Abstract
Rickettsia species are bacteria that may cause multiple diseases in animals and humans, via transmission through multiple arthropod vectors. Routine surveillance of Rickettsia spp. within vectors is critical to determine their presence and risk to mammalian hosts within human populations. Therefore, to better characterize the circulating Rickettsia species in an understudied region we targeted pet dogs to survey. Ticks were collected from pet dogs in three populations of the Yucatan where we tested for the presence of Rickettsia spp. by PCR in metagenomic DNA. In these ticks removed from pet dogs we detected Rickettsia amblyommatis and Rickettsia bellii in Amblyomma auriculatum, Amblyomma ovale and Amblyomma mixtum ticks obtained in a rural community in the Mexican state of Yucatan. This is the first report detecting both species for this state in Mexico, underpinning the importance of more routine surveillance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rickettsia spp. are obligate intracellular bacteria, many of which are the causative agents of zoonotic diseases around the world. These bacteria are usually transmitted to humans and animals through the bite of infected arthropod vectors (ticks, fleas, mites, and lice) or via infectious products such as flea dirt. Some of the known diseases caused by Rickettsia spp. include Rocky Mountains spotted fever (RMSF), murine typhus fever (MTF), epidemic typhus fever (ETF), and scrub typhus fever (STF). Such diseases cause a myriad of adverse clinical outcomes and fatal complications, reaching a case-fatality rate (CFR) ranging from 8 to 10%, but even going to 30% among children and other vulnerable individuals. Such CFR are mainly associated with the delay (> 5 days after onset of symptoms) of the specific antibiotic (i.e., doxycycline), particularly for RMSF (Buckingham et al. 2007; Nogueira Angerami et al. 2009; Alvarez-Hernandez et al. 2015; Álvarez-López et al. 2021). Stray and house dogs and cats play a significant role in transmitting these diseases, as fleas and ticks infest them, and have been proved to be a problem in Mexico (Alvarez-Hernandez et al., 2020). In addition to dogs and cats, other small mammals (e.g., Didelphis virginiana, known as tlacuaches in many Mexican regions) may host various species of Rickettsia and vectors of Rickettsia (Peniche-Lara et al. 2014; Charles et al. 2021; Lugo-Caballero et al. 2021; Salceda-Sánchez et al. 2023; Sánchez-Montes et al. 2023), but it is unknown if wildlife experiences negative health outcomes when infected with Rickettsia species. In human landscapes where there is also a lot of wildlife in addition to stray dogs and cats, humans and domestic animals are at high risk of exposure to Rickettsia due to the vector population blood feeding on both wildlife (such as opossums and capybaras) and domestic hosts (i.e., cats and dogs) (Miranda et al. 2017; Salceda-Sánchez et al. 2023; Premaratna 2022).
In Mexico, it is mandatory to report detected rickettsiosis to the health authorities, but this is not always the case as its diagnosis is mainly centralized in Mexico City making it difficult and expensive to perform the tests. A 2021 report from InDRE (National Diagnosis and Reference Laboratory) shows that this disease has a 0.0969 mortality rate (per 100,000 people) in Mexico and that Sonora (a state in the northwest part of the country) has the highest mortality rate in the country (186 per 100,000 people) (Dirección General de Epidemiología, 2021). In Yucatan, five Rickettsia species have been reported in humans: Rickettsia felis, Rickettsia rickettsii, Rickettsia typhi, Rickettsia akari and Rickettsia parkeri (Peniche-Lara et al. 2015; Martínez-Ortiz et al. 2016; Ulloa-García et al. 2020; Torres-Castro et al. 2022a, b; Peniche-Lara and Lara-Perera 2022). The cat flea (Ctenocephalides felis) and R. felis have been documented in both home courtyards and pet animals in the Yucatan (Peniche-Lara et al. 2015). In the above-mentioned epidemiology report, Yucatan has a mortality rate similar to the national average (0.09 per 100,000 people) suggesting that this is not an important health issue though this might be a misconception (Dirección General de Epidemiología, 2021). Historically throughout Mexico several species of hard ticks have been known vectors of Rickettsia spp., such as Amblyomma cajennense (Peniche-Lara et al. 2014), Amblyomma mixtum (Peniche-Lara et al. 2018; Martínez-Ortiz et al. 2019), Rhipicephalus sanguineus sensu lato (Peniche-Lara et al. 2018; Martínez-Ortiz et al. 2019), Amblyomma imitator, Amblyomma aureolatum, Dermacentor andersoni, and Dermacentor variabilis; these tick species have also been reported as transmitting Rickettsia rickettsii to domestic animals, wild animals, and humans (Rodríguez Vivas et al. 2019).
The Yucatan Peninsula located in the southeast of Mexico, is a geographic region with a warm sub-humid tropical climate, and is made up of three states: Campeche, Quintana Roo and Yucatan. The climate, together with wildlife and the fact that many poor persons live in isolated rural and peri-urban populations increase the chances of these people to get in contact and be parasitized by arthropods that can transmit diseases. Moreover, infected people with symptoms have a hard time to get to hospitals or clinics to get proper attention. In this sense, reports describing the multiple tick species and the bacteria colonizing them are in need to know the circulating pathogens in these areas.
