Abstract
During pregnancy, apoptosis is a physiological event critical in the remodeling and aging of the placenta. Increasing evidence has pointed towards the relevance of endocannabinoids (ECs) and hypoxia as modulators of trophoblast cell death. However, the relation between these factors is still unknown. In this report, we evaluated the participation of ECs in placental apoptosis induced by cobalt chloride (CoCl2), a hypoxia mimicking agent that stabilizes the expression of hypoxia inducible factor-1 alpha (HIF-1α). We found that HIF-1α stabilization decreased FAAH mRNA and protein levels, suggesting an increase in ECs tone. Additionally, CoCl2 incubation and Met-AEA treatment reduced cell viability and increased TUNEL-positive staining in syncytiotrophoblast layer. Immunohistochemical analysis demonstrated Bax and Bcl-2 protein expression in the cytoplasm of syncytiotrophoblast. Finally, HIF-1α stabilization produced an increase in Bax/Bcl-2 ratio, activation of caspase 3 and PARP cleavage. All these changes in apoptotic parameters were reversed with AM251, a CB1 antagonist. These results demonstrate that HIF-1α may induce apoptosis in human placenta via intrinsic pathway by a mechanism that involves activation of CB1 receptor suggesting a role of the ECs in this process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endogenous cannabinoids (Endocannabinoids, ECs) are an emerging group of bioactive lipids mediators that include amides, esters, and ethers of long-chain polyunsaturated fatty acids. These compounds act as ligands for the cannabinoid receptors (CB1 and CB2), a seven transmembrane G-protein coupled receptor that triggers different signaling pathways. Two members are of utmost importance in several reproductive processes: N-arachidonoylethanolamide (anandamide, AEA) and 2-arachidonoylglycerol (2-AG) [1]. AEA was the first endogenous ligand isolated and its biological activity has been widely characterized.
ECs are produced on demand by different biosynthetic pathways, principally by action of N-acylphosphatidylethanolamide-phospholipase D (NAPE-PLD), which releases AEA and phosphatidic acid from its membrane precursor [2]. ECs availability is regulated through degradation by a fatty acid amide hydrolase (FAAH), principal enzyme controlling the magnitude and the duration of AEA signaling [3]. ECs together with their receptors and the enzymes involved in their metabolism constitute the endocannabinoid system (ES). Previous studies demonstrated expression of FAAH and CB1 [4] as well as high levels of AEA production [5] in human term placenta. In addition, our group reported that several components of the ES were differentially expressed in pathological pregnancy conditions such as preeclampsia [6].
As a developing organ, the placenta undergoes constant tissue remodeling. Cell turnover is characterized by the functional loss of trophoblast that occurs through apoptosis, a carefully regulated process of cell death. In the placenta, apoptosis is a homeostatic mechanism that participates in syncitialization and degeneration of syncytiotrophoblast [7] and it is part of the physiological villous remodeling that occurs in all normal pregnancies. However, an aberrant cell turnover as well as increased levels of trophoblast apoptosis was detected in many pregnancy disorders such as intrauterine growth restriction and preeclampsia [8, 9].
Hypoxia inducible factor 1 (HIF-1) is a transcription factor that regulates the expression of a large number of genes including those involved in cell cycle arrest and apoptosis [10]. In the placenta, HIF-1 plays a key role in homeostasis and development of this tissue [11]. HIF-1 is a heterodimer consisting of two subunits: a constitutively expressed HIF-1β and a HIF-1α subunit that is regulated in an oxygen-dependent manner, being rapidly inactivated and degraded in normoxia. Even if hypoxia is the main mechanism by which HIF-1 is activated, there are several non-hypoxic factors that are capable of turning on this transcription factor. Such stimuli include hormones, cytokines and growth factors, many of which are expressed in the placenta or are increased in the maternal circulation during pregnancy [12]. HIF-1α was detected in cytotrophoblast and syncytiotrophoblast of first and third trimester placentas [13] and it expression is exacerbated in pathological conditions [14].
In several tissues ECs have an apoptotic effect and impair cell growth. AEA could promote apoptosis in a high number of cell systems through activation of CB1, CB2 and/or TRPV-1 receptors; or independent of receptor activation [15].
It is well established that HIF-1α is relevant in the transcriptional regulation of several genes. However, there is a paucity of data available on the role of this protein as a regulator of the ES. Therefore, in the present study we use cobalt chloride (CoCl2), a well-known hypoxia-mimetic agent, to investigate the participation of HIF-1α in the induction of apoptosis in human term placenta focusing on the characterization of the role of ECs in this process.
