Abstract
Predatory mite species (Acari: Phytoseiidae) are essential tools in the biological control of greenhouses pests. The natural enemies can be released directly into a crop. A better, partly preventive system is to place slow-release sachets on the plants. Inside such sachets is a factitious prey’s food substrate—which also acts as refuge—and the predator. The objective of this study was to develop a new methodology to evaluate the population dynamics of this sachet system, based on the factitious prey Carpoglyphus lactis and the predatory mite Amblyseius swirskii. Through two tests carried out under laboratory conditions, the sachets were first compared to the traditional extraction method that uses Berlese-Tullgren funnels and an extraction method using flotation in hexane. The latter method proved more effective at sampling the motile states (larvae, nymphs, and adults), both for the predatory species and for the factitious prey, extracting up to 3.7 × more mites than the Berlese-Tullgren funnel. Second, the population dynamics of both mite species was studied in a laboratory test, both inside and outside the sachets. In this way, a positive correlation was demonstrated between the number of predatory mites and the number of prey mites inside the sachets. Conversely, no correlation was found between the interior population of predatory mites and the number that venture outside. We can conclude that hexane extraction is very useful both in quality control of predatory mites and in studying how the sachets behave when faced with various factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The worldwide use of predatory mites (mainly the family Phytoseiidae) constitutes one of the basic pillars of integrated pest management in greenhouse crops (Knapp et al. 2018). There are several commercial formulations for manipulating and releasing them in crops, all of which have food substrate for factitious prey (usually astigmatid mites) and/or carrier substances (Vila and Cabello 2014).
One of the factors determining the success of a biological control programme is the quality of the predators supplied by the manufacturers (Vila and Cabello 2014). In addition to biological parameters, such as, among others, fecundity, sex ratio, and search capacity, the number of predatory mites and prey present in the substrate is very important to the success of the control used in the crop (van Lenteren et al. 2003). Releases made with low-quality material, or with fewer individuals than necessary, lead to failures and uncertainty in the augmentative biological control (O’Neil et al. 1998; van Lenteren et al. 2003).
Detailed guidelines for various species of natural enemies have been developed by the International Organization for Biological Control and are used worldwide for the quality control of phytoseiid mites (van Lenteren et al. 2003). Thresholds for quantity, longevity, sex ratio and fecundity are included in these guidelines to set the requisite quality standards in certain mite species of the family Phytoseiidae. Regarding the quantification of mites present in commercial substrate, three methodologies are detailed for four species: Neoseiulus cucumeris (Oudemans), Iphiseius degenerans (Berlese), Phytoseiulus persimilis Athias-Henriot, and Neoseiulus californicus (McGregor). Quantifications are performed on the living material by hot water sieving (N. californicus), direct counting (I. degenerans and P. persimilis) and the Berlese-Tullgren funnel technique (N. cucumeris).
On the other hand, since 2005, the marketing of Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae) and its implementation in biological control programmes has represented a great advance in the treatment of protected crops in Europe (Calvo et al. 2015). The application of mites on greenhouse crops is performed using mechanical blowers or slow-release sachets (Midthassel et al. 2014; Vila and Cabello 2014; Calvo et al. 2015). These sachets mainly contain a wheat bran and other food sources are present, such as yeast and wheat germ. The wheat bran acts mainly as carrier material (Vila and Cabello 2014; Calvo et al. 2015).
These types of sachets allow the phased dispersion of the predator mites from the sachets to the crop, especially important when there is no alternative food source (usually pollen) other than the target pest available for crop colonisation by predator. However, the predator–prey dynamics inside the sachets can be affected by various factors that might impact the success of the biological control. Intrinsic factors include the spatial complexity between the substrate, the quality of the prey’s feed substrate, the initial predator–prey density, and the intra- and inter-specific interactions (Midthassel et al. 2014). Extrinsically, the mite populations can be affected by environmental conditions, such as temperature, humidity, water availability and phytosanitary treatments (Ghazy et al. 2016; Shimoda et al. 2017; San et al. 2021).
The aim of the present work was to develop a system that could be more adequate and timesaving to assess the populations of the prey mite Carpoglyphus lactis (Acari: Carpoglyphidae) and the predatory mite A. swirskii in the formulations currently available in slow-release sachets.
