Abstract
Tetranychus urticae is an important pest worldwide. The auto-dissemination of spores of entomopathogenic fungi from an infected individual to conspecifics may be important for controlling pests that can build high populations. The current study was carried out to determine the auto-dissemination of the entomopathogenic fungus Cordyceps fumosorosea strain PFs-1 (Priority®) between T. urticae females. The study consisted of four experiments. First, the efficacy of entomopathogenic fungus bioassays was assessed in Petri dishes (experiment 1) and on potted bean plants (experiment 2). In the auto-dissemination trials (experiments 3 and 4, in Petri dishes and on potted plants, respectively), contaminated adult females (1–5) were released among uncontaminated females (10 individuals). All experiments were carried out separately, and observations were made on days 3, 5, and 7. In exp. 1, the control was different from Priority on all observation days. In exp. 2, the average number of surviving individuals in the control was significantly higher than in the Priority treatment. In the auto-dissemination experiments, as the number of contaminated individuals increased, the mortality rate of uncontaminated individuals also increased, in exp. 3 (Petri dishes) on all observation days, and in exp. 4 (potted plants) only on days 5 and 7. The median lethal time (LT50) decreased as the number of individuals contaminated with Priority increased in both Petri dish and pot trials. Consequently, the effectiveness of biological control may increase with the occurrence of indirect contamination from infected to uncontaminated individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tetranychus urticae Koch (two-spotted spider mite) (Acari: Tetranychidae) is a polyphagous pest that feeds on more than 1100 plant species and varieties, including numerous commercial fruits and vegetables as well as ornamental plants (Migeon and Dorkeld 2010; Atalay and Kumral 2013). This pest has developed resistance to many pesticides. Its high fertility, short life cycle, and haplodiploid reproductive characteristics accelerate the development of resistance (Denholm et al. 1998; Carrière 2003; Van Leeuwen et al. 2010; Dermauw et al. 2012). In addition, these features cause them to reach population levels that cause significant economic losses in cultivated plants (Sato et al. 2007; El-Saiedy and Fahim 2021). Accordingly, it becomes increasingly difficult to control spider mites with only the use of pesticides. The phytophagous feeding feature and short life cycle of this pest allow it to develop resistance to many acaricides after a few applications (Cranham and Helle 1985; Keena and Granett 1990; Devine et al. 2001; Stumpf and Nauen 2001; Sato et al. 2005). Besides, individuals of this species can live individually or in groups. Tetranychus urticae females can establish colonies by arrhenotokous parthenogenetic reproduction and subsequently mate with their own sons (Yano 2008). In addition, T. urticae exhibits various forms of social behavior such as gathering or building a communal web (Saito 1983; Clotuche et al. 2009; Le Goff et al. 2009). Two-spotted spider mite builds complex three-dimensional webs on plant leaves (Saito 1983; Oku et al. 2009). A denser and more protective web provides continuity in group living characteristics, and their control in production areas may become harder as the dense webbing protects them from acaricides and also predators (Le Goff et al. 2010; Clotuche et al. 2011).
Cordyceps (formerly Isaria) fumosorosea (Wize) (Hypocreales: Cordycipitaceae) blastospores have been used extensively in pest management programs around the world. The blastospores are easily produced and require only 6–8 h to germinate (Vega et al. 1999; Avery 2002; Lozano-Contreras et al. 2007; Avery et al. 2009). This entomopathogenic fungus is eco-friendly with minimal impact on beneficial non-target arthropods (Sterk et al. 1995a, b; Avery et al. 2009). Some strains of C. fumosorosea were proven to be pathogenic to multiple mite pests in various habitats and highly virulent against active individuals of T. urticae (Alves et al. 2002; Shi and Feng 2004, 2009; Kim et al. 2008; El-Sharabasy 2015).
Entomopathogenic fungi are important and selective in the control of arthropod species. The inclusion of entomopathogenic fungi in pest control practices is increasing due to environmental awareness and concerns about food safety and pesticide use that lead to an increase in the number of insecticide-resistant species (Rai et al. 2014). Food safety, protection of biodiversity, minimization of pesticide residues, and the safety of non-target organisms are some of the advantages of microbial control agents over conventional chemical pesticides (Shahid et al. 2012). Mycoinsecticides are the first-choice organisms among entomopathogens in various ecosystems. Characteristics of entomopathogenic fungi such as high host specificity, negligible effect on non-target organisms, and easy mass production make them preferred in pest control (Rai et al. 2014; Singh et al. 2017). Entomopathogenic fungi are known to help regulate insect and mite populations through epizootics (Singh et al. 2017). The transfer of conidia from infected individuals to others is crucial as it causes the spread of infections and the onset of epizootics (Lacey et al. 1994; Hesket et al. 2010). Transmission of entomopathogenic fungi to healthy individuals is also achieved with the use of disseminating devices designed for attracting insects and infecting them through entomopathogenic fungi (Baverstock et al. 2010). Arthropods can mediate the horizontal transmission of disease to susceptible hosts either through direct physical contact or indirectly by releasing infective propagules (fungal spores) in the habitat (Lin et al. 2019). Infected individuals remaining in the environment can be beneficial for the spread of fungal pathogens and transmission to healthy conspecifics (Long et al. 2000; Avery et al. 2009, 2010). Reaching dense populations of host individuals increases the possibility of contact between them. Cordyceps fumosorosea has an important potential in the management of T. urticae. This present study aimed to determine the dissemination of this entomopathogenic fungus (Priority®) to uncontaminated (healthy) individuals through infected T. urticae individuals.
