Abstract
The green synthesized nanoparticles have been determined as a novel pesticide against arthropod pests. This study was designed to evaluate the in vitro acaricidal activity of green synthesized nickel oxide nanoparticles (NiO NPs) using aqueous extract of Melia azedarach ripened fruits against different developmental stages of the camel tick Hyalomma dromedarii in addition to their toxic effect on laboratory animals. The synthesized NiO NPs were characterized by UV–visible (UV–Vis) spectroscopy, Fourier transforms infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and energy dispersive X-ray spectroscopy (EDS). The UV–Vis spectra of the NiO NPs showed an absorption peak at 307 nm. FTIR analysis showed the possible functional groups used for capping and stabilization of NiO NPs with strong bands at 3416.2 and 1626.6 cm−1. The SEM images of the NiO NPs exhibited a size ranging from 21 to 35 nm. The immersion test was used for the in vitro application of the synthesized NiO NPs on the various tick stages (egg, nymph, larva, and adult). Mortality percentages and LC50 values of each tick stage were calculated. The oviposition and hatchability of the engorged females were monitored for the survived tick after treatment. The LC50 values for NiO NPs on embryonated eggs, larvae, and engorged nymphs were 5.00, 7.15, and 1.90 mg/mL, respectively. The egg productive index (EPI), egg number, and hatchability (%) were lower in females treated with the NiO NPs than in control ticks. The toxicity of the NiO NPs on laboratory animals was also investigated using Swiss albino mice by oral dose of 500 mg/kg/day administration for five consecutive days. The hematological, biochemical, and histopathological changes were evaluated. The hematological analysis showed significant increase in the level of white blood cells (WBC) and hemoglobin (Hb). Biochemical analysis showed non-significant decrease in alkaline phosphatase (ALP) and alanine amino transferase (ALT). We concluded that NiO NPs have a significant acaricidal activity as demonstrated on eggs, larvae, engorged nymphs, and fully fed females of H. dromedarii. From a toxicological point of view further in vivo investigations are needed to determine the mechanism of toxic effect of NiO NPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ticks are hematophagous ectoparasites of vertebrate animals. They transmit a variety of infectious diseases including babesiosis, anaplasmosis, ehrlichiosis, borreliosis, and rickettsioses (Abdel-Shafy et al. 2012; Guglielmone et al. 2014; Abdullah et al. 2016; Hassan et al. 2017). Ticks represent an important problem for farmers and veterinarians as they carry diseases that negatively impact the livestock industry (Shimizu et al. 2000). Hyalomma dromedarii is one of the most important tick species that affects camels in Egypt (Abdel-Shafy 2000). In its parasitic phase, it causes severe blood loss which results in anemia, weight loss, and a reduction in meat and milk production.
In general, parasite control relies on the use of chemical acaricides. However, continuous and indiscriminate use of these agents has many drawbacks including the development of chemical resistance in the tick population, contamination of food and the environment, and negative bystander effects on non-target organisms such as humans. Concerns about the safety and environmental impact of chemical acaricides warrant a search for alternative and sustainable methods for biological tick control using plant-derived materials (Chandler et al. 2000; Massoud et al. 2005; Abdel-Shafy et al. 2007).
Currently, nanotechnology is considered a novel approach for the treatment of animal parasites (Irache et al. 2011; Rai and Ingle 2012; Underwood and van Eps 2012). Nanoparticles (NPs) may be synthesized by a variety of methods (i.e., biologically, physically, and chemically). Physical and chemical methods require expensive equipment, a large area for equipment setup, high pressure and temperature, and toxic chemicals for capping and stabilizing the NPs, which is hazardous to the environment (Dhandapani et al. 2014; Chandrasekaran et al. 2016). To overcome these problems, green methods have recently been applied for the biocompatible synthesis of metal NPs (Sharmila et al. 2019).