Dogs function as effective sentinels to detect circulation of several species of rickettsiae and some other epidemiologically important tick-borne pathogens (e.g., Ehrlichia spp., Anaplasma spp., Babesia spp.) (Ortuño et al. 2009; Salomon et al. 2022; Torres-Castro et al. 2022). Because dogs and humans exist in the same landscapes, similar pathogen prevalences of dogs can be detected via serological and molecular tests. For example, simultaneous cases of infection have been documented in dogs and people living together in the same household (Arroyo-Ramírez et al. 2022). Therefore, human populations that live in close contact with high dog populations are at a higher risk of Rickettsia spp. infections (Martínez-Ortiz et al. 2019; Salomon et al. 2022; Torres-Castro et al. 2022).
Currently, many diseases including vector-borne diseases are shifting their frequency and distribution to peri-urban and non-endemic areas as a result of global factors such as: globalization, land use changes (e.g., urban sprawl, destruction of wildlife habitats, agriculture expansion), parasites evolution of antibiotic resistance, privatization of public health services, and finally environmental changes such as global warming (Cortés 2010; Caminade et al. 2019; Swei et al. 2020). Therefore, more epidemiological surveys in humans, domestic and wild animals should be performed on a regular basis to understand the distribution of ectoparasites and the pathogens present in them.
The aim of this survey was to identify Rickettsia spp. in metagenomic DNA from ticks collected from pet dogs in the southern region of Mérida city, and two other populations in the southeastern region of the Yucatan. This region currently lacks any routine surveillance of Rickettsia spp. within arthropod vectors or sylvatic hosts but has reported human cases (Peniche-Lara and Lara-Perera 2022), the results from this survey will establish a baseline of pathogen prevalence and present tick species, which will strengthen public health response and instigate further research.
Materials and methods
Collection of ticks
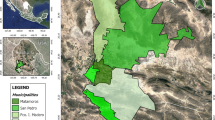
Adult hard ticks were collected from 27 pet dogs in three regions in the Mexican state of Yucatan (Fig. 1): the Dzununcán population is part of the Merida municipality (20°53’37.5"N, 89°39’23.1"W), and despite its nearness to the capitol city (Merida city) it is a poor semi-rural population (2,380 persons by the 2020 national census) where people is partially dedicated to growing crops but mostly work in the city, therefore we classified it as peri-urban; the Chenché de las Torres population is part of the Temax municipality (21°07’49.1"N, 88°58’56.2"W) (257 persons by the 2020 national census) and the Cholul population is part of the Cantamayec municipality (20°26’34.6"N, 89°09’16.9"W) (462 persons by the 2020 national census), both populations are isolated, poor and rural. These populations were selected by convenience as follows: Chenché and Cholul were chosen because three of the authors (G.C. Reyes-Solís, A.E. Cano-Ravell, and C. Machain-Williams) have social, health and community projects and ticks are a main concern for the residents who asked them to do a deeper search into this issue; Dzununcán was selected because these authors also have an educational program at a local high school and, again, they were asked to look into the arthropod problem.
Location of the communities where the ticks were collected from pet dogs. A) Map of Yucatan (south-east Mexico) showing the approximate location of each population: Merida (1), Temax (2) and Cantamayec (3) municipalities. The figure is an original map from INEGI, (with public domain map data from INEGI https://www.inegi.org.mx/app/mapas/) (accessed on March 27, 2023) and modified with Microsoft PowerPoint. Pictures shown are representatives of Dzununcán (1), Chenché (2) and Cholul (3)
Dogs’ enrollment included a verbal consent from the owners. Out of the 27 dogs, only two (nos. 7 and 14) made regular visits to the veterinarian and had a few vaccines, but none of them received treatment against fleas or ticks. Collections were done between September of 2018 and June of 2019 because during these periods the projects mentioned above were done. Each dog was examined following the specifications described by Peniche-Lara et al. (2014); briefly, only ticks were removed with entomological forceps and ticks were deposited in 15-mL tubes containing 5 mL of 70% ethanol duly labeled and stored in a cooler containing ice packs and ice to preserve them and for transferring them to our laboratory. Finally, the ticks were stored in an ultra-freezer at -70 ºC.
Tick identification by morphological and molecular methods
All the ticks were observed under an SZM-LED2 Optika stereomicroscope with magnification up to 90×. The dichotomous keys by Brinton and Beck (1963), Keirans and Litwak (1989) and Guzmán-Cornejo et al. (2011) were used for morphological identification. Molecular identification was performed only to those ticks with a positive PCR for Rickettsia spp. as described in the following sections.
DNA isolation
Ticks were frozen overnight at -70 °C and thawed at room temperature, placed individually in sterile 1.5-mL microtubes. DNA was isolated from individual ticks by salt extraction as described by Black and DuTeau (1997) and suspended in 200 µL of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0). DNA concentration and purity was assessed for each sample by using a Nanodrop. In order to determine that the purified DNA was suitable for a PCR, amplification of an internal 200-bp fragment of the gene coding for the bacterial 16 S rRNA was done as a control for each sample as described in the following section.
