Abstract
The poultry red mite, D. gallinae has been involved in the transmission of many pathogenic agents, responsible for serious diseases both in animals and humans. Nowadays, few effective methods are available to control the ectoparasite in poultry farms. Consequently, this is an emerging problem which must be taken into account to maintain good health in commercial egg production. This paper addresses the vector capacity of the ectoparasite with special emphasis on salmonellae, pathogenic agents responsible for many of the most important outbreaks of food-borne diseases worlwide. It has been experimentally shown that D. gallinae could act as a biological vector of S. enteritidis and natural carriage of these bacteria by the mite on poultry premises has also been reported. It was also found that D. gallinae carried other pathogens such as E. coli, Shigella sp., and Staphylococcus, thus increasing the list of pathogenic agents potentially transmitted by the mite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been shown that Acari can be implicated in the vectorial transmission of diseases. However, their role in the natural transmission cycles of pathogenic agents is poorly known and the Acari, particularly mites, are largely ignored as vectors of human or animal diseases.
Within the order Acari, the Dermanyssoidea superfamily represents a vast group of ubiquitous organisms, they also have a very broad range of hosts and they can easily parasitise other species including farm animals and man. Among the superfamily, Dermanyssus gallinae (the poultry red mite) and Ornithonyssus bacoti are the only two mites for which the vectorial transmission of pathogens seems to be highly complex, i.e. not limited simply to a mechanical role of a vector that simply carries a microorganism without replication occurring (Valiente Moro et al. 2005). There are few reported studies which deal with the vectorial role of D. gallinae and the studies which have been published are often incomplete which could lead to an underestimation of the importance of this agent in the transmission of bacteria and viruses, responsible for animal infections and zoonoses (Table 1). For some of them, only the isolation of pathogens from field samples has been reported. However, the simple detection of a vector-borne bacterial agent in an ectoparasite does not demonstrate vector competence. It is the case for the bacteria Erysipelothrix rhusiopathiae, Salmonella gallinarum and Listeria monocytogenes and the Newcastle disease virus (NDV); (Grebenyuk et al. 1972; Zeman et al. 1982; Arzey 1990; Chirico et al. 2003; Valiente Moro et al. 2009). For other pathogens which have been associated with D. gallinae, only experimental transmissions under laboratory conditions have been carried out. Natural carriage of the pathogens by the mite was not investigated by the authors. Some assays remained fruitless as D. gallinae was unable to be infected or to transmit the pathogens, supporting the relative unimportance of this mite in the transmission of active pathogen infection. This is the case for viruses responsible for tick-borne encephalitis and Saint-Louis encephalitis (Chamberlain et al. 1957; Zemskaya and Pchelkina 1962; Wegner 1976). Concerning Pasteurella multocida, the bacterium causing pasteurellosis, microbiological studies and biological experiments revealed that bacteria persisted in the body of D. gallinae mites after they engorged on infected birds (Petrov 1975). For Coxiella burnetii, a bacterium responsible for Q fever, Zemskaya and Pchelkina (1967) showed that D. gallinae could acquire infection, while feeding on infected animals. The rickettsiae survived in the mites, which subsequently fed on healthy birds, for about 6 months, and for about 1 year in dead mites. The role of the poultry red mite was also studied in the transmission of spirochaetosis, a disease caused by the bacterium Borrelia anserine which can infect chickens, turkeys, geese, ducks, pheasants, grouse and canaries with morbidity and mortality up to 100%. It is usually transmitted by Argas persicus ticks and occasionally by infected faeces. Ciolca et al. (1968) observed that spirochetes were regularly transmitted to healthy hens, provided that the mites fed on the healthy hens within 48 h after the mites had become infected. The spirochaetes were usually eliminated in the excreta shortly after ingestion suggesting that the mite was only an occasional vector of them. With the species Spirochaeta gallinarum, Reshetnikov (1967) observed similar results except that the interval between blood meals should not exceed 48 h in order to reproduce the disease in the host. Several laboratory studies have also concerned experimental transmissions with equine encephalitis viruses. Chamberlain and Sikes (1955) and Durden et al. (1993) showed that D. gallinae which engorged on chicks infected with east equine encephalitis (EEE) virus remained carriers for at least a month. Given the chronology of mean viral titres in the mite samples and the prolonged persistence of virus in the mites (30d), some viral replication may have occurred at a low level. Moreover, authors showed that mites were able to transmit the virus to other chicks by bite when taking a blood meal. Cockburn et al. (1957) obtained an infestation from D. gallinae which had fed on chickens infected with west equine encephalitis (WEE), but were not able to demonstrate either transmission to healthy birds or transovarian transmission in the acarian. Interesting results were also obtained by Shirinov et al. (1972), where samples of D. gallinae collected from poultry farms known to have birds infected with fowl pox virus were also found to harbour the virus. When naturally-infected mites were kept in the laboratory, the virus survived inside them up to 300 days. Transovarian transmission was demonstrated and the disease was transmitted to healthy fowl by the bite of infected mites.