Inorganic metal ions may be converted into metal NPs by secondary metabolites and proteins that are present in plants (Makarov et al. 2014). Secondary metabolites found in plants including polysaccharides, polyphenols, alkaloids, terpenoids, and other carbohydrates, play a vital role in reducing metal ions into NPs to maintain their stability (Aromal and Philip 2012). Therefore, using plant extracts for the bio-reduction of metal ions by adjusting reaction temperature and pH provides selective control of the shape and size of NPs, which results from a variety of metabolites.
Melia azedarach L. (Meliaceae) is one of the most important medicinal plants available and widely distributed in tropical and subtropical countries including African and Arab countries (Rubae 2009). Its leaves have been traditionally used to treat snake bites and skin infections (Handa et al. 2006). In addition, it has been reported to exhibit antiparasitic activity (Sousa et al. 2008, 2011; Wandscheer et al. 2004). Melia azedarach leaf extracts have been successfully used to synthesize various NPs including silver NPs (Mehmood et al. 2017; Chinnasamy et al. 2019; Jebril and Dridi 2020), CuO NPs (Khan and Mateen 2018), Pallidum NPs (Bhakyaraj et al. 2017), and ZnO NPs (Manokari et al. 2016). Furthermore, plant mediated synthesis of NiO NPs has been achieved from various medicinal plants including Nephelium lappaceum, Moringa oleifera, Callistemon viminalis, Tamarix serotine, and Geranium wallichianum (Yuvakkumar et al. 2014; Ezhilarasi et al. 2016; Sone et al. 2016; Nasseri et al. 2016; Abbasi et al. 2019).
Nickel NPs possess antiparasitic activities against the larvae of Rhipicephalus (Boophilus) microplus, Hyalomma anatolicum anatolicum, as well as the fourth larval instars of Culex quinquefasciatus, Culex gelidus, and Anopheles subpictus (Rajakumar et al. 2013). The rapid development and increased use of NPs has prompted toxicologists to evaluate their safety (Ahamed et al. 2008; Singh et al. 2009). It is important to evaluate NP toxicity to avoid any adverse effects on human health and the environment prior to embarking on widespread industrial application. Because of their small size, NPs are distributed to various regions following administration. They can pass through the intestine and further disseminate throughout the blood, brain, heart, lung, spleen, liver, and kidney upon oral administration (Hillyer and Albrecht 2001). Furthermore, after NP administration into the nasal cavity using the intratracheal instillation method, they accumulate in the lung, liver, and heart tissues (Adeel et al. 2020). NPs that accumulate in the liver caused toxicity in mice following oral administration (Chen et al. 2006; Wang et al. 2007). Several studies have evaluated the toxicity of NiO NPs and their biochemical effects after oral administration of 125, 250, and 500 mg/kg dose in female Wister rats. At high doses, a significant inhibition of red blood cells (RBCs) and brain acetylcholinesterase (AchE) was observed in the treated rats (Dumala et al. 2018). Dumala et al. (2019) demonstrated that repeated administration of 50, 100, and 200 mg/kg/day NiO NPs by oral gavage in Wister rats for 28 days resulted in a significant increase in platelets and white blood cell (WBC) count. Ajdari and Ghahnavieh (2014) studied the histopathological effects of nickel nanoparticles on the liver, lungs, and spleen tissues of male mice following an intraperitoneal injection of NPs at 75 ppm for 7 days. The liver experienced hyperemia, the spleen exhibited severe congestion, and interstitial fibrosis occurred in the lungs.
In the present study, we evaluated the acaricidal activity of green synthesized nickel oxide nanoparticles (NiO NPs) using aqueous extract of M. azedarach ripened fruits against the developmental stages of the camel ticks, H. dromderaii. The toxicological effects of biosynthesized nickel oxide nanoparticles in healthy adult mice were also evaluated.
