Abstract
Centipedes, a kind of arthropod, have been reported to produce antimicrobial peptides as part of an innate immune response. Scolopendin 2 (AGLQFPVGRIGRLLRK) is a novel antimicrobial peptide derived from the body of the centipede Scolopendra subspinipes mutilans by using RNA sequencing. To investigate the intracellular responses induced by scolopendin 2, reactive oxygen species (ROS) and glutathione accumulation and lipid peroxidation were monitored over sublethal and lethal doses. Intracellular ROS and antioxidant molecule levels were elevated and lipids were peroxidized at sublethal concentrations. Moreover, the Ca2+ released from the endoplasmic reticulum accumulated in the cytosol and mitochondria. These stress responses were considered to be associated with yeast apoptosis. Candida albicans cells exposed to scolopendin 2 were identified using diagnostic markers of apoptotic response. Various responses such as phosphatidylserine externalization, chromatin condensation, and nuclear fragmentation were exhibited. Scolopendin 2 disrupted the mitochondrial membrane potential and activated metacaspase, which was mediated by cytochrome c release. In conclusion, treatment of C. albicans with scolopendin 2 induced the apoptotic response at sublethal doses, which in turn led to mitochondrial dysfunction, metacaspase activation, and cell death. The cationic antimicrobial peptide scolopendin 2 from the centipede is a potential antifungal peptide, triggering the apoptotic response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the development of major antimicrobial drugs that are currently used in the clinic, microbial resistance to most antibiotics has increased drastically in recent times [1]. Pathogenic microorganisms have acquired resistance to various antibiotics by means of efflux of the antibiotic from the cell, interaction of the drug with its target, or direct destruction or modification of the antibiotics [2]. The discovery of broad-spectrum, novel antibiotics with rapid bactericidal activity and the ability to limit the induction of microbial resistance might not be keeping up with the pace at which pathogens are developing resistance [3, 4].
Antimicrobial peptides (AMPs) secreted by numerous living organisms are novel antimicrobial agents with diverse functions such as the induction of innate immunity effectors in mammalians cells [5], stimulation of epithelial cell production [6], low cytotoxicity, and broad antimicrobial properties. They mostly cause a disruption of the membrane integrity of the pathogen and are unlikely to initiate the development of resistance. Owing to these properties, positively charged AMPs have been used as a part of the polyelectrolyte multilayer architecture to defend bacterial or fungal infection [7]. Studies on industrial or medicinal applications of AMPs are consistently increasing because of their antipathogenic potential and lower predisposition to induce resistance.
Peptides from venomous animals have been highlighted owing to their structural diversity and multiple pharmacological activity [8]. These peptides have not been studied in-depth and have only been well-explored in a few species, including snakes, spiders, cone snails, and scorpions [8]. Centipedes are the oldest venomous arthropods using toxins as their defense system [8]. The venom of the centipede bears no resemblance to any characterized protein or peptide family [9] and was recently demonstrated to be a unique, disulfide-rich peptide modulating the activity of mammalian voltage-gated ion channels [10]. Peptides from the body and venom of centipede, such as scolopendrisin I and scolopin I and 2, have been reported [11]. The novel antimicrobial peptide scolopendin 2 was recently identified in the centipede body, and it exhibits broad antimicrobial activity by inducing membrane disruption [12]. In the present study, intracellular stress response to scolopendin 2 from the centipede Scolopendra subspinipes mutilans was investigated in Candida albicans.
Materials and methods
C. albicans strain and growth conditions
C. albicans (ATCC 90028) was obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA) and cultured in aerated YPD (Yeast extract-peptone Dextrose) broth (BD) at 28 °C. Transcriptome sequencing and AMP selection were performed as described by Choi et al. [13]. Peptide synthesis was performed by AnyGen Co. (Gwangju, Korea). Melittin was used as a positive control for comparison in terms of the physiological responses elicited by scolopendin 2 [14].
Measurement of cell viability
C. albicans cells (2 × 106 cells/mL) were incubated with scolopendin 2 at three concentrations, including the minimum inhibitory concentration (MIC). The concentrations were chosen on the basis of a published report [12]. The treatment concentrations of scolopendin 2 used were 1.6 μM (1/4 MIC), 3.1 μM (1/2 MIC), and 6.3 μM (MIC) [12]. The cultures were acquired after incubation for 4 h and spread onto YPD agar plates. Colony-forming units were counted after incubation for 24 h at 28 °C [15]. The percentage survival was determined relative to the untreated cells. All experiments were performed three times independently. Statistical significance was determined by Student’s t test.