Materials and methods
Tissue collection
The experimental procedures reported here were approved by the Ethics Committee of CEMIC. (Center for Medical Education and Clinical Research, Norberto Quirno). After obtaining informed consent, term placentas (38–40 weeks of gestation) from non labor healthy normotensive women (n = 22) who underwent elective cesarean section were collected.
Villous biopsies were gently separated by sterile dissection from different cotyledons, excluding chorionic and basal plates, minced with scalpel blades, and washed repeatedly with 0.9 % sodium chloride to remove blood from intervillous space. Placental tissue was cut into small pieces and residual blood clots were carefully removed.
Villous explants culture
For experiments, placental explants were cultured in RPMI 1640 medium (Microvet, Bs. As, Argentina) supplemented with 10 % fetal bovine serum (Natocor, Córdoba, Argentina), 1 % penicillin/streptomycin and 1 % gentamicin (Microvet, Bs. As., Argentina) at 37 °C in humidified atmosphere of 5 % CO2 for 20 h.
Explants were incubated in absence or presence of 100 or 250 μM CoCl2 (Sigma Chemical Co., St. Louis, MO), R-(+)-Methanandamide (Met-AEA) (Enzo Life Sciences, Ann Arbor, MI) (0.01 to 10 µM). In other sets of experiments, villous explants were pre-incubated for 30 min with 0.1 μM AM251 (CB1 antagonist) (Tocris Cookson Inc., Ellisville, MO), 0.1 μM SR141716A (Tocris Cookson Inc., Ellisville, MO), 300 µM ZnCl2 (Sigma Chemical Co., St. Louis, MO), 1 µM 6-aminoflavone (Sigma Chemical Co., St. Louis, MO). Then, placental explants were incubated alone or with 250 μM CoCl2.
MTT viability
Tissue viability was determined by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; thiazolyl blue) (Sigma Chemical Co., St. Louis, MO) assay as described previously [16]. Briefly, after treatments, villous explants were incubated with a MTT solution for 2 h at 37 °C. At the end of the incubation period, formation of formazan product of MTT was measured at 540 nm in a microplate spectrophotometer. Determinations were made by duplicate.
Western blot
After treatments, placental samples were processed as detailed previously [6]. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Blots were treated with blocking solution (5 % non-fat powdered milk) and then were incubated overnight with anti HIF-1α (1:500) (Abcam, MA); anti Bax (1:600), anti β-actin (1:500) (Sigma Chemical Co., St. Louis, MO); anti caspase 3 (1:500) (Cell Signaling, Danvers, MA); anti Bcl-2 (1:500), anti PARP (1:500), anti Fas (1:200) (Santa Cruz Biotech, Santa Cruz, CA); anti CB1 (1:250) (Cayman Chemical, Ann Arbor, MI), anti FAAH (1:200) (anti FAAH antibody was a gift from Dr. Benjamin Cravatt). Blots were washed with buffer Tween 20 (10 mmol/l Tris, 100 mmol/l NaCl and 0.1 % (v/v), pH 7.5) followed by incubation with secondary antibody horseradish peroxidase-conjugated IgG (Jackson Inmuno Research, PA) and developed by Enhanced Chemiluminescence. Blots were scanned using a scanning densitometer and the intensity of bands was determined using the Image J (NIH) program.
Western blot reagents were obtained in Bio-Rad Laboratories (Alfatron SRL, Bs. As., Argentina).
RNA extraction and RT-PCR
After incubation, total RNA was isolated from placental explants using Trizol Reagent (Life Technologies, Carlsbad, CA) according to manufacturer’s instructions. Quantification, purity and integrity were evaluated based on determination of the ratio of the absorbance at 260 and 280 nm. RNA quality was determined by using Gel Red-stained gels (Biotium, Corporate Place Hayward, CA).
For cDNA synthesis, 3 µg of total RNA were reverse transcribed using Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV-RT) (Promega, WI) and random primers in presence of ribonuclease inhibitor.
Then, 5 µl of cDNA were subjected to PCR with specific primers for human NAPE-PLD, FAAH and β-actin genes. Sequence of primers was FAAH antisense (5′–3′): CAAGGCTCTGTTTGGTCTCC, FAAH sense (5′–3′): GCCTGAAGGGCTGTGTCTAT; NAPE-PLD antisense (5′–3′): GGTTCATAAGCTCCGATGGG, NAPE-PLD sense (5′–3′): TGGACTGGTGGGAGGAG; and β-actin antisense (5′–3′): CAGCGGAACCGCTCATTGCCAATGG, β-actin sense (5′–3′): TCACCCACACTGTGCCCATCTACGA.