Materials and methods
Preparation of plant materials
Aqueous extracts of M. azedarach fruits were used to synthesize nickel (II) oxide nanoparticles. The dried ripened fruits of M. azedarach were obtained from the Genetics and Cytology Department of the National Research Centre (Dokki, Giza, Egypt) and identified according to El-Hadidi and Boulos (1988). The fruits were ground using a stainless-steel knife mill. Fifteen g of fruit was soaked in 100 mL double-distilled water in a 250-mL Erlenmeyer flask. The solution was heated to70 ºC for 30 min (Osuntokun et al. 2019). Finally, the extract was cooled at room temperature and filtered through Whatman filter paper (#1) and stored at 4 °C until further use.
Green synthesis of nickel oxide nanoparticles
The green synthesis of NiO NPs was done according to the method described by Iqbal et al. (2019) with some modifications. Nickel nitrate hexahydrate [Ni(NO3)26H2O] was freshly prepared (0.1 M) using distilled water. Then, 80 mL of nickel nitrate solution was mixed with 20 mL of M. azedarach aqueous solution. The pH of the resulting solution mixture was adjusted to 7 and the mixture was incubated under continuous stirring at 60 °C and 1200 rpm for 5 h. A dark green color appeared which indicated the completed synthesis of NiO NPs. The solution was allowed to cool followed by washing and centrifugation three times at 3,000 rpm for 25 min using double-distilled water to remove impurities. The resulting pellets were dried in an oven at 80 °C for 6 h. The dried particles were collected in a ceramic crucible and calcined in a Muffle Furnace (Carbolite, CWF-1200) at 500 °C for 3 h. The samples were ground into fine powder and preserved in air-tight bottles until further characterization.
Characterization of green synthesized nanoparticles
The synthesized NiO NPs were characterized by various techniques including UV–Vis spectroscopy, scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS), and Fourier transform infrared spectroscopy (FTIR).
UV–visible spectroscopy
The biosynthesized NiO NPs were also characterized using a spectrophotometric assay. The form of the NiO NPs was determined by UV–visible spectroscopy (Jasco V-730 UV–Vis spectrophotometer) through a scanning wavelength range of 200–800 nm. The UV–visible spectral determination provides insight into the actual formation of the metal oxide nanoparticles through a surface plasmon resonance effect.
Fourier transform infrared spectroscopy (FTIR)
The green synthesized NiO NPs were analyzed by FTIR to determine the actual functional groups in the aqueous extract of M. azedarach which are involved in the capping and stabilization of NiO NPs. The analysis was done by mixing KBr salt with the samples. Infrared spectra were then recorded within a scan range of 400–4000 cm−1 resolution using a Jasco 6100-FTIR (National Research Centre) (Sharmila et al. 2019).
SEM and EDS
SEM (Quanta FEG-250) at the National Research Centre was used to detect the shape and size of the NiO NPs. The samples were placed on a carbon-coated copper grid and coated with a very thin layer of gold using a S15OA Sputter Coater to make the sample surface conductive and to prevent charge accumulation for obtaining a better contrast. EDS analysis was also performed to evaluate the elemental composition and purity of the NiO NPs.
Tick colony
Hyalomma dromedarii engorged females were collected from camels (Camelus dromidarius) from Berkash village (30°09′31.8″N, 31°01′17.0″E) in Giza, Egypt. These females were identified according to Walker et al. (2003) and maintained in plastic cups (one female/cup) at 25 ± 1 °C and 75–80% r.h. for oviposition in an incubator (Friocell, MMM, Germany). The eggs were collected daily and kept in the incubator. A portion of the eggs were used for the immersion test and the remainder were placed into labeled plastic cups, closed with nylon gauze, and maintained under the same conditions to hatch. A portion of the larvae were used for the larval immersion test, whereas the remainder were used to feed on rabbits using a capsule technique (Abdel-Shafy 2008) for obtaining engorged nymphs. The nymphs were divided into two groups: one group was used for the nymph immersion test and another group of nymphs was kept under the same conditions to obtain unfed adults. A portion of the unfed adults (males and females) was used for the adult immersion test, whereas the others were used to feed on rabbits to obtain engorged females for the bioassays.