PCR amplification
For the molecular characterization of ticks, PCR targeting the 16S + 1 gene on metagenomic DNA was performed with ticks that were positive for the Rickettsia detection by PCR. PCR reactions were done by using the primers 16s + 1 (5’-CTGCTCAATGATTTTTTAAATTGC-3’) and 16s-1 (5’-CCGGTCTGAACTCAGATCATGTA-3’), the enzyme used was DreamTaq Green PCR Master Mix (2×) (Thermo Scientific) and using amplification conditions as previously described (Black and Piesman 1994; Bermudez et al. 2017). As positive control we used metagenomic DNA from Ornithodoros turicatae used for another project (Vázquez-Guerrero et al., in preparation) and as a negative control we used bacterial DNA isolated from Escherichia coli DH10B. Amplicons with the expected size of 475 bp were observed in a 1.5% agarose gel; then, these fragments were purified and sequenced using both primers at Macrogen (https://dna.macrogen.com/) to yield a 2× coverage. Sequences were trimmed and assembled with ChromasPro (Technelysium) (https://technelysium.com.au/wp/chromaspro/). Assembled sequences obtained were compared with available sequences in GenBank using BlastN (https://blast.ncbi.nlm.nih.gov). We used a criterion of 100% query cover and the species was considered with a minimum or 98% for ticks.
Tick metagenomic DNA samples were used to perform two PCR assays for bacteria and the pathogen detection. The latter was to ensure the DNA extraction of bacterial DNA. Amplification of bacterial DNA used a 200-bp fragment using primers Eub338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and Eub518R (5’- ATTACCGCGGCTGCTGG-3’) (Guo et al. 2008). Cycling conditions used were as described by Guo et al. (2008) and the PCR reagents used were DreamTaq Green PCR Master Mix (2×) (Thermo Scientific). To amplify Rickettsia spp. specifically, we targeted a 374 bp fragment of the promoter and the structural regions of Rickettsia gltA; this was initially described for qPCR (Labruna et al. 2004) with primers CS-78 (5’-GCAAGTATCGGTGAGGATGTAAT-3’) and CS-323 (5’-GCTTCCTTAAAATTCAATAAATCAGGAT-3’) but here we used it for a regular PCR reaction as has been done in other reports (Bermúdez et al. 2021; Cicuttin et al. 2022; Sebastian et al. 2022). For this, the positive control included a positive fragment obtained from a patient confirmed with the diagnosis of RMSF caused by R. rickettsii (M. Leyva-Gastélum, data not shown). This fragment was purified and sequenced as described above (by Macrogen) and later cloned by using a TOPO-TA kit (Invitrogen), preserved in an E. coli DH10B strain, and used in further amplifications. Briefly, once this fragment was verified by sequencing to belong to R. rickettsii a new PCR reaction was done, and the obtained fragment cloned into the pCR2.1TOPO vector following the manufacturer instructions; plasmids from candidate clones were characterized by digesting with EcoRI (Thermo Scientific) and by sequencing using the universal primer M13Fw. Obtained sequence was analyzed with BLASTn to corroborate it. All the amplicons were observed in 1.5% agarose gels stained with either ethidium bromide or SYBR Green (Thermo Scientific) in an Eagle-eye UV transilluminator. For the phylogenetic analysis, the amino acid sequences were aligned with Muscle (https://www.ebi.ac.uk/Tools/msa/muscle/). The phylogenetic tree was generated in MEGA11 (Tamura et al. 2021) with maximum likelihood using the model of amino acids substitution Blosum62.
Results
Morphological classification of the collected ticks
We collected and morphologically characterized 171 engorged ticks from 27 mixed breed pet dogs whose owners live in either of the three assessed populations (Fig. 1). Ticks removed from these dogs were identified as: Rhipicephalus sanguineus (s.l.) (134), Amblyomma dissimile (2), Amblyomma tuberculatum (1), Amblyomma sp. (3), Amblyomma cajennense (9), Amblyomma auriculatum (14), Amblyomma maculatum (3), Amblyomma parvum (3), Amblyomma ovale (1), and Hyalomma sp. (1) (Table 1). Out of the 171 ticks, 148 were adults (58 males and 90 females) and 23 were nymphs. The tick burden per dog was represented as described before (Alvarez-Hernandez et al. 2020); Table 2 shows that the tick burden per dog is moderate with all the sampled dogs having at least one tick. Dogs sampled from Cholul had a higher burden than those in Dzununcán and Chenché. Molecular characterization was done only for those ticks with a positive PCR test for gltA.