Each PCR cycle included a denaturation step at 94 °C for 5 min, annealing for 30 s (62 °C for FAAH, 60 °C for NAPE-PLD and 55 °C for β-actin) and extension at 72 °C for 1 min.
Each primer pair was designed using the Primer 3 input program. Negative control was performed in absence of cDNA in the mix reaction.
PCR products were resolved on 2 % agarose gel and stained with Gel Red®. Photographs were taken using a digital camera and densitometry of the bands was analyzed using the Image J (NIH) program. Results were expressed as optical density of each product respect to optical density of β-actin.
TUNEL assay
Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling (TUNEL) assay was used to detect apoptotic cells. Tissue paraffin sections were performed with a fluorescein-based cell death detection kit (In Situ Cell Death Detection kit, Roche Applied Science, Indianapolis, IN) according to the manufacturer’s instructions. Nuclei were counterstained with DAPI (Sigma Chemical Co., St. Louis, MO). A negative control was generated by the omission of the TdT enzyme. Slices were observed under a fluorescence microscope (Nikon Eclipse E200, NY).
To quantify the number of apoptotic cells, TUNEL positive cells were counted manually by assessing ten random fields per experimental condition. The apoptotic index was calculated as the percentage of trophoblast nuclei stained TUNEL-positive divided by the total number of DAPI-stained in each section.
Immunohistochemistry
Placental tissue was fixed overnight in 4 % formaldehyde 0.1 mol/l sodium phosphate buffer (PBS), pH 7.4, dehydrated, and embedded in paraffin. Tissue sections were cut and mounted on 2 % silanized slices, dried, dewaxed, and rehydrated. Then, slides were permeabilized (Triton X-100 2 % in PBS), washed and blocked in blocking solution (2 % non-fat powdered milk in PBS). Tissue sections were incubated in 3 % hydrogen peroxide (H2O2)/methanol (v/v) to block endogenous peroxidase and washed three times with PBS. Finally, slices were incubated with anti CB1, anti CB2 (Abcam, MA), anti Bax, anti Bcl-2, anti Fas and anti FasL (Santa Cruz Biotech, Santa Cruz, CA) primary antibodies (1:100). After overnight incubation, slides were thrice rinsed in PBS and incubated for 1 h at room temperature with the appropriate 1:200 diluted biotinylated secondary antibodies (Vector Labs, Peterborough, UK). After further washing in PBS, sections were incubated for 30 min with 1:100 diluted streptavidin-peroxidase complexes (ABC kit, Vector Labs, UK). Sections were then washed twice with PBS, and development of peroxidase activity was performed with 0.05 % 3,3′-diaminobenzidine (w/v) and 0.1 % H2O2 (v/v) in Tris–HCl. Finally, sections were washed with distilled water and mounted in Canada balsam (Biopack, Buenos Aires, Argentina).
Staining was done in at least three different experiments. Negative control was performed omitting incubation with primary antibody. Sections were viewed and photographs were taken under a Nikon microscope.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism Software (San Diego, CA). Comparisons between values of different treatments were performed using one-way analysis of variance (ANOVA). Significance was determined using Tukey’s multiple comparison tests for unequal replicates. The results presented in this study represent mean ± SEM of independent experiments performed. Differences between means were considered significant when p < 0.05.
Results
Cobalt Chloride increase HIF-1α expression in human placenta
In the present study, chemical hypoxia was induced by incubation with CoCl2, a hypoxia mimicking agent that inhibits HIF-1α degradation. First, we evaluated the induction of chemical hypoxia analyzing HIF-1α expression by Western Blot. Figure 1 shows a specific band of 120 kDa, the expected size for this protein, in control and treated placentas. Densitometric analysis indicated that incubation with CoCl2 for 20 h increased HIF-1α expression in both assessed concentrations.
Expression of HIF-1α protein in placental explants treated with CoCl2 for 20 h (n = 6). Representative Western blot and graph of relative expression of this protein (fold of change compared to control) are shown. Data are expressed as the mean values ± SEM. (* p < 0.05 vs control, ** p < 0.01 vs control)
Time of incubation was selected based on our previous findings where we observed that biochemical parameters of placental tissue such as production of beta—human chorionic gonadotrophin (β-hCG) and Lactate dehydrogenase (LDH) remained unchanged after incubation with CoCl2 for 20 h [16].
Cobalt Chloride modulates the expression of the endocannabinoid system
Since previously we demonstrate the expression of NAPE-PLD, FAAH and CB1 in human placenta at term [6], we decided to analyze the localization of CB2 receptor. Immunohistochemical studies showed that CB2 was mainly localized in blood cells and no label was observed in villous tissue (Fig. 2a).
