Abstract
The hematophagous mite Dermanyssus gallinae poses a serious sanitary problem in the Brazilian laying poultry industry. Its control is typically performed with acaricides, either in powder or liquid form. However, the intensive use of these products has caused populations of this species to develop tolerance and even resistance. The aim of the present study is to evaluate the response of eggs and adults of D. gallinae to products in aqueous suspension according to commercial indication and as per the recommendations of the Brazilian Ministry of Agriculture, Livestock and Supply. The study used four acaricide products (product 1: cypermethrin, chlorpyrifos, and piperonyl butoxide; product 2: alkyl-benzyl-dimethyl ammonium chloride, glutaraldehyde, deltamethrin; product 3: dichlorvos; product 4: fluralaner) tested in vitro using the contact method. Distilled water was used in the control group. The effectiveness of each of the products differed significantly between eggs and adults. Products 2, 3, and 4 caused 100% of adult mortality up to day 5 after start of treatment, product 1 97.5%. The corrected mortality (non-viability) of eggs was 21.4% (product 1) 39.4% (product 2), 47.8% (product 3), and 14.4% (product 4). Although the products evaluated were effective against adults of D. gallinae, their effectiveness against eggs was lower under the same conditions. This finding might be directly related to frequent D. gallinae reinfestations in poultry houses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Various arthropods are a threat to the poultry industry due to the direct and indirect effects they have on bird health and well-being (Sparagano et al. 2009); additionally, commercial laying hens have been affected by mite infestations in Brazil for a long time (Rezende et al. 2013; Faleiro et al. 2015; Horn et al. 2016). One of the species that poses a threat to the hen population, and that represents a serious sanitary problem for the laying poultry industry, is the hematophagous mite Dermanyssus gallinae (De Geer) (Mesostigmata: Dermanyssidae) (Cunha et al. 2009; Cencek et al. 2011).
Dermanyssus gallinae may cause irritation, anaemia, bloodstained eggs, aggressive behaviour, cannibalism, and in some severe cases, even death of laying hens (Chauve 1998; Sparagano et al. 2009; Cunha 2013; Flochlay et al. 2017; Oliveira 2017). This mite is also related to low yield, decreased egg quality, and host immune alterations, which leads this species to attain pest status (Taylor et al. 2007; Oliveira 2017). The high number of specimens of this mite in poultry houses with recurring Salmonella infections also raises the issue of the potential role played by D. gallinae as a vector for this disease (and other diseases) in poultry farms (Valiente Moro et al. 2007, 2009; Sparagano et al. 2014). Dermanyssus gallinae spend the majority of their life cycle away from the host, and they suck blood mostly during the night. When they are not feeding, they form colonies in cracks and crevices that are used as hiding places (Cunha et al. 2009). Adults of this species might survive away from hens without feeding for several months, or even up to a year, which explains their persistence in poultry houses (Taylor et al. 2007; Cencek et al. 2011).
Once D. gallinae populations are established, control is in present-day poultry production systems is typically performed with acaricides, in either powder or liquid form (Taylor 2001; Abbas et al. 2014). However, the availability of chemical acaricides has decreased in many countries due to legislation and the options for controlling this mite are somewhat limited due to food safety regulations (Brännström et al. 2008; Abbas et al. 2014; Sparagano et al. 2014). In addition, these chemical compounds have been suffering drawbacks caused by mite resistance and concerns with human, animal, and environmental health (Taylor 2001).
In Brazil, the use of agrochemicals is monitored by the National Plan for the Control of Residues in Products of Animal Origin, which is the risk management tool adopted by the Brazilian Ministry of Agriculture, Livestock and Supply (in Portuguese: Ministério da Agricultura, Pecuária e Abastecimento—MAPA). This national plan aims at knowing and preventing the violation of the residual safety levels of authorized substances, as well as monitoring the occurrence of any levels of residues of chemical compounds banned in the country (Brasil 1999).
