Abstract
Tetranychus urticae is a widespread polyphagous mite, found on a variety of fruit crops. Tetranychus urticae feeds on the underside of the leaves perforating plant cells and sucking the cell contents. Foliar damage and excess webbing produced by T. urticae can reduce fruit yield. Assessments of T. urticae populations while small provide reliable and accurate ways of targeting control strategies and recording their efficacy against T. urticae. The aim of this study was to evaluate four methods for extracting low levels of T. urticae from leaf samples, representative of developing infestations. These methods were compared to directly counting of mites on leaves under a dissecting microscope. These methods were ethanol washing, a modified paraffin/ethanol meniscus technique, Tullgren funnel extraction and the Henderson and McBurnie mite brushing machine with consideration to: accuracy, precision and simplicity. In addition, two physically different leaf morphologies were compared; Prunus leaves which are glabrous with Malus leaves which are setaceous. Ethanol extraction consistently yielded the highest numbers of mites and was the most rapid method for recovering T. urticae from leaf samples, irrespective of leaf structure. In addition the samples could be processed and stored before final counting. The advantages and disadvantages of each method are discussed in detail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tetranychus urticae (Acari: Tetranychidae) is a widespread polyphagous mite, found on economically important fruit crops including walnut, strawberry, blackcurrant, gooseberry, raspberry, apple, pear and plum amongst others (Alford 2007). Tetranychus urticae feeds on the underside of the leaves perforating the cells of the plant and sucking the cell contents. The direct impact of T. urticae feeding is reduction in fruit yield (number, size or quality of fruit) due to reduced photosynthetic ability of the plants (Wise et al. 1999). In addition, webbing produced by T. urticae can thus contaminate fruit making it uneconomical to harvest.
For the implementation of biological control techniques to be successful populations of T. urticae need to be detected whilst numbers are low (two mites per leaf action threshold). These levels are such that direct observations in the field can easily miss this level of infestation. Whereas mature infestations are easy to spot due to the conspicuous webbing, but these established populations are difficult to control. Reliable assessments of mite populations provide an accurate way of assessing initial levels of infestation and evaluating the efficacy of pesticide, mechanical or biological control treatments. This is particularly important as T. urticae has developed resistance to commonly used pesticides (Van Leeuwen et al. 2010). A robust method for the extraction of T. urticae from leaf samples may also be of benefit in that it may allow the direct detection of predatory mites used in the control of T. urticae.
The aim of this study was to determine which mite extraction technique would maximize the number of mites recovered from field collected samples where mites were present but not yet manifesting symptoms of infestation, with consideration to simplicity, efficiency and accuracy. The four methods evaluated are those most commonly used by the scientific community for convenient extraction of small organisms like mites from substrate. Tullgren funnel extraction (Tullgren 1918) uses heat and light to drive mites from the leaves and into a collecting vessel. It relies on the supposed migration of mites due to phototropism (Krantz 1978). However, Nef (1971) observed that Oribatida mites migrate through the sample due to decreasing substrate humidity.
Ethanol washing, first suggested by Jones and Prendergast (1937), produced reliable estimates of mites (e.g., Hossain 1992). Repeatedly rinsing and washing the leaves in ethanol provided clean samples for easy mite identification.
In a relatively new method developed by Faraji et al. (2004) the mites remain suspended by their lipophilic cuticle in the meniscus between a layer of paraffin and ethanol. A benefit of this system is the lack of contamination by leaf remnants. It has been successful on several mite species other than T. urticae (e.g., Faraji et al. 2004). The potential for this method to remove T. urticae from leaves in a clean and efficient manner make this method attractive. However, there are several difficulties in the application of this method as it requires the manufacture of a specialist counting dish which is not readily available. If it were possible to modify this method to use readily available equipment it could become a useful method.
Mite brushing has proved very effective at removing mites that accumulate on the underside of leaves in the crevices adjacent to the midrib and lateral veins of the leaf (Henderson and McBurnie 1943). However, leaf remnants contaminate brushing samples, reducing the ease and reliability of mite identification (Morgan et al. 1955).
All of these methods were compared to direct counting of mites on leaf samples under a dissecting microscope, with the aim of establishing which technique is the most efficient and robust for the collection of population data of T. urticae from Malus and Prunus leaves.