Reactive oxygen species assessment
Intracellular reactive oxygen species (ROS) accumulation was assessed using 2′,7′-dichlorodihydrofluorescein diacetate (H2-DCFDA) (Molecular probes, Eugene, OR, USA) [16]. To detect ROS, C. albicans cells (2 × 106 cells/mL) were treated with 1.6, 3.1, and 6.3 μM scolopendin 2 and 2.5 μM melittin for 4 h. The treatment concentration was chosen on the basis of a previous report [17]. After the cells were washed in PBS, they were stained with H2-DCFDA (excitation [Ex.] 495 nm, emission [Em.] 525 nm) for analysis by spectrofluorophotometry (Shimadzu RF-5301PC, Shimadzu, Japan). Fluorescence quantum yields were obtained using RFPC software. Statistical significance was determined by Student’s t-test.
Lipid peroxidation analysis
Lipid peroxidation was quantified based on malondialdehyde (MDA) levels. After treatment with 1.6, 3.1, and 6.3 μM scolopendin 2 for 4 h, the C. albicans cell suspension was centrifuged at 12,000 rpm for 5 min, after which the pellet was sonicated twice on ice with lysis buffer (2 % Triton-X 100, 1 % SDS, 100 mM NaCl, 10 mM Tris–HCl, 1 mM EDTA [pH 8.0]). The mixture was centrifuged, and the supernatant was added to an equal volume of 0.5 % (w/v) thiobarbituric acid (TBA) solution in 5 % trichloroacetic acid (TCA). The mixture was heated at 95 °C for 30 min and then cooled on ice. The absorbance of the reaction mixture was measured at 532 and 600 nm [18], and each experiment was performed in triplicate. Statistical significance was determined by Student’s t-test.
Glutathione quantification
C. albicans cells (2 × 106 cells/mL) were treated with 1.6 μM scolopendin 2 and 2.5 μM melittin for 4 h, after which samples were added to 3 volumes of 5 % 5-sulfosalicylic acid (Sigma-Aldrich) before being sonicated. Deproteinated supernatants were analyzed using a glutathione assay kit (Sigma-Aldrich). Oxidized glutathione (GSSG) was specifically determined by derivatizing reduced glutathione (GSH) with 2-vinylpyridine. The absorbance of the reaction mixture was monitored at 415 nm with a microtiter ELISA reader (Molecular Devices Emax, Sunnyvale, CA, USA) [19].
Analysis of cytosolic and mitochondrial Ca2+ levels
Fura-2AM (Molecular Probes) and Rhod-2AM (Molecular Probes) were used to analyze cytosolic and mitochondrial Ca2+ levels, respectively. After incubation of C. albicans cells (2 × 106 cells/mL) with 1.6 μM scolopendin 2 and 2.5 μM melittin for 4 h, the cells were washed twice in Krebs buffer (pH 7.4) and treated with 0.01 % pluronic F-127 (Molecular Probes) and 1 % BSA. The cells were stained with 5 μM Fura-2AM or 10 μM Rhod-2AM and incubated at 37 °C for 40 min. Two washes were carried out with calcium-free Krebs buffer at 37 °C for 30 min. The fluorescence intensities of Fura-2AM (Ex. = 335 nm, Em. = 505 nm) and Rhod-2AM (Ex. = 550 nm, Em. = 580 nm) were monitored with a spectrofluorophotometer [20]. The fluorescence intensities were obtained using the calculation software (RFPC software) installed in the spectrofluorophotometer. Statistical significance was determined by Student’s t-test.
Phosphatidylserine externalization and DNA and nuclear fragmentation
To prepare protoplasts, C. albicans cells were incubated with 0.1 M potassium phosphate buffer (pH 6.0) containing 1 M sorbitol and 20 mg/mL lysing enzyme (Sigma) for 2 h at 28 °C. The protoplasts were incubated with 1.6 μM scolopendin 2 and 2.5 μM melittin for 2 h, and an FITC Annexin V apoptosis detection kit (BD Pharmingen) was used according to the manufacturer’s instructions. The stained cells were analyzed with a FACSVerse flow cytometer (Becton–Dickinson, Franklin Lakes, NJ, USA) [21]. Nuclear fragmentation and condensation were examined using a TUNEL assay [22] as follows. Cells (2 × 106 cells/mL) treated with 1.6 μM scolopendin 2 and 2.5 μM melittin for 6 h were washed in PBS (pH 7.4) and then suspended in permeabilization solution (0.1 % Triton X-100 and 0.1 % sodium citrate) for 2 min on ice. After the cells were washed again in PBS, they were stained using an in situ cell death detection kit (Roche) for 1 h at 37 °C and were assessed by fluorescence microscopy (Nikon Eclipse Ti-S) and spectrofluorophotometry (Ex. 495 nm, Em. 519 nm). Nuclear fragmentation and condensation were examined with 4′-6-diamidino-2-phenylindole (DAPI) staining [23] as follows. After incubation with 1.6 μM scolopendin 2 and 2.5 μM melittin, the cells were washed in PBS, stained with 1 μg/mL DAPI for 20 min, and examined by fluorescence microscopy and spectrofluorophotometry (Ex. 340 nm, Em. 488 nm). The fluorescence intensity was obtained using the RFPC software installed in the RF-5301 PC. Statistical significance was determined by Student’s t-test.