Effect of green synthesized nickel oxide nanoparticles
The effect of NiO NPs on the developmental stages of H. dromedarii [embryonated eggs, engorged nymph, larvae, unfed adults (males and females) and engorged females] was evaluated. The nanoparticle solutions were diluted with 2% Tween 80 as a solvent to obtain the target concentrations. To avoid precipitation of the synthesized nanoparticles, particularly at higher concentrations, they were sonicated for 5 min before treatment. Initially, a pilot test was performed for each tick stage to select suitable concentrations for the experiments.
Egg immersion test (EIT)
To determine the acaricidal activities of the NiO NPs on H. dromedarii eggs, 300 embryonated eggs were used for each concentration and the control group (Abdel-Ghany et al. 2019). The eggs were divided into three replicates (100 eggs per replicate) and immersed for 1 min in 1 mL of NiO NP at a concentration of 32, 16, 8 and 4 mg/mL. Eggs immersed in 2% Tween 80 for 1 min were considered as a solvent control. The eggs were also immersed for 1 min in the reference acaricide, deltamethrin (Butox® 5%), at 1 mL/L. Subsequently, the solutions were removed, the tubes were closed with nylon gauze, and incubated at 25 ± 1 °C at 75–80% r.h. for 14 days. Dead eggs and hatched larvae were counted by using a binocular dissecting microscope and the percentage of mortality in the eggs was calculated.
Larval immersion test (LIT)
To determine the acaricidal activities of the NiO NPs on H. dromedarii larvae, approximately 300 larvae were divided into three replicates (100/replicate) and used for each concentration or control group. Then, the larvae were immersed in 1 mL of the NiO NPs (32, 16, 8 and 4 mg/mL) for 1 min. Larvae immersed in 2% Tween 80 for 1 min were considered as a solvent control. Furthermore, larvae were immersed in the reference acaricide, deltamethrin, at 1 mL/L for 1 min. The dead larvae were counted and mortality percentages after 24 h were calculated. The larvae unable to move after stimulation by breathing or with ataxia were considered dead.
Nymphal immersion test (NIT)
To evaluate the effect of NiO NPs on H. dromedarii nymphs, 30 engorged nymphs were divided into three replicates (10 per replicate). The nymphs were immersed in 5 mL of NiO NP solution (8, 4, 2, 1 mg/mL) for 1 min. Nymphs immersed in 2% Tween 80 for 1 min were considered as a control. The nymphs were also immersed in deltamethrin at 1 mL/L for 1 min. The nymphs were incubated under the previously described conditions. Nymphs that failed to molt were counted and their mortality percentages were calculated.
Adult immersion test (AIT)
Unfed adults
An adult immersion test was used to evaluate the acaricidal activity of NiO NPs on unfed adults (10-day-old ticks). Only the highest concentration of NiO NPs (32 mg/mL) was used based on the results of a pilot test. Thirty unfed adults were divided into three replicates (10 per replicate, five females and five males) for the used concentration or as a control group. The adults were immersed in 5 mL of the NiO NPs solution for 1 min. Unfed adults immersed in 2% Tween 80 for 1 min were used as a solvent control. Furthermore, the unfed adults were immersed for 1 min in deltamethrin at 1 mL/L. The solutions were removed, the ticks were dried on filter paper, placed in labeled cups, and placed into an incubator at 25 ± 1 °C and 75–80% r.h. The cups were monitored daily for 7 days and mortality was determined.
Engorged females
The acaricidal potential of NiO NPs on engorged H. dromedarii females fed on rabbits was determined by the adult immersion test according to Drummond et al. (1973) with slight modification. Three replicates were used for each concentration and each replicate consisted of three engorged females. The initial weight of each female was recorded before treatment and then immersed in 10 mL of NiO NPs solution (32, 16, 8, 4 mg/mL) for 1 min. Engorged females immersed in 2% Tween 80 for 1 min were considered solvent controls. Engorged females were also immersed in deltamethrin at 1 mL/L for 1 min.