Materials and methods
Sample preparation
Low levels of T. urticae are difficult to detect in the field, and could result in highly variability of mite numbers between samples, because of this it was decided to use leaves from trees that were thought to be mite free due to their history of pesticide applications and to then artificially infest them with T. urticae.
Four hundred and eighty Malus (apple) leaves (cv. Jupiter) and Prunus (cherry) leaves (cv. Sweetheart) were collected into polyethylene bags from orchards at NIAB EMR, Kent, UK, on 21 July 2014 for experiment 1 and on 28 July 2014 for experiment 2. Leaves were selected based on their size, shape, age and level of damage so that all the leaves within the sample were uniform. Leaves were not picked from the same branch to ensure that leaves were fully representative of the tree.
The 480 Malus leaves were randomly distributed into 24 polythene sample bags, each with 20 leaves. The same was repeated with the Prunus leaves. Runner bean leaves of the same size and age were used to inoculate the samples. The inoculum leaves were collected from a glasshouse culture of runner bean plants heavily infested with T. urticae. The inoculum leaves were collected into a single bag and the population of mites allowed 24 h to distribute evenly over the bean leaves. Each sample of 20 fruit tree leaves was then artificially inoculated with a bean leaf. The true number of mites present on the experimental leaves was never know. It would have been interesting to have inoculated all leaves manually with a standardized number of mites to compare the accuracy across all extraction methods and leaf types, especially since there is interest in evaluating the performance of extraction techniques when there are naturally low levels of T. urticae.
The bags were securely sealed with tape to ensure that no mites escaped and left for 48 h at a fixed temperature of 20 °C. This ensured that the mites were given sufficient time to distribute themselves over the leaf samples. After inoculation, all bags were randomly assigned to the different extraction methods to ensure that six Prunus and six Malus replicates were used for each treatment. The bean leaf was kept in the samples during extraction for experiment 1. The bean leaf was removed after 48 h in experiment 2. Each mite extraction technique was repeated 4× (twice on Prunus leaves and twice on Malus).
Extraction methods
Four methods commonly used for the collection of T. urticae from leaf samples were evaluated and compared to direct counting. In experiment 1 the inoculation bean leaf was left in the sample for processing, as it was unknown whether the mites would successfully migrate from it to the sample leaves. Based on the results from experiment 1 it was found that the mites did migrate between the species they were reared on and the leaves of the experimental sample. The physical structure of the bean leaf is very different to that of either the Malus or Prunus leaves and may behave differently when processed, i.e., be more friable and so contaminate the sample with irrelevant bean been leaf fragments, because of this in experiment 2 the bean leaf was removed.
Ethanol washing
Ethanol washing was developed for extracting T. urticae from samples of strawberry leaves by Nordengen and Klingen (2006), adapted from work done by Newell (1947) and Hossain (1992). These methods were originally designed to use water and detergent, but this was replaced with 70% ethanol as it allows samples to be collected and stored for a longer period of time.
Samples were placed into 2-l plastic jars with 700 ml of 70% ethanol so that leaves were submerged. Mites become stiff if preserved at too high an ethanol concentration (Evans et al. 1961) and it was vital to adjust the size of the jar to the size of the sample as too small a container will not allow for free movement of leaves. The samples were then shaken thoroughly to dislodge mites from the leaves. Samples were left to soak for 24 and 2 h for experiments 1 and 2, respectively.
The samples were then shaken again to ensure no mites were clinging to the sides of the jar. The sample was tipped through a course sieve (2.5 mm), leaving the leaves in the jar, and removing small leaf fragments and other detritus that would otherwise contaminate the final sample. Three hundred ml of fresh 70% alcohol was added to the jar, shaken and the sieving process repeated. A further 300 ml was added and the process repeated again. Individual leaves were swirled around in the washing alcohol to dislodge any remaining mites. When all the leaves were washed, a small amount of fresh alcohol was used to rinse the original container and lid into the sample. The sample was now approximately 1.5 l of 70% ethanol.
The liquid was then passed through a 50-μm sieve leaving the mites on the mesh. Using fresh alcohol, the sample container was triple rinsed and the alcohol passed through the sieve. Using a wash bottle of 70% alcohol the sieve was gently washed back into the original sample container. The sample could now be stored if necessary.