Materials and methods
Mites were used from a 5-year-old laboratory colony of T. urticae, that was collected from Antalya vegetable greenhouses in 2018 and that has since then been reared on bean plants, Phaseolus vulgaris L. (Fabaceae), and maintained at 25 ± 1 °C, 60–70% RH, and L8:D16 photoperiod. The identification of T. urticae was made using the keys in Bolland et al. (1998) and Jeppson et al. (1975). Bean leaves infested with T. urticae were cut and placed on non-contaminated plants to ensure the production of mites continued. Two commercial pesticides were used in the experiments: the mycopesticide Priority (1.5% C. fumosorosea strain PFs-1, 108 CFU/mL; MRFC [maximum field recommended concentration] = 250 mL/decare) and the acaricide Torpedo (EC 18 g/L abamectin; MFRC = 25 mL/100 L water), obtained from Agrobest (İzmir, Turkey) and Hektaş (Antalya, Turkey), respectively.
The study consisted of four experiments. When bean plants are treated with entomopathogenic fungi (1.5% C. fumosorosea strain PFs-1), the lethal effect on T. urticae females was determined in Petri dish conditions in exp. 1 and on bean plants in the climate room in exp. 2. In these two experiments, sterile distilled water was used as the negative control and abamectin as the positive control. Priority, abamectin, and the water control were sprayed onto leaf discs (6 cm diameter) with an apparatus (glass material that sprays the liquid under pressure at the tip of a motorized insect aspirator), with the ability to mist at 4 atm pressure for 10 s (0.6 mL).
In order to determine the dissemination of the same entomopathogenic fungus by contaminated individuals to conspecific individuals, varying numbers of contaminated individuals (in the range of 1–5 contaminated adults) were transferred to Petri dishes in exp. 3 and bean plants in exp. 4. In these two experiments, 10 uncontaminated females (per replication) were previously transferred to leaf discs prepared in both Petri dishes and pots. For exp. 3 and 4, the entomopathogenic fungus was sprayed with a misting apparatus at 4 atm pressure for 10 s (0.6 mL) onto leaf discs. Then, 20 T. urticae adults were transferred with a fine brush on the bean leaf disc (6 cm diameter) in Petri dishes with 10 replications. After 24 h, the contaminated individuals (a range of 1–5 contaminated females) were used. In exp. 3 and 4, the bean plants were germinated in pots (11.5 cm diameter) containing sterile soil in a climatic chamber.
Petri dishes and pot trials (exp. 1–4) were carried out under climate chamber conditions (25 ± 1 °C, 60 ± 10% RH, and L16:D8 photoperiod). In exp. 1–4, a complete randomized block design was used, and sterile distilled water (dH2O) was applied in the control. The number of deaths was recorded on days 3, 5, and 7 after the applications (DAA). In all experiments, cadavers of T. urticae were placed on potato dextrose agar (PDA) in Priority treatments, and fungal growth was observed in glass Petri dishes (9 cm diameter) by the method of Wraight et al. (1998) with some modifications. All experiments were repeated separately per observation day.
Experiment 1
Exp. 1 was conducted in Petri dishes with bean leaf discs (6 cm diameter) on moist cotton (Fig. 1). Priority, Torpedo, or distilled water were sprayed on leaf discs for 10 s. Afterwards, 10 uncontaminated females were transferred with a brush onto the leaf discs (10 replications).
Experiment 2
In exp. 2, 2-week-old bean plants – with two leaves, in plastic pots (11.5 cm diameter) with sterilized soil mixture (soil + organic matter) – were used in the climatic chamber. For the applications in these plants, a leaf disc area of 6 cm diameter was constructed on each leaf with a Vaseline ring (it was placed around the leaf discs to prevent the escape of T. urticae) (Al-Azzazy et al. 2020) (Fig. 1). One leaf selected on each plant (with two leaves) was evaluated as one replication (total of 10 replications). The distilled water was sprayed on leaf discs selected per plant in the control (with 10 replications).
