerical modeling approach. Ecotox Environ Safe 217:112238. https://doi.org/10.1016/j.ecoenv.2021.112238
Alvarez-Pellitero P (2008) Diseases caused by flagellates. In: Eiras JC, Segner H, Wahli T, Kapoor BG (eds) Fish diseases, vol 1. Science Publishers Enfield NH, pp 421–515
Ananda Raja R (2022) Parasitoses in brackishwater aquaculture. In: Alavandi SV, Saraswathy R, Muralidhar M, Vijayan KK (eds) Perspectives on Brackishwater Aquauclture in India, vol 3. ICAR-Central Institute of Brackishwater Aquaculture & Society of Coastal Aquaculture and Fisheries, Chennai, India, pp 215–252
Baticados MCL, Quinition GF (1984) Occurrence and pathology of an amyloodinium-like protozoan parasite on gills of grey mullet, Mugil cephalus. Helgoliinder Meeresunters 37:595–601
Bessat M, Fadel A (2018) Amyloodiniosis in cultured Dicentrarchus labrax: parasitological and molecular diagnosis, and an improved treatment protocol. Dis Aquat Org 129:41–51. https://doi.org/10.3354/dao03237
Byadgi O, Massimo M, Dirks RP, Pallavicini A, Bron JE, Ireland JH, Volpatti D, Galeotti M, Beraldo P (2021) Innate immune-gene expression during experimental amyloodiniosis in European seabass (Dicentrarchus labrax). Vet Immunol Immunopathol 234110217. https://doi.org/10.1016/j.vetimm.2021.110217
Cobb CS, Levy MG, Noga EJ (1998) Development of Immunity by the Tomato Clownfish, Amphiprion frenatus to the Dinoflagellate Parasite Amyloodinium ocellatum. J Aquat Anim 10:259–263. https://doi.org/10.1577/1548-8667(1998)010%3C0259:DOIBTT%3E2.0.CO;2
Cruz-Lacierda ER, Maeno Y, Pineda AJT, Matey VE (2004) Mass mortality of hatchery-reared milkfish (Chanos chanos) and mangrove red snapper (Lutjanus argentimaculatus) caused by Amyloodinium Ocellatum (Dinoflagellida). Aquaculture 236:85–94. https://doi.org/10.1016/j.aquaculture.2004.02.012
Dhayanithi NB, Sudhagar A, Kumar TTA, Lal KK (2022) Study on amyloodiniosis outbreak in captive-bred percula clownfish (Amphiprion percula) and improved control regimens. J Parasit Dis 46(4):1103–1109. https://doi.org/10.1007/s12639-022-01530-1
Eissa AE, Moustafa M, Abumhara A, Hosni M (2016) Future prospects of biosecurity strategies in Egyptian fish farms. J Fish Aquat Sci 11(2):100–107. https://doi.org/10.3923/jfas.2016.100.107
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. https://doi.org/10.2307/2408678
Fishar MR (2006) Red swamp crayfish (Procambarus clarkia) in river Nile, Egypt: Case study. Biodiversity monitoring and assessment project (BioMap), Nature conservation sector. Egyptian environmental affairs Agency, Ministry of State for Environmental affairs, p 32
Francis-Floyd R, Floyd M (2011) Amyloodinium ocellatum, an important parasite of cultured marine fish. SRAC Publication 4705, Southern Regional Aquaculture Center, Stoneville, MS, pp 11
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp 41:95–98
Kizhakudan JK, Praveena EP, Maran BAV, Otta SK, Bhuvaneswari T, Rajan JJS, Krishnamoorthi S, Jithendran KP (2015) Investigation on the mortality of juveniles in captive stock of the Indian halibut, Psettodes erumei. Indian J Mar Sci 44:1217–1223. http://nopr.niscpr.res.in/handle/123456789/34895
Kumar R, Nazar AKA, Jayakumar R, Tamilmani G, Sakthivel M, Kalidas C, Balamurugan V, Sirajudeen S, Thiagu R, Gopakumar G (2015) Amyloodinium Ocellatum infestation in the broodstock of silver pompano Trachinotus blochii (Lacepede, 1801) and its therapeutic control. Indian J Fish 62(1):131–134