Expression of CBs receptors. a Localization of CB2 receptor was analyzed by immunohistochemistry in human placenta at term with no treatment. A negative control was included. Bar 50 µm. b Placental explants were treated with 250 µM CoCl2 for 20 h or pre-treated with 0.1 µM AM251 for 30 min and then CoCl2 was added (n = 7) or c treated only with 1 µM AF or 300 µM ZnCl2. Then, CB1 protein expression was determined by Western Blot
Then, we analyzed if CoCl2 treatment could affect the expression of CB1 receptor. Incubation with CoCl2 did not produce significant changes in CB1 expression (Fig. 2b). Additionally, Fig. 2c shows that incubation with HIF-1α inhibitors, ZnCl2 and 6-aminoflavone (AF), per se do not affect the expression of this protein.
To investigate the effects of mimicking hypoxic conditions on the expression of NAPE-PLD and FAAH, mRNA levels were determined by RT-PCR after CoCl2 treatment. Stabilization of HIF-1 did not induce any significantly change in NAPE-PLD. However, when explants were treated with 250 μM CoCl2, a decrease in FAAH mRNA levels was observed (Fig. 3a). In addition, we also found a decrease in FAAH protein expression (Fig. 3b).
Effect of CoCl2 treatment in NAPE-PLD and FAAH expression. Placental explants were treated with 100 µM and 250 µM CoCl2 for 20 h. a mRNA levels of both genes were evaluated by RT-PCR. b FAAH protein expression was analyzed by Western Blot. c Villous tissue was incubated with 300 µM ZnCl2 or 1 µM 6-aminoflavone (AF) for 30 min before CoCl2 addition. β-actin was used to normalize the signals of each product. Representative results are shown. Relative values for each product are expressed in optical density (OD) units, (n = 7). Data are expressed as the mean values ± SEM. (* p < 0.05 vs control, **p < 0.01 vs control)
To confirm that this effect was mediated by HIF-1α, placental tissue was incubated with ZnCl2 or AF and then FAAH mRNA levels were analyzed. As shown in Fig. 3c, both treatments reversed the decrease in FAAH transcript produced by CoCl2, suggesting that downregulation of FAAH may be mediated by HIF-1α. Figure 3d shows that HIF-1 inhibitors per se do not modify FAAH protein expression.
Effects of ECs and HIF-1α on placental viability
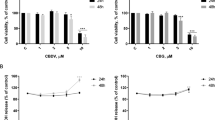
To investigate the effect of ECs on viability of term placenta, MTT assay was performed. Placental explants were incubated with different concentrations of Met-AEA, a stable AEA analogous. A decrease in cell viability was detected when explants were treated with a concentration of 10 μM Met-AEA (Fig. 4a). Furthermore, we studied the effect of HIF-1α. Results of MTT assay indicated that stabilization of HIF-1α produce a significant decrease in placental explants viability. Subsequently, we investigated if ECs could be related to this effect. Placental explants were pre-incubated with AM251, a CB1 receptor antagonist, and then CoCl2 was added. We observed that co-incubation with the antagonist reversed the inhibitory effect on cell viability produced by CoCl2 suggesting a participation of ECs (Fig. 4b).
Effect of Met-AEA and CoCl2 in placental tissue viability. Placental explants were incubated with different concentrations of Met-AEA (0.01–10 µM) (a), 250 µM CoCl2 or pre-treated with 0.1 µM AM251 for 30 min and then CoCl2 was added (b) (n = 6). Data are expressed as the mean values ± SEM (* p < 0.05 vs control)
HIF-1α induce apoptosis in human placenta
Since MTT assay does not provide information about the processes involved in the alteration of viability in placental explants, we studied if this effect was associated with apoptosis induction. Apoptotic cells were detected by TUNEL assay. After Met-AEA and CoCl2 treatments, we detected that percentage of apoptotic cells number (DAPI stained TUNEL-positive cells) was increased compared with untreated group. Specifically, TUNEL-positive cells were found mainly in the syncytiotrophoblast. Incubation with AM251 or SR141716A, two CB1 antagonists, blocked the increase in TUNEL-positive cells caused by CoCl2 treatment. Representative images and quantification from different experimental conditions are shown in Fig. 5.