The samples were prepared for examination by collecting the mites on black filter paper (Whatman International). The paper was divided in to 12 radial segments with a white chinagraph pencil to enable counting the mites. A Buchner funnel and vacuum pump were used to draw the liquid sample through the filter paper and collect the mites (Krantz 1978). Using a wash bottle of 70% alcohol the original sample container and lid were triple rinsed into the funnel. The prepared samples were then counted manually under a dissecting microscope.
Paraffin washing
The paraffin/ethanol meniscus technique was a slight modification of the technique developed by Faraji et al. (2004). The specialist counting vessel was not available. Two-litre plastic jars were filled with 1.5 l of water and 7.5 ml of detergent added to reduce the surface tension and allow the mites to sink. This setup ensured leaves were separated and thoroughly washed. Samples were left to soak for 24 h in experiment 1 and 2 h in experiment 2.
Samples were then vigorously shaken and swirled to ensure the leaves did not stick together and mites were washed off. After washing, each sample was shaken again to re-suspend the mites and passed through a course 2.5-mm sieve to remove leaf remnants and then through a fine 50-µm sieve to capture the mites. The leaves were washed with water. This rinsing technique was repeated 9×.
The sieve was then rinsed with 5 ml of ethanol using a wash bottle to transfer the mites from each sample into a test tube via a funnel. An equal amount of paraffin to the ethanol was then added along with two drops of methyl blue. Methyl blue is a dye that colours the ethanol without affecting the mites. This has the effect of improving mite recognition on the meniscus layer as white/green mites stand out from the dark blue.
The test tubes were then closed and shaken thoroughly and left to settle into two layers with paraffin at the top, ethanol at the bottom and the mites presented on the meniscus. Once the solutions had settled into two distinct layers (minimum 30 min), the samples were carefully transferred into Petri dishes and mites counted under a microscope. The Petri dish lids were divided into 12 segments with a permanent black marker to assist with counting.
Brushing
The brushing machine consisted of two contra-rotating brushes powered by a small electric motor which physically removes mites by repeated insertion of the leaves between the brushes. Detergent (3 ml) was added to a 12.7-cm-diameter glass disc and spread out to create a thin layer to capture the mites. This was placed under the leaf brushing machine (Henderson and McBurnie 1943) and each leaf from a sample was individually brushed 3× on each side, held by the petiole then repeated with the leaf held by the tip (e.g., Herbert and Butler 1973). This was to ensure that the lateral veins protected by the hairs of the midrib were thoroughly brushed because many mites accumulate in these specific regions of the leaf. This machine has proven effective at removing several species of mites (Morgan et al. 1955).
The mite brushing machine provided a target made up of 132 sections (comprising of 12 radial segments each sub divided in to 11 sections) to be placed under the glass slide (Morgan et al. 1955). The black and white segments aid counting. The drying of the detergent means these samples could not be preserved.
Tullgren
Each leaf sample (20 leaves) was placed into a Tullgren sieve and leaves were positioned centrally away from the edges of the sieve to prevent mites from escaping. The sieves (3 mm) were then placed over the funnels and an incandescent light of fixed intensity (25 W) was placed above each sample, so that each sample was exposed to the same conditions. The leaves were placed under heat and light for 48 h and a test tube of 10 ml of 70% ethanol was placed under each funnel. This method works uses heat and light to reduce the moisture levels in the leaves. The drying process forces the mites to migrate lower into the Tullgren funnel where they fall into the test tube. This method works only when the mites are alive.
The ethanol was then transferred to a Petri dish for mite counting under a microscope. The test tubes from the Tullgren funnel were rinsed with 70% ethanol to ensure that all mites are transferred into the Petri dish. A segmented Petri dish lid was used to assist mite counting with one segment being marked to denote the starting point as for the ethanol washing method.
Direct counting
Direct counting was used as a control to compare the efficacy of extraction of the other methods. Both sides of each leaf were viewed under a microscope. Most mites situated themselves on the undersides of the leaves and under the leaf hairs adjacent to the mid vein of the leaf. Fine forceps and a mounted needle were used to tease the mites out of their refugia in order to count them accurately. Mite eggs were not counted.