Experiment 3
The treatments were conducted in Petri dishes (9 cm diameter) in which bean leaf discs were placed on moist cotton. Contaminated females (1–5) were released with a brush to Petri dishes containing 10 non-contaminated individuals. Each Petri dish was one replication (in total 10 replications).
Experiment 4
In this experiment, 24 h after Priority application, contaminated individuals (1–5 females per application) were transferred onto leaves of bean plants in pots (1–5 contaminated females per application). After that, 10 uncontaminated individuals were released to these leaves to determine whether the entomopathogenic fungus could infect healthy individuals. Each plant was a replication and in total 10 replications were made per application.
Statistical analysis
One-way ANOVA was applied to the number of individuals surviving obtained from exp. 1 and 2 followed by Tukey’s honestly significant difference (HSD) test (α = 0.05) (IBM SPSS v.20.0). Mortality rates obtained from exp. 3 and 4 were calculated using Abbott’s formula (Abbott 1925). One-way ANOVA was applied to the data followed by Tukey’s HSD test. The median lethal time (LT50) was calculated using mortality rates in exp. 3 and 4. The independent samples t-test was separately applied to compare the results of the Petri dish and pot trials with each other for mortality rates at each time point. Also correlation and regression analyses were done (α = 0.01). In addition, second order polynomials were used depending on the correlation coefficient appropriate to the obtained mortality rates.
Results
Experiment 1
The ANOVA indicated that treatments had a strong effect on mite survival on each observation day (3 DAA: F2,27 = 4.734, P = 0.017; 5 DAA: F2,27 = 24.927; 7 DAA: F2,27 = 47.499, both P < 0.001). The average number of surviving T. urticae females treated with Priority was not different from that treated with abamectin on 3 and 5 DAA, but it was significantly higher at 7 DAA (Fig. 2). Survival in the water control was higher than in the treatments on all observation days (Fig. 2).
Mean (± SE) number of surviving Tetranychus urticae females treated with a mycopesticide (Priority), abamectin (Torpedo) or distilled water (control) at 3, 5 and 7 days after application (DAA), on bean leaf either in Petri dishes (exp. 1) or on potted plants (exp. 2). Means within a day capped with different letters are significantly different (Tukey’s test: P < 0.005)
Experiment 2
ANOVA indicated a strong effect of treatments on mite survival on each observation day (3 DAA: F2,27 = 14.289; 5 DAA: F2,27 = 30.465; 7 DAA: F2,27 = 65.498, all P < 0.001). There was no statistical difference between Priority and abamectin on any observation day. The average number of surviving individuals in the control was higher than in the other applications (Fig. 2).
Experiment 3
In exp. 3, it was evaluated whether different numbers of Priority-contaminated T. urticae (1–5) would have an effect on the mortality rate in uncontaminated individuals in Petri dishes. ANOVA indicated strong effects of treatments on mite survival on each observation day (3 DAA: F4,45 = 43.321; 5 DAA: F4,45 = 23.573; 7 DAA: F4,45 = 14.616, all P < 0.001).
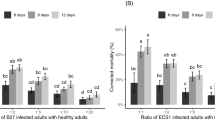
At 3 DAA, the mortality rates were 8.2–29.3% in the trial with 1–5 contaminated adults released, respectively. These values were 17.3–44.7% on 5 DAA, and 29.6–56.4% on 7 DAA (Fig. 3). Overall, mortality rates increased with increasing mite density (Fig. 3). The highest mortality rates were achieved in the trials with five contaminated individuals released on all observation days (Fig. 3).
Mean (± SE) mortality rates (%) of Tetranychus urticae females treated with 1–5 mycopesticide-contaminated individuals at 3, 5 and 7 days after application (DAA), on bean leaf either in Petri dishes (exp. 3) or on potted plants (exp. 4). Means within a day capped with different letters are significantly different (Tukey’s test: P < 0.005)
Experiment 4
In exp. 4, it was investigated whether different numbers of Priority-contaminated T. urticae (1–5) would have an effect on the mortality rate of uncontaminated individuals on potted plants. Here, ANOVA indicated strong effects of treatments on mite survival on 5 and 7 days after application (5 DAA: F4,45 = 134.720; 7 DAA: F4,45 = 29.957, both P < 0.001), but not on 3 DAA (F4,45 = 1.336, P = 0.27).
At 3 DAA, the mortality rates were 8.2–11.7% in the trial with 1–5 contaminated adults released, at 5 DAA these values were 10.9–40% and at 7 DAA 14.6–40.7% (Fig. 3). Also here, overall mortality rates increased with increasing mite density (Fig. 3).