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Kumar TS, Makesh M, Alavandi SV, Vijayan KK (2022) Clinical manifestations of White feces syndrome (WFS), and its association with Enterocytozoon hepatopenaei in Penaeus vannamei grow-out farms: a pathobiological investigation. Aquaculture 547:737463. https://doi.org/10.1016/j.aquaculture.2021.737463
Kuperman B, Matey VE (1999) Massive infestation by Amyloodinium Ocellatum (Dinoflagellida) of fish in a highly saline lake, Salton Sea, California. Dis Aquat Org 39:65–73. https://doi.org/10.3354/dao039065
Levy MG, Poore MF, Colorni A, Noga EJ, Vandersea MW, Litaker RW (2007a) A highly specific PCR assay for detecting the fish ectoparasite, Amyloodinium Ocellatum. Dis Aquat Org 73(3):219–226. https://doi.org/10.3354/dao073219
Levy MG, Litaker RW, Goldstein RJ, Dykstra MJ, Vandersea MW, Noga EJ (2007b) Piscinoodinium, a fish-ectoparasitic dinoflagellate, is a member of the class dinophyceae, subclass gymnodiniphycidae: convergent evolution with Amyloodinium. J Parasitol 93(5):1006-15. https://doi.org/10.1645/GE-3585.1. PMID: 18163333
Lieke T, Meinelt T, Hoseinifar SH, Pan B, Straus DL, Steinberg C (2019) Sustainable aquaculture requires environmental friendly treatment strategies for fish diseases. Rev Aquac 12:943–965. https://doi.org/10.1111/raq.12365
Marques CL, Medeiros A, Moreira M, Quental-Ferreira H, Mendes AC, Pousao-Ferreira P, Soares F (2019) Report and genetic identification of Amyloodinium ocellatum in a sea bass (Dicentrarchus labrax) broodstock in Portugal. Aquac Rep 14:100191. https://doi.org/10.1016/j.aqrep.2019.100191
Masson I, Lotz JM, Blaylock RB (2013) Population model for Amyloodinium ocellatum infecting the spotted seatrout Cynoscion nebulosus and the red snapper Lutjanus campechanus. Dis Aquat Org 106(2):139–148. https://doi.org/10.3354/dao02637
Menconi V, Pastorino P, Cavazza G, Santi M, Mugetti D, Zuccaro G, Prearo M (2019) The role of live fish trade in the translocation of parasites: the case of Cystidicola farionis in farmed rainbow trout (Oncorhynchus mykiss). Aquac Int 27:1667–1671. https://doi.org/10.1007/s10499-019-00422-1
Moreira M, Schrama D, Soares F, Wulff T, Pousao-Ferreira P, Rodrigues P (2017) Physiological responses of reared sea bream (Sparus aurata Linnaeus, 1758) to an Amyloodinium ocellatum outbreak. J Fish Dis 40:1545–1560. https://doi.org/10.1111/jfd.12623
Moreira M, Costas B, Rodrigues PM et al (2023) Amyloodiniosis in aquaculture: a review. Rev Aquac 1–27. https://doi.org/10.1111/raq.12883
Noga EJ, Levy MG (1995) Dinoflagellida (Phylum Sarcomastigophora). In: Woo PTK (ed) Fish diseases and disorders, vol 1. CAB International, Wallingford, pp 1–25
Noga EJ, Levy MG (2006) Phyllum Dinoflagellata. In: Woo PTK (ed) Fish diseases and disorders, vol 1. CAB International Oxford, UK, pp 16–45
Oestmann DJ, Lewis DH, Zettler BA (1995) Communications: Clearance of Amyloodinium Ocellatum dinospores by Artemia salina. J Aquat Anim 7:257–261. https://doi.org/10.1577/1548-8667(1995)007%3C0257:CCOAOD%3E2.3.CO;2
Paperna I (1984) Reproduction cycle and tolerance to temperature and salinity of Amyloodinium Ocellatum (Brown, 1931) (Dinoflagellida). Ann Parasit Hum Comp 59:7–30
Rajaprabhu G, Kirubagaran R, Santhanakumar J, Sendhil Kumar R, Dharani G (2021) Short term culture of wild caught juvenile rabbit fishes (Siganus javus) in open sea cages at Palk Bay. Indian J Sci Technol 14(12):990–998. https://doi.org/10.17485/IJST/v14i12.415
