Abstract
Mass rearing of the predatory mite Neoseiulus californicus (McGregor) (Acari: Phytoseiidae) using natural (prey) methods is costly and laborious, limiting its application in the biological control of pests. A high-production, low-cost method using a prey substitute would help to relieve this problem. Oulenziella bakeri Hughes (Acari: Winterschmidtiidae) could be an alternative prey source, but studies on the reproductive parameters of N. californicus under rearing conditions are lacking. This study evaluated the potential of O. bakeri as an alternative prey in N. californicus rearing by comparing developmental parameters among N. californicus reared on three diets based on an age-stage two-sex life table. We found that the preoviposition period and developmental time of N. californicus did not vary based on diet. The fecundity of N. californicus adults reared on O. bakeri was 29.8 eggs per female, which was lower than that of adults reared on Tetranychus urticae Koch (Acari: Tetranychidae) (42.9 eggs per female); there was no significant difference between O. bakeri and apple pollen (30.2 eggs per female). The oviposition rate of mites fed on O. bakeri was 69% of that fed on T. urticae. Neoseiulus californicus reared on O. bakeri and apple pollen showed the same intrinsic rate of increase (0.25 per day), which was 86% of the rate of those fed on T. urticae. Compared with predatory mites reared on natural prey, N. californicus reared on O. bakeri had a high survival rate and good oviposition and population growth parameters, suggesting that O. bakeri is suitable for the rearing of N. californicus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An increasing number of biocontrol agents is being introduced to agricultural systems, and it is important that they are mass produced in a suitable and sustainable manner as part of the foundation of enhanced biological control (King 1993). Of the predatory mite species that have been successfully commercialized (McMurtry and Scriven 1965; McMurtry and Croft 1997), more than 20 are used to control spider mites, thrips, whiteflies and aphids (van Lenteren 2012). Maintaining large-scale, low-cost production of predatory mites is crucial for their application as biocontrol agents (Song et al. 2019).
There are some established methods for the mass rearing of predatory mites. The first is the natural prey breeding method, which involves breeding natural prey and using them as a food source. This approach is mainly used for predatory mites that prey exclusively or mainly on spider mites, such as Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae). The second method is alternative (factitious) prey breeding, which uses wheat bran and other diets to rear alternative prey, including some astigmatid mites, lepidopteran eggs, and the pupae of small insects (Altieri 1992; Pina et al. 2012; Barbosa and de Moraes 2015; Ji et al. 2015). For example, Carpoglyphus lactis (L.) (Acari: Astigmata) are often used as a food source in rearings of predatory mites such as Neoseiulus barkeri (Hughes), Amblyseius swirskii Athias-Henriot, Amblyseius gossipi Elbadry (all Acari: Phytoseiidae), and Stratiolaelaps scimitus (Womersley) (Acari: Laelapidae) (Rasmy et al. 1987; Bolckmans and van Houten 2006). The third method is pollen breeding – some mites are specialized pollen feeders/generalist predators (so-called ‘type IV’ predators in the terminology of McMurtry et al. 2013). Finally, artificial diets may be used as substitute food sources in mass cultures of predatory mites, such as honey, tryptone, sucrose, egg yolk, and yeast extract (Nguyen et al. 2014; Janssen and Sabelis 2015).
Neoseiulus californicus (McGregor) is a predatory mite used as a biocontrol agent against the two-spotted spider mite, Tetranychus urticae Koch, throughout a wide range of climatic and management conditions (Greco et al. 2005; Fraulo and Liburd 2007; Fraulo et al. 2008; Palevsky et al. 2008). Based on their food habits, biological traits, and morphological traits, phytoseiid mites have been classified into four categories (McMurtry and Croft 1997): type I mites are specialist predators of Tetranychus, type II mites are selective (specialist) predators of tetranychid mites, type III mites are generalist predators, and type IV mites are specialized pollen feeders/generalist predators. Although N. californicus feeds on tetranychid mites in the field, they can either selectively prey on spider mites [selective (specialist) predators] or feed on alternative foods such as pollen or small arthropods when field prey mite populations are low [generalist predators] (Rhodes and Liburd 2005; Saber 2013; Vacacela Ajila et al. 2019). Furthermore, some non-prey foods, such as fungal spores and hyphae, pollen, artificial diets and even plant tissues, may be used as supplementary or alternative foods in N. californicus rearing (Overmeer et al. 1985; van Rijn and Tanigoshi 1999). Therefore, in the commercial production of N. californicus, a range of alternative food sources may be used.