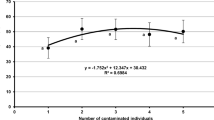
The LT50, a measure of the virulence of the entomopathogenic fungus, was calculated separately for experiments 3 and 4. In exp. 3, the LT50 decreased from 10.49 to 5.94 days, with 1 and 5 contaminated individuals released in Petri dishes, respectively (Fig. 4). In exp. 4, the LT50 decreased from 29.38 to 7.50 days, with 1 and 5 contaminated individuals released on potted plants, respectively (Fig. 4).
Comparison of Petri dish and pot trials (experiments 3 and 4)
Mortality rates in Petri dish and pot experiments, with 1–5 contaminated individuals, were compared per observation day. When 1 contaminated individual was released, mortality rates in Petri dishes and pot trials were the same on 3 and 5 DAA, but higher in Petri dishes on 7 DAA (Fig. 5). Almost all other comparisons – 2–5 contaminated individuals released, 3–7 DAA – indicated higher mortality rates in Petri dishes than in pot trials; only at 5 DAA, the differences in mortality rate were not significantly different with 3 and 5 contaminated individuals released (Fig. 5).
Mean (± SE) mortality rates (%) of Tetranychus urticae in Petri dish vs. potted plant trials, compared at 3, 5 and 7 days after application (DAA). Mortality rates within a day and within a mite density marked with different letters are significantly different (t-test: P < 0.01; equations of the fitted lines are based on correlation and regression analyses)
Discussion
One of the major disadvantages of continuous chemical control against T. urticae is the fact that it gains resistance to pesticides over time (Herron and Rophail 1998; Van Leeuwen et al. 2004). In order to overcome the resistance problem, it is preferred to use alternative control methods in pest management (Yeşilayer 2018). Auto-dissemination of entomopathogenic fungi has an important role in integrated pest management. This strategy can be used to disseminate microbial organisms against pests that can coexist in the same habitat with natural enemies (Vega et al. 2000). Auto-dissemination can be provided using devices containing both entomopathogens and semiochemicals; pests enter the device, get infected by the pathogen, exit from the device, and transfer the inoculum (Vega et al. 2007; Baverstock et al. 2010; Lacey et al. 2015; Gonzalez et al. 2016). Dispersal of conidia of entomopathogenic fungi with devices has been reported to be simple, economical, and effective (Maniania and Ekesi 2013). Klein and Lacey (1999) and Maniania (2002) determined 95 and 100% infection in Popilia japonica Newman and Glossina fuscipes fuscipes Newstead, respectively, by conidia of Metarhizium anisopliae Petch.
An insect’s or mite’s behavioral response to a fungal pathogen has an important impact on the entomopathogenic fungus’ efficacy. In the literature, the effects of insect and mite behavior on the preference of fungal pathogens have been studied (Baverstock et al. 2010; Parker et al. 2011; Vezilier et al. 2015; Zélé et al. 2020). However, the effectiveness of entomopathogens was associated with the contact between the pest and the entomopathogenic fungus (Wraight et al. 2001; FAO/IAEA 2019). A 3-min contact of M. brunneum (GranMet®) with an adult P. japonica was sufficient to infect the insect and eventually kill it (Benvenuti et al. 2019). In the current study, individuals considered contaminated were also exposed to entomopathogenic fungus (1.5% C. fumosorosea) for 24 h.
An increase in the number of contaminated T. urticae adults in this study showed a significant effect on the mortality rate of healthy adults in Petri dishes as well as on potted plants. Yet, on the potted plants it took a bit longer for this effect to become significant, and overall the mortality rate was higher in the Petri dishes than on the potted plants – for instance, the mortality rate was 56.4% on day 7 when five contaminated individuals were released into Petri dishes, whereas this value was 40.7% on potted plants. Amjad et al. (2012) determined a mortality rate of 79% of adult T. urticae females on day 8 after direct application of C. fumosorosea (n32) at 108 conidia/mL.
The virulence of an entomopathogenic fungus can be determined by the LT50, the average lethal time from exposure to the pathogen to the death of an infected insect (FAO/IAEA 2019). The virulence of each entomopathogenic fungus species depends on the host from which the fungus is isolated and the susceptibility of the target for which it is evaluated (Hajek and St. Leger 1994; De la Rosa et al. 2002; Roberts and Leger 2004; Rehner 2005; Meyling and Eilenberg 2007; Zélé et al. 2020). Differences between entomopathogenic fungal strains and their effects on pest species may result from host–pathogen relationships (Lecuona 1996; FAO/IAEA 2019). In the present study, the LT50s obtained at all observation times in the pot trials were higher than in the Petri dishes. In addition, it was determined that the LT50 decreased as the number of contaminated individuals increased in both experiments 3 and 4. Amjad et al. (2012) reported that the LT50 of C. fumosorosea (n32) was 4.6 days in 108 conidia/mL in Petri dishes. In this study, the LT50 value was determined as 5.9 days when five contaminated individuals were released into Petri dishes. In another study, the LT50 for T. urticae adults treated with Beauveria bassiana Bb101, another entomopathogenic fungus, has been detected as 81.8 h (3.4 days) (Saranya et al. 2013). Hence, the LT50 values in the current study were found to be close to those reported in other studies.