Detection of apoptosis in placental explants by TUNEL assay. Placental explants were treated with 250 µM CoCl2 or 10 µM Met-AEA for 20 h. In other sets of experiments villous explants were pre-incubated for 30 min with 0.1 μM AM251 or 0.1 μM SR141716A, two different CB1 antagonists. a TUNEL-positive cells were stained in red and nuclei were stained with DAPI (blue). Negative control shows no staining (data not shown). b Graph shows percentage of TUNEL-positive cells undergoing apoptosis when placental explants were treated. Data are presented as mean ± SEM. Bar 50 µM **p < 0.01 vs control, ***p < 0.001
Then, we analyzed several proteins implicated in intrinsic and extrinsic pathways. By Western Blot, we detected a weak band corresponding to Fas protein. However, no changes in protein expression were observed between control and treated placentas (Fig. 6a). Immunohistochemical studies revealed a scarcely label corresponding to Fas occasionally localized in cytoplasm of syncytiotrophoblast. FasL staining was weak in syncytiotrophoblast layer of treated and untreated placentas while only scattered positive label was observed in endothelial cells (Fig. 6b).
Detection of Bax, Bcl-2, Fas and FasL in human placenta after CoCl2 treatment. Fas expression was analyzed by Western Blot in control and treated placentas (a). Villous tissue was stained with specific antibodies for Fas, FasL (b) Bax and Bcl-2 (c) in control and treated placentas. Black arrows indicate immunopositive cells. Negative controls were realized in absence of primary antibody. Representative images are shown. b, c Bar 10 µM
Bax immunoreactivity was found in blood cells in control and treated tissue (Fig. 6c). After CoCl2 treatment, positive staining for Bax was observed in cells of the villous core, frequently adjacent to vessels and it was barely detected in the syncytiotrophoblast layer. For Bcl-2, a clear and strong immunoreactivity was predominantly noted in syncytiotrophoblast cytoplasm of both normal and treated placentas (Fig. 6c).
HIF-1 activate the intrinsic pathway through CB1 receptor
To investigate about the intracellular signaling pathway involved in HIF-1α-induced apoptosis, expression of different apoptotic proteins was analyzed by Western Blot.
Quantitative densitometric analysis showed an upregulation of Bax and a decrease in Bcl-2 expression, leading to an increase in Bax/Bcl-2 ratio (Fig. 7a). Additionally, HIF-1α also had an effect in activation of caspase 3 producing an increase in the expression of their cleaved and active form (Fig. 7b). A raised expression of cleaved poly ADP-ribose (PARP), substrate of caspase 3, was detected (Fig. 7c).
Expression of pro- and anti-apoptotic proteins after CoCl2 treatment. Bax, Bcl-2 (a), cleaved caspase 3 (b) and cleaved PARP (c) expression was analyzed by Western Blot. Representative gel and graph of relative expression of each protein (fold of change compared to control) are shown. Explants were pre-treated with AM251 and then CoCl2 was added (n = 6). Data are expressed as the mean values ± SEM. (** p < 0.01 vs control, # p < 0.05 vs 250 µM CoCl2)
To determine if ECs participates in the activation of apoptosis through CB1 receptor, villous explants were co-incubated with AM251 and then apoptotic proteins were evaluated.
When villous tissues were treated with the CB1 antagonist, the apoptotic process was blocked and no changes in Bax/Bcl-2 ratio, cleaved caspase 3 and PARP expression relative to control were detected (Fig. 7).
Discussion
In this study, we investigated the participation of ECs in placental apoptosis mediated by HIF-1α and the mechanisms involved in this process.
For this, placental villous were cultured with CoCl2, a chemical compound that mimics a hypoxic environment by causing the stabilization of HIF-1α. The use of placental explants offer an excellent model to study trophoblast function in a context that retains the cellular architecture of the tissue in vivo and provides a tool to investigate how the placenta behaves in late gestation [17]. In mammalian systems, CoCl2 has been widely used to analyze the molecular mechanisms involved in hypoxia-linked cell death [18].
A critical balance of ECs is necessary to modulate several processes in human reproduction. The effect of ECs is dependent upon their half-life in the extracellular matrix which is a product of the rate of synthesis and degradation mediated by the metabolic enzymes, NAPE-PLD and FAAH. In the present work, after HIF-1α stabilization, we did not detect any changes in transcript levels of NAPE-PLD. However, a decrease in FAAH mRNA and protein levels were observed. It is well known that FAAH is a critical regulator in the metabolic control of ECs levels in normal and pathological pregnancy. In women with reproductive failures, decreased plasma FAAH expression and activity [19] as well as enhanced FAAH expression in trophoblast cells was previously reported [20]. Furthermore, increased levels of plasma AEA were found in women with early pregnancy loss [21]. Based on this background, we speculate that FAAH downregulation mediated by HIF-1α could increase the ECs tone. A similar observation was reported in other systems [22, 23] and is in agreement with our data where a decrease in FAAH expression and activity was detected in preeclamptic placentas [6].