Neoseiulus californicus has been successfully reared on a variety of astigmatid mites (Castagnoli et al. 2006; Simoni et al. 2006; Barbosa and de Moraes 2015), including the dust mites Dermatophagoides farinae (Hughes) and Lepidoglyphus destructor (Schrank). Some astigmatid mites can be easily reared in large numbers on bran, flour or similar substrates, which greatly reduces the cost of predatory mite mass-rearing compared with the use of phytophagous mites as prey (Ramakers and van Lieburg 1982; Gerson et al. 2003). However, some astigmatid mites are not practicable food sources for predatory mites because they may trigger allergies in those that rear them (Castagnoli et al. 2006). It is well known that using spider mite-infested plants to rear phytoseiid mites has several disadvantages, such as the need for a large space, high costs and harvesting difficulties (Nguyen et al. 2015; Vangansbeke et al. 2016; Su et al. 2019). The use of an artificial food source to rear predatory mites is also costly compared with alternative prey such as Oulenziella bakeri Hughes (Acari: Winterschmidtiidae) (De Clercq et al. 2010). Hence, a cost-effective alternative prey for mass rearing is an essential requirement.
The fungivorous mite O. bakeri inhabits the leaves and fruits of rubber, jute, and citrus trees in tropical regions (Fan et al. 2012). Liu and Zhang (2016) studied the biological characteristics of O. bakeri and found that populations fed on yeast can become established at 25 °C. Studies have demonstrated that large numbers of O. bakeri can be reared on yeast alone or on combinations of yeast, flour, wheat husk, vermiculite, sawdust and other components (Jiang 2014). This is a cost-effective breeding method to maintain an O. bakeri population as prey. Similar to the commercial predatory mite breeding method using astigmatid mites (Ramakers and Van Lieburg 1982; Rasmy et al. 1987), most food sources of O. bakeri may be obtained in large numbers, are inexpensive and are easy to procure. Zhu et al. (2019) found that adult female N. californicus displays a type II functional response when fed on O. bakeri eggs and nymphs.
Although O. bakeri may be an alternative prey species in the large-scale commercial production of N. californicus (Liu and Zhang 2016; Zhu et al. 2019), to date there are no detailed data on the reproduction and development of N. californicus reared on O. bakeri. Therefore, we aimed to understand the effects of diet on N. californicus populations reared on O. bakeri, apple pollen and T. urticae.
Materials and methods
Mite source and rearing
The initial populations of N. californicus and O. bakeri used in this study were purchased from Fuzhou Guannong Biological Science and Technology (Fuzhou, China), and reared in the laboratory in a 60 × 62 × 172-cm climate cabinet at 27 ± 1 °C, 75 ± 5% RH, and L16:D8 photoperiod. Yeast was purchased from Angel Yeast Company, China, and used to rear O. bakeri. The arenas used to rear N. californicus and O. bakeri consisted of square plastic boxes (18 × 18 cm, 8 cm high) with a sponge placed at the bottom and a 12-cm-diameter Petri dish on top of the sponge. Distilled water was added to fill the box to the bottom of the Petri dish to prevent mites from escaping. Colonies of T. urticae were established from specimens originally collected in Guiyang city, China (26°55′N, 107°17′E). Tetranychus urticae colonies were reared on bean plants (Phaseolus vulgaris L.) in a greenhouse at 27 ± 1 °C under natural humidity and photoperiod conditions.