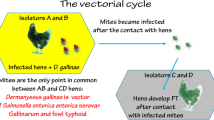
As a result, the role of the poultry red mite as a potential mechanical vector has been clearly shown for some pathogens even if its precise role in the epidemiology of the associated pathology remains to be determined. Most of the studies are incomplete for conclusions to be made about the precise role of D. gallinae in the circulation of pathogens, as complete transmission ways remain to be proven as shown in Fig. 1. To compensate for this lack, a complete study was recently undertaken, exploring in detail the role of D. gallinae as a vector of Salmonella, responsible for the most often encountered zoonotic diseases in man. In 2004, 192,703 salmonellosis cases were reported in the European Union corresponding to an increase of 22% compared to 2003 and the most important increase in incidence since 1999 (European Food Safety Autority 2005). Poultry products are among the most important sources of Salmonella that can be transmitted through the food chain to humans (Lacey 1993). This particular serovar, strongly associated with the production of eggs for human consumption, has replaced S. typhimurium as the primary cause of salmonellosis worlwide (Baumler et al. 2000). Consequently, many food safety laws and hygiene control methods are aimed at preventing its transmission (Rodrigue et al. 1990). A recent hypothesis suggests that the eradication of S. gallinarum, a bacterium which does not affect man, would be the origin of the implantation of S. enteritidis in fowls which can infect man (Velge et al. 2005). These Enterobacteriaceae take part from normal flore within the chicken and, although not necessarily harmful for them, they are responsible for some of the most widespread zoonoses in the world. They are particularly resistant in the environment, probably due to their capacity to survive dessication better than other coliforms (Morse and Duncan 1974). Salmonellas, and in particular the serotype Enteritidis, survive in various wildlife reservoirs, and their presence in arthropods as litter beetles, houseflies and cockroaches has been recorded and found to be of significant importance in their transmission (Olsen and Hammack 2000; Davies and Breslin 2003; Fischer et al. 2003; Skov et al. 2004). Zeman et al. (1982) have already shown that D. gallinae could shelter salmonellas (serotype Gallinarum) for more than 4 months. However, authors did not investigate further by evaluating vectorial competence from the red mite for Salmonella. Consequently, these preliminary results raised the question of the vectorial role of D. gallinae for salmonella. Indeed, D. gallinae often hide under the dry droppings of the hens which are also frequently contaminated by the salmonellas (Morse and Duncan 1974). Moreover, mites can feed several times in each life stage on birds which increases the chance of exposure to blood-borne bacterial agents and it has been observed that the birds ingest mites (Kilpinen 2005). So the recurring problems of salmonellosis in housing systems for laying hens associated with the simultaneous presence of D. gallinae in these buildings, lead to a preliminary study of the role of D. gallinae in the transmission of salmonella, particularly S. enteritidis, serotype usually found in collective alimentary toxi-infections (Valiente Moro et al. 2007a, b).
Due to their haematophagous behaviour and ability to fast for long periods of time, these mites are particularly well adapted candidates for pathogen transmission. Moreover, the current lack of effective measures to control this mite, partly due to increasing acaricide resistance, causes reoccurring mite problems in poultry facilities once they have become infested by this parasite. Consequently, mites can persist in the poultry house between flock cycles and may also act as reservoir hosts for pathogenic agents such as bacteria giving a source of infection for the replacement pullets. As a result, the ubiquitous presence of D. gallinae in poultry breeding farms worldwide raises the question of their role in the colonisation, survival and propagation of pathogenic agents.