Analysis of mitochondrial membrane potential
5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolyl carbocyanine iodide (JC-1; Molecular Probes) was used to examine changes in mitochondrial membrane potential [24]. C. albicans cells (2 × 106 cells/mL) incubated with 1.6 μM scolopendin 2 and 2.5 μM melittin for 4 h were stained with 2.5 μg/mL JC-1 and incubated in warm PBS for 20 min. The mean of the fluorescence intensities at FL1 and FL2 was analyzed with a FACSVerse flow cytometer. The ratio of the fluorescence intensities of aggregated JC-1 (FL2) to monomer (FL1) was calculated.
Cytochrome c release assay
C. albicans cells (2 × 106 cells/mL) incubated with 1.6 μM scolopendin 2 and 2.5 μM melittin for 4 h were subsequently homogenized in buffer A (50 mM Tris, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [pH 7.5]), after which 2 % glucose was added, and the mixture was centrifuged at 20,000×g for 10 min. The supernatants were collected for the quantification of released cytoplasmic cytochrome c. To obtain pure mitochondria, the pellet was centrifuged in buffer B (50 mM Tris, 2 mM EDTA [pH 5.0]) at 12,000 rpm for 30 s and was then suspended in 2 mg/mL Tris–EDTA buffer. After treatment with 500 mg/mL ascorbic acid for 5 min, the cytochrome c content in the cytoplasmic or mitochondrial samples was determined by spectrophotometric analysis at 550 nm (DU530; Beckman) [22]. The protein content was quantified using the standard Bradford method [25]. Statistical significance was determined by Student’s t-test.
Metacaspase activation assay
Metacaspase activation was investigated using the CaspACE™ FITC-VAD-FMK in situ marker (Promega). C. albicans cells (2 × 106 cells/mL) treated with 1.6 μM scolopendin 2 and 2.5 μM melittin for 4 h were centrifuged at 12,000 rpm for 5 min and stained with 2.5 μM CaspACE FITC-VAD-FMK for 30 min at 28 °C. The resulting fluorescence levels were assessed using a FACSCalibur flow cytometer (Becton–Dickinson, San Jose, CA, USA) [22].
Results
Cell growth and intracellular oxidative stress response
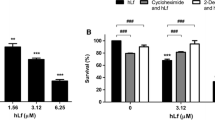
To determine whether the decrease in scolopendin 2-treated cells reflected growth inhibition or loss in cell viability and possibly cellular death, we measured cell viability. For a comparison of antifungal activities, melittin was used as a positive control. Untreated C. albicans cells was observed 94.6 % plating efficiency, the percentage of forming colonies per inoculated cells. The result of this test showed that scolopendin 2 exerts anticandidal activity in a dose-dependent manner compared to untreated cells (Fig. 1). Furthermore, to assess the effect of scolopendin 2 on intracellular ROS production, the fluorescent H2-DCFDA probe was used as a ROS indicator, where the fluorescent probe is oxidized by ROS [26]. The results of the ROS assessment showed that scolopendin 2 induced ROS production in a dose-dependent manner (Fig. 2a), where the peptide concentrations used were based on a previous study [12]. To measure lipid peroxidation, MDA is routinely measured by reaction with TBA-reactive substances (TBARS) [27]. The MDA levels in the scolopendin 2-treated cells were found to be 1.02, 0.86, and 0.65 nM at 1/4 MIC, 1/2 MIC, and MIC, respectively (Fig. 2b). The intracellular levels of GSH and GSSG were quantified using spectrophotometry. The concentrations of GSH and GSSG, as well as the ratio of GSH to GSSG (GSH/GSSG), were used to assess the redox balance in the C. albicans cells (Table 1). A general decrease in the GSH/GSSG ratio was observed in scolopendin 2-treated cells, indicating that the peptide induced an oxidative stress response.
a C. albicans cells were treated with scolopendin 2 (1.6, 3.1, and 6.3 μM) and melittin (2.5 μM) for 4 h at 28 °C with 10 μM H2-DCFDA dye. The fluorescence intensities of the stained cells were measured using a spectrofluorophotometer. b Dose-dependent increase in MDA level (lipid peroxidation). Cells were treated with 1.6, 3.1, and 6.3 μM scolopendin 2. MDA concentration of cells were determined using the TBARS assay after treatment. p values are compared to the untreated cells. The data represent the average, standard deviation (SD), and p values of three independent experiments (*p < 0.1; **p < 0.05; ***p < 0.01)
Effects on free cytoplasmic Ca2+ levels
To determine whether calcium signaling is involved in cell death, intracellular calcium levels were measured with the membrane-permeable derivatives of the ratiometric calcium indicators Fura-2AM and Rhod-2AM. The free cytoplasmic Ca2+ concentration in C. albicans was found to be significantly higher in the scolopendin 2-treated group than in the untreated group. The fluorescence intensity of Rhod-2AM was also shown to increase in cells treated with scolopendin 2 (Fig. 3). The Ca2+ levels in the cytoplasm, as well as in the mitochondria, were higher in the cells treated with scolopendin 2 than in the untreated cells (Fig. 3), indicating that calcium signaling is involved in fungal cell death.