Materials and methods
Biological material
Swirski ulti-mite© (Koppert, Almeria, La Mojonera, Spain) slow-release sachets with a 1.85 ± 0.15 g net weight were used, containing A. swirskii and C. lactis mites, in all life stages, and wheat bran as the main dispersion and feeding element of the prey (C. lactis). The material was used within 24 h of reception and following the handling instructions given by the company, to avoid decrease of quality. Two motile mite extraction methods were used, as described below.
Extraction methods
Extraction based on the Berlese-Tullgren funnel
The methodology proposed by Kim et al. (2001) and van Lenteren et al. (2003) was followed for N. cucumeris, and that proposed by Lopez and Smith (2016) for A. swirskii. To do this, the contents of the sachets were homogenized carefully with a spoon for 1 min and then 0.5 g samples were taken. The samples were put on a 6-cm-diameter sieve with a 750-µm mesh and successively placed at two heights under a 60-W incandescent bulb to achieve an ascending temperature ramp. The initial moisture content of the sachets was 23.07 ± 0.01%; hence, prior to the tests, the times and the optimal distance at which the sieve was placed with respect to the bulb were determined to achieve the greatest possible extraction, as sachet moisture contents greater than 16.5–19% require more exposure time (van Lenteren et al. 2003). The sieve was set at a distance of 20 cm from the bulb for 10 min, then it was placed at 10 cm for 5 min. The motile mites (predators and prey) that fell through the sieve were collected on a plate containing a liquid soap film and counted under a binocular magnifying glass (larvae, nymphs, and adults). The number of replications was four sachets, taken at random from the same commercial batch.
Extraction by flotation in hexane
Hexane extraction was performed by modifying the methodology of Geurs et al. (1991) proposed for the extraction of microarthropods in soil samples using heptane flotation. To do this, after homogenizing the sachet, a 0.5-g sample was taken and divided into 4 sub-samples of 0.125 g each. Separation by hexane (Hexanux Nazza, Industrias Químicas Eurotex, El Viso del Alcor, Seville, Spain) was carried out in three phases. In phase I, the material was fixed with 20 ml of 96° alcohol; then, 20 ml distilled water was added to create an aqueous phase along with a drop of Triton X-100 (A4975, Panreac Quimica, Barcelona, Spain) to decrease the surface tension and facilitate the bran deposition at the bottom of the beaker, as well as to prevent the formation of fat drops from the bran. In phase II, 20 ml hexane was added and stirred with a spoon to facilitate the disconnecting of mites that are trapped in the bran fragments. In phase III, the mites were extracted at the interface between the hexane and the alcohol and water using a glass pipette, after which the motile A. swirskii and C. lactis mites were counted under a binocular magnifying glass. Specimens that showed symptoms of dehydration and/or collapsed were not counted. The number of replications was four sachets, taken at random from the same commercial batch.
Population dynamics of mites inside the sachet and their release to the outside
Variation in the motile forms of the mites was evaluated by sampling the individuals present both outside and inside the sachets. For the external evaluation, the methodology of Shipp and Wang (2003) was modified by placing the sachets on wooden sticks (8 cm high) punctured into the centre of gummed plates (20 × 24 cm) that had a rectangle of ungummed dark paper (5 × 7 cm) and a Vaseline edge around the plate to prevent possible escape of the arena. The plates were changed on days 3, 7, 13 and 21, and the moving mites present on the plate were counted using a binocular magnifying glass. These represented the releases from the sachets. To count the population inside the sachets, destructive sampling was performed after positioning the sachets (T = 0 days) and on the same days as the external population was determined. To do this, the number of mites present inside four sachets for each day of sampling was quantified following the hexane method.
The test was conducted in a ICP 600 climate chamber (Memmert, Schwabach, Germany) at 25 ± 1 °C, 60–70%RH and L16:D8 photoperiod.
Statistical analysis
In the extraction method assay, the experimental design was univariate totally randomized with only one factor or treatment (with sub-sampling per sachets). This factor had two levels: extraction by Berlese-Tullgren and extraction by flotation in hexane. The total number of motile mites within the sachets, extracted using the two methods, was analysed using Generalized Linear Models with the gamma distribution and the logarithmic link function, since these have been reported as the best approach for describing the population size (Dennis and Costantino 1998; Benton et al. 2002). The mean values were compared using the Wald test.