The repeated use of veterinary pesticides for long periods of time, as well as their incorrect application or application without a clear management program, or even the use of illegal chemical acaricides (off-label) have led D. gallinae to develop resistance to these compounds, frequently rendering their effectiveness uncertain (Marangi et al. 2009; Sparagano et al. 2009, 2014; Abbas et al. 2014; Gay et al. 2020). Control is also hampered because these mites hide in inaccessible places, due to their ability to remain long periods without feeding, and to their high fertility (Cencek et al. 2011). Therefore, the aim of the present study is to evaluate the response of eggs and adults of D. gallinae to acaricidal products in aqueous suspension according to commercial indications and as per recommended by the Brazilian Ministry of Agriculture, Livestock and Supply (MAPA).
Materials and methods
Experimental design
Mites and eggs were collected in a commercial laying poultry house situated in the municipality of Salvador do Sul (RS, Brazil), inserted in plastic bags, which were sealed, and taken to Laboratório de Acarologia/Tecnovates/Univates, where they were immediately screened to begin the experiment, separating eggs and visibly engorged adults; as described by Sparagano et al. (2014), engorged mites are intense red.
Four acaricide products in aqueous suspension were used, tested in vitro through the contact method, according to the amount of product per area indicated on their labels (Table 1). These products are: (1) cypermethrin, chlorpyrifos, and piperonyl butoxide; (2) alkyl-benzyl-dimethyl ammonium chloride, glutaraldehyde, and deltamethrin; (3) dichlorvos; and (4) fluralaner. The total volume applied per arena was 0.5 ml of prepared solution. Distilled water was used in the control group. Eggs and visibly engorged adults of D. gallinae were used in the test, and the methodology was adapted from Alves et al. (2019).
Experimental unit
The arenas were comprised of Petri dishes (6 cm diameter, area 28.26 cm2) with Whatman filter paper discs (80 g/m2) on the bottom and petroleum jelly on the edges, as a barrier to prevent mites from escaping (Alves et al. 2019). Ten D. gallinae adults were distributed on each arena. Five replicates/treatment were performed, with 0.5 ml of solution sprayed on each replicate, using a professional SW-775 airbrush (working pressure of 10 to 45 psi) at a distance of 15 cm inside an Exhaust Cabin. After drying under ambient conditions, the dishes were sealed with plastic wrap and maintained in a climate chamber at 25 ± 1 °C, 70 ± 5% relative humidity, and L14:D10 photoperiod (Alves et al. 2019).
Mites were evaluated on a daily basis for 5 days using a Leica stereomicroscope (S6E—LED 2500; Leica Microsystems, Wetzlar, Germany), and were considered dead if no movement was seen after touching them with a fine-tipped brush. In order to assess ovicidal activity, the same procedure was repeated with eggs of D. gallinae, with 0.5 ml of solution applied to each dish. Five replicates were performed for each treatment and for the control. Evaluations were performed on a daily basis for 5 days, counting the eggs that hatched, and live and dead mites (adults) by using a stereomicroscope.
Data analysis
Mite mortality (%) was calculated as: (sum of dead mites/total number of mites) × 100. Corrected mortality (Mc, mortality relative to the control) of adults and eggs was calculated using Abbott's formula (1925):
where Mo is the observed mortality in each treatment and Mt is the mortality observed in the control (Silva et al. 2007; Locher et al. 2010). Acaricide lethal activity was classified according to Kim et al. (2007), where mortality > 80% is considered strong, 80–61% is moderate, 60–40% is weak, and mortality < 40% is considered little or no activity.
Data analysis was done with two tests using BioEstat v.5.0 (Ayres et al. 2007). Mean corrected mortalities of adults and eggs were compared among treatments using the non-parametric Kruskal–Wallis test, followed by Dunn’s test (α = 0.05). The Mann–Whitney test was performed on corrected mortalities between adults and eggs within each pesticide.
Results
Effects on eggs
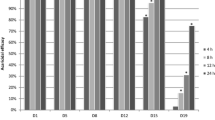
After spraying the products, the mean corrected mortality (non-viability) of eggs was 21.4% with product 1, 39.4% with product 2, and 47.8% with product 3 (Table 2). Product 4 had the lowest egg non-viability after treatment application: 14.4%. Products 1–4 had no significant difference from each other; however, products 2 and 3 differed from the control sample (Table 2).
Effects on adults
Corrected mortality was significantly different from the control with all products tested (Table 2). Products 2–4 caused 100% mortality up to the 5th day, product 1 97.5%. Following Kim et al. (2007), the lethal activity of all products tested was considered strong (> 80%). There was no significant difference among treatments (Table 2). The effectiveness of all four products was clearly higher against adults than against eggs (Table 2).