Materials and methods
Dermanyssus gallinae as an experimental vector of Salmonella enteritidis
Dermanyssus gallinae populations were collected from laying hen breeding facilities known to be free of Salmonella infection. Two methods of infecting the mites were tested: infection via the blood meal and via cuticular contact. The methodology of Bruneau et al. (2001) was modified slightly to create an in vitro feeding device to infect the mites during the blood meal. To ensure that any bacteria subsequently detected were those located inside the mite, we cleaned them following the protocol described by Zeman et al. (1982) using 4% w/v paraformaldehyde followed by rinsing with sterile distilled water. A total of 50 mites were fed on blood containing 108 CFU/ml during 4 h feeding periods. Cuticular infection of the mites was achieved by leaving them on a dry Salmonella coating for 48 h at room temperature (by putting a Whatman paper in contact with a saturated salmonella culture on a petri plate, then the mite was left on the contaminated paper). To test the presence of Salmonella within the mites immediately after the infection, 100 and 40 mites infected, respectively by the oral route and cuticular contact were individually washed as described above and analysed by microbiological culture. To test whether Salmonella survived and multiplied within D. gallinae, bacteria were counted overtime by analysing mites at 1, 3, 7 and 14 days after infection. Concerning the infection through the blood meal, we considered that all mites with a Salmonella count five times higher (statistically determined) than the theoretical value of 20,000 bacteria proved bacterial multiplication. This latter value was determined by considering that the number of enterobacteriaceae inside freshly engorged mites was approximately 2 × 104 CFU, since a mite absorbs about 0.2 μl of blood, containing in our experiment a bacterial level of 108 CFU/ml. Similarly, to have an idea of the threshold value in mites infected after cuticular contact, the average population of salmonella inside the mites 1 day after the infection was estimated as equal to 7.6 × 103 CFU/ml. All mites with a bacterial count five times higher than this threshold value i.e. 3.8 × 104 CFU/ml proved bacterial multiplication. The effect of Salmonella on mite oviposition, transovarial and transstadial passages was only tested for those mites, which acquired Salmonella during the blood meal because it would have been necessary to wash the cuticule of contaminated mites to avoid external contaminations. In this case, the mites would have been killed during the washing process. To test whether the presence of the pathogen inside engorged females reduced the number of laying females as well as their fertility rate, comparisons of the number of eggs laid were made using 165 females engorged on either infected or uninfected blood. Assessing how D. gallinae could contaminate birds has been investigated in two different ways. The possibility that D. gallinae could contaminate the blood during a blood meal after acquiring the bacteria during a previous blood meal or contact with Salmonella, was first tested using the in vitro feeding device. As the ingestion of D. gallinae by birds has been frequently reported, the assumption that hens become contaminated with S. enteritidis after ingesting infected mites was also assessed (Valiente Moro et al. 2007b). In this aim, 98 1-day-old chicks were inoculated orally by 10 mites: 34 chicks received mites contaminated during the blood meal, 34 received mites contaminated by the cuticular route, and 30 received uncontaminated mites. Following oral administration of contaminated mites to chicks, the ability of S. enteritidis to colonise the digestive tract and to invade the internal organs of the chicks was investigated. Using direct plating, S. enteritidis was counted from the spleen and the liver of all birds.
Natural carriage of Salmonella by Dermanyssus gallinae in poultry farms
The second step was to assess the natural carriage of Salmonella by D. gallinae in poultry farms (Valiente Moro et al. 2007d). Preliminary field studies have been undertaken using a molecular detection tool associating a simple filter-based DNA preparation with a specific 16S rDNA Salmonella sp. polymerase chain reaction (PCR) amplification (Desloire et al. 2006; Valiente Moro et al. 2007c). The presence of Salmonella was tested in D. gallinae collected in two types of laying farms: six farms were currently declared positive for Salmonella by the French DSV (Direction des Services Vétérinaires) while 10 others had been previously declared positive. For each farm, 6–20 pools of 15 mites (a total of 249 pools) were analysed for the presence of S. enteritidis as described above.