Subsequently, the females were dried using Whatman filter paper and placed in separate labeled cups. The cups were tightly closed with nylon gauze and incubated under the aforementioned conditions. The tested female ticks were then incubated for 15 days and the mortality rate was recorded. After oviposition, the eggs were collected, weighed, and kept under the same conditions until hatching occurred. After 15 days, the tubes were monitored to observe the hatched larvae for both treatments and controls in order to estimate the hatching rates. The Egg Productive Index (EPI) and hatchability of laid eggs were calculated according to Abuowarda et al. (2020).
Toxicological effects of nickel oxide nanoparticles on Swiss albino mice
Mice were obtained from the National Research Centre and housed in the Departmental Animal House in plastic laboratory animal cages in a ventilated room at 26 ± 2 °C, 44–56% r.h., and L14:D10 h photocycle, respectively. The mice were provided with a standard rodent pellet diet. Food and water were provided ad libitum for all control and experimental groups.
Thirty adult male mice (20–25 g) were divided into three groups of 10 mice each. The first group represented a negative control group, the second group was a vehicle control group, and the third group was the NiO NPs treated group. The negative control group received no treatment in order to measure basic parameters and the vehicle control group received 2% Tween 80 by oral gavage. The treatment group received 500 mg/kg/day of nickel oxide nanoparticles for five successive days by oral gavage. This dose was selected based on a previous study conducted by Dumala et al. (2018). The NiO NPs were suspended in 2% Tween 80 and dispersed by ultrasonic vibration for 5 min before treatment. Behavioral changes, including food and water intake, as well as the appearance of symptoms in the mice were carefully monitored each day after NiO NPs administration. At day 10, blood samples were collected from the retro-orbital plexus. A portion of the fresh blood was collected in an EDTA solution and analyzed by counting erythrocytes, platelets, and total leukocytes using an automatic hematology counter (Medonic, NRC). Additional blood samples were collected without EDTA, kept at room temperature for 1 h, and centrifuged at 3000 rpm for 15 min at 4 °C to obtain serum. The serum was analyzed using an automatic biochemical analyzer for the determination of various biochemical parameters including alanine amino transferase (ALT), alkaline phosphatase (ALP), and creatinine (CR). A small piece of liver and kidney was fixed with 10% formalin, subjected to a dehydration procedure, processed in a tissue processor, and embedded in paraffin wax (El-Nasr for Chemicals and Medicals, Egypt). Sections of 5–8 µm thickness were prepared, stained with hematoxylin–eosin stain (Biodiagnostic, Giza, Egypt), and observed under a light microscope.
Statistical analysis
Data were analyzed by a one-way ANOVA test followed by Duncan’s test using IBM-SPSS v. 20. The lethal concentration (LC50) values were calculated by applying regression equation analysis to the probit transformed data of mortality. The dose response data were analyzed by the probit method (Finney 1962).
Results
Characterization of synthesized NiO NPs
UV–visible spectroscopy
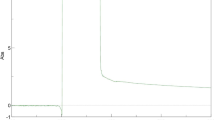
NiO NPs characterization were performed by UV–Vis spectral scanning at a wavelength of 200–800 nm. Figure 1 shows the results of the prepared NiO NPs. The UV–Vis spectra of the biosynthesized NiO NPs showed an absorption peak maximum at 307 nm which indicates the characteristic signature of NiO NPs.