The technique for searching each leaf was as follows: the top part of the leaf was examined along the midrib twice and then once all around that side of the leaf. The underside was then extensively checked along the midrib and lateral veins and then elsewhere for thorough counting.
Data analysis
Data were subject to ANOVA, and pairwise multiple comparisons using Fisher’s protected least significant difference were used to determine which extraction technique(s), if any, provided a significantly higher recovery of mites from infested leaves. The count data met the assumptions of normality and equal variance and consequently required no transformation (GENSTAT v.13).
Results
Experiment 1
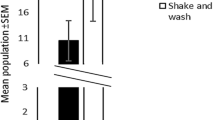
The mean number of mites on the control (direct counting) was 242 on Malus leaves. Ethanol extraction yielded significantly higher mean numbers of T. urticae (391) than all other methods including direct counting. The next most efficient treatment was brushing (283). The lowest numbers of T. urticae were extracted using the paraffin/ethanol meniscus technique (149) and the Tullgren funnel (154) (Fig. 1).
There were significant differences in mite numbers between the methods on Prunus (Fig. 2), with ethanol washing extraction with the highest numbers of mites (393). The brushing and Tullgren methods produced average mite counts of 248 and 221, respectively, and this result was similar to direct counting (250). Although there was variation in these three techniques, they were not statistically different. The paraffin/ethanol meniscus technique produced significantly lower mite counts (88) compared to all other extraction methods on Prunus leaves.
Experiment 2
The Paraffin/ethanol meniscus technique proved to be impractical without the specialist counting dish yielding low numbers of T. urticae from both Malus and Prunus leaves in the first experiment. The method could not be readily modified to improve its practicality, so was not used in the second experiment.
For this trial the bean leaf used to inoculate the Malus and Prunus samples with T. urticae was removed before the mite counts were made. In this experiment ethanol washing was not statistically different to brushing or direct counting in either Prunus or Malus (Fig. 3).
Mean (±SE; n = 6) number of Tetranychus urticae per 20 leaves for each extraction method on Malus and Prunus leaves. Different upper case letters indicate a significant difference between extraction methods (SED = 19.68, df = 30; P < 0.05). Different lower case letters indicate a significant difference between leaves types (SED = 8.09, df = 5; P < 0.05)
The Tullgren method produced significantly lower mite counts, particularly in Prunus (58), supported by the results from experiment 1. The reproducibility of results, regardless of the presence of the bean leaf strongly supports that the Tullgren method is not effective in collecting T. urticae from Malus and Prunus leaves.
In Prunus the highest mite counts were obtained by direct counting per 20 leaves (249), whereas brushing and ethanol washing were very similar with 174 and 179 mites on average, respectively, these were not significantly different from each other.
Malus leaves showed the lowest variation in mite counts as the mite counts ranged from 154 (Tullgren count) to 288 (ethanol washing). Direct counting (208) and mite brushing (252) were not significantly different from the ethanol washing.
For all methods there were significant differences between Malus and Prunus leaves, with significantly more mites being removed from Malus leaves with the brushing, ethanol washing and Tullgren funnel methods, whereas Prunus leaves yielded significantly more mites than Malus for direct counting.
Discussion
The aim of this study was to determine which mite extraction technique would maximize the number of mites recovered from field collected samples with consideration to simplicity, efficiency and accuracy on both Malus and Prunus leaves.
The Paraffin/ethanol meniscus extraction method consistently yielded low numbers of T. urticae. This was mainly caused by the samples remaining cloudy and the lack of the specialist counting dish. This method relies on the availability of a specialist counting dish designed and built by Faraji et al. (2004) and is difficult to conduct without it as it reduces the movement of the sample while counting and presents the meniscus in such a way as to facilitate easy counting, hence efficiency may have been impaired. This method has several practical advantages as shown by previous studies (Faraji et al. 2004) in that samples can be processed and stored indefinitely before counting, leaf detritus and contaminates tend to stay in the ethanol rather than the paraffin while the lipophilic cuticle of the mites adheres to the paraffin. However, this method is time consuming, taking nearly as long as directly counting mites on leaves (Table 1). The addition of methyl blue dye provided a way to increase the visibility of mites; however, in practice, the dye made recognition more difficult. Several attempts were made in house to manufacture a counting dish, none of which produced a satisfactory dish, It was hoped that this method could be modified to facilitate it use without the counting dish. It is hoped that in future an adequate counting dish can be obtained and this method can be given the validation it deserves.