In the population dynamics assay, the relationships between the motile forms of the predator and the prey, inside and outside the sachets, were determined using Pearson’s correlation coefficient. In both cases, the analyses were carried out with IBM SPSS v.26 statistical software.
Results
Extraction methods
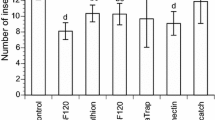
Figure 1 shows the number of motile mites extracted, their different states and species, using the two methods. In the statistical analyses, the models were significantly adjusted to the data according to the Omnibus tests in all the categories analysed and highly significant statistical differences were found between the two methods (Table 1). More motile individuals, both adult and immature of both species, were extracted by hexane flotation than by the Berlese-Tullgren funnel (Fig. 1).
Population dynamics of mites inside the sachet and their release to the outside
Figure 2 shows the number of motile immature and adult A. swirskii and C. lactis present inside the sachets throughout the sampling period. The maximum number of motile forms of A. swirskii inside the sachets was reached after 7 days (Fig. 2A), whereas the maximum population of C. lactis was recorded on the 3rd day (Fig. 2B). In both cases, the number of immature forms was higher than that of adults. Compared to the start of the test, a gradual decrease was recorded in the number of adults of both species present inside the sachets (Fig. 2). Inside the sachet, the initial predator–prey ratio was 1:4.9 for the first 3 days, after which it gradually decreased until day 13, when they were practically equal (1:1.2). At 21 days, the populations of both species inside the sachet were close to 0 under the conditions tested.
Mean (± SE; n = 4 for each sampling day) number of motile immature, adult and total A Amblyseius swirskii and B Carpoglyphus lactis mites present inside the slow-release sachets under controlled environmental conditions as a function of time since start of the sampling. Note the differences in scale on the vertical axes
The number of A. swirskii and C. lactis individuals coming out of the sachets are shown in Fig. 3. It has been established that the cumulative number of predators over the test period exceeded the product specifications for use in crops (325.25 ± 50.76 vs. 250 predators/sachet). The maximum number of predators leaving the sachets occurred between days 7 and 13 (approximately 33 predators per day and per sachet) (Fig. 3A). With regard to C. lactis, it was observed that it is mostly the motile immature states leaving the sachets, and this occurs during the first 7 days, decreasing practically to 0 over the following days (Fig. 3B).
Mean (± SE; n = 4) number of motile immature, adult, and total A Amblyseius swirskii and B Carpoglyphus lactis mites exiting the slow-release sachets under controlled environmental conditions, accumulated at the time intervals indicated on the x-axis. Note the differences in scale on the vertical axes
Regarding the interior contents of the sachets, Pearson’s correlation coefficients revealed that the number of A. swirskii motile immature was positively correlated with the number of immature prey (C. lactis) (Table 2). The Pearson’s correlation coefficient was highly significant regarding the number of A. swirskii adults to adult prey (C. lactis) inside the sachets. On the other hand, the number of motile immature predators that left the sachets was negatively correlated with the interior content of C. lactis adults, and positively correlated with the number of motile immature predators present inside the sachets (Table 2). The number of adult predators that left the sachets was negatively correlated with the number of C. lactis immature motile and adult contents inside the sachet. Conversely, this correlation was positive with respect to the number of immature co-specifics that came out of the sachets (Table 2).
Discussion
The hexane flotation method extracted a higher number of both motile immature and adult A. swirskii and C. lactis mites than the method based on the Berlese-Tullgren funnel proposed for N. cucumeris (Kim et al. 2001; van Lenteren et al. 2003). Moreover, this new application has allowed us to verify the extraction far more rapidly and to dissociate the sampling over time with the extraction of the mites following their fixation. This enables mite populations to be studied in detail when a large number of sachets need to be analysed simultaneously, as is the case in biofactory quality control and/or in field tests. However, it should be noted that this technique does not allow the extraction of mite eggs.