Apple pollen was chosen as a natural food source because the survival rate and fecundity of N. californicus on apple pollen were better than on other pollen types (including apricot, camellia and loofah) according to our preliminary evaluations. Fresh apple pollen was purchased from Shandong Qingdao Jinbaolun Agricultural Technology, China, and stored at −20 °C for no more than 6 months. During the experiments, the pollen was stored in a refrigerator at ca. 4 °C for no more than 2 weeks.
Life table study
Low-quality food leads to nutrient imbalances and can affect biological parameters (such as fecundity, among others) (Lee 2007). To avoid the effects of nutrient imbalance, an experimental population of N. californicus was reared for five generations on T. urticae, O. bakeri and apple pollen before the initiation of the experiments, and then the newly emerged generation were used in the following experiments. The experimental unit contained a 3-cm-diameter bean leaf disc placed on top of a wet sponge in a 10-cm-diameter Petri dish containing water. The edges of the bean leaf were covered with absorbent cotton to provide moisture and prevent predators from escaping. Water was added to the tray daily to keep the cotton moist. At the beginning of the experiments, to obtain N. californicus eggs of the same-age, 50 pairs of males and females in the same period were transferred from the conditioned colonies onto a bean leaf disc. After 24 h, the deposited eggs were individually transferred to the experimental units, with up to 60 replicates per treatment. Sixty N. californicus eggs were used for each diet. After hatching, 0.1 mg of apple pollen per experimental unit or a mixture of different stages of O. bakeri or T. urticae was offered as food.
Each experimental unit was checked daily, and the survivorship and development of the various immature stages of the predators were recorded. When the predatory mites reached the adult stage, females were paired with males obtained in the same experiment. Couples were kept together through the end of the study. The number of eggs laid was recorded, and eggs were removed daily after oviposition until all the adult mites died. In the pollen tests, pollen was changed and removed every 5 days. In the mite prey tests, the amount of O. bakeri and T. urticae were observed daily and supplemented, if necessary, to ensure adequate prey for the predator. All tests were carried out at 27 ± 1 °C, 75 ± 5% RH and L16:D8 photoperiod.
Data analysis
The raw data of N. californicus individuals were analysed according to developmental stage and the two-sex life table using the Two Sex-MSChart program (Chi and Liu 1985; Chi 1988, 2017). The age-stage specific survival rate (Sxj), age-specific survival rate (lx), age-specific fecundity (mx), age-stage specific fecundity (fxj), age-stage life expectancy (exj) and growth parameters, including intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R0), and mean generation time (T), were calculated according to Chi and Liu (1985) and Chi (1988).
Huang and Chi (2012) indicated that the jackknife method leads to substantial errors in the pseudo-values of the net reproductive rate (R0) and overestimates their differences. Therefore, bootstrapping with 100,000 iterations was used to estimate the means and standard errors of the life table parameters. The paired bootstrap test was used to compare differences in developmental duration in various life stages, adult longevity, total preoviposition period (TPOP; the time from hatching to the first oviposition in females), adult preoviposition period (APOP; the time between adult emergence and the first oviposition in females), oviposition days, preadult survival rate, fecundity and population parameters (r, λ, R0 and T) among the three treatments. The significance of differences between treatments was calculated above 100,000 bootstrap and then evaluated based on the 95% confidence interval (Goodman 1982; Brandstätter 1999; Hesterberg et al. 2005). All figures were generated using SigmaPlot v.14.0 software (Systat Software, San Jose, CA, USA).