Rickettsia spp. molecular detection in ticks
First, all the samples were positive for the control 16 S rRNA fragment, indicating that bacterial DNA was included in the metagenomic DNA. Out of all the analyzed metagenomic DNA samples, three (1.8%) were positive via a portion of gltA PCR, indicating that these ticks were infected with Rickettsia species. Analysis of the fragment of mitochondrial DNA of the infected ticks by BLAST-n indicated that two were A. parvum (99.1% identity), and one was A. mixtum (99.3% identity), all of them obtained from the same dog of Cholul (Table 1). When morphological and molecular identifications were compared for these three ticks, discrepancies were observed for two of them (A. auriculatum/A. ovale vs. A. parvum) whereas for one it suggested that the species was A. mixtum.
Identification of Rickettsia species
The three gltA amplicons obtained from the metagenomic DNA were sequenced and analyzed to determine the species they belong to. These sequence were deposited in GenBank with the accession numbers OQ709151 for g6, OQ709152 for g7 and OQ709153 for g8. As seen in Fig. 2, control DNA (g0) clustered with R. rickettsii, as expected. The other sequences aligned with Rickettsia amblyommatis (g6 and g7) and Rickettsia bellii (g8). The outgroup was taken as the bacteria with the lowest identity percentage. These results show that multiple species Rickettsia were detected in the analyzed ticks.
Maximum-likelihood tree constructed from gltA partial sequences of various Rickettsia species. The analysis was performed with the amino acid substitution model Blosum62. Sequences generated in this study are noted as R. rickettsii g0 (positive control), R. amblyommatis g6, R. amblyommatis g7 and R. bellii g8. Numbers represent bootstrap support generated from 1000 replications. The bar represents 0.050 different nucleotides between sequences
Discussion
Our results show that ticks collected from pet dogs in Yucatan are infected with R. amblyommatis and R. bellii and shows that these dogs and the humans and other domestic animals are at risk of exposure to these bacteria. To the best of our knowledge this is the first report of these Rickettsia species in ticks in this Mexican state. The presence of Rickettsia species has already been documented in Mexico in both urban and wild environments (Peniche-Lara et al. 2014; Delgado-de la Mora et al. 2019; Merino et al. 2020; Lugo-Caballero et al. 2021; Sánchez-Montes et al. 2021; Salomon et al. 2022; Torres-Castro et al. 2022b). Here, the populations where the dogs reside are poor, one is peri-urban (Dzununcán) and two are rural and isolated (Cheché and Cholul).
To contribute to a better understanding of the distribution of Rickettsia spp. in Mexico, we report the identification of R. amblyommatis and R. bellii in metagenomic samples obtained from A. parvum and A. mixtum, respectively. To the best of our knowledge and based on a recent compilation (Sanchez-Montes et al. 2021), these two Rickettsia species have not been widely described in studies done in Mexico (Medina 2007; Salomon et al. 2022). Rickettsia bellii has been described as a non-pathogenic member of this genus that diverged from the pathogenic ones (Ogata et al. 2006). However, a recent report has shown that R. bellii causes a subclinical infection in a guinea pig model, though it is not clear whether it is able to be transmitted by a tick bite to humans or animals (Snellgrove et al. 2021). This bacterium has been detected and isolated from multiple Amblyomma species but, to the best of our knowledge this is the first report detecting it in A. mixtum.
Rickettsia amblyommatis has been isolated from Amblyomma ticks in the Americas, including Amblyomma americanum and A. cajennense in the USA (Karpathy et al. 2016) and from Amblyomma longirostre in Brazil (Labruna et al. 2004), Amblyomma neumanni and Amblyomma hadanii in Argentina (Labruna et al. 2007; Mastropaolo et al. 2016), amongst others in other Latin American countries (quoted by Karpathy et al. 2016; Charles et al. 2021; Richardson et al. 2023). Thus, this species is widely distributed in many ticks along the Americas. Salomon et al. (2022) detected it in ticks parasitizing dogs although these were R. sanguineus in the northern state of Tamaulipas. Rickettsia amblyommatis pathogenicity is not yet fully characterized, but it is considered to be an opportunistic pathogen for humans (Yen et al. 2021; Richardson et al. 2023). Rickettsia amblyommatis has been detected in multiple species of ticks including those described here (A. parvum) (Richardson et al. 2023).
Regarding the identification of the ticks, all were first identified by their morphological characteristics and then, only those infected with Rickettsia sp. were identified molecularly. In this way, we were able to determine with more accuracy the species these ticks belong to, although molecular identification would better be based on a multilocus sequence analysis (MLSA) or by sequencing the mitochondrial genome to perform a phylogenomic analysis. In any case, detection of R. amblyommatis in Yucatan was not previously described and indicates that more research and surveillance should be done in domestic animals and wildlife fauna in this Mexican state.
These results shed light on a potential public health problem in similar settings across the country as dogs are interacting with humans and may serve as amplification hosts to species of Rickettsia. Moreover, widespread co-feeding of ticks suggests that known vector ticks could amplify the number of infected ticks on suitable hosts such as pet dogs. An important note is to remark that most reports of Rickettsia spp. have found R. sanguineus s.l. ticks on domestic dogs and here is shown that other ticks are also able to infest these hosts and transmit these bacteria. Even when the number of dogs was small, we saw that multiple tick species parasitized them, and we suggest that this is the norm for pet dogs in these populations that are so near to the jungle. Of course, to test this hypothesis more studies are needed in populations like the one studied here. This raises the possibility of an eventual spillover of pathogens to novel vectors and another matter to search into. This is a matter of interest in several groups for this and other tick-borne and insect-borne transmitted diseases (reviewed in Laukaitis and Macaluso 2021).