Discussion
Product 1, composed of cypermethrin, chlorpyrifos, and piperonyl butoxide, had a strong lethal activity (Kim et al. 2007) against adults, but did not show ovicidal action. Considering hens cannot be inside the poultry house when this product is used (Ouro Fino 2020), the low effectiveness against eggs of D. gallinae can cause the establishment of new populations of this species even before the chickens repopulate the aviary. Additionally, D. gallinae might survive long enough to infest a new flock, especially because they may live up to several months without feeding (Taylor et al. 2007; Cencek et al. 2011).
Both cypermethrin and chlorpyrifos are widely used to control arthropods and animal parasites. Chlorpyrifos is an inhibitor of acetylcholinesterase (AChE) affinity, whereas cypermethrin blocks sodium channels. Cypermethrin and piperonyl butoxide are classified as synergist components of pesticide formulations, especially pyrethroids (Beckel et al. 2006; Campos et al. 2017). However, the ineffectiveness against eggs of D. gallinae might indicate that the product probably does not penetrate the eggshell, but there is no information so far to help understand how the eggs are protected to the products.
Some studies found that even low doses of cypermethrin caused immunotoxicity, oxidative stress, and apoptosis of poultry lymphocytes (e.g., Eraslan et al. 2017; Ambwani et al. 2018). Data such as these show the importance of the correct application of products, in absence of hens and observing the specified withholding period, especially at sites populated by animals that shall subsequently be used for human consumption.
Product 2 is a disinfectant/insecticide composed of alkyl-benzyl-dimethyl ammonium chloride, glutaraldehyde, and deltamethrin. The disinfectant effect of this compound derives from benzalkonium chloride and glutaraldehyde. Deltamethrin is a pyrethroid, which is a synthetic adaptation of pyrethrins, and provides excellent knockdown, despite its low residual activity due to instability (Casida et al. 1983; Taylor 2001; Abbas et al. 2014). Similar to product 1, product 2 cannot be applied while hens are inside the poultry house (Ouro fino 2020; Theseo 2007). Dermanyssus gallinae resistance to pyrethroids has already been widely reported and observed in Europe, for instance in UK, Italy, France, and Sweden (Mul et al. 2009; Sparagano et al. 2014). Thomas et al. (2018) reported apparent resistance of D. gallinae to deltamethrin (part of product 2) as well as cypermethrin (present in product 1).
Product 3 (dichlorvos) is an organophosphate that acts by inhibiting AChE function, which consequently affects the transmission of nervous impulses and ultimately leads to pest paralysis and death (Taylor 2001). Organophosphates were pioneers among chemical groups used for the control of arachnids, which include bird mites (Beesley 1963; Abbas et al. 2014). Still, studies such as Nordenfors and Höglund (2000) have already mentioned the limited effect of organophosphates, which only provide temporary suppression of mite populations.
The red poultry mites used in the present study proved sensitive: after in vitro application the product caused mortality of 100% of adults. These findings corroborate Beugnet et al. (1997), who found that dichlorvos was effective against adults of D. gallinae. Although effective against adults, the dichlorvos-based treatment had much less effect against eggs.
Also product 4 (fluralaner) had strong lethal activity against adults, but was much less effective against eggs (only 14.4% nonviable eggs). Unlike the other poultry acaricides, this product acts systemically and its administration occurs via drinking water. After ingestion by the hen, fluralaner inhibits the mites’ nervous system, leading to paralysis and death (Thomas et al. 2018). Thomas et al. (2018) tested three application methods against D. gallinae using fluralaner: spray application (used for traditional contact acaricides), immersion, and a feeding test. They found that fluralaner was active in all three methods, although the highest activity was reported in the feeding treatment. They only studied effects on mite adults, not on eggs.
In many countries, the use of synthetic products has been increasingly limited due to a progressively stricter legislation regarding components and their impacts. Restraints to the use of these products also include egg withholding periods or restrictions preventing treatments while hens are inside poultry houses, in order to mitigate risks of residues in the products, and consequently, minimize risks to human health (Roy et al. 2009; Sparagano et al. 2014).