Dermanyssus gallinae as the carrier of other bacteria in poultry farms
Five samples of D. gallinae were collected from one farm in the north-east of UK, the surface sterilised before the mites were crushed as described in De Luna et al. (2008). Serial dilutions were performed up to 10−3 and the dilutions were put directly into culture on several non-specific media such as LB agar, blood sheep agar, and brain heart infusion agar. Specific media for enterobacteria were also used (BPL agar and XLT-4 agar). Colonies were harvested directly and DNA was extracted using a tissue protocol of a commercial kit following the manufacturer’s instructions (Qiagen, UK). This was used as the template DNA for the subsequent PCR reactions. Partial amplification of the 16S rRNA gene was undertaken using a universal primer pair: 27-F′-AGAGTTTGATCMTGGCTCAG-3′, and 1513-R 5′-AGGGYTACCTTGTTACGACTT-3′ (Weisburg et al. 1991). PCR products (of around 1,200 pb) obtained from specific PCR were sequenced by dye-labelling using the BigdyeTM dideoxy technique and either the forward or reverse primers of the original PCR reactions were used. Sequences were run on an automated DNA sequence system at the IRES Genomics service at Newcastle University, UK. The BLASTN (Basic Local Alignment Search Tool) search option of the National Centre for Biotechnology Information (NCBI) internet site (http://www.ncbi.nlm.nih.gov) was used to identify close evolutionary relatives in the GenBank database. When the similarity percent between our sequences and previously described sequences exceeded 97%, the sequences were considered as corresponding to the same species as that in the GenBank reference (Stackebrandt and Goebel 1994).
Results and discussion
The results showed that immediately after the experimental infection, Salmonella was found in 29% of mites infected by a bloodmeal and in 55% of mites infected by cuticular contact. Given the efficiency of paraformaldehyde in eliminating all external contaminations (Zeman et al. 1982), two hypotheses can be proposed for cuticular infection: either transcuticular passage of the bacteria and/or entry of the microorganisms through the stigmata. By either infection route, a significant increase in the number of mites carrying Salmonella was observed in comparison to day 0 (Fig. 2a). The fact that the number of infected mites was greater 3 or 7 days after infection than the day after infection suggests that the bacteria may multiply inside the mites. Regarding the oral route, bacterial multiplication was shown for 21 and 24% of mites at D7 and D14, respectively (Fig. 2b). For the other mites, the population of bacterial was either stable or decreased over time. Similarly, multiplication was demonstrated for mites infected by cuticular contact for 42 and 25% of mites, respectively, at D7 and D14 (Fig. 2c). Cases of decreasing number of bacteria may result from an antibacterial response of mites similar to that observed for ticks, or even from the destruction of bacteria by the digestive system of the mite (Weyer 1975; Nakajima et al. 2003). Effect of Salmonella on mite oviposition was also demonstrated as the number of ovipositing females was significantly lower when mites were engorged on contaminated blood than when they were fed on uncontaminated blood (31 vs. 68%, respectively, P < 0.05). Moreover, the fecundity rate was slightly lower for mites engorged on contaminated blood (1.31 vs. 1.92 for uninfected blood) but this difference was not significant. This decrease in fertility of D. gallinae could be explained by the presence of enterobacteriaceae in the reproductive organs of the mites. Of the 74 ovipositing females, 37 showed transovarial passage: in vitro females engorged on contaminated blood produced infected protonymphs. The transovarial passage has often been reported for ticks (Macaluso et al. 2001; Rennie et al. 2001), although it is less frequent in other mites. For example, Liponyssoides sanguineus, the main vector of Rickettsia akari, the bacteria responsible for vesicular fever, can transmit the bacteria to its progeny. Furthermore, out of a total of 22 N1 nymphs obtained from uncontaminated females and subsequently fed on contaminated blood, three deutonymphs were detected as positive for Salmonella demonstrating transstadial passage. Although passage between the deutonymph and adult stages was not tested, we can assume that it does occur, allowing bacteria to persist throughout the entire life cycle of the mite. Concerning