This is not the first time that the affinity between arthropod cuticles and petroleum derivatives has been used to extract them from different media; indeed, it has been successfully used in diverse substrates such as soils, debris, stream substrates, moss and leaves (Barmuta 1984; Walters et al. 1987; Geurs et al. 1991; Kethley 1991; Belascoain et al. 1998; Andrew and Rodgerson 1999; Proctor 2001; Faraji et al. 2004; Rolland and Laroque 2007; Harris et al. 2017). In our case, hexane flotation extracted 3.7 × the total number of motile mites in a totally organic medium composed almost entirely of wheat bran (Fig. 1) compared to the conventional method based on the Berlese-Tullgren funnel. Similar values (up to 4.1 × more individuals) were obtained by Walter et al. (1987) in soil samples employing the same principle but using heptane flotation versus a Macfadyen-type funnel with a temperature gradient. A greater quantity and diversity of mites and other arthropods was obtained in stream substrates and debris samples when comparing flotation in kerosene versus direct extraction under a dissection microscope (Barmuta 1984; Proctor 2001) and even in cephalic capsules of chironomid dipterans (Rolland and Laroque 2007). Extraction has also been higher in other arthropods, such as springtails (Collembola) and Diptera, when this technique is compared to sugar flotation (based on density differences) (Andrew and Rodgerson 1999). Only Harris et al. (2017) reported a lower rate of mite recovery from a sample when using paraffin to extract Tetranychus urticae Koch (Acari: Tetranychidae) from apple and cherry leaves compared to other methods, including the Berlese-Tullgren funnel.
From the biological control standpoint, the dispersion rate of A. swirskii from the inside of the sachet to the crop is the reason for using this release technique. In predator–prey mite systems, two types of dispersal strategies can be distinguished: the ‘killer-strategy’ in which the predators remain in the ‘patch’ while prey are available, and the ‘milker-strategy’ in which predators disperse from the patch at a constant rate while the interaction lasts (Pels and Sabelis 1999). Furthermore, it is assumed that the predator emigration rate decreases as a function of prey density and is also influenced by the increased density of predators in the patch (Eveleigh and Chant 1982; Bernstein 1984). In the context of slow-release sachets in controlled conditions, if one compares the predator and prey populations, the maximum number of prey (motile forms) inside the sachet was recorded on day 3, from which point the population of C. lactis gradually decreased (Fig. 2B), whereas the maximum predator population inside the sachet was recorded on day 7 (Fig. 2A). With regard to the predator population leaving the sachet, the largest number of A swirskii exits was recorded within 5 days of recording the maximum number inside the sachet, most being adult forms (Fig. 2). Based on these results, A. swirskii might use a strategy similar to the ‘killer’ type, as supported by the negative correlation found between the density of adult predators present in the slow-release sachets and the proportion of the population that went outside (Table 2). However, this should be viewed with caution as there may be differences among populations within a species (Pels and Sabelis 1999; Revynthi et al. 2018). Despite the above, it should be noted that a sachet such as the one we used, cannot exactly be considered a ‘patch’ due to the dispersion limitations that the sachet presents, having a single exit hole. On the other hand, the predator–prey relationship is not the only dispersion factor; at the individual level, there are numerous local conditioners and interactions that influence dispersion, as documented by Revynthi et al. (2018), all of which might influence the predator dispersion rate. Likewise, the sachets do not provide a watertight system; they will suffer variations in temperature, moisture content and relative humidity depending on the external environmental conditions. These physical factors are the most important influencing the development of mite populations (Collins 2012), affecting the dispersion rate from inside the sachet.
Regardless of the population fluctuations that occur inside the sachet, it was observed that the initial number of predators packaged in the biofactory was much higher than the total number leaving the sachet during the trial (1216.67 ± 94.50 A. swirskii individuals packaged in the biofactory compared to a total of 325.25 ± 50.76 predators leaving the sachet under the test conditions) and the amount of A. swirskii indicated by the manufacturer. These data suggest that the sachet performance may be improved. Given the complexity of comparing the inner content of the sachets and the number of predators that emerge, future research is required that investigates the most influential variables and that can model these interactions in a way that allows their behaviour to be explained.