Similar to this study, Demirözer (2019) investigated the dissemination of Fusarium subglutinans 12 A through females of Frankliniella occidentalis Pergande. Mortality rates were 39.1, 51.8, 51.6, 48.2, and 50.1% on day 8 in Petri dishes in which, respectively, 1–5 inoculated thrips were released together with 10 uninoculated females. In the present study, mortality rates were 29.6–56.4% on day 7 in Petri dishes in which 1–5 T. urticae adults were contaminated with C. fumosorosea strain PFs-1. In addition, Demirözer (2019) found that the mortality rate on day 7 remained < 30% in cell cage treatments where five inoculated females were used. In the pot trial of the current study, the mortality rate was 40.7% on day 7 after release of five contaminated adults. In both studies, the mortality rates in pot trials were lower than in Petri dish trials.
In the current study, the disseminating ability of individuals exposed to an entomopathogenic fungus to uninfected individuals of the same species was evaluated. As the number of contaminated individuals increased, the mortality rate also increased. Thus, the effectiveness of biological control may increase with the occurrence of indirect contamination from infected to non-contaminated individuals. This study may encourage similar studies on other harmful arthropods that have a high probability of contact with each other.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Al-Azzazy MM, Alsohim AS, Yoder CE (2020) Biological effects of three bacterial species on Tetranychus urticae (Acari: Tetranychidae) infesting eggplant under laboratory and greenhouse conditions. Acarologia 60(3):587–594
Alves SB, Rossi LS, Lope RB, Tamai MA, Pereira RM (2002) Beauveria bassiana yeast phase on agar medium and its pathogenicity against Diatraea saccharalis (Lepidoptera: Crambidae) and tetranychus urticae (Acari: Tetranychidae). J Invertebr Pathol 81:70–77
Amjad M, Bashir MH, Afzal M, Sabri MA, Javed N (2012) Synergistic effect of some entomopathogenic Fungi and synthetic pesticides, against two spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae). Pak J Zool 44(4):977–984
Atalay E, Kumral NA (2013) Tetranychus urticae (Koch) (Acari: Tetranychidae)’nin farklı sofralık domates çeşitlerinde biyolojik özellikleri ve yaşam çizelgeleri. Turk Entomol Derg 37(3):329–341
Avery PA, Queele GL, Faull J, Simmonds MSJ (2010) Effect of photoperiod and host distribution on the Horizontal transmission of Isaria fumosorosea (Hypocreales: Cordycipiticeae) in Greenhouse Whitefly assessed using a Novel Model Bioassay. Biocontrol Sci Technol 20:1097–1111
Avery PB (2002) Tritrophic Interactions among Paecilomyces fumosoroseus, Encarsia formosa and Trialeurodes vaporariorum on Phaseolus vulgaris and Pelargonium spp. Ph.D. Dissertation, Univ. London, Birkbeck College, London, UK
Avery PB, Hunter WB, Hall DG, Jackson MA, Powell CA, Rogers ME (2009) Diaphorina citri (Hemiptera: Psyllidae) infection and dissemination of the entomopathogenic fungus isaria fumosorosea (Hypocreales: Cordycipitaceae) under laboratory conditions. Fla Entomol 92(4):608–618
Baverstock J, Roy HE, Pell JK (2010) Entomopathogenic fungi and insect behaviour: from unsuspecting hosts to targeted vectors. Biocontrol 55:89–102
Benvenuti C, Barzanti GP, Marianell L, Sabbatini Peverieri G, Paoli F, Bosio G, Venanzio D, Giacometto E, Roversi P (2019) A new device for auto-disseminating entomopathogenic fungi against Popillia japonica: a study case. Bull Insectology 72(2):219–225
Bolland HR, Gutierrez J, Flechtmann CHW (1998) World Catalogue of the Spider Mite Family (Acari: Tetranychidae). BRILL
Carrière Y (2003) Haplodiploidy, sex, and the evolution of pesticide resistance. J Econ Entomol 96(6):1626–1640
Clotuche G, Le Goff G, Mailleux AC, Deneubourg JL, Detrain C, Hance T (2009) How to visualize the spider mite silk? Microsc Res Tech 72:659–664
Clotuche G, Mailleux AC, Astudillo Fernandez A, Deneubourg JL, Detrain C, Hance T (2011) The formation of collective silk balls in the spider mite Tetranychus urticae Koch. PLoS ONE 6(4):e18854