Salmonella retransmission to birds, D. gallinae was able to contaminate the blood during a blood meal after acquiring the bacteria during a previous blood meal or contact with Salmonella. In cases of oral acquisition, only one Salmonella transmission was observed among the 18 separate assays performed. In cases of cuticular contact, blood was infected in 5 cases out of a total of 12 separate experiments. The blood on which uninfected mites were fed remained negative in each experiment. Even if a few cases of salmonellosis were observed, it may be noteworthy in infested poultry facilities due to the large number of D. gallinae which are often present in commercial housing conditions (Nordenfors and Chirico 2001). Concerning oral transmission of contaminated mites to chicks, faecal samples from both sets of infected chicks were positive for Salmonella at 6 days after inoculation while the control corresponding to chick inoculated with uncontaminated mites remained negative. On D 12 post-inoculation, S. enteritidis was isolated from the caecum of all birds that had received contaminated mites with an average number of S. enteritidis of above 8.5 × 104 MNP Salmonella/g (Table 2). Statistical analysis did not show any significant differences between the infection routes. The level of infection obtained in both the infection models tested shows that previously infected mites could represent a source of Salmonella sp. infection when eaten by 1-day-old chicks. The invasion of organs such as the liver and the spleen is an indication of systemic infection. So the reproductive organs could be also contaminated and thus it could introduce a risk for humans when eggs are consumed. Presently, it is difficult to quantify how many D. gallinae are ingested by hens each day and therefore it is difficult to assess the real impact of D. gallinae in laying hen system facilities. However, it has been shown that this mode of infection is possible in 1-day-old chicks, even if it would be interesting to confirm whether such infections takes place in older birds. Although D. gallinae can both acquire S. enteritidis either by contaminating the blood under experimental conditions or chicks by oral ingestion, this does not mean that the mite is a natural vector. To rule out the possibility of this type of contamination in the field, it was necessary to study the level of Salmonella sp. in poultry facilities both before and after the arrival of a new flock. Of the six currently infected farms, only one farm had four positive pools (out of six) for Salmonella sp. Of the 10 farms that had previously been declared infected, only one farm had one positive pool (out of six). Thus, despite limited sampling, we have demonstrated that D. gallinae can be naturally infected in a contaminated poultry environment. Interestingly, Salmonella was detected in mites collected from a farm that was not currently contaminated, which suggests that mites infected during a previous outbreak survived the sanitation periods as well as the cleaning and disinfection programmes and may be a source of infection for replacement birds. D. gallinae could therefore act as a potential reservoir and will certainly play a role in the epidemiology of avian salmonellosis, as previously suggested by Zeman et al. (1982). This preliminary field study shows that D. gallinae can naturally harbour the pathogen and allows its persistence between outbreaks. In order to accurately evaluate the prevalence of D. gallinae carrying Salmonella in poultry farms on a national scale, much more widespread testing of facilities would be required. Other data would need to be considered in order to understand the role of D. gallinae in the epidemiology of avian salmonellosis, such as the vectorial capacity or the extrinsic incubation. In an epidemiological context, it is possible that infected mites crushed or eaten by chicks may be the main source of infection rather than the mite blood meal. Carriage of Salmonella by arthropods has already been reported in previous studies. Most recorded examples refer to litter beetles (Alphitobius diaperinus) or cockroaches but their role in the transmission of infection remains unproven (Davies and Wray 1955; McAllister et al. 1994; Gray et al. 1999). Ash and Greenberg (1980) showed that the German cockroach, Blatella germanica, is an effective mechanical transmitter of S. typhimurium via faeces, although the bacteria were recoverable from its gut for about 10 days longer than from the faeces.