Results
Development time, preadult survival, adult longevity, and lifespan
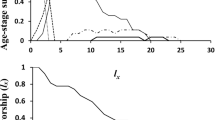
The mean development times of the various immature life stages of N. californicus reared on O. bakeri, apple pollen, and T. urticae are summarized in Table 1. The durations of egg, larva, protonymph and deutonymph were not different among the three diet groups (Table 1). Female and male adult longevity in N. californicus reared on apple pollen were 32.15 and 25.96 days, respectively; the values were significantly higher than those of N. californicus fed on O. bakeri (female and male adult longevity were 20.85 and 18.92 days, respectively) and those reared on T. urticae (21.52 and 19.64 days, respectively) (Table 1). Diet had no influence on the preadult survival rate in N. californicus (Fig. 1). The total numbers of eggs laid by N. californicus reared on T. urticae was 42.87 per female, which was significantly higher than those fed on O. bakeri (29.78 eggs per female) and apple pollen (30.18 eggs per female) (Table 2).
No difference in the TPOP was observed among the mites fed the different diets (Table 2). However, the APOP was significantly prolonged in the mites fed on apple pollen compared to the other two diets (Table 2).
Age-specific survivorship and fecundity
The overlap observed in the age-stage specific survival rates (Sxj) clearly showed a variable developmental rate among individuals (Fig. 2). The average daily egg production by N. californicus females reared on T. urticae was higher than those in mites reared on O. bakeri and apple pollen. Females reared on O. bakeri, apple pollen, and T. urticae achieved peak oviposition at 6, 5 and 8 days, with maximum daily egg production per female of 2.92, 2.89, and 3.48 eggs, respectively (Fig. 3).
The life expectancy at age zero (e01) in individuals reared on apple pollen (30.20 days) was higher than the corresponding values for individuals reared on O. bakeri (22.86 days) and T. urticae (23.30 days) (Fig. 4). The reproductive values (vxj) at age zero were 1.30, 1.28, and 1.33 day−1 for N. californicus reared on O. bakeri, apple pollen, and T. urticae, respectively (Fig. 5).
Life table parameters
The intrinsic rate of increase (r), net reproductive rate (R0), mean generation time (T), and finite rate of increase (λ) values are listed in Table 3. There were no differences in T among N. californicus mites reared on the different diets (Table 3). Higher values of λ (1.34 day−1), r (0.29 day−1), and R0 (22.17 offspring) of N. californicus were recorded on T. urticae as compared to O. bakeri (λ = 1.28 day−1, r = 0.25 day−1, R0 = 13.40 offspring) and apple pollen (λ = 1.28 day−1, r = 0.25 day−1, R0 = 13.58 offspring).
Discussion
The aim of this study was to assess the use of O. bakeri in the rearing of N. californicus as an alternative food source compared to T. urticae and apple pollen. Neoseiulus californicus was reared for five generations on all three food sources, with an immature survival rate > 96%. Neoseiulus californicus reared on O. bakeri and apple pollen had similar development times and population growth. There were no significant differences in female and male total longevity between N. californicus reared on O. bakeri and T. urticae. Oulenziella bakeri mites as a food source yielded a 69% oviposition rate of N. californicus (Table 2), approximately 60% net reproductive rate (R0), 86% intrinsic rate of increase (r) and > 95% finite rate of increase (λ) (Table 3) compared to predatory mites reared on T. urticae. These results showed that O. bakeri holds a promise in N. californicus rearing.
Pollen may positively affect multiple characteristics of predatory mites, including survival rates, body size, and/or fecundity (Castagnoli et al. 1999a, b; Khanamani et al. 2017). In this study, rearing N. californicus on apple pollen resulted in significantly longer total longevity in both sexes and longer APOPs in females than rearing N. californicus on O. bakeri and T. urticae. However, the total number of eggs laid per female reared on apple pollen was significantly lower than that on T. urticae. The number of eggs laid by N. californicus females reared on maize pollen (34.89) was lower than that laid by females reared on T. urticae (38.31) (Khanamani et al. 2017). Typhlodromus bagdasarjani females fed almond pollen laid 27.59 eggs, which was more than females fed T. urticae (21.50 eggs per female) (Riahi et al. 2016). These differences in fecundity may be due to nutritional variability in pollen from different plant species, and predatory mite species differ in their ability to utilize pollen (Khanamani et al. 2017). Pollen, as an alternative food source, may be used to maintain the predator population when primary prey is unavailable, or to produce predatory mites on a small scale for experimental use. Nevertheless, the pollen rearing method is not feasible for large-scale rearing because pollen needs to be replaced frequently due to the potential for fungal growth during rearing.