Cranham JE, Helle W (1985) Pesticide resistance in Tetranychidae. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies, and control. Elsevier, Amsterdam
De la Rosa WF, López L, Liedo P (2002) Beauveria bassiana as a pathogen of the mexican fruit fly (Diptera: Tephritidae) under laboratory conditions. J Econ Entomol 95:36–43
Demirözer O (2019) Target-oriented dissemination of the entomopathogenic fungus fusarium subglutinans 12A by the Western Flower Thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Phytoparasitica 47(4):1–11
Denholm I, Cahill M, Dennehy TJ, Horowitz AR (1998) Challenges with managing insecticide resistance in agricultural pests, exemplified by the whitefly Bemisia tabaci. Philos Trans R Soc B 353(1376):1757–1767
Dermauw W, Wybouw N, Rombauts S, Menten B, Vontas J, Grbic M, Clark RM, Feyereisen R, Van Leeuwen T (2012) A link between host plant adaptation and pesticide resistance in the polyphagous spider mite tetranychus urticae. PNAS 17:E113–E122
Devine GJ, Barber M, Denholm I (2001) Incidence and inheritance of resistance to METI-acaricides in european strains of the two-spotted spider mite (Tetranychus urticae) (Acari: Tetranychidae). Pest Manag Sci 57:443–448
El-Saiedy ESM, Fahim SF (2021) Evaluation of two predatory mites and acaricide to suppress Tetranychus urticae (Acari: Tetranychidae) on strawberry. Bull Natl Res Cent 45(97):1–9
El-Sharabasy HM (2015) Laboratory evaluation of the effect of the entomopathogenic fungi, Hirsutella thompsonii and Paecilomyces fumosoroseus, against the citrus brown mite, Eutetranychus orientalis (Acari: Tetranychidae). Plant Prot Sci 51:39–45
FAO/IAEA (Food and Agriculture Organization of the United Nations International Atomic Energy Agency) (2019) Use of conidia disseminators. In A. Villaseñor S, Flores SE, Campos J, Toledo P, Montoya P, Liedo W, Enkerlin (Eds.), Use of Entomopathogenic Fungi for Fruit Fly Control in Area-Wide SIT Programmes (pp. 16–18), Vienna, Austria
Gonzalez F, Tkaczuk C, Dinu MM, Fiedler Z, Vidal S, Zchori-Fein E, Messelink GJ (2016) New opportunities for the integration of microorganisms into biological pest control systems in greenhouse crops. J Pest Sci 89:295–231
Hajek AE, St Leger RJ (1994) Interactions between fungal pathogens and insect hosts. Ann Rev Entomol 39:293–322
Herron GA, Rophail (1998) Tebufenpyrad (pyranica(R)) resistance detected in two-spotted spider mite Tetranychus urticae Koch (Acarina: Tetranychidae) from Apples in Western Australia. Exp Appl Acarol 22:633–641
Hesket H, Roy HE, Eilenberg J, Pel JK, Hail RS (2010) Challenges in modelling complexity of fungal entomopathogens in semi-natural populations of insects. Bio-Control 55(1):55–73
Jeppson LR, Keifer HH, Baker EW (1975) Mites injurious to economic plants. University of California Press, Berkeley, CA
Keena MA, Granett J (1990) Genetic analysis of propargite resistance in Pacific spider mites and two spotted spider mite (Acari: Tetranychidae). J Econ Entomol 83:655–661
Kim JS, Roh JY, Choi JY, Shin SC, Jeon MJ, Je YH (2008) Insecticidal activity of Paecilomyces fumosoroseus SFP-198 as a multi targeting biological control agent against the greenhouse whitefly and the two spotted spider mite. Int J Indust Entomol 17:181–187
Klein MG, Lacey LA (1999) An attractant trap for the autodissemination of entomopathogenic fungi into populations of the japanese beetle, Popillia japonica (Coleoptera: Scarabaeidae). Biocontrol Sci Technol 9:151–158
Lacey LA, Grzywacz D, Shapiro-Ilan DI, Frutos R, Brownbridge M, Goettel MS (2015) Insect pathogens as biological control agents: back to the future. J Invertebr Pathol 132:1–41
Lacey LA, Martins A, Ribeiro C (1994) The pathogenicity of Metarhizium anisopliae and Beauveria bassiana for adults of the japanese beetle, Popillia japonica (Coleoptera: Scarabeidae). Eur J Entomol 91:313–319
Lecuona R (1996) Microorganismos patógenos empleados en el control microbiano de insectos plaga. Imyza–Cica–Inta Castelar, Buenos Aires