Although the products evaluated in the current study were effective against adults of D. gallinae, their effectiveness against eggs was much lower under the same conditions. This finding might be directly related to frequent D. gallinae reinfestations in poultry houses. According to Beugnet et al. (1997), reinfestation in poultry houses occurs within 4–8 weeks after acaricidal treatments, and the apparent treatment failure might be related to rapid parasite reproduction, short interval between depopulating and repopulating the poultry house, or even due to acaricide resistance. The present study corroborates this information, despite the products having an effect on the adults, the eggs showed tolerance to the products tested.
Since the ineffectiveness of acaricides against eggs of these ectoparasites might lead to concerning effects on poultry farm systems and affect their economic viability, further studies aiming to evaluate side effects on immatures mites derived from treated hatched eggs are recommended, as well as field tests in order to confirm the acaricidal activity of these compounds in these environments.
Data availability
All data and materials are available for publication.
Code availability
Not applicable.
References
Abbas RZ, Colwell DD, Iqbal Z, Khan A (2014) Acaricidal drug resistance in poultry Red Mite (Dermanyssus gallinae) and approaches to its management. Worlds Poult Sci J 70:113–124. https://doi.org/10.1017/S0043933914000105
Abbott WS (1925) A method of computing the effectiveness of insecticide. J Econ Entomol 18:265–267
Alves LFA, Oliveira DGP, Kasburg CR, Nardelli MS (2019) Acaricidal activity of inert powders against the poultry red mite Dermanyssus gallinae (De Geer, 1778) (Mesostigmata: Dermanyssidae). Arch Vet Sci 24:81–92. https://doi.org/10.5380/avs.v24i2.62775
Ambwani S, Ambwani TK, Singh R (2018) Immunotoxic effects of cypermethrin in mitogen stimulated chicken lymphocytes due to oxidative stress and apoptosis. J Entomol Zool Stud 6:37–42
Ayres M, Ayres Junior M, Ayres DL, Santos ADAD (2007) BioEstat: aplicações estatísticas nas áreas das ciências biomédicas. Ong Mamiraua. Belém
Beckel HS, Lorini I, Lazzari SMN (2006) Efeito do Sinergista Butóxido de Piperonila na Resistência de Oryzaephilus surinamensis (L.) (Coleoptera, Silvanidae) a Deltametrina e Fenitrotiom. Rev Bras Entomol 50:110–114
Beesley WN (1963) The effect of three organo-phosphorus insecticides on certain arthropods which infest livestock. Ann Appl Biol 52:295–303. https://doi.org/10.1111/j.1744-7348.1963.tb03751.x
Beugnet FC, Chauve M, Gauthey BL (1997) Resistance of the Red Poultry Mite to pyrethroids in France. Vet Rec 140:577–579. https://doi.org/10.1136/vr.140.22.577
Brännström S, Morrison DA, Mattsson JG, Chirico J (2008) Genetic differences in internal transcribed spacer 1 between Dermanyssus gallinae from wild birds and domestic chickens. Med Vet Entomol 22:152–155. https://doi.org/10.1111/j.1365-2915.2008.00722.x
Brasil. Ministério da Agricultura e do Abastecimento - MAPA. Instrução Normativa nº 42, de 20 de dezembro de 1999. Brasília, DF (1999) http://www.agricultura.gov.br/assuntos/inspecao/produtos-animal/plano-de-nacional-de-controle-de-residuos-e-contaminantes/documentos-da-pncrc/instrucao-normativa-sda-n-o-42-de-20-de-dezembro-de-1999.pdf. Accessed 01 June 2021
Campos DR, Avelar BR, Oliveira GF, Alves MSR, Borges DA, Medeiros MT, Scott FB (2017) Eficácia da associação de cipermetrina, clorpirifós, butóxido de piperonila e fluazuron contra larvas de Dermatobia hominis em bovinos naturalmente infestados. Braz J Vet Med 39:28–32
Casida JE, Gammon DW, Glickman AH, Lawrence LJ (1983) Mechanisms of selective action of pyrethroids insecticides. Ann Rev Pharmacol Toxicol 23:413–438. https://doi.org/10.1146/annurev.pa.23.040183.002213