Reyes-Becerril M, Ascencio-Valle F, Alamillo E, Hirono I, Kondo H, Jirapongpairoj W, Angulo C (2015) Molecular cloning and comparative responses of toll-like receptor 22 following ligands stimulation and parasitic infection in yellowtail (Seriola lalandi). Fish Shellfish Immunol 46(2):323–333. https://doi.org/10.1016/j.fsi.2015.06.020
Roberts-Thomson A, Barnes AC, Fielder DS, Lester RJ, Adlard RD (2006) Aerosol dispersal of the fish pathogen, Amyloodinium Ocellatum. Aquaculture 257:118–123. https://doi.org/10.1016/j.aquaculture.2006.02.058
Saitou N, Nei M (1987) The neighbor-joining method: A new method for reconstructing phylogenetic trees. M Biol and Evol 4: 406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454. PMID: 3447015
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a Laboratory Manual. Harbor Laboratory, Cold Spring Harbor, New York
Smit SA, Levy MG, Noga EJ (1994) Communications: detection of Anti-Amyloodinium ocellatum antibody from cultured hybrid striped bass (Morone saxatilis × M. chrysops) during an epizootic of Amyloodiniosis. J Aquat Anim 6:79–81. https://doi.org/10.1577/1548-8667(1994)006%3C0079:CDOAAO%3E2.3.CO;2
Sudhagar A, Sundar Raj N, Mohandas SP, Serin S, Sibi KK, Sanil NK, Raja Swaminathan T (2022) Outbreak of parasitic dinoflagellate piscinoodinium sp. Infection in an endangered fish from India: Arulius Barb (Dawkinsia Arulius). Pathogens 14(11):1350. https://doi.org/10.3390/pathogens11111350
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101:11030–11035. https://doi.org/10.1073/pnas.0404206101
Thomas D, Krishnan AN, Praveena PE, Angel RJ, Kailasam M, Jithendran KP (2020) Amyloodinium Sp (Brown, 1931) (dinoflagellida) infestation in captive stock of silver moony, Monodactylus argenteus (Linnaeus, 1758). Indian J Fish 67(4):154–159. https://doi.org/10.21077/ijf.2020.67.4.88605-1
Zhang Y, Hu J, Li Y, Zhang M, Jacques KJ, Gu W, Sun Y, Sun J, Yang Y, Xu S, Wang Y, Yan X (2021) Immune response of silver pomfret (Pampus argenteus) to Amyloodinium ocellatum infection. J Fish Dis 44(12):2111–2123. https://doi.org/10.1111/jfd.13524
Acknowledgements
The authors are grateful to the Director, ICAR-CIBA, for providing institutional facilities to complete the research program.
Funding
The research was funded by Indian Council of Agriculture Research (ICAR), New Delhi, India.
Author information
Authors and Affiliations
Contributions
R vidya has contributed to concept, study design, and formal analysis. Satheesha Avunje, KP Kumaraguru Vasagam, T Sathish Kumar, T Bhuvaneswari and R Aravind were contributed to sampling and analysis. R. Anand Raja contributed to histology reading, JAJ Raymond contributed illustrations, M Poornima corrected and revised the previous versions of manuscript, KP Jithendran involved in overall administration and management of project. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The research undertaken complies with the current animal welfare laws in India and obtained approval from the statutory authorities of the Institute Ethical Committee of ICAR-CIBA (CIBA/IAEC/2022/06 dated 26-03-2022). Fish were sacrificed following the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forests (Animal Welfare Division), Govt. of India.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vidya, R., Raja, R.A., Avunje, S. et al. A report on outbreak of Amyloodinium ocellatum infestation in broodstock of Java rabbitfish, Siganus javus (Linnaeus, 1766). J Parasit Dis (2024). https://doi.org/10.1007/s12639-024-01710-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12639-024-01710-1