Le Goff G, Mailleux AC, Detrain C, Deneubourg JL, Clotuche G, Hance T (2010) Group effect on fertility, survival and silk production in the web spinner Tetranychus urticae (Acari: Tetranychidae) during colony foundation. Behaviour 147:1169–1184
Le Goff G, Mailleux AC, Detrain C, Deneubourg JL, Hance T (2009) Spatial distribution and inbreeding in Tetranychus urticae? C R Biol 332:927–933
Lin G, Guertin C, Di Paolo SA, Todorova S, Brodeur J (2019) Phytoseiid predatory mites can disperse entomopathogenic fungi to prey patches. Sci Rep 9:19435
Long DW, Groden E, Drummond FA (2000) Horizontal transmission of Beauveria bassiana (bals.) Vuill. Agric for Entomol 2:11–17
Lozano-Contreras MG, Elías-Santos M, Rivasmorales C, Luna-Olvera A, Galán-Wong LJ, Maldonado-Blanco MG (2007) Paecilomyces fumosoroseus blastospore production using liquid culture in a bioreactor. Afr J Biotechnol 6:2095–2099
Maniania NK (2002) A low-cost contamination device for infecting adult tsetse, Glossina spp., with the entomopathogenic fungus metarhizium anisopliae in the field. Biocontrol Sci Technol 12:59–66
Maniania NK, Ekesi S (2013) The use of entomopathogenic fungi in the control of tsetse flies. J Inverteb Pathol 112:583–588
Meyling NV, Eilenberg J (2007) Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biol Control 43: 145–155.
Migeon A, Dorkel F (2010) Spider Mites Web: a Comprehensive Database for the Tetranychidae. http://www.montpellier.inra.fr/CBGP/spmweb. Accessed 2 February 2023
Oku K, Magalhaes S, Dicke M (2009) The presence of webbing affects the oviposition rate of two-spotted spider mites, Tetranychus urticae (Acari: Tetranychidae). Exp Appl Acarol 49(3):167–172
Parker BJ, Barribeau SM, Laughton AM, de Roode JC, Gerardo NM (2011) Non-immunological defense in an evolutionary framework. Trends Ecol Evol 26:242–248
Rai D, Updhyay V, Mehra P, Rana M, Pandey AK (2014) Potential of entomopathogenic fungi as biopesticides. Indian J Sci Res 2(5):7–13
Rehner SA (2005) Phylogenetics of the insect pathogenic genus Beauveria. In F. E. Vega, M. Blackwell (eds), Insect-fungal associations: Ecology and evolution, (pp. 3–27) NY: Oxford University Press, New York.
Roberts DW, Leger RJ S (2004) Metarhizium spp., cosmopolitan insect-pathogenic fungi: Mycological aspects. In A. I. Laskin, J. W. Bennet, G. M. Gadd (eds), Advances in applied microbiology (pp. 1–70) CA: Academic Press, San Diego.
Saito Y (1983) The concept of life types in Tetranychinae. An attempt to classify the spinning behaviour of Tetranychinae. Acarologia 24:377–392
Saranya S, Ramaraju K, Jeyarani S (2013) Pathogenicity of entomopathogenic fungi to two spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae). Biopestic Int 9(2):127–131
Sato ME, da Silva MZ, de Souza Filho MF, Matioli AL, Raga A (2007) Management of Tetranychus urticae (Acari: Tetranychidae) in strawberry fields with Neoseiulus californicus (Acari: Phytoseiidae) and acaricides. Exp Appl Acarol 42:107–120
Sato ME, Da Silva MZ, Raga A, De Souza Filho MF (2005) Abamectin Resistance in Tetranychus urticae Koch (Acari: Tetranychidae): Selection, Cross-Resistance and Stability of Resistance. Neotrop Entomol 34(6):991–998
Shahid AA, Rao AQ, Bakhsh A, Tayyab H (2012) Entomopathogenic fungi as biological controllers: new insights into their virulence and pathogenicity. Arch Biol Sci 64(1):21–42
Shi WB, Feng MG (2004) Lethal effect of Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces fumosoroseus on the eggs of Tetranychus cinnabarinus (Acari: Tetranychidae) with a description of a mite egg bioassay system. Biol Control 30:165–173
Shi WB, Feng MG (2009) Effect of fungal infection on reproductive potential and survival time of Tetranychus urticae (Acari: Tetranychidae). Exp Appl Acarol 48:229–237
Singh D, Raina TK, Singh J (2017) Entomopathogenic Fungi: an effective Biocontrol Agent for Management of Insect populations naturally. J Pharm Sci Res 9(6):830–839
Sterk G, Bolckmans K, De Jonghe R, De Wael L, Vermeule J (1995a) Side-effects of the microbial insecticide PreFeRal WG (Paecilomyces fumosoroseus, strain Apopka 97) on Bombus terrestris. Meded Fac Landbouww Rijksuniv 60:713–717