Cencek T, Karamon J, Sroka J, Zdybel J (2011) New in vitro method for determination of Acaricide efficiency against Dermanyssus gallinae mites. Bull Vet Inst Pulawy 55:657–662
Chauve C (1998) The poultry red mite Dermanyssus gallinae (De Geer, 1778): current situation and future prospects for control. Vet parasitol 79:239–245. https://doi.org/10.1016/s0304-4017(98)00167-8
Cunha LM (2013) Aspectos epidemiológicos relacionados à ocorrência de ácaros hematófagos em granjas comerciais de postura no estado de Minas Gerais e avaliação de armadilhas para captura de Dermanyssus gallinae (Acari: Dermanyssidae) (de Geer, 1778). PhD dissertation, Universidade Federal de Minas Gerais. http://hdl.handle.net/1843/BUBD-9BGJZE
Cunha LM, Cunha MM, Leite RC, Silva IJ, Oliveira PR (2009) Comparação da eficiência de diferentes armadilhas utilizadas para a captura de Dermanyssus gallinae (Acari: Dermanyssidae) (de Geer, 1778). Rev Bras Parasitol Vet 18:59–62. https://doi.org/10.4322/rbpv.01804011
Eraslan G, Tekeli MY, Karabacak M (2017) Toxicokinetic of cypermethrin in broiler chickens. Fresenius Environ Bull 26:4704–4710
Faleiro DCC, Toldi M, Da Silva GL, Ferla NJ (2015) The ectoparasites Dermanyssus gallinae and Megninia ginglymura: bioecology and natural enemies in commercial egg-laying hens. Syst Appl Acarol 20:861–874. https://doi.org/10.11158/saa.20.8.3
Flochlay AS, Thomas E, Sparagano O (2017) Poultry red mite (Dermanyssus gallinae) infestation: a broad impact parasitological disease that still remains a significant challenge for the egg-laying industry in Europe. Parasites Vectors 10:1–6. https://doi.org/10.1186/s13071-017-2292-4
Gay M, Lempereur L, Francis F, Megido RC (2020) Control of Dermanyssus gallinae (De Geer 1778) and other mites with volatile organic compounds, a review. Parasitology 147:731–739. https://doi.org/10.1017/S0031182020000530
Horn TB, Korbes JH, Granich J, Senter M, Ferla NJ (2016) Influence of laying hen systems on the mite fauna (Acari) community of commercial poultry farms in southern Brazil. Parasitol Res 115:355–366. https://doi.org/10.1007/s00436-015-4756-9
Kim SI, Na YE, Yi JH, Kim BS, Ahn YJ (2007) Contact and fumigant toxicity of oriental medicinal plant extracts against Dermanyssus gallinae (Acari: Dermanyssidae). Vet Parasitol 145:377–382. https://doi.org/10.1016/j.vetpar.2006.12.021
Locher N, Al-Rasheid KA, Abdel-Ghaffar F, Mehlhorn H (2010) In vitro and field studies on the contact and fumigant toxicity of a neem-product (Mite-Stop®) against the developmental stages of the poultry red mite Dermanyssus gallinae. Parasitol Res 107:417–423. https://doi.org/10.1007/s00436-010-1882-2
Marangi M, Cafiero MA, Capelli G, Camarda A, Sparagano OAE, Giangaspero A (2009) Evaluation of the poultry red mite, Dermanyssus gallinae (Acari: Dermanyssidae) susceptibility to some acaricides in field populations from Italy. Exp Appl Acarol 48:143–155. https://doi.org/10.1007/s10493-008-9224-0
Mul M, Van Niekerk T, Chirico J, Maurer V, Kilpinen O, Sparagano OAE (2009) Control methods for Dermanyssus gallinae in systems for laying hens: results of an international seminar. Worlds Poult Sci J 65:589–600. https://doi.org/10.1017/S0043933909000403
Nordenfors H, Hoglund J (2000) Long term dynamics of Dermanyssus gallinae in relation to mite control measures in aviary systems for layers. Br Poult Sci 41:533–540. https://doi.org/10.1080/713654991
Oliveira TM (2017) Caracterização epidemiológica e avaliação de risco associada a presença de ectoparasitos em granjas de postura comercial em Minas Gerais. PhD dissertation, Universidade Federal de Minas Gerais. http://hdl.handle.net/1843/SMOC-AKGPFG
Ouro fino (2020) Colosso Avicultura. https://www.ourofinosaudeanimal.com/produtos/aves/ectoparasiticidas/colosso-avicultura/ Accessed 15 Dec 2020