Other investigators demonstrated that HIF-1α regulates gene expression by increasing the transcription rate. However, HIF-1α could also decrease gene expression by two different mechanisms; direct recognition and binding of HIF-1α to a specific sequence in their target genes [24] and the participation of other factors that, indirectly, downregulate gene expression [25]. Nevertheless, the exact mechanism by which HIF-1α regulates FAAH transcription in human placenta remains unknown.
Several reports have demonstrated that AEA exert an antiproliferative effect and could potentially cause cell death in trophoblast [26, 27]. Here, a reduction in cell viability was detected in placental tissue treated with Met-AEA. In addition, same effect was observed after CoCl2 treatment. This assay suggests that an increase in ECs levels produce a toxic effect on placental explants. When we analyze the apoptotic effect, enhanced TUNEL positive staining was found principally in the syncytiotrophoblast layer after HIF-1α stabilization and Met-AEA incubation. An increase in apoptosis may alter the homeostasis of the syncytiotrophoblast and modify the normal functionality of this layer.
It has been demonstrated that Fas/FasL system in trophoblast cells could activate apoptotic signaling in human term placenta. Here, we analyze the localization of these proteins in control and treated tissue. In agreement with Roh [28], we detected Fas protein by Western Blot. However, Fas was barely localized in some sections of villous tissue. Additionally, we observed FasL principally in syncytiotrophoblast layer. Nevertheless, these findings are contradictory to previous reports describing the presence of both proteins in term placenta [29, 30]. Despite exist a controversy about Fas/FasL detection in placenta at term, we suppose that differential Fas and FasL immunomarcation does not appear to be related to apoptosis induction in trophoblast. It has been demonstrated that Fas/FasL signaling induces apoptosis as a protecting mechanism against trophoblast rejection during early pregnancy [31] and this could explain why this apoptotic pathway is not functional at term.
Accordingly with previous reports, Bcl-2 protein was localized in syncitiotrophoblast layer of normal placenta [32]. In placentas treated with CoCl2, immunomarcation was detected in the same layer, demonstrating that HIF-1α stabilization did not induce changes in protein localization. The presence of Bcl-2 in trophoblast tissue could contribute to maintain a homeostatic state within the placenta [33]. In fact, some investigators suggest that Bcl-2 may be involved in limiting and regulating apoptosis in the syncytiotrophoblast [34].
Bax protein was not expressed in syncytiotrophoblast from normal term villi; however, after HIF-1α stabilization, positive immunostaining of this protein was detected. A continuous incorporation of RNA transcripts and other molecules from cytotrophoblast by syncitial fusion is necessary to maintain functional activity of syncytiotrophoblast. This process also contributes to transfer the molecular machinery required to complete the apoptosis cascade in the syncytiotrophoblast [35] and could explain the change in Bax localization. This suggests that the expression of Bax may contribute to apoptosis progression in hypoxia mimicking conditions.
Additionally, data indicate that HIF-1α induce an increase of different apoptotic parameters such as Bax/Bcl-2 ratio, cleavage of caspase 3 and PARP, demonstrating the activation of the intrinsic pathway. Although ECs could induce apoptosis by independent receptor mechanism that involves synthesis of ceramides, most of the biological effects of these mediators are produced by activation of cannabinoid receptors [36]. In our work, participation of CB1 was demonstrated since pharmacological blockade of this receptor abrogates the effect on viability as well as the induction of different apoptotic parameters produced by HIF-1α. Apoptosis induced by CB1 activation is a process implicated in different reproductive events and occurs in trophoblast and decidual cells [27, 37]. Although we demonstrate that HIF-1α may produce the activation of the intrinsic pathway through CB1 receptor, we do not discard the possibility that ECs and HIF-1 could induce apoptosis in hypoxic conditions through other alternative pathways.
A successful progression of pregnancy requires highly regulated levels of AEA and a tight regulation in the expression of the ECS [21]. First trimester and term placenta trophoblast have different characteristics respect to their capacity of proliferation and invasion and this fact may explain the difference in the expression of CBs receptors.
Recently, it was demonstrated that ECs act as modulators of cell survival or death and that alterations in their balance could impair the functionality of the placenta [27]. Furthermore, it was demonstrated the critical role that exerts the apoptotic process in villous trophoblast cell turnover [38]. Here, we describe a new mechanism that demonstrates a connection between HIF, the ECs and the apoptotic process in human placenta. However, we do not exclude the possibility that HIF and ECs activate independent pathways that contribute to the induction of placental apoptosis.