Alternative non-mite foods, such as artificial diets, have been evaluated for their use in low-cost, effective commercial rearing of N. californicus. Song et al. (2019) found that the artificial diet enriched with Ephestia kuehniella eggs (that is, 20% E. kuehniella eggs, 4% honey, 4% sucrose, 4% tryptone, 4% yeast extract, 8% egg yolk, and 56% distilled water) was adequate to support long-term rearing of N. californicus. However, using artificial diets to rear predatory mites often leads to reduced fecundity, a reduced lifespan, an inability to locate hosts, and poor survival. Ogawa and Osakabe (2008) reared N. californicus on an artificial diet based on yeast, sugar, and fresh egg yolk and found that the mites successfully developed to the adult stage, but they laid few eggs. Another artificial diet consisting of honey, sucrose, tryptone, yeast extract, fresh egg yolks, and the shelled egg extract of brine shrimp, Artemia franciscana (Kellogg) (Nguyen et al. 2015), was used to rear N. californicus, yielding mites that developed to the adult stage and laid some eggs, but their offspring was unable to develop to the adult stage. Neoseiulus californicus is considered a selective predator of tetranychid mites, which may explain its relatively poor performance on some artificial diet (McMurtry and Croft 1997; Nguyen et al. 2015).
Previous studies on predatory mite mass rearing on other saprophagous mites has reported encouraging results. For example, N. barkeri reared on the synanthropic mite Tyrophagus putrescentiae (Schrank) (Acari: Acaridae) laid 18.20 eggs per female (Li et al. 2017), to the fecundity of predators reared on T. urticae. Saprophagous mites include Tyrolichus casei Oudemans, T. putrescentiae and Acarus farris (Oudemans) (all Acari: Astigmata), which have been used as prey in the mass production of Agistemus exsertus Gonzalez (Acari: Stigmaeidae), Amblyseius cucumeris Oudemans (Acari: Phytoseiidae) and N. barkeri (Schliesske 1981).
Several other astigmatid mites have been screened as alternative prey for N. californicus. Castagnoli et al. (2006) found that L. destructor was the most suitable prey species among five astigmatid mites, as there was no phytoseiid mortality, and the development time was relatively short. In females reared on L. destructor at 25 °C and 80% RH, the daily fecundity rate was 2.16 eggs per female. The fecundity of N. californicus reared on D. farinae at 25 °C and 80–90% RH was 30.50 eggs per female (Castagnoli et al. 1999a). Barbosa and de Moraes (2015) reported that the fecundity of N. californicus reared on Blomia tropicalis Bronswijk and Austroglycyphagus lukoschusi (Fain) at 25 °C and 90% RH was 17.8 and 21.7 eggs per female, respectively. Our results showed that the fecundity of N. californicus reared on O. bakeri was 29.78 eggs per female, similar to the fecundity rates in females reared on astigmatid mites under similar environmental conditions. Li et al. (2016) assessed the dried fruit mite C. lactis as an alternative prey species for N. californicus, but this yielded a fecundity and intrinsic rate of increase (r) of only 36.7 and 56%, respectively, compared to natural prey at 25 °C and 75% RH. As mentioned above, we found that the oviposition rate and r in N. californicus reared on O. bakeri were 69 and 86% of those in N. californicus reared on T. urticae, respectively – much higher than those reported previously and high enough to sustain rapid population growth under similar environmental conditions.
Compared with those in other astigmatid mites used in the rearing of N. californicus, large-scale producing of O. bakeri with wheat bran or yeast costs very little (Jiang 2014), and no studies or reports have shown that O. bakeri causes human allergies. The results suggest the possible use of O. bakeri as an alternative prey for the mass rearing of N. californicus, potentially reducing production costs and enabling its widespread application. Despite these promising results, complementary studies are warranted because many factors interact during mass rearing.