Rezende LDC, Cunha LM, Teixeira CM, Oliveira PRD, Martins NRDS (2013) Ácaros de importância para a avicultura de postura: algumas considerações aplicadas à realidade da indústria avícola brasileira. Ciência Rural 43:1230–1237. https://doi.org/10.1590/S0103-84782013005000088
Roy L, Chauve C, Delaporte J, Inizan G, Buronfosse T (2009) Exploration of the susceptibility of AChE from the poultry red mite Dermanyssus gallinae (Acari: Mesostigmata) to organophosphates in field isolates from France. Exp Appl Acarol 48:19–30. https://doi.org/10.1007/s10493-009-9249-z
Silva WC, Ribeiro JD, Souza HEM, Corrêa RS (2007) Atividade inseticida de Piper aduncum L. (Piperaceae) sobre Aetalion sp. (Hemiptera: Aetalionidae), praga de importância econômica no Amazonas. Acta Amazônica 37:293–298. https://doi.org/10.1590/S0044-59672007000200017
Sparagano O, Aleksander P, Murano T, Camarda A, Sahibi H, Kilpinen O, Mul M, Emous R, Bouquin S, Hoel K, Cafiero MA (2009) Prevalence and key figures for the poultry red mite Dermanyssus gallinae infections in poultry farm systems. Exp Appl Acarol 48:3–10. https://doi.org/10.1007/978-90-481-2731-3_2
Sparagano O, George DR, Harrington DWJ, Giangaspero A (2014) Significance and control of the poultry red mite, Dermanyssus gallinae. Annu Rev Entomol 59:447–466. https://doi.org/10.1146/annurev-ento-011613-162101
Taylor MA (2001) Recent developments in ectoparasiticides. Vet J 161:253–268. https://doi.org/10.1053/tvjl.2000.0549
Taylor MA, Coop RL, Wall RL (2007) Vet parasitol, 3rd edn. Blackwell Publishing, London
Theseo (2007) Désinfectant bactéricide, virucide, acaricide et insecticide. Fiche Technique. https://www.hyprodis.fr/F-FICHIER_LIE_A_1024.pdf-mefisto-shock.aspx. Accessed 16 Dec 2020.
Thomas E, Zoller H, Liebisch G, Alves LFA, Vettorato L, Chiummo RM, Sigognault-Flochlay A (2018) In vitro activity of fluralaner and commonly used acaricides against Dermanyssus gallinae isolates from Europe and Brazil. Parasites Vectors 11:361. https://doi.org/10.1186/s13071-018-2956-8
Tucci EC, Prado AP, Araujo RP (2008) Thermal requirements of Dermanyssus gallinae (De Geer, 1778) (Acari: Dermanyssidae). Rev Bras Parasitol Vet 17:67–72. https://doi.org/10.1590/S1984-29612008000200002
Valiente Moro C, Chauve CM, Zenner L (2007) Experimental infection of Salmonella Enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol 146:329–336. https://doi.org/10.1016/j.vetpar.2007.02.024
Valiente Moro C, De Luna CJ, Tod A, Guy JH, Sparagano OAE, Zenner L (2009) The poultry red mite (Dermanyssus gallinae): a potential vector of pathogenic agents. Exp Appl Acarol 48:93–104. https://doi.org/10.1007/s10493-009-9248-0
Acknowledgements
The authors are grateful to Universidade do Vale do Taquari—Univates for its financial support and for providing the material required to conduct this study.
Funding
No specific funding is associated with this case report.
Author information
Authors and Affiliations
Contributions
AS, LJ, GLS, and FRS designed the study; AS, DM performed the research and laboratory activities; AS, LJ, GLS, NJF, and FRS contributed to diagnosis and writing; AS, LJ, and GLS drafted the paper with contributions from all other authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Consent to participate
All authors participated voluntarily in the research.
Consent for publication
All authors read and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sulzbach, A., Mallmann, D., Silva, F.R. et al. In vitro evaluation of the response of Dermanyssus gallinae to products in aqueous suspension. Exp Appl Acarol 86, 201–209 (2022). https://doi.org/10.1007/s10493-022-00697-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-022-00697-9