Sterk G, Bolckmans K, Van De Veire M, Sel B, Stepman W (1995b) Side-effects of the microbial insecticide PreFeRal WG (Paecilomyces fumosoroseus, strain Apopka 97) on different species of beneficial arthropods. Meded Fac Landbouww Rijksuniv 60:719–724
Stumpf N, Nauen R (2001) Cross-resistance, inheritance, and biochemistry of mitochondrial electron transport inhibitor-acaricide resistance in Tetranychus urticae (Acari: Tetranychidae). J Econ Entomol 94:1577–1583
Van Leeuwen T, Stillatus V, Tirry L (2004) Genetic analysis and cross-resistance spectrum of a laboratory-selected chlorfenapyr resistant of two spotted spider mite (Acari: Tetranychidae). Exp Appl Acarol 32:249–261
Van Leeuwen T, Vontas J, Tsagkarakou A, Dermauw W, Tirry L (2010) Acaricide resistance mechanisms in the two-spotted spider mite tetranychus urticae and other important Acari: a review. Insect Biochem Mol Biol 40(8):563–572
Vega FE, Dowd PF, Lacey LA, Pell JK, Jackson DM, Klein MG (2007) Dissemination of beneficial microbial agents by insects. In: Lacey LA, Kaya HK (eds) Field manual of techniques in invertebrate pathology. Springer, Dordrecht, pp 127–146
Vega FE, Jackson MA, Mcguire MR (1999) Germination of conidia and blastospores of Paecilomyces fumosoroseus on the cuticle of the silverleaf whitefly, Bemisia argentifolii. Mycopathologia 147:33–35
Vega FE, Klein MG, Dowd PF, Michael Jackson D, Lacey LA, Pell JK (2000) Dissemination of beneficial microbial agents by insects. In: Lacey LA, Kaya HK (eds) Field Manual of Techniques in Invertebrate Pathology: application and evaluation of pathogens for control of insects and other Invertebrate Pests. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 153–177
Vezilier J, Nicot A, Gandon S, Rivero A (2015) Plasmodium infection brings forward mosquito oviposition. Biol Lett 11:20140840
Wraight SP, Carruthers RI, Bradley CA, Jaronski ST, Lacey LA, Wood P, Galaini-Wraigh S (1998) Pathogenicity of the Entomopathogenic Fungi Paecilomyces spp. and Beauveria bassiana against the Silverleaf Whitefly, Bemisia argentifolii. J Invertebr Pathol 71:217–226
Wraight SP, Jackson MA, de Kock SL (2001) Production, stabilization and formulation of Fungal Biocontrol Agents. In: Butt TM, Jackson C, Magan N (eds) Fungi as Biocontrol Agents: Progress, problems and potential. CAB International, London, UK, pp 253–288
Yano S (2008) Collective and solitary behaviors of Twospotted Spider Mite (Acari: Tetranychidae) are Induced by Trail following. Ann Entomol Soc Am 101(1):247–252
Yeşilayer A (2018) Efficiency of two different entomopathogen fungi Beauveria bassiana and purpureocillium lilacinum tr1 against Tetranychus urticae. Appl Ecol Environ Res 16(5):6077–6086
Zélé F, Altıntaş M, Santos I, Cakmak I, Magalhães S (2020) Inter- and intraspecific variation of spider mite susceptibility to fungal infections: implications for the long-term success of biological control. Ecol Evol 10:3209–3221
Acknowledgements
The author is grateful to Dr. Emre İnak (Ankara University, Turkey) for providing the diagnosed population of Tetranychus urticae. Special thanks to Dr. Ozan Demirözer (Isparta University of Applied Sciences, Turkey) for all contributions and support to the study. The author thanks Dr. Ali Kemal Birgücü (Isparta University of Applied Sciences, Turkey) for assisting with the statistical analysis, and Agrobest (İzmir, Turkey) and Hektaş (Antalya, Turkey) for providing pesticides.
Funding
The author did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Study conception and experimental design were performed by AUY, data collection was carried out by AUY, The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uzun Yiğit, A. Auto-dissemination of Cordyceps fumosorosea amongst adult females of the two-spotted spider mite. Exp Appl Acarol 91, 279–290 (2023). https://doi.org/10.1007/s10493-023-00845-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-023-00845-9