Fourier transforms infrared spectroscopy (FTIR) analysis
FTIR analysis identifies the biomolecules used for capping and efficient stabilization of the NiO NPs that were synthesized using an aqueous extract of M. azedarach. It revealed intense peaks at 3416.28, 1626.66, 1421.28, 1334.5, 1245.7, 1147.4, 1101.15, 1016.3, 958.4, 891.9, 767.5, 643.1, and 534.1 cm−1 (Fig. 2). The peak at 3416.2 cm−1 indicated the presence of O–H stretching corresponding to poly phenols. The peak at 1626.6 cm−1 was attributed to the C=O stretching vibration of primary amines. The sharp peak at 1421.2 cm−1 indicated a C–C stretch (in ring) in aromatics. The peak at 1334.5 cm−1 indicated C-H stretching vibrations of the alkene group. The peak at 1101.15 cm−1 corresponded to a C-O bond attributed to the characteristic group of alcohols and carboxylic acids. The peak at 1016 cm−1 resulted from a stretching amine and the five peaks at 958.4, 891.9, 767.5, 643.1, and 534.1 cm−1 resulted from a C–N stretching amine group. Thus, the FTIR analysis revealed the presence of C=O, C–N, C–H, and O–H groups, corresponding to the presence of metabolites and proteins that are responsible for the reduction and stabilization of the synthesized nanoparticles.
SEM and EDS
The synthesized NiO NPs were further characterized by SEM analysis. SEM determines the size and surface morphology of the formed nanoparticles. The SEM images of the NiO NPs exhibited a size ranging from 21 to 35 nm (Fig. 3a, b) with EDS (Fig. 3c). The particles were predominantly spherical in shape. Moreover, the purity of the NiO NPs was estimated by SEM–EDS. EDS analysis of NiO NPs revealed two peaks, one for nickel and another for oxygen. EDS results confirmed that the NiO NPs were free from any impurities and composed of 12.25% oxygen and 87.75% nickel.
Effect of synthesized nickel oxide nanoparticles.
Embryonated eggs
Table 1 shows the acaricidal effects of green synthesized NiO NPs on the embryonated eggs. The results indicated a significant effect of synthesized NiO NPs on the embryonated eggs compared with the reference acaricide (deltamethrin) and the control. Mortality increased concomitantly with increased NiO NPs concentration. NiO NPs exhibited strong ovicidal effects compared with deltamethrin. At the highest concentration (32 mg/mL) of NiO NPs, mortality reached 100%, whereas at the lowest NiO NPs concentration (4 mg/mL), the mortality rate was 45%. At concentrations of 16 and 8 mg/mL NiO NPs, the mortality rate was 92.1 and 72.7%, respectively. The calculated LC50 and LC90 values for the embryonated eggs exposed to NiO NPs were 5.00 and 15.90 mg/mL, respectively (Table 1).
Larvae
The results indicated that there was a significant effect of NiO NPs on H. dromedarii larvae after 24 h of treatment compared with the reference acaricide (deltamethrin) and the control as shown in Table 1. The mortality percentage increased in a dose-dependent manner. NiO NPs exhibited a significant larvicidal effect, especially at the highest concentration (32 mg/mL) and the mortality percentage reached 100%. This finding is similar to that of the reference acaricide (deltamethrin). At a concentration of 16, 4, and 8 mg/mL NiO NPs, the mortality percentage was 75.9, 52.5, and 30.2%, respectively. The calculated LC50 value for NiO NPs was 7.15 mg/mL and the LC90 was 25.88 as shown in Table 1.
Nymphs
A significant effect of NiO NPs on the molting of engorged nymphs of H. dromedarii was observed compared with the reference acaricide (deltamethrin) and control as shown in Table 1. The synthesized NiO NPs exhibited a higher effect compared with deltamethrin, especially at concentrations higher than 2 mg/mL. At the highest NiO NPs concentration (8 mg/mL), the mortality rate reached 92.5%. At the other concentrations (4, 2, and 1 mg/mL NiO NPs), the mortality percentage was 82.5, 50, and 23.3%, respectively. The LC50 and LC90 values for NiO NPs were 1.90 and 5.12 mg/mL, respectively (Table 1).
Unfed adults
Unfed H. dromedarii adults did not show mortality even at the highest concentration of NiO NPs (32 mg/mL).
Reproductive performance of engorged females
The reproductive performance (including EPI, egg number, and hatchability) of H. dromedarii engorged females exposed to NiO NPs was evaluated (Table 2). The EPI of the treated females increased as particle