Changes in the fluorescence intensity of Fura-2AM (a) and Rhod-2AM (b) were expressed as arbitrary fluorescence units, after the cell suspensions were treated with 1.6 μM of scolopendin 2 and 2.5 μM of melittin. The data represent the mean ± SD of three independent experiments. The data represent the average, SD, and p values of three independent experiments (*p < 0.1; **p < 0.05; ***p < 0.01)
Detection of yeast apoptotic features
To examine if the C. albicans cell death induced by scolopendin 2 occurs via the induction of apoptosis and/or necrosis, annexin V and PI assays were carried out to detect the extent of phosphatidylserine (PS) externalization. The cell population in the lower right quadrant, which represents the early apoptotic cells (annexin V-positive/PI-negative) was found to increase to 12.6 % following scolopendin 2 treatment and to 37.0 % following melittin treatment at the lowest test concentration (Fig. 4A). The fungal cell death caused by scolopendin 2 was therefore shown to exhibit features of early apoptotic cell death. To confirm that scolopendin 2 also induces late-stage apoptotic cell death, morphological changes in the DNA were assessed using a TUNEL assay in which apoptotic DNA breakage is monitored by labeling free 3′-OH termini with fluorescent dUTP catalyzed by terminal deoxynucleotidyl transferase [18, 21]. C. albicans cells exposed to scolopendin 2 exhibited green fluorescent spots, indicating increased DNA fragmentation (Fig. 4B, C). Morphological changes in the nuclei were further monitored by DAPI staining [22, 28]. Both fluorescence microscopy analyses revealed that cells treated with scolopendin 2 exhibited higher levels of fluorescence compared to untreated cells (Fig. 4B, D), indicating nuclear condensation and fragmentation.
A Phosphatidylserine externalization of plasma membranes was detected by annexin V-FITC fluorescence. a Untreated, b 1.6 μM scolopendin 2, c 2.5 μM melittin. B DNA and nuclear damage during late-stage apoptosis was visualized by fluorescence microscopy after C. albicans cells were treated with 1.6 μM of scolopendin 2 and 2.5 μM of melittin. a–c DAPI indicates nuclear condensation and fragmentation. d–f DNA fragmentation was observed by the TUNEL assay. Fluorescence intensity of C DAPI and D TUNEL staining by spectrofluorophotometry. The error bars represent the SD for three independent experiments. The data represent the average, SD, and p values of three independent experiments (*p < 0.1; **p < 0.05; ***p < 0.01)
Mitochondrial dysfunction
To assess changes in mitochondrial function, JC-1 staining was used. In the mitochondrial matrix of normal cells, the lipophilic cationic JC-1 dye appears as red aggregates. In contrast, in apoptotic cells with depolarized mitochondria, JC-1 remains in the cytoplasm and appears green [29]. The ratio of FL2/FL1 was shown to be lower in cells treated with both scolopendin 2 (FL2/FL1 = 0.8) and melittin (FL2/FL1 = 0.1) than in untreated cells (FL2/FL1 = 1.4) (Fig. 5A). These findings reveal that mitochondrial depolarization is involved in scolopendin 2-induced cell death. Cytochrome c release from the mitochondria to the cytoplasm is a crucial step in apoptotic cell death. Hence, the translocation of cytochrome c from the mitochondria to the cytoplasm was examined. The results of this experiment revealed that, compared with untreated cells, cells treated with scolopendin 2 exhibited decreased cytochrome c levels in the mitochondria and increased levels in the cytoplasm (Fig. 5B).