a Detection of Salmonella enteritidis inside D. gallinae infected through a blood meal or cuticular contact. Vertical bars are standard errors. Significance of difference between D0 and others dates is indicated (**P < 0.01, *P < 0.05). bS. enteritidis multiplication in infected D. gallinae after a blood meal (theoretical value = 2.104 bacteria in freshly engorged mites and multiplication if the number of bacteria on SM ID = 5 × 2.104 equal to 1 × 105). cS. entetitidis multiplication in infected D. gallinae after cuticular contact (theoretical value = average at D1 = 7.6 × 103 and multiplication if the number of bacteria on SM ID = 5 × 7.6.103 equal to 3.8 × 104)
Finally it was also found that D. gallinae was carrying E. coli, Streptomyces sp. and Staphyloccocus sp. (Table 3), the latter has been associated with infections in starlings (Berger et al. 2003). E. coli has been associated with Avian Pathogenic E. coli and Uropathogenic E. coli. However, there was no conclusive evidence of these organisms acting as disease causing at the poultry farm at the moment the mites were sampled.
Conclusions
In view of these results, D. gallinae certainly plays an important role in the transmission of salmonellosis and other diseases in poultry farms, particularly between successive flocks. It would be of further interest to quantify the level of competence of D. gallinae for Salmonella and other bacterial genera and to assess the relationships between pathogen/vector. Another perspective would be to analyse the development cycle of the pathogen within the mite by studying the crossing of intestinal and salivary barriers. The nature of the immune response of birds and its impact on the vectorial competence of mites would also be interesting to aspects to investigate. The literature reviewed and the new data presented in this paper suggest that effective control of D. gallinae during downtime sanitation periods and before the introduction of new birds are key areas to target to reduce the persistence of pathogens such as Salmonella between successive poultry flocks.
References
Arzey G (1990) Mechanism of spread of Newcastle disease. Technical bulletin—New South Wales, Agriculture and Fisheries 42, Sydney, 12 pp
Ash N, Greenberg B (1980) Vector potential of the German cockroach (Dictyoptera: Blatellidae) in dissemination of Salmonella enteritidis serotype Typhimurium. J Med Entomol 17:417–423
Baumler AJ, Hargis BM, Tsolis RM (2000) Tracing the origins of Salmonella outbreaks. Science 287:50–52. doi:10.1126/science.287.5450.50
Berger S, Disko R, Gwinner H (2003) Bacteria in starling nests. J Fur Ornit 144:317–322
Bruneau A, Dernburg A, Chauve C, Zenner L (2001) First in vitro cycle of the chicken mite, Dermanyssus gallinae (DeGeer 1778), utilizing an artificial feeding device. Parasitology 123:583–589. doi:10.1017/S0031182001008836
Chamberlain RW, Sikes RK (1955) Laboratory investigations of the role of bird mites in the transmission of eastern and western equine encephalitis. Am J Trop Med Hyg 4:106–118
Chamberlain RW, Sikes RK, Sudia RW (1957) Attempted laboratory infection of bird mites with the virus of Saint Louis encephalitis. Am J Trop Med Hyg 6:1047–1053
Chirico J, Eriksson H, Fossum O, Jansson D (2003) The poultry red mite, Dermanyssus gallinae, a potential vector of Erysipelothrix rhusiopathiae causing erysipelas in hens. Med Vet Entomol 17:232–234. doi:10.1046/j.1365-2915.2003.00428.x
Ciolca AL, Tanase I, May I (1968) Role of the poultry red mite, Dermanyssus gallinae, in the transmission of spirochaetosis. Arch Vet Pol 5:207–215
Cockburn TA, Sooter CA, Langmuir AD (1957) Ecology of western Equine and Saint Louis Encephalitis viruses. A summary of field investigations in weld county, Colorado 1949–1953. Am J Hyg 65:130–146