In general, it has been shown that the extraction methods must be effective in recovering most of the population, be efficient in extracting all the species and states present and be capable of rapidly producing clean samples that allow arthropod identification (McSorley and Walter 1991). Another desirable feature is that they allow the indefinite storage of the samples prior to counting, as with this new proposed methodology. Considering all that is mentioned above, we believe that the hexane flotation extraction methodology proposed in this work offers an important advantage over dynamic and direct extraction methods, especially in the context of slow-release sachets used in biological control programmes.
Data availability
Raw data will be made available upon request.
References
Andrew N, Rodgerson L (1999) Extracting invertebrates from bryophytes. J Insect Conserv 3:53–55. https://doi.org/10.1023/A:1009682523054
Barmuta LA (1984) A method for separating benthic arthropods from detritus. Hydrobiologia 112:105–107. https://doi.org/10.1007/BF00006913
Belascoain C, Ariño AH, Jordana R (1998) A new integrated extraction method for microarthropods and nematodes from the same soil samples. Pedobiologia 42:165–170
Benton TG, Lapsley CT, Beckerman AP (2002) The population response to environmental noise: population size, variance and correlation in an experimental system. J Anim Ecol 71:320–322. https://doi.org/10.1046/j.1365-2656.2002.00601.x
Bernstein C (1984) Prey and predator emigration responses in the acarine system Tetranychus urticae-Phytoseiulus persimilis. Oecologia 61:134–142. https://doi.org/10.1007/BF00379099
Calvo FJ, Knapp M, van Houten YM, Hoogerbrugge H, Belda JE (2015) Amblyseius swirskii: what made this predatory mite such a successful biocontrol agent? Exp Appl Acarol 65(4):419–433. https://doi.org/10.1007/s10493-014-9873-0
Collins DA (2012) A review on the factors affecting mite growth in stored grain commodities. Exp App Acarol 56:191–208. https://doi.org/10.1007/s10493-012-9512-6
Dennis B, Costantino RF (1998) Analysis of steady-state populations with the gamma abundance model: application to Tribolium. Ecology 69:1200–1213. https://doi.org/10.2307/1941275
Eveleigh ES, Chant DA (1982) Experimental studies on acarine predator-prey interactions: the effects of predator density on prey consumption, predator searching efficiency, and the functional response to prey density (Acarina: Phytoseiidae). Can J Zool 60:611–629. https://doi.org/10.1139/z82-091
Faraji F, Bruin J, Bakker F (2004) A new method for mite extraction from leaf samples. Exp Appl Acarol 32:31–39. https://doi.org/10.1023/B:APPA.0000018227.71296.65
Geurs M, Bongers J, Brussaard L (1991) Improvements of the heptane flotation method for collecting microarthropods from silt loam soil. Agric Ecosyst Environ 34:213–221. https://doi.org/10.1016/0167-8809(91)90108-A
Ghazy NA, Osakabe M, Negm MW, Schausberger P, Gotoh T, Amano H (2016) Phytoseiid mites under environmental stress. Biol Control 96:120–134. https://doi.org/10.1016/j.biocontrol.2016.02.017
Harris AL, Ullah R, Fountain MT (2017) The evaluation of extraction techniques for Tetranychus urticae (Acari: Tetranychidae) from apple (Malus domestica) and cherry (Prunus avium) leaves. Exp Appl Acarol 72:367–377. https://doi.org/10.1007/s10493-017-0154-6
Kethley J (1991) A procedure for extraction of microarthropods from bulk soil samples with emphasis on inactive stages. Agric Ecosyst Environ 34:192–200. https://doi.org/10.1016/0167-8809(91)90105-7
Kim JH, Broadbent AB, Lee SG (2001) Quality control of the mass-reared predatory mite, Amblyseius cucumeris (Acarina: Phytoseiidae). J Asia-Pacific Entomol 4:175–179. https://doi.org/10.1016/S1226-8615(08)60120-X
Knapp M, van Houten Y, van Baal E, Groot T (2018) Use of predatory mites in commercial biocontrol: current status and future prospects. Acarologia 58:72–82. https://doi.org/10.24349/acarologia/20184275
Lopez L, Smith HA (2016) Quality assessment of the commercially available predator Amblyseius swirskii (Acari: Phytoseiidae). Plant Health Progress 17(3):206–210. https://doi.org/10.1094/PHP-RS-16-0040