Although practical, the Tullgren funnel method also yielded poor mite extraction for T. urticae on Prunus and Malus leaves. The extraction of mites by light and heat has several advantages. Firstly, the mites are trapped in 70% ethanol and preserved, so immediate observation is not necessary. In terms of efficiency, the setup of this extraction method took less than half the time of the next quickest method (brushing) (Table 1). After set up, the Tullgren requires minimum maintenance and no leaf fragments contaminate the mite samples due to the sieve. One challenge is that this method requires the active movement of mites to move away from the light. Consequently mite eggs are not captured and many mites probably die before reaching the ethanol. Care needs to be taken when counting the sample to rotate the sample slowly so as not to move the mites with in the ethanol. It would have been ideal to know the numbers of mites in the initial inoculation; even though this was not done it may have been possible to examine the leaf debris remaining in the sieve of the Tullgren funnel looking for the remains of mites, in order to obtain another measure of extraction accuracy.
Compared to all the other methods, ethanol washing provided excellent recovery of mites from tree fruit leaves regardless of the type of leaf (Malus or Prunus). The simplicity of mite collection and recognition make ethanol washing comparably the most effective extraction technique as shown by past authors (Nordengen and Klingen 2006). The success of this extraction method can be attributed to the repetitive rinsing and sieving of the leaves ensured that the majority of mites are extracted and transferred into the sample without contamination of leaf fragments. Although this also applies to the Paraffin/ethanol meniscus method, ethanol extraction was faster and yielded more mites. The use of vacuum filtration speeds up the extraction process and although leaf hairs (trichomes) occasionally clump together and end up in the sample, the procedure to thoroughly check them with forceps and a mounted needle is effortless and does not affect counting significantly. The removal of the liquid from the sample also means that movement of the mites within the sample is not a hindrance. Furthermore, filtering samples through black segmented filter paper improved the visibility and counting of the mites. In addition, this method ensures leaves are individually mixed in ethanol so that more mites are extracted.
The numbers of mites in the ethanol washing extraction method did not differ significantly from direct counting or brushing in the 2nd experiment. This suggests that the bean leaf in experiment 1 may have influenced mite counts; more evidence that leaf morphology affects mite recovery. Since the bean leaf was removed, it suggests that the mites may be exhibiting host plant preference with mites remaining on the bean leaf when given the option of Malus leaves but readily moving on to the Prunus when given that option or that mites are more difficult to remove from Malus leaves.
Overall the brushing and direct counting did not differ significantly in the number of mites recovered. However, a major limitation with brushing is the friability of the target leaf. The brushing machine needs to be set up for each type of leaf and increased mite recovery from fragile leaves goes together with increased numbers of leaf fragments within the sample. This increase in leaf remnants makes counting more difficult and time consuming. Additionally this method requires samples to be checked immediately as the detergent dries up quickly, particularly under the light of the microscope.
The pressure of the brushes on the leaves can be changed, but needs to be firm enough to remove the mites but not so firm as to damage the leaves this would also change with the age of the leaf and leads to a lot of initial adjustments to get the ideal settings before experimental samples are processed.
Direct counting is very thorough if the midrib and lateral veins are teased apart. However, it is time consuming and takes approximately 9 h to check 12 replicates, compared to 6 h for ethanol washing. It was used in this study as a control to ensure the extraction methods had a good recovery rate. Hence it can be assumed that any differences in results are purely due to the extraction methods ability to extract mites. The most important concept to consider is that the effectiveness of an extraction technique is strongly influenced by the leaf the mites are extracted from. Leaves come in a variety of thickness, shapes, textures, ornamentation, physiological state and ‘hairiness’, each of these factors can alter the amount of protection the leaf offers the mites. In cereals and smooth-leaved crops it is possible to collect spider mites from crops using adhesive tape with 80% accuracy (Nansen et al. 2010). Hence, there may be no universal extraction method for all leaf types but specific methods for certain types of leaves.
The efficacy of an extraction method is also dependant on the time it takes. If the methodology extracts all mites but takes days to extract then it may not be suitable for all studies.