Davies RH, Breslin M (2003) Persistence of Salmonella enteritidis phage type 4 in the environment and arthropod vectors on an empty free-range chicken farm. Environ Microbiol 5:79–84. doi:10.1046/j.1462-2920.2003.00387.x
Davies RH, Wray C (1955) The role of the lesser mealworm beetle (Alphitobius diaperinus) in carriage of Salmonella enteritidis. Vet Rec 137:407–408
De Luna CJ, Valiente Moro C, Guy JH, Zenner L, Sparagano OAE (2008) Endosymbiotic bacteria living inside the poultry red mite (Dermanyssus gallinae): a possible tool for biological control? Exp Appl Acarol. doi:10.1007/s10493-008-9230-2
Desloire S, Valiente Moro C, Chauve C, Zenner L (2006) Comparison of four methods of extracting DNA from D. gallinae (Acari: Dermanyssidae). Vet Res 37:725–732. doi:10.1051/vetres:2006031
Durden LA, Linthicum KJ, Turell MJ (1992) Mechanical transmission of Venezuelan equine encephalomyelitis by hematophagous mites (Acari). J Med Vet Entomol 9:118–121
Durden LA, Linthicum KJ, Monath P (1993) Laboratory transmission of eastern equine encephalomyelitis virus to chickens by chicken mites (Acari: Dermanyssidae). J Med Entomol 30:281–285
European Food Safety Autority (2005) The community summary report on trends and sources of zoonoses, zoonotic agents and antimicrobial resistance in the European union in 2004. 25 December 2005. Part 3.1. Salmonella, pp 1–67
Fischer OA, Matlova L, Dvorska L, Svastova P, Pavlik I (2003) Nymphs of the oriental cockroach (Blatta orientalis) as passive vectors of causal agents of avian tuberculosis and paratuberculosis. Med Vet Entomol 17:145–150. doi:10.1046/j.1365-2915.2003.00417.x
Gray JP, Maddox CW, Tobin PC, Gummo JD, Pitts CW (1999) Reservoir competence of Carcinops pumilio for Salmonella enteritidis. J Med Entomol 36:888–891
Grebenyuk RV, Chirov PA, Kadysheva AM (1972) The role of wild animals and blood-sucking arthropods in the epizootiology of infection with Listeria. Rol’ Dikikh Zhivotnykh i Krovososushchikh Chlenistonogikh v Epizootologii Listerioza. Frunze, Kirghiz SSR; Izdatel’stvo Ilim. Institut Biologii, Akademiya Nauk Kirgizskoi SSR, Frunze, Kirghiz, SSR. 124 pp
Kilpinen O (2005) How to obtain a bloodmeal without being eaten by a host: the case of poultry red mite, Dermanyssus gallinae. Physiol Entomol 30:232–240. doi:10.1111/j.1365-3032.2005.00452.x
Lacey RW (1993) Food-borne bacterial infections. Parasitology 107:S75–S93
Macaluso KR, Sonenshine DE, Ceraul SM, Azad AF (2001) Infection and transovarial transmission of rickettsiae in Dermacentor variabilis ticks acquired by artificial feeding. Vector-Borne Zoonot 1:45–53. doi:10.1089/153036601750137660
McAllister JC, Steelman CD, Skeeles JK (1994) Reservoir competence of the lesser mealworm (Coleoptera: Tenebrionidae) for Salmonella typhimurium (Eubacteriales: Enterobacteriaceae). J Med Entomol 31:369–372
Morse EV, Duncan MA (1974) Salmonellosis-an environmental health problem. JAMA 175:1015–1019
Nakajima Y, Ishibashi J, Yukuhiro F, Asaoka A, Taylor D, Yumakawa M (2003) Antibacterial activity and mechanism of action of tick defensin against Gram-positive bacteria. Biochim Biophys Acta 1624:125–130
Nordenfors H, Chirico J (2001) Evaluation of a sampling trap for Dermanyssus gallinae (Acari: Dermanyssidae). J Econ Entomol 94:1617–1621
Olsen AR, Hammack T (2000) Isolation of Salmonella sp. from the housefly, Musca domestica, and the dumpfly (Hydrotaea aenescens) (Wiedmann) (Diptera:Muscidae) at caged-layer houses. J Food Prot 63:958–960
Petrov D (1975) Study of Dermanyssus gallinae as a carrier of Pasteurella multocida. Veterinarno—Mededitsinski Nauki 12:32–36
Rennie L, Wilkinson PJ, Mellor PS (2001) Transovarial transmission of African swine fever virus in the argasid tick Ornithodoros moubata. Med Vet Entomol 15:140–146. doi:10.1046/j.1365-2915.2001.00282.x