References
Altieri MA (1992) Agroecological foundations of alternative agriculture in California. Agr Ecosyst Environ 39:23–53
Barbosa MFC, de Moraes GJ (2015) Evaluation of astigmatid mites as factitious food for rearing four predaceous phytoseiid mites (Acari: Astigmatina; Phytoseiidae). Biol Control 91:22–26
Bolckmans KJF, van Houten YM (2006) Mite composition, use thereof, method for rearing the phytoseiid predatory mite Amblyseius Swirskii, rearing system for rearing said phytoseiid mite and methods for biological pest control on a crop. WO Patent WO/2006/057552
Brandstätter E, Kepler J (1999) Confidence intervals as an alternative to significance testing. Methods Psychol Res Online 4:33–46
Castagnoli M, Simoni S, Biliotti N (1999a) Mass-rearing of Amblyseius californicus (Acari: Phytoseiidae) on two alternative food sources. In: Bruin J, van der Geest LPS, Sabelis MW (eds) Ecology and evolution of the acari. Series Entomologica, vol 55. Springer, Dordrecht, pp 425–431
Castagnoli M, Liguori M, Simoni S (1999b) Effect of two different host plants on biological features of Neoseiulus californicus (McGregor). Internat J Acarol 25:145–150
Castagnoli M, Nannelli R, Tarchi F, Simoni S (2006) Screening of astigmatid mites for mass rearing Neoseiulus californicus (McGregor) (Acari: Phytoseiidae). Redia 89:55–58
Chi H (1988) Life-table analysis incorporating both sexes and variable development rates among individuals. Environ Entomol 17:26–34
Chi H (2017) TWOSEX-MSChart: A computer program for the age-stage, two-sex life table analysis. National Chung Hsing University, Taichung, Taiwan, China. Available: http://140.120.197.173/Ecology/
Chi H, Liu H (1985) Two new methods for the study of insect population ecology. Bull Inst Zool Acad Sin 24:225–240
De Clercq P, Bonte M, Van Speybroeck K, Bolckmans K, Deforce K (2010) Development and reproduction of Adalia bipunctata (Coleoptera: Coccinellidae) on eggs of Ephestia kuehniella (Lepidoptera: Phycitidae) and pollen. Pest Manag Sci 61:1129–1132
Fan QH, George S, Kumarasinghe L (2012) Redescription of Oulenzia arboricola (Oudemans, 1928), type species of Oulenzia Radford, 1950 (Acari: Astigmata: Winterschmidtiidae). Syst Appl Acarol 17:333–338
Fraulo AB, Liburd OE (2007) Biological control of two-spotted spider mite, Tetranychus urticae, with predatory mite, Neoseiulus californicus, in strawberries. Exp Appl Acarol 43:109–119
Fraulo AB, Mcsorley R, Liburd OE (2008) Effects of the biological control agent Neoseiulus californicus (Acari: Phytoseiidae) on arthropod community structures in north Florida strawberry fields. Fla Entomol 91:436–445
Gerson U, Smiley RL, Ochoa R (2003) Mites (acari) for pest control. Blackwell, Science, Oxford
Goodman D (1982) Optimal life histories, optimal notation, and the value of reproductive value. Am Nat 119:803–823
Greco NM, Sanchez NE, Liljesthrom GG (2005) Neoseiulus californicus (Acari: Phytoseiidae) as a potential control agent of Tetranychus urticae (Acari: Tetranychidae): effect of pest/predator ratio on pest abundance on strawberry. Exp Appl Acarol 37:57–66
Hesterberg TD, Moore S, Monaghan S, Clipson A, Epstein R (2005) Bootstrap methods and permutation tests. In: Moore DS, McCabe GP, Duckworth WM, Sclove SL (eds) The practice of business statistics, 2nd edn. W. H. Freeman and Company, New York, NY, pp 18–38
Huang YB, Chi H (2012) Agestage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci 19:263–273