It would be very interesting to extend this research to detect antibodies specific to R. amblyommatis in the isolated population of Cholul, and to extend the search for other Rickettsia species. As in other studies, dogs and other domestic animals could be used as sentinels for these and other pathogens and also help to study the cross-reactivity observed between R. amblyommatis and other SFG rickettsiae (reviewed in Richardson et al. 2023).
References
Alvarez-Hernandez G, Murillo-Benitez C, Candia-Plata Mdel C, Moro M (2015) Clinical profile and predictors of fatal Rocky Mountain spotted fever in children from Sonora, Mexico. Pediatr Infect Dis J 34(2):125–130. https://doi.org/10.1097/INF.0000000000000496
Alvarez-Hernandez G, Drexler N, Paddock CD, Licona-Enriquez JD, la Mora JD, Straily A, Del Carmen Candia-Plata M, Cruz-Loustaunau DI, Arteaga-Cardenas VA (2020) Community-based prevention of epidemic Rocky Mountain spotted fever among minority populations in Sonora, Mexico, using a one health approach. Trans R Soc Trop Med Hyg 114(4):293–300. https://doi.org/10.1093/trstmh/trz114
Álvarez-López DI, Ochoa-Mora E, Nichols Heitman K, Binder AM, Álvarez-Hernández G, Armstrong PA (2021) Epidemiology and clinical features of Rocky Mountain spotted fever from enhanced surveillance, Sonora, Mexico: 2015–2018. Am J Trop Med Hyg 104(1):190–197. https://doi.org/10.4269/ajtmh.20-0854
Arroyo-Ramírez A, Lugo-Caballero C, Bolio-González M, Rodríguez-Vivas RI, Reyes Novelo E, Panti-May JA, Torres-Castro M (2022) El género Rickettsia y reportes de infección en perros de Yucatán. Bioagrociencias 15(1):65–76
Bermúdez SE, Castillo E, Pohlenz TD, Kneubehl A, Krishnavajhala A, Domínguez L, Suárez A, López JE (2017) New records of Ornithodoros puertoricensis Fox 1947 (Ixodida: Argasidae) parasitizing humans in rural and urban dwellings, Panama. Ticks Tick Borne Dis 8(4):466–469. https://doi.org/10.1016/j.ttbdis.2017.02.004
Bermúdez SE, Félix ML, Domínguez AL, Kadoch N, Muñoz-Leal S, Venzal JM (2021) Molecular screening for tick-borne bacteria and hematozoa in Ixodes cf. boliviensis and Ixodes tapirus (Ixodida: Ixodidae) from western highlands of Panama. Curr Res Parasitol Vector Borne Dis 1:100034. https://doi.org/10.1016/j.crpvbd.2021.100034
Black WC, DuTeau NM (1997) RAPD-PCR and SSCP analysis for insect population genetic studies. In: Crampton JM, Beard CB, Louis C (eds) Molecular Biology of Insect Disease vectors: a Methods Manual. Chapman & Hall, London, pp 362–363
Black WC, Piesman J (1994) Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc Natl Acad Sci USA 91(21):10034–10038. https://doi.org/10.1073/pnas.91.21.10034
Brinton EP, Beck DE (1963) Hard-bodied ticks of the Western United States. Part I. Brigh Young Univ Sci Bull 2(3):1–28
Buckingham SC, Marshall GS, Schutze GE, Woods CR, Jackson MA, Patterson LE, Jacobs RF (2007) Tick-borne infections in children Study Group. Clinical and laboratory features, hospital course, and outcome of Rocky Mountain spotted fever in children. J Pediatr 150(2):180–184. https://doi.org/10.1016/j.jpeds.2006.11.023
Caminade C, McIntyre KM, Jones AE (2019) Impact of recent and future climate change on vector-borne diseases. Ann NY Acad Sci 1436(1):157–173. https://doi.org/10.1111/nyas.13950
Charles RA, Bermúdez S, Banović P, Obregon D, Díaz-Sánchez AA, Corona-González B, Etter E, Rodríguez I, Ghafar A, Jabbar A, Moutailler S, Cabezas-Cruz A (2021) Ticks and Tick-Borne Diseases in Central America and the Caribbean: a One Health Perspective. Pathogens 10(10):1273. https://doi.org/10.3390/pathogens10101273
Cicuttin GL, De Salvo MN, Venzal JM, Nava S (2022) Rickettsia spp., Ehrlichia sp. and Candidatus Midichloria sp. associated to ticks from a protected urban area in Buenos Aires City (Argentina). Exp Appl Acarol 86:271–282. https://doi.org/10.1007/s10493-022-00684-0
Cortés JA (2010) Cambios en la distribución y abundancia de las garrapatas y su relación con el calentamiento global. Revista de la Facultad de Medicina Veterinaria y de Zootecnia 57(1):48–57