In summary, we showed that ECs mediate apoptosis induced by HIF-1α through activation of CB1 receptor. In the placenta, increased apoptosis [39], higher expression of HIF-1α [40] and a deregulation in the expression of ES [6] are associated with pathological pregnancy conditions. Evidence presented in this work allows proposing a possible mechanism by which these events might be associated. Further studies are required to support this concept.
References
Habayeb OM, Taylor AH, Evans MD, Cooke MS, Taylor DJ, Bell SC, Konje JC (2004) Plasma levels of the endocannabinoid anandamide in women–a potential role in pregnancy maintenance and labor? J Clin Endocrinol Metab 89(11):5482–5487
Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D (1994) Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372(6507):686–691
Cravatt BF, Lichtman AH (2002) The enzymatic inactivation of the fatty acid amide class of signaling lipids. Chem Phys Lipids 121(1–2):135–148
Park B, Gibbons HM, Mitchell MD, Glass M (2003) Identification of the CB1 cannabinoid receptor and fatty acid amide hydrolase (FAAH) in the human placenta. Placenta 24(10):990–995
Marczylo TH, Lam PM, Amoako AA, Konje JC (2010) Anandamide levels in human female reproductive tissues: solid-phase extraction and measurement by ultraperformance liquid chromatography tandem mass spectrometry. Anal Biochem 400(2):155–162
Aban C, Leguizamon GF, Cella M, Damiano A, Franchi AM, Farina MG (2013) Differential expression of endocannabinoid system in normal and preeclamptic placentas: effects on nitric oxide synthesis. Placenta 34(1):67–74
Heazell AE, Crocker IP (2008) Live and let die—regulation of villous trophoblast apoptosis in normal and abnormal pregnancies. Placenta 29(9):772–783
Smith SC, Baker PN, Symonds EM (1997) Increased placental apoptosis in intrauterine growth restriction. Am J Obstet Gynecol 177(6):1395–1401
Leung DN, Smith SC, To KF, Sahota DS, Baker PN (2001) Increased placental apoptosis in pregnancies complicated by preeclampsia. Am J Obstet Gynecol 184(6):1249–1250
Harris AL (2002) Hypoxia–a key regulatory factor in tumour growth. Nat Rev Cancer 2(1):38–47
Pringle KG, Kind KL, Sferruzzi-Perri AN, Thompson JG, Roberts CT (2010) Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Hum Reprod Update 16(4):415–431
Déry MA, Michaud MD, Richard DE (2005) Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol 37(3):535–540
Rajakumar A, Conrad KP (2000) Expression, ontogeny, and regulation of hypoxia inducible transcription factors in the human placenta. Biol Reprod 63(2):559–569
Rajakumar A, Michael HM, Daftary A, Jeyabalan A, Gilmour A, Conrad KP (2008) Proteasomal activity in placentas from women with preeclampsia and intrauterine growth restriction: implications for expression of HIF-alpha proteins. Placenta 29(3):290–299
Costa MA (2016) The endocannabinoid system: a novel player in human placentation. Reprod Toxicol 61:58–67
Castro-Parodi M, Szpilbarg N, Dietrich V, Sordelli M, Reca A, Aban C, Maskin B, Farina MG, Damiano AE (2013) Oxygen tension modulates AQP9 expression in human placenta. Placenta 34(8):690–698
Miller RK, Genbacev O, Turner MA, Aplin JD, Caniggia I, Huppertz B (2005) Human placental explants in culture: approaches and assessments. Placenta 26(6):439–448
Lee JH, Choi SH, Baek MW, Kim MH, Kim HJ, Kim SH, Oh SJ, Park HJ, Kim WJ, Jung JY (2013) CoCl2 induces apoptosis through the mitochondria- and death receptor-mediated pathway in the mouse embryonic stem cells. Mol Cell Biochem 379(1–2):133–140
Maccarrone M, Bisogno T, Valensise H, Lazzarin N, Fezza F, Manna C, Di Marzo V, Finazzi-Agro A (2002) Low fatty acid amide hydrolase and high anandamide levels are associated with failure to achieve an ongoing pregnancy after IVF and embryo transfer. Mol Hum Reprod 8(2):188–195
Chamley LW, Bhalla A, Stone PR, Liddell H, O’Carroll S, Kearn C, Glass M (2008) Nuclear localization of the endocannabinoid metabolizing enzyme fatty acid amide hydrolase (FAAH) in invasive trophoblasts and an association with recurrent miscarriage. Placenta 29(11):970–975
Habayeb OM, Taylor AH, Finney M, Evans MD, Konje JC (2008) Plasma anandamide concentration and pregnancy outcome in women with threatened miscarriage. JAMA 299(10):1135–1136