A Mitochondrial depolarization was detected with the fluorescent dye JC-1. C. albicans cells (2 × 106 cells/mL) were incubated with 1.6 μM scolopendin 2 and 2.5 μM melittin for 4 h at 28 °C. a Untreated, b scolopendin 2, c melittin. B Cytochrome c release from mitochondria to the cytosol in C. albicans was determined by measuring the absorbance at 550 nm. The data represent the average, SD, and p values of three independent experiments (*p < 0.1; **p < 0.05; ***p < 0.01). C Metacaspase activation in C. albicans after treatment with peptides for 4 h at 28 °C using 10 μM CaspACE FITC-VAD-FMK in situ marker. a Untreated, b 1.6 μM scolopendin 2, c 2.5 μM melittin
Activation of metacaspase
The FITC-VAD-FMK FITC-conjugated peptide pan-caspase inhibitor was used to assess the activation of caspases during scolopendin 2-induced cell death. The FITC-labeled caspase inhibitor VAD-FMK irreversibly binds to activated metacaspases in apoptotic cells [29]. Compared with untreated cells, the scolopendin 2-treated cells exhibited a 12.1 % increase in fluorescence, indicating that scolopendin 2 induced metacaspase activation (Fig. 5C).
Discussion
The mechanisms of the antimicrobial activities exerted by agents such as amphotericin B, melittin, and caspofungin are well studied. However, the physiological mechanisms by which they cause cell death are not clear [17, 30, 31]. Other mechanisms of action were recently proposed for these agents, including effects on cell viability and apoptosis [17, 23, 31]. Natural AMPs acts various effect including membrane disruption [32, 33], interaction with intracellular target, resulted antimicrobial [34], anti-inflammation [35], and anticancer activity [36]. Among them, scolopendin 2 (AGLQFPVGRIGRLLRK), a peptide identified in centipedes, reportedly exhibits broad-spectrum antimicrobial activity against pathogenic bacteria and fungi via membrane permeabilization [12]. Lower concentrations, known to induce membrane damage, cause significant cell death. However, the suggested mechanism is insufficient to explain the mechanism of cell death induction.
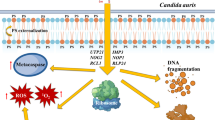
While intracellular ROS induced by scolopendin 2 may leak out of cells at high concentrations, intracellular ROS levels might increase the intracellular response at low concentrations. The toxic effects of ROS are crucial in the destruction of intracellular pathogens by macrophages and reflect the ability to damage essential cellular components such as nucleic acids, lipids, and proteins [37]. To investigate lipid damage, the levels of MDA, a biomarker of lipid peroxidation and a decomposition product of polyunsaturated fatty acid hydroperoxidase, were assessed [38]. Lipid peroxidation occurs as a result of oxidative damage induced by exposure to sublethal dose of scolopendin 2. However, even slight detection of lipid peroxidation under high doses is thought to induce membrane damage. Under conditions of ROS-induced stress, glutathione has been shown to play an antioxidant role in yeast, and the ratio of GSH to GSSG is used as a marker of oxidative stress [39]. Reduced glutathione is the main non-enzymatic antioxidant agent of defense, and its importance in the elimination of organic peroxides has already been emphasized in different organisms [40]. The present data demonstrating glutathione depletion, ROS generation, and lipid peroxidation following treatment with scolopendin 2 indicate that the peptide induces oxidative stress.
The oxidative stress observed in C. albicans cells treated with scolopendin 2 is associated with calcium ion levels, which play important roles in the regulation of cellular processes. ROS production by mitochondria is closely related to Ca2+ homeostasis [41]. In C. albicans cells exposed to sublethal concentrations of scolopendin 2, Ca2+ from the endoplasmic reticulum leaked into the cytosol, and the mitochondria imported this Ca2+, resulting in disrupted Ca2+ homeostasis. Increased cytosol Ca2+ promotes elevated ROS production by the respiratory chain [41]. Ca2+ is furthermore considered one of the most important messengers in organisms as it is involved in a series of biological processes, including cell growth, proliferation, apoptosis, and mating morphogenesis [42]. Intracellular calcium overload has always been regarded as an important pathological link in cell damage. The mode of cell death induced by scolopendin 2 seems to involve features of apoptotic cell death, including ROS accumulation, lipid peroxidation, glutathione depletion, and disruption of Ca2+ homeostasis.
The apoptotic events relating to multiple key markers of the early and late stages were assessed to investigate a potential mechanism of scolopendin 2-induced cell death. The influx of Ca2+, which activates a scramblase with subsequent PS externalization, is induced by ROS at the cell surface [43]. PS is known to play an important role in the regulation of apoptosis in response to particular calcium-dependent stimuli [44]. Fungal cell death caused by scolopendin 2 also exhibits early apoptotic features. Our findings suggest that low concentrations of scolopendin 2 induce apoptotic mechanisms, while high concentrations induce the membrane damage mechanism. Apoptotic response also involves a number of cellular processes such as chromatin condensation, DNA fragmentation, and PS externalization to the outer leaflet of the plasma membrane [45]. DNA fragmentation and nuclear condensation can be observed as typical features of late stages of apoptotic death [46]. TUNEL and DAPI staining demonstrated that scolopendin 2 treatment induced DNA fragmentation, as well as nuclear fragmentation and condensation, indicating early progress to the late stage of apoptosis. Taken together, these data indicate that scolopendin 2 induces the hallmark features of apoptotic cell death.