Reshetnikov PT (1967) The mite Dermanyssus gallinae, a vector of fowl spirochaetosis. Veterinariia 44:48
Rodrigue DC, Tauxe RV, Rowe B (1990) International increase in Salmonella enteritidis: a new pandemic. Epidemiol Infect 105:21–27
Shirinov FB, Ibragimova AI, Misirov ZG (1972) The dissemination of the fowl-pox by the mite Dermanyssus gallinae. Veterinarya 4:48–49
Skov MN, Spencer AG, Hald B, Petersen L, Nauerby B, Carstensen B, Madsen M (2004) The role of litter beetles as potential reservoir for Salmonella enterica and thermophilic Campylobacter spp. between broiler flocks. Avian Dis 48:9–18. doi:10.1637/5698
Stackebrandt E, Goebel BM (1994) Taxonomic note: a place for DNA–DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol 44:846–849
Valiente Moro C, Chauve C, Zenner L (2005) Vectorial role of some Dermanyssoid mites (Acari, Mesostigmata, Dermanyssoidea). Parasite 12:99–109
Valiente Moro C, Chauve C, Zenner L (2007a) Experimental infection of Salmonella enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol 146:329–336. doi:10.1016/j.vetpar.2007.02.024
Valiente Moro C, Fravalo P, Amelot C, Chauve C, Salvat G, Zenner L (2007b) Colonization and organ invasion in chicks experimentally infected with Dermanyssus gallinae contaminated by Salmonella enteritidis. Avian Pathol 36:307–311. doi:10.1080/03079450701460484
Valiente Moro C, Desloire S, Vernozy-Rozand C, Chauve C, Zenner L (2007c) Comparison of the VIDAS system, FTA filter-based PCR and culture on SM ID for detecting Salmonella in Dermanyssus gallinae. Lett Appl Microbiol 44:431–436. doi:10.1111/j.1472-765X.2007.02119.x
Valiente Moro C, Desloire S, Chauve C, Zenner L (2007d) Detection of Salmonella sp. in Dermanyssus gallinae using an FTA filter-based PCR. Med Vet Entomol 21:148–152. doi:10.1111/j.1365-2915.2007.00684.x
Valiente Moro C, Normand P, Thioulouse J, Chauve C, Zenner L (2009) Bacterial taxa associated with the hematophagous mite, Dermanyssus gallinae detected by 16S rDNA PCR amplification and TTGE fingerprint. Res Microbiol 160:63–70. doi:10.1016/j.resmic.2008.10.006
Velge P, Cloeckaert A, Barrow P (2005) Emergence of Salmonella epidemics: the problems related to Salmonella enterica serotype Enteritidis and multiple antibiotic resistance in other major serotypes. Vet Res 36:267–288. doi:10.1051/vetres:2005005
Wegner Z (1976) Laboratory study on some parasitic hematophagous arthropods as possible subsidiary links of the biocenosis of tick-borne encephalitis. Bull Inst Marit Trop Med Gdynia 27:75–85
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173(2):697–703
Weyer F (1975) Beobachtungen uber das Verhalten des Q-Fieber-Erregers (Coxiella burneti) in der Lederzecke Ornithodoros moubata. Tropenmed Parasitol 26:219–231
Zeman P, Stika V, Skalka B, Bartik M, Dusbabek F, Lavickova M (1982) Potential role of Dermanyssus gallinae (De Geer, 1778) in the circulation of the agent of pullurosis-typhus in hens. Folia Parasitol (Praha) 29:371–374
Zemskaya AA, Pchelkina AA (1962) The relation of gamasid mites to the virus of tick-borne encephalitis. Medskaya Parazitology 31:439–442
Zemskaya AA, Pchelkina AA (1967) Gamasoid mites and Q fever. In: Markevich (ed) Problemy Parazitologii, Kiev, pp 258–259
Acknowledgments
This work was partly supported financially by the European Commission through the STREP project “RESCAPE”, contract no. 036018, under the 6th Framework Programme, priority 5, food quality and safety.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valiente Moro, C., De Luna, C.J., Tod, A. et al. The poultry red mite (Dermanyssus gallinae): a potential vector of pathogenic agents. Exp Appl Acarol 48, 93–104 (2009). https://doi.org/10.1007/s10493-009-9248-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-009-9248-0