Endsley MP, Thill R, Choudhry I, Williams CL, Kajdacsy-Balla A, Campbell WB, Nithipatikom K (2008) Expression and function of fatty acid amide hydrolase in prostate cancer. Int J Cancer 123(6):1318–1326
Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, Defonseca FR, Piomelli D, Ciccocioppo R (2008) Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology 198(4):449–460
Jian H, Liu B, Zhang J (2014) Hypoxia and hypoxia-inducible factor 1 repress SEMA4B expression to promote non-small cell lung cancer invasion. Tumour Biol 35(5):4949–4955
Zheng J, Sun X, Wang W, Lu S (2010) Hypoxia-inducible factor-1alpha modulates the down-regulation of the homeodomain protein CDX2 in colorectal cancer. Oncol Rep 24(1):97–104
Costa MA, Fonseca BM, Keating E, Teixeira NA, Correia-da-Silva G (2014) Transient receptor potential vanilloid 1 is expressed in human cytotrophoblasts: induction of cell apoptosis and impairment of syncytialization. Int J Biochem Cell Biol 57:177–185
Costa MA, Fonseca BM, Teixeira NA, Correia-da-Silva G (2015) The endocannabinoid anandamide induces apoptosis in cytotrophoblast cells: involvement of both mitochondrial and death receptor pathways. Placenta 36(1):69–76
Roh CR, Lee JW, Kang BH, Yang SH, Kim BG, Bae DS, Kim JH, Lee JH (2002) Differential expressions of Fas and Fas ligand in human placenta. J Korean Med Sci 17(2):213–216
Pongcharoen S, Searle RF, Bulmer JN (2004) Placental Fas and Fas ligand expression in normal early, term and molar pregnancy. Placenta 25(4):321–330
Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T (2002) Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol 186(1):158–166
De Falco M, Penta R, Laforgia V, Cobellis L, De Luca A (2005) Apoptosis and human placenta: expression of proteins belonging to different apoptotic pathways during pregnancy. J Exp Clin Cancer Res 24(1):25–33
Ratts VS, Tao XJ, Webster CB, Swanson PE, Smith SD, Brownbill P, Krajewski S, Reed JC, Tilly JL, Nelson DM (2000) Expression of BCL-2, BAX and BAK in the trophoblast layer of the term human placenta: a unique model of apoptosis within a syncytium. Placenta 21(4):361–366
Huppertz B, Frank HG, Kingdom JC, Reister F, Kaufmann P (1998) Villous cytotrophoblast regulation of the syncytial apoptotic cascade in the human placenta. Histochem Cell Biol 110(5):495–508
Abumaree MH, Stone PR, Chamley LW (2012) Changes in the expression of apoptosis-related proteins in the life cycle of human villous trophoblast. Reprod Sci 19(6):597–606
Huppertz B, Kingdom J, Caniggia I, Desoye G, Black S, Korr H, Kaufmann P (2003) Hypoxia favours necrotic versus apoptotic shedding of placental syncytiotrophoblast into the maternal circulation. Placenta 24(2–3):181–190
Tomiyama K, Funada M (2014) Cytotoxicity of synthetic cannabinoids on primary neuronal cells of the forebrain: the involvement of cannabinoid CB1 receptors and apoptotic cell death. Toxicol Appl Pharmacol 274(1):17–23
Fonseca BM, Correia-da-Silva G, Teixeira NA (2009) Anandamide-induced cell death: dual effects in primary rat decidual cell cultures. Placenta 30(8):686–692
Huppertz B, Kadyrov M, Kingdom JC (2006) Apoptosis and its role in the trophoblast. Am J Obstet Gynecol 195(1):29–39
Sharp AN, Heazell AE, Crocker IP, Mor G (2010) Placental apoptosis in health and disease. Am J Reprod Immunol 64(3):159–169
Caniggia I, Winter JL (2002) Adriana and Luisa Castellucci Award lecture 2001 Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies–a review. Placenta 23(Suppl A):S47–S57
Acknowledgments
We thank Mrs. Ramona Morales and Ph.D. Maximiliano Cella for technical assistance and Mrs. Rossana Santarelli for English corrections.
Funding
This work was supported by PICT 2010 N 1974 (CONICET), Fundación Florencio Fiorini and Fundación Roemmers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abán, C., Martinez, N., Carou, C. et al. Endocannabinoids participate in placental apoptosis induced by hypoxia inducible factor-1. Apoptosis 21, 1094–1105 (2016). https://doi.org/10.1007/s10495-016-1274-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-016-1274-x