Delgado-de la Mora J, Sánchez-Montes S, Licona-Enríquez JD, Delgado-de la Mora D, Paddock CD, Beati L, Colunga-Salas P, Guzmán-Cornejo C, Zambrano ML, Karpathy SE, López-Pérez AM, Álvarez-Hernández G (2019) Rickettsia parkeri and Candidatus Rickettsia andeanae in Tick of the Amblyomma maculatum Group, Mexico. Emerg Infect Dis 25(4):836–838. https://doi.org/10.3201/eid2504.181507
Dirección General de Epidemiología, Sistema Epidemiológico y Estadístico de las Defunciones 2021 (SEED), Secretaría de Salud
Guo X, Xia X, Tang R (2008) Real-time PCR quantification of the predominant bacterial divisions in the distal gut of Meishan and Landrace pigs. Anaerobe 14(4):224–228. https://doi.org/10.1016/j.anaerobe.2008.04.001
Guzmán-Cornejo C, Robbins RG, Guglielmone AA, Montiel-Parra G, Pérez TM (2011) The Amblyomma (Acari: Ixodida: Ixodidae) of Mexico: Identification Keys. Distribution and Hosts Zootaxa 2998(1):16–38. https://doi.org/10.11646/zootaxa.2998.1.2
Karpathy SE, Slater KS, Goldsmith CS, Nicholson WL, Paddock CD (2016) Rickettsia amblyommatis sp. nov., a spotted fever group Rickettsia associated with multiple species of Amblyomma ticks in North, Central and South America. Int J Syst Evol Microbiol 66(12):5236–5243. https://doi.org/10.1099/ijsem.0.001502
Keirans JE, Litwak TR (1989) Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), east of the Mississippi river. J Med Entomol 26(5):435–448. https://doi.org/10.1093/jmedent/26.5.435
Labruna MB, Whitworth T, Horta MC, Bouyer DH, McBride JW, Pinter A, Popov V, Gennari SM, Walker DH (2004) Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of São Paulo, Brazil, where brazilian spotted fever is endemic. J Clin Microbiol 42(1):90–98. https://doi.org/10.1128/JCM.42.1.90-98.2004
Labruna MB, Pacheco RC, Nava S, Brandão PE, Richtzenhain LJ, Guglielmone AA (2007) Infection by Rickettsia bellii and Candidatus “Rickettsia amblyommii” in Amblyomma neumanni ticks from Argentina. Microb Ecol 54(1):126–133. https://doi.org/10.1007/s00248-006-9180-3
Laukaitis HJ, Macaluso KR (2021) Unpacking the intricacies of Rickettsia-vector interactions. Trends Parasitol 37(8):734–746. https://doi.org/10.1016/j.pt.2021.05.008
Lugo-Caballero C, Torres-Castro M, López-Ávila K, Hernández-Betancourt S, Noh-Pech H, Tello-Martín R, Puerto-Manzano F, Dzul-Rosado K (2021) Molecular identification of zoonotic Rickettsia species closely related to R. typhi, R. felis, & R. rickettsii in bats from Mexico. Indian J Med Res 154(3):536–538. https://doi.org/10.4103/ijmr.IJMR_1083_19
Martínez-Ortiz D, Torres-Castro M, Koyoc-Cardeña E, López K, Panti-May A, Rodríguez-Vivas I, Puc A, Dzul K, Zavala-Castro J, Medina-Barreiro A, Chablé-Santos J, Manrique-Saide P (2016) Molecular evidence of Rickettsia typhi infection in dogs from a rural community in Yucatán. México Biomed 36(0):45–50. https://doi.org/10.7705/biomedica.v36i2.2913
Martínez-Ortiz D, Torres-Castro M, López-Ávila K, Koyoc-Cardeña E, Manrique-Saide P (2019) Rickettsia spp. en garrapatas (Acari: Ixodidae) que infestan perros de una comunidad rural con antecedentes de rickettsiosis, Yucatán, México. Biomédica 30(2). https://www.revistabiomedica.mx/index.php/revbiomed/article/view/650/678
Mastropaolo M, Tarragona EL, Silaghi C, Pfister K, Thiel C, Nava S (2016) High prevalence of “Candidatus Rickettsia amblyommii’ in Amblyomma ticks from a spotted fever endemic region in north Argentina. Comp Immunol Microbiol Infect Dis 46:73–76. https://doi.org/10.1016/j.cimid.2016.05.001
Medina A, Guevara E, Alcantara V, Garza C, Hunt F, Davis J, Gonzalez R, Bouyer D, Walker D (2007) Isolation of Rickettsia amblyommii and seroprevalence of Rickettsia in the state of Veracruz, Mexico. Abstract #132. 21st Meeting of the American Society for Rickettsiology; Colorado Springs, CO