McSorley R, Walter DE (1991) Comparison of soil extraction methods for nematodes and microarthropods. Agric Ecosyst Environ 34:201–207. https://doi.org/10.1016/0167-8809(91)90106-8
Midthassel A, Leather SR, Wright DJ, Baxter IH (2014) The functional and numerical response of Typhlodromips swirskii (Acari: Phytoseiidae) to the factitious prey Suidasia medanensis (Acari: Suidasiidae) in the context of a breeding sachet. Biocontrol Sci Technol 24:361–374. https://doi.org/10.1080/09583157.2013.863270
O’Neil RJ, Giles KL, Obrycki JJ, Mahr DL, Legaspi JC, Katovich K (1998) Evaluation of the quality of four commercially available natural enemies. Biol Control 11(1):1–8. https://doi.org/10.1006/bcon.1997.0570
Pels B, Sabelis MW (1999) Local dynamics, overexploitation and predator dispersal in an acarine predator–prey system. Oikos 86:573–583. https://doi.org/10.2307/3546662
Proctor HC (2001) Extracting aquatic mites from stream substrates: a comparison of three methods. Exp Appl Acarol 25:1–11. https://doi.org/10.1023/A:1010677700404
Revynthi AM, Egas M, Janssen A, Sabelis MW (2018) Prey exploitation and dispersal strategies vary among natural populations of a predatory mite. Ecol Evol 8:10384–10394. https://doi.org/10.1002/ece3.4446
Rolland N, Larocque I (2007) The efficiency of kerosene flotation for extraction of chironomid head capsules from lake sediments samples. J Paleolimnol 37:565–572. https://doi.org/10.1007/s10933-006-9037-2
San PP, Tuda M, Takagi M (2021) Impact of relative humidity and water availability on the life history of the predatory mite Amblyseius swirskii. Biocontrol 66:497–510. https://doi.org/10.1007/s10526-021-10081
Shimoda T, Kagawa Y, Mori K, Hinomoto N, Hiraoka T, Nakajima T (2017) A novel method for protecting slow-release sachets of predatory mites against environmental stresses and increasing predator release to crops. Biocontrol 62:495–503. https://doi.org/10.1007/s10526-017-9800-5
Shipp JL, Wang K (2003) Evaluation of Amblyseius cucumeris (Acari: Phytoseiidae) and Orius insidiosus (Hemiptera: Anthocoridae) for control of Frankliniella occidentalis (Thysanoptera: Thripidae) on greenhouse tomatoes. Biol Control 28:271–281. https://doi.org/10.1016/S1049-9644(03)00091-4
van Lenteren JC, Hale A, Klapwijk JN (2003) Guidelines for quality control of commercially produced natural enemies. In: van Lenteren JC (ed) Quality control and production of biological control agents. Theory and testing procedures. CAB International, Wallingford, pp 265–303
Vila E, Cabello T (2014) Biosystems engineering applied to greenhouse pest control. In: Torres I, Guevara R (eds) Biosystems engineering: biofactories for food production in the XXI Century. Springer, Berlin, pp 99–128
Walter DE, Kethley J, Moore JC (1987) A heptane flotation method for recovering microarthropods from semiarid soils, with comparison to the Merchant-Crossley hrgh-gradient extraction method and estimates of microarthropod biomass. Pedobiologia 30:221–232
Acknowledgements
We would like to thank J.E. Belda (Kopper Biological System, Spain) for kindly providing the biological materials and supplies.
Funding
This research was funded by the Junta de Andalucia, (Spain), grant number A1122062E0: AgroMIS Project: ceiA3 strategic instrument towards a Modern, Innovative and Sustainable Agrifood productive fabric: driving force of the Andalusian rural territory. The funding bodies played no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
JRG, BT, YSR and TC conceived and designed the experiments. BT and YSR performed the experiments. JRG, MG, and TC analysed the data. JRG and TC wrote the manuscript. TC acquired funding and supervised the work. All the authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gallego, J.R., Solano-Rojas, Y., Tiseyra, B. et al. Population dynamics of mites in slow-release sachets used in biological control: a new study methodology. Exp Appl Acarol 87, 325–335 (2022). https://doi.org/10.1007/s10493-022-00739-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-022-00739-2