Mitochondria play a pivotal role in yeast apoptosis since they represent the major site of ROS production and contain many pro-apoptotic factors [47]. The dissipation of mitochondrial membrane potential is a key cellular event during early apoptosis and leads to the opening of the transition pores of the mitochondrial membrane and the subsequent release of apoptotic factors into the cytosol [31]. During the apoptotic response in scolopendin 2-treated C. albicans, mitochondrial dysfunction results in the depolarization of mitochondrial membrane potential and translocation of cytochrome c, the main apoptotic factor, from the mitochondria to the cytosol. When cytochrome c is released into the cytosol, metacaspases are activated, which in turn play an important role in yeast apoptosis [48]. Caspases are a class of cysteine-aspartic acid proteases regulated at the posttranslational level. When cleaved, caspases convey a signal via a proteolytic cascade that induces apoptotic cell death and leads to cell death [31]. In C. albicans, a putative caspase encoded by metacaspase has been shown to play a role in apoptotic cell death [49]. Recently, the existence of caspase-like proteases and other unidentified caspases, named metacaspases, in C. albicans was reported [50]. Cell death of C. albicans cells exposed to scolopendin 2 proceeds, at least in part, in a metacaspase-dependent manner. Cytochrome c release from mitochondria following treatment with scolopendin 2 is suggestive of caspase activation. In each of the assays, an increase in oxidative damage was evident at sublethal concentrations of scolopendin 2. Glutathione is required to minimize Ca2+-induced ROS-dependent damage to mitochondria and mitochondrial membrane permeabilization [41]. The accumulated ROS triggered mitochondrial dysfunction, causing mitochondrial depolarization and cytochrome c release from the mitochondria to the cytosol. Eventually, the released cytochrome c stimulated yeast metacaspase activation.
In conclusion, scolopendin 2 was identified as a novel cationic AMP from S. s. mutilans, and it facilitated mitochondrial- and caspase-mediated apoptotic mechanisms at sublethal concentrations.
Abbreviations
- AMP:
-
Antimicrobial peptide
- YPD:
-
Yeast extract-peptone dextrose
- ROS:
-
Reactive oxygen species
- H2-DCFDA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- MDA:
-
Malondialdehyde
- TCA:
-
Trichloroacetic acid
- TBA:
-
Thiobarbituric acid
- SSA:
-
5-Sulfosalicylic acid
- GSSG:
-
Oxidized glutathione
- GSH:
-
Reduced glutathione
- PS:
-
Phosphatidylserine
- DAPI:
-
4′-6-Diamidino-2-phenylindole
- JC-1:
-
5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide
References
Khan A, Ahmad A, Khan LA, Manzoor N (2014) Ocimum sanctum (L.) essential oil and its lead molecules induce apoptosis in Candida albicans. Res Microbiol 165:411–419
Davies J, Davies D (2010) Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 74:417–433
Wright GD (2005) Bacterial resistance to antibiotics: enzymatic degradation and modification. Adv Drug Deliv Rev 57:1451–1470
Nguyen LT, Haney EF, Vogel HJ (2011) The expanding scope of antimicrobial peptide structures and their mode of action. Trends Biotechnol 29:464–472
Franzenburg S, Walter J, Künzel S, Wang J, Baines JF, Bosch TC, Fraune S (2013) Distinct antimicrobial peptide expression determines host species-specific bacterial associations. Proc Natl Acad Sci USA 110:E3730–E3738
Ogawa Y, Kawamura T, Matsuzawa T, Aoki R, Gee P, Yamashita A, Moriishi K, Yamasaki K, Koyanagi Y, Blauvelt A, Shimada S (2013) Antimicrobial peptide LL-37 produced by HSV-2-infected keratinocytes enhances HIV infection of Langerhans cells. Cell Host Microbe 13:77–86
Cado G, Aslam R, Séon L, Garnier T, Fabre R, Parat A, Chassepot A, Voegel JC, Senger B, Schneidr F, Frère Y, Jierry L, Schaaf P, Kerdjoudj H, Metz-boutigue MH, Boulmedais F (2013) Self-defensive biomaterial coating against bacteria and yeast: polysaccharide multilayer film with embedded antimicrobial peptide. Adv Funct Mater 23:4801–4809