Merino O, De la Cruz NI, Martinez J, Pérez de León AA, Romero-Salas D, Esteve-Gassent MD, Lagunes-Quintanilla R (2020) Molecular detection of Rickettsia species in ticks collected in the Mexico-USA transboundary region. Exp Appl Acarol 80(4):559–567. https://doi.org/10.1007/s10493-020-00483-5
Miranda RJ, Mattar VS, González TM (2017) Rickettsiosis. Revista MVZ Córdoba 22(supl) 6118–6133. https://doi.org/10.21897/rmvz.1080
Nogueira Angerami R, Oliveira Morais E, Katz G, Jacintho da Silva L (2009) Brazilian spotted fever in the paediatric age-segment in the state of São Paulo, southeastern Brazil, 2003–2006. Clin Microbiol Infect 15(Suppl 2):205–206. https://doi.org/10.1111/j.1469-0691.2008.02728.x
Ogata H, La Scola B, Audic S, Renesto P, Blanc G, Robert C, Fournier PE, Claverie JM, Raoult D (2006) Genome sequence of Rickettsia bellii illuminates the role of Amoebae in gene exchanges between intracellular pathogens. PLoS Genet 2(5):e76. https://doi.org/10.1371/journal.pgen.0020076
Ortuño A, Pons I, Nogueras MM, Castellà J, Segura F (2009) The dog as an epidemiological marker of Rickettsia conorii infection. Clin Microbiol Infect 15 Suppl 2:241–242. https://doi.org/10.1111/j.1469-0691.2008.02158.x
Peniche-Lara G, Lara-Perera V (2022) Rickettsiosis caused by Rickettsia parkeri, Mexico. Emerg Infect Dis 28(2):478–479. https://doi.org/10.3201/eid2802.210454
Peniche-Lara G, Dzul-Rosado K, Jiménez-Delgadillo B, Vado-Solís I, Pérez-Osorio C, Zavala-Castro J (2014) Identificación de Rickettsia spp. en garrapatas Amblyomma cajennense parasitando bovinos en ranchos del estado de Yucatán. Ciencia y Humanismo en la Salud 1(1):23–27
Peniche-Lara G, Dzul-Rosado K, Pérez-Osorio C, Zavala-Castro J (2015) Rickettsia typhi in rodents and R. felis in fleas in Yucatán as a possible causal agent of undefined febrile cases. Rev Inst Med Trop Sao Paulo 57(2):129–132. https://doi.org/10.1590/S0036-46652015000200005
Peniche-Lara G, Jimenez-Delgadillo B, Munoz-Zanzi C, Cárdenas-Marrufo M, Pérez-Osorio C, Arias-León J (2018) Presence of Rickettsia Species in a Marginalized Area of Yucatan, Mexico. J Trop Med. 2018:7675828. https://doi.org/10.1155/2018/7675828
Premaratna R (2022) Rickettsial illnesses, a leading cause of acute febrile illness. Clin Med (Lond) 22(1):2–5. https://doi.org/10.7861/clinmed.2021-0790
Richardson EA, Roe RM, Apperson CS, Ponnusamy L (2023) Rickettsia amblyommatis in Ticks: a review of distribution, pathogenicity, and Diversity. Microorganisms 11(2):493. https://doi.org/10.3390/microorganisms11020493
Rodríguez Vivas RI, Ojeda Chi M, Bolio González M, Rosado Aguilar JA (2019) Las garrapatas como vectores de enfermedades zoonóticas en México. Bioagrociencias 12(1):19–26
Salceda-Sánchez B, Gasca-Zarate CM, Jiménez-Soto K, Grostieta E, López-Sánchez CG, Soto-Gutiérrez JJ, Lammoglia-Villagómez MA, Huerta-Peña J, Hernández-Carbajal GR, Chagoya-Fuentes JL, Jácome-Sosa E, Pérez-Brígido CD, Ballados-González GG, Becker I, Sánchez-Montes S (2023) Molecular detection of Rickettsia felis in fleas and ticks collected from dogs and cats of Puebla, Mexico. Zoonoses Public Health 70(2):176–183. https://doi.org/10.1111/zph.13011
Salomon J, Fernandez Santos NA, Zecca IB, Estrada-Franco JG, Davila E, Hamer GL, Rodríguez Pérez MA, Hamer SA (2022) Brown Dog Tick (Rhipicephalus sanguineus Sensu Lato) infection with endosymbiont and human pathogenic Rickettsia spp., in northeastern México. Int J Environ Res Public Health 19(10):6249. https://doi.org/10.3390/ijerph19106249
Sánchez-Montes S, Colunga-Salas P, Lozano-Sardaneta YN, Zazueta-Islas HM, Ballados-González GG, Salceda-Sánchez B, Huerta-Jiménez H, Torres-Castro M, Panti-May JA, Peniche-Lara G, Muñoz-García CI, Rendón-Franco E, Ojeda-Chi MM, Rodríguez-Vivas RI, Zavala-Castro J, Dzul-Rosado K, Lugo-Caballero C, Alcántara-Rodríguez VE, Delgado-de la Mora J, Licona-Enríquez JD, Delgado-, de la Mora D, López-Pérez AM, Álvarez-Hernández G, Tinoco-Gracia L, Rodríguez-Lomelí M, Ulloa-García A, Blum-Domínguez S, Tamay-Segovia P, Aguilar-Tipacamú G, Cruz-Romero A, Romero-Salas D, Martínez-Medina MA, Becker I (2021) The genus Rickettsia in Mexico: Current knowledge and perspectives. Ticks Tick Borne Dis 12(2):101633. https://doi.org/10.1016/j.ttbdis.2020.101633