Conclusion
No extraction method is universally suited to all leaf morphologies. The structure of leaves is so diverse that setae, texture, prominence of the veins and leaf structural strength can all impact on the collection and counting of mites. The concept behind the paraffin/ethanol meniscus method is interesting and offers many advantages; however, without the specialised counting dish designed by Faraji et al. (2004) the method was difficult to use and time consuming. Collection of T. urticae by Tullgren funnel was the quickest to set up but the efficiency of collections of mites were low collecting approximately half of the mites of the most effective method. Mite brushing was also relatively quick but the numbers of mites collected was variable depending on the structure of the leaves used. Ethanol washing was quick, consistent and lends itself to being able to store samples before final counting and appears to be less influenced by leaf structure than brushing.
References
Alford DV (2007) Pests of fruit crops—a colour handbook. Academic Press (an imprint of Elsevier, 30 Corporate Drive, Suite 400, Burlington, MA 01803, USA, pp 425–426)
Evans GO, Sheals JG, Macfarlane D (1961) The terrestrial Acari of the British Isles. An introduction to their morphology, biology and classification. Trustees of the British museum, London, p 219
Faraji F, Bruin J, Bakker FM (2004) A new method for mite extraction from leaf samples. Exp Appl Acarol 32:31–39
Henderson CF, McBurnie HV (1943) Sampling techniques for determining populations of the citrus red mite and its predators. U.S. Department of Agriculture, Circulars no. 671. Washington, pp 1–11
Herbert HJ, Butler KP (1973) Sampling systems for European red mite, Panonychus ulmi (Acarina: Tetranychidae), eggs on Malus in Nova Scotia. Can Entomol 105:1519–1523
Hossain SKM (1992) Comparison of sampling techniques for the European red mite, Panonychus ulmi (Koch) (Acari: Tetranychidae) and the Malus rush mite, Aculus schlechtendali (Nalepa) (Acari: Eriophyidae). Acta Agric Scand Sect B Soil Plant Sci 42:128–132
Jones JL, Prendergast DT (1937) Method of obtaining an index to density of field populations of citrus red mite. J Econ Entomol 30:934–940
Krantz GW (1978) A manual of acarology, 2nd edn. Oregon S.U. Book Stores Inc., Corvallis, pp 77–80
Morgan CVG, Chant DA, Anderson NH, Ayre GL (1955) Methods for estimating orchard mite populations, especially with the mite brushing machine. Can Entomol 87(5):189–200
Nansen C, Sidumo AJ, Gharalari AH, Vaughn K (2010) A new method for sampling spider mites on field crops. Southwest Entomol 35:1–10
Nef L (1971) Influence de l’humiditẻ sur le gẻotactisme des Oribates (acarina) dans l’extractor de Berlese-Tullgren. Pedobiologia 11:433–445
Newell IM (1947) Quantative methods in biological and control studies of orchard mites. J Econ Entomol 40:683–689
Nordengen I, Klingen I (2006) Comparison of methods for estimating the prevalence of Neozygites floridana in Tetranychus urticae populations infesting strawberries. J Invertebr Pathol 92:1–6
Tullgren A (1918) Ein Sehr einfacher ausleseapparat furterricole tierfauned. Z Angew Entomol 4:149–150
Van Leeuwen T, Vontas J, Tsagkarakou A, Dermauw W, Tirry L (2010) Acaricide resistance mechanisms in the two-spotted Tetranychus urticae and other important Acari: a review. Insect Biochem Mol Biol 40:563–572
Wise JC, Gut LJ, Thornton G (1999) Cherry, control of spider mites and European red mites. In: Saxena KN (ed) Arthropod management tests, vol 24. Entomological Society of America, p 71
Acknowledgements
We would like to thank the Agriculture and Horticulture Development Board for supporting this study and the Nuffield Research Placements for their assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harris, A.L., Ullah, R. & Fountain, M.T. The evaluation of extraction techniques for Tetranychus urticae (Acari: Tetranychidae) from apple (Malus domestica) and cherry (Prunus avium) leaves. Exp Appl Acarol 72, 367–377 (2017). https://doi.org/10.1007/s10493-017-0154-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-017-0154-6