Rong M, Yang S, Wen B, Mo G, Kang D, Liu J, Lin Z, Jiang W, Li B, Du C, Yang S, Jiang H, Feng Q, Xu X, Wang J, Lai R (2015) Peptidomics combined with cDNA library unravel the diversity of centipede venom. J Proteomics 114:28–37
Undheim EA, Fry BG, King GF (2015) Centipede venom: recent discoveries and current state of knowledge. Toxins 7:679–704
Yang S, Xiao Y, Kang D, Liu J, Li U, Undheim EA, Klint JK, Rong M, Lai R, King GF (2013) Discovery of a selective NaV1.7 inhibitor from centipede venom with analgesic efficacy exceeding morphine in rodent pain models. Proc Natl Acad Sci USA 110:17534–17539
Yoo WG, Lee JH, Shin Y, Shim JY, Jung M, Kang BC, Oh J, Seong J, Lee HK, Kong HS, Song KD, Yun EY, Kim IW, Kwon YN, Lee DG, Hwang UW, Park J, Hwang JS (2014) Antimicrobial peptides in the centipede Scolopendra subspinipes mutilans. Funct Integr Genomics 14:275–283
Lee H, Hwang JS, Lee J, Kim JI, Lee DG (2015) Scolopendin 2, a cationic antimicrobial peptide from centipede, and its membrane-active mechanism. Biochim Biophys Acta 1848:634–642
Choi H, Hwang JS, Lee DG (2014) Identification of a novel antimicrobial peptide, scolopendin 1, derived from centipede Scolopendra subspinipes mutilans and its antifungal mechanism. Insect Mol Biol 23:788–799
Wang P, Bang JK, Kim HJ, Kim JK, Shin Y (2009) Antimicrobial specificity and mechanism of action of disulfide-removed linear analogs of the plant-derived Cys-rich antimicrobial peptide Ib-AMP1. Peptides 30:2144–2149
Klepser M, Ernst EJ, Lewis RE, Ernst ME, Pfaller MA (1998) Influence of test conditions on antifungal time-kill curve results: proposal for standardized method. Antimicrob Agents Chemother 42:1207–1212
Jakubowski W, Biliński T, Bartosz G (2000) Oxidative stress during aging of stationary cultures of the yeast Saccharomyces cerevisiae. Free Radic Biol Med 28:659–664
Park C, Lee DG (2010) Melittin induces apoptotic features in Candida albicans. Biochem Biophys Res Commun 394:170–172
Aubron C, Glodt J, Matar C, Huet O, Borderie D, Dobrindt U, Duranteau J, Denamur E, Conti M, Bouvet O (2012) Variation in endogenous oxidative stress in Escherichia coli natural isolates during growth in urine. BMC Microbiol 12:120
Slavi N, Rubinos C, Li L, Sellitto C, White TW, Mathias R, Srinivas M (2014) Connexin 46 (cx46) gap junctions provide a pathway for the delivery of glutathione to the lens nucleus. J Biol Chem 289:32694–32702
Arduino DM, Esteves AR, Domingues AF, Pereira CM, Cardoso SM, Oliveira CR (2009) ER-mediated stress induces mitochondrial-dependent caspases activation in NT2 neuron-like cells. BMB Rep 42:719–724
Madeo F, Herker E, Maldener C, Wissing S, Lachelt S, Herlan M, Fehr M, Lauber K, Sigrist SJ, Wesselborg S, Frolich KU (2002) A caspase-related protease regulates apoptosis in yeast. Mol Cell 9:911–917
Madeo F, Frohlich E, Fröhlich KU (1997) A yeast mutant showing diagnostic markers of early and late apoptosis. J Cell Biol 139:729–734
Kapuscinski J (1995) DAPI: a DNA-specific fluorescent probe. Biotech Histochem 70:220–233
Almeida A, Almeida J, Bolanos JP, Moncada S (2001) Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc Natl Acad Sci USA 98:15294–15299
Lowry OH, Rosebrough NJ, Farr AJ, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Arnt L, Rennie JR, Linser S, Willumeit R, Tew GN (2006) Membrane activity of biomimetic facially amphiphilic antibiotics. J Phys Chem B 110:3527–3532
Vargas-Sánchez RD, Torrescano-Urrutia GR, Acedo-Félix E, Carvajal-Millán E, González-Córdova AF, Vallejo-Galland B, Torres-Llanez MJ, Sánchez-Escalante A (2014) Antioxidant and antimicrobial activity of commercial propolis extract in beef patties. J Food Sci 79:C1499–C1504
Munoz AJ, Wanichthanarak K, Meza E, Petranovic D (2012) Systems biology of yeast cell death. FEMS Yeast Res 12:249–265
Sandoval CM, Salzameda B, Reyes K, Williams T, Hohman VS, Plesniak LA (2007) Anti-obesity and anti-tumor pro-apoptotic peptides are sufficient to cause release of cytochrome c from vesicles. FEBS Lett 581:2463–5468
Chudzik B, Koselski M, Czuryło A, Trębacz K, Gagoś M (2015) A new look at the antibiotic amphotericin B effect on Candida albicans plasma membrane permeability and cell viability functions. Eur Biophys J 44:77–90