Sebastian PS, Winter M, Abate SD, Tarragona EL, Nava S (2022) Molecular detection of Candidatus Rickettsia andeanae and Ehrlichia sp. in Amblyomma pseudoconcolor Aragão, 1908 (Acari: Ixodidae) from the argentinian Patagonia. Animals 12(23):3307. https://doi.org/10.3390/ani12233307
Snellgrove AN, Krapiunaya I, Scott P, Levin ML (2021) Assessment of the pathogenicity of Rickettsia amblyommatis, Rickettsia bellii, and Rickettsia montanensis in a Guinea Pig Model. Vector Borne Zoonotic Dis 21(4):232–241. https://doi.org/10.1089/vbz.2020.2695
Swei A, Couper LI, Coffey LL, Kapan D, Bennett S (2020) Patterns, drivers, and Challenges of Vector-Borne Disease Emergence. Vector Borne Zoonotic Dis 20(3):159–170. https://doi.org/10.1089/vbz.2018.2432
Tamura K, Stecher G, Kumar S (2021) MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol 38(7):3022–3027. https://doi.org/10.1093/molbev/msab120
Torres-Castro M, Reyes-Novelo E, Noh-Pech H, Sánchez-Montes S, Colunga-Salas P, Lugo-Caballero C, Rodríguez-Vivas RI (2022a) Rickettsia rickettsii and Rickettsia typhi in inhabitants from a rural community of southeast Mexico. Rev Peru Med Exp Salud Publica. 39(1):124–125. https://doi.org/10.17843/rpmesp.2022.391.10519
Torres-Castro M, Reyes-Novelo E, Bolio-González M, Lugo-Caballero C, Dzul-Rosado K, Colunga-Salas P, Sánchez-Montes S, Noh-Pech H, Puerto FI, Rodríguez-Vivas RI (2022b) Epidemiological study of the occurrence of Typhus Group Rickettsia Natural infection in Domiciliated Dogs from a Rural Community in South-Eastern Mexico. Anim (Basel) 12(20):2885. https://doi.org/10.3390/ani12202885
Ulloa-García A, Dzul-Rosado K, Bermúdez-Castillero SE, López-López N, Torres-Monzón JA (2020) Detección de Rickettsia typhi en Rhipicephalus sanguineus s.l. y Amblyomma mixtum en el sur de México [Detection of Rickettsia typhi in Rhipicephalus sanguineus s.l. and Amblyomma mixtum in South of Mexico]. Salud Publica Mex. 62(4):358–363. https://doi.org/10.21149/10160. PMID: 32549080
Yen WY, Stern K, Mishra S, Helminiak L, Sanchez-Vicente S, Kim HK (2021) Virulence potential of Rickettsia amblyommatis for spotted fever pathogenesis in mice. Pathog Dis 79(5):ftab024. https://doi.org/10.1093/femspd/ftab024
Acknowledgements
We would like to thank the owners of the dogs and their families for their support and permission to check their beloved pets. We also would like to thank members of our respective laboratories for their invaluable support. Specially to Javier Solís, Jose Hernández, and Sofía Luna (Laboratorio de Genética Microbiana at ENCB), and Martha Guadalupe Zacarias Pérez (Universidad Autónoma de Yucatán). We are also in great debt to both reviewers for all their critical comments and suggestions.
Funding
This work was supported by Secretaría de Investigación y Posgrado del Instituto Politécnico Nacional (SIP-IPN) (grant numbers 2022 − 0679 and 2023 − 0850).
Author information
Authors and Affiliations
Contributions
Conceptualization: JAI, GCR-S, EV-G; Methodology: EV-G, GCR-S, AEC-R, CM-W, ML-G, PE-DLS, GA-H; Formal analysis and investigation: JAI, GCR-S, EV-G, GA-H; Writing - original draft preparation: JAI, EV-G, GCR-S; Funding acquisition: JAI, GCR-S; Resources: EV-G, GCR-S, AEC-R, CM-W, ML-G, PE-DLS, GA-H; Software: PE-DLS; Supervision: JAI, GCR-S, GA-H; Validation: PE-DLS, GA-H, ML-G; Visualization: JAI, GCR-S, EV-G; Writing - review and editing: EV-G, GCR-S, AEC-R, CM-W, ML-G, PE-DLS, GA-H, JAI.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vázquez-Guerrero, E., Reyes-Solís, G.C., Cano-Ravell, A.E. et al. Detection of Rickettsia amblyommatis and Rickettsia bellii in ticks collected from pet dogs in peri-urban and rural areas in Yucatan, Mexico. Exp Appl Acarol 90, 441–453 (2023). https://doi.org/10.1007/s10493-023-00825-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-023-00825-z