Janssen A, Sabelis MW (2015) Alternative food and biological control by generalist predatory mites: the case of Amblyseius swirskii. Exp Appl Acarol 65:413–418
Ji J, Zhang Y, Lin J, Chen X, Sun L, Saito Y (2015) Life histories of three predatory mites feeding upon Carpoglyphus lactis (Acari, Phytoseiidae; Carpoglyphidae). Syst Appl Acarol 20:491–496
Jiang HB (2014) Breeding method and application of Oulenziella bakeri Hughes, China. Patent, application No.CN103875609A. State Intellectual Property Office, Beijing, China
Khanamani M, Fathipour Y, Talebi AA, Mehrabadi M (2017) Linking pollen quality and performance of Neoseiulus californicus (Acari: Phytoseiidae) in two-spotted spider mite management programmes. Pest Manag Sci 73:452–461
King EG (1993) Augmentation of parasites and predators for suppression of arthropod pests. In: Lumsden RD, Vaughn JL (Eds) Pest management: biologically based technologies. Conference Proceedings Series, American Chemical Society, Washington, DC, pp 90–100
Lee KP (2007) The interactive effects of protein quality and macronutrient imbalance on nutrient balancing in an insect herbivore. J Exp Biol 210:3236–3244
Li Y, Lu JL, Wang ED, Xu XN (2016) Impact of crude protein and amino acid levels in yeast on development and reproduction of Carpoglyphus lactis and its predator Neoseiulus californicus (Acari: Phytoseiidae). Chin J Bio Control 32:25–32
Li YY, Zhang GH, Tian CB, Liu MX, Liu YQ, Liu H, Wang JJ (2017) Does long-term feeding on alternative prey affect the biological performance of Neoseiulus barkeri (Acari: Phytoseiidae) on the target spider mites? J Econ Entomol 110:915–923
Liu JF, Zhang ZQ (2016) Effects of short-term exposure to low temperature on survival, development and reproduction of banana associated Oulenziella bakeri (Acari: Winterschmidtiidae). Syst Appl Acarol 21:1078–1086
McMurtry JA, Croft BA (1997) Life-styles of phytoseiid mites and their roles in biological control. Annu Rev Entomol 42:291–321
McMurtry JA, Scriven GT (1965) Insectary production of phytoseiid mites. J Econ Entomol 58:282–284
McMurtry JA, De Moraes GJ, Sourassou NF (2013) Revision of the life styles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst Appl Acarol 18:297–320
Nguyen DT, Vangansbeke D, De Clercq P (2014) Artificial and factitious foods support the development and reproduction of the predatory mite Amblyseius swirskii. Exp Appl Acarol 62:181–194
Nguyen DT, Vangansbeke D, De Clercq P (2015) Performance of four species of phytoseiid mites on artificial and natural diets. Biol Control 80:56–62
Ogawa Y, Osakabe M (2008) Development, long-term survival, and the maintenance of fertility in Neoseiulus californicus (Acari: Phytoseiidae) reared on an artificial diet. Exp Appl Acarol 45:123–136
Overmeer WPJ (1985) Alternative prey and other food resources. In: Helle W, Sabelis MW (Eds) Spider mites: their biology, natural enemies and control. World Crop Pests 1B. Elsevier, Amsterdam, The Netherlands, pp. 131–139
Palevsky E, Walzer A, Gal S, Schausberger P (2008) Evaluation of dry-adapted strains of the predatory mite Neoseiulus californicus for spider mite control on cucumber, strawberry and pepper. Exp Appl Acarol 45:15–27
Pina T, Argolo PS, Urbaneja A, Jacas JA (2012) Effect of pollen quality on the efficacy of two different life-style predatory mites against Tetranychus urticae in citrus. Biol Control 61:176–183