Hao B, Cheng S, Clancy CJ, Nguyen MH (2013) Caspofungin kills Candida albicans by causing both cellular apoptosis and necrosis. Antimicrob Agents Chemother 57:326–332
Silvestro L, Weiser JN, Axelsen PH (2000) Antibacterial and antimembrane activities of cecropin A in Escherichia coli. Antimicrobial Agents Chemother 44:602–607
Andrä J, Berninghausen O, Leippe M (2001) Cecropins, antibacterial peptides from insects and mammals, are potently fungicidal against Candida albicans. Med Microbiol Immunol 189:169–173
Park CB, Yi KS, Matsuzaki K, Kim MS, Kim SC (2000) Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: the proline hinge is responsible for the cell-penetrating ability of buforin II. Proc Natl Acad Sci USA 97:8245–8250
Ruan Y, Shen T, Wang Y, Hou M, Li J, Sun T (2013) Antimicrobial peptide LL-37 attenuates LTA induced inflammatory effect in macrophages. Int Immunopharmacol 15:575–580
Wang C, Tian LL, Li S, Li HB, Zhou Y, Wang H, Yang QZ, Ma LJ, Shang DJ (2013) Rapid cytotoxicity of antimicrobial peptide tempoprin-1CEa in breast cancer cells through membrane destruction and intracellular calcium mechanism. PLoS One 8:e60462
Alvarez-Peral FJ, Zaragoza O, Pedreno Y, Argüelles JC (2002) Protective role of trehalose during severe oxidative stress caused by hydrogen peroxide and the adaptive oxidative stress response in Candida albicans. Microbiology 148:2599–2606
Singh V, Pal A, Darokar MP (2015) A polyphenolic flavonoid glabridin: oxidative stress response in multidrug-resistant Staphylococcus aureus. Free Radic Biol Med 87:48–57
Guo Z, Olsson L (2014) Physiological response of Saccharomyces cerevisiae to weak acids present in lignocellulosic hydrolysate. FEMS Yeast Res 14:1234–1248
Candiracci M, Citterio B, Piatti E (2012) Antifungal activity of the honey flavonoid extract against Candida albicans. Food Chem 131:493–499
Perrone GG, Tan SX, Dawes IW (2008) Reactive oxygen species and yeast apoptosis. Biochim Biophys Acta 1783:1354–1368
Makovitzki A, Avrahami D, Shai Y (2006) Ultrashort antibacterial and antifungal lipopeptides. Proc Natl Acad Sci USA 103:15997–16002
Li H, Jiang W, Liu Y, Jiang J, Zhang Y, Wu P, Zhao J, Duan X, Zhou X, Feng L (2016) The metabolites of glutamine prevent hydroxyl radical-induced apoptosis through inhibiting calcium ion involved pathway in fish erythrocytes. Free Radic Biol Med 92:126–140
van den Eijnde SM, Boshart L, Baehrecke EH, De Zeeuw CI, Reutelingsperger CP, Vermeij-Keers C (1998) Cell surface exposure of phosphatidylserine during apoptosis is phylogenetically conserved. Apoptosis 3:9–16
Léger T, Garcia C, Ounissi M, Lelandais G, Camadro JM (2015) The metacaspase (Mca1p) has a dual role in farnesol-induced apoptosis in Candida albicans. Mol Cell Proteomics 14:93–108
Kyrylkova K, Kyryachenko S, Leid M, Kioussi C (2012) Detection of apoptosis by TUNEL assay. Methods Mol Biol 887:41–47
Reiter J, Herker E, Madeo F, Schmitt MJ (2015) Viral killer toxins induce caspase mediated apoptosis in yeast. J Cell Biol 168:353–358
Pereira C, Camougrand N, Manon S, Sousa MJ, Côrte-Real M (2007) ADP/ATP carrier is required for mitochondrial outer membrane permeabilization and cytochrome c release in yeast apoptosis. Mol Microbiol 66:571–582
Shirtliff ME, Krom BP, Meijering RA, Peters BM, Zhu J, Scheper MA, Harris ML, Jabra-Rizk MA (2009) Farnesol-induced apoptosis in Candida albicans. Antimicrobial Agents Chemother 53:2392–2401
Aerts AM, Carmona-Gutierrez D, Lefevre S, Govaert G, François IE, Madeo F, Santo RR, Cammue BP, Thevissen K (2009) The antifungal plant defensin RsAFP2 from radish induces apoptosis in a metacaspase independent way in Candida albicans. FEBS Lett 583:2513–2516
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (Project No. PJ01104303), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Heejeong Lee and Jae-Sam Hwang have contributed equally to this work and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Lee, H., Hwang, JS. & Lee, D.G. Scolopendin 2 leads to cellular stress response in Candida albicans . Apoptosis 21, 856–865 (2016). https://doi.org/10.1007/s10495-016-1254-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-016-1254-1