Ramakers PMJ, Van Lieburg MJ (1982) Start of commercial production and introduction of Amblyseius mekenziei Sch. & Pr. (Acarina: Phytoseiidae) for the control of Thrips tabaci Lind. (Thysanoptera: Thripidae) in glasshouses. Med Fac Landbouwwet Rijksuniv Gent Bel 47:541–545
Rasmy AH, Elbagoury ME, Reda AS (1987) A new diet for reproduction of two predaceous mites Amblyseius gossipi and Agistemus exsertus [Acari: Phytoseiidae, stigmaeidae]. Entomophaga 32:277–280
Rhodes EM, Liburd OE (2005) Predatory mite. Neoseiulus californicus (Mcgregor) (Arachnida: Acari: Phytoseiidae). IFAS Extension. IN639. University of Florida, Gainesville, FL
Riahi E, Fathipour Y, Talebi AA, Mehrabadi M (2016) Pollen quality and predator viability: life table of Typhlodromus bagdasarjani on seven different plant pollens and two-spotted spider mite. Syst Appl Acarol 21:1399–1413
Saber SA (2013) Survival, fecundity and reproductive recovery period of Neoseiulus californicus (McGregor) during long-term preservation on maize pollen and after switch to Tetranychus urticae Koch. Arch Phytopathol Plant Prot 46:789–795
Schliesske J (1981) Ueber die Technik Der Massenanzucht Von Raubmilben (Acari: Phytoseiidae) unter kontrollierten bedingungen. Med Fac Landbouwwet Rijksuniv Gent Bel 46:511–517
Simoni S, Nannelli R, Goggioli D, Guidi S, Castagnoli M (2006) Biological and demographic parameters of Neoseiulus californicus (McGregor) (Acari: Phytoseiidae) reared on two astigmatid mites. Redia 89:59–63
Song ZW, Nguyen DT, Li DS, De Clercq P (2019) Continuous rearing of the predatory mite Neoseiulus californicus on an artificial diet. BioControl 64:125–137
Su J, Dong F, Liu S, Lu Y, Zhang J (2019) Productivity of Neoseiulus bicaudus (Acari: Phytoseiidae) reared on natural prey, alternative prey, and artificial diet. J Econ Entomol 112:2604–2613
Vacacela Ajila HE, Colares F, Lemos F, Marques PH, Franklin EC, Santos do Vale W, Oliveira EE, Venzon M, Pallini A (2019) Supplementary food for Neoseiulus californicus boosts biological control of Tetranychus urticae on strawberry. Pest Manag Sci 75:1986–1992
van Lenteren JC (2012) The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. BioControl 57:1–20
van Rijn PC, Tanigoshi LK (1999) Pollen as food for the predatory mites Iphiseius degenerans and Neoseiulus cucumeris (Acari: Phytoseiidae): dietary range and life history. Exp Appl Acarol 23:785–802
Vangansbeke D, Nguyen DT, Audenaert J, Verhoeven R, Gobin B, Tirry L, De Clercq P (2016) Supplemental food for Amblyseius swirskii in the control of thrips: feeding friend or foe? Pest Manag Sci 72:466–473
Zhu R, Guo JJ, Yi TC, Xiao R, Jin DC (2019) Functional and numerical responses of Neoseiulus californicus (McGregor) to eggs and nymphs of Oulenziella bakeri and Tetranychus urticae. Syst Appl Acarol 24:1225–1235
Funding
This work was supported by the National Key R & D Program of China (2017YFD0201000), the Provincial Science Fund for Distinguished Young Scholars [(2016)5641], the Scientific Research Foundation of Guiyang Healthcare Vocational University (No. Guikangdak2023-4), and the Government Procurement Public Services for Control of Pests, Disease and Rats, Ministry of Agriculture and Rural Affairs of the People’s Republic of China (15190061).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, R., Guo, Jj., Yi, Tc. et al. Potential of a winterschmidtiid prey mite for the production of the predatory mite Neoseiulus californicus (Acari: Phytoseiidae). Exp Appl Acarol 91, 571–584 (2023). https://doi.org/10.1007/s10493-023-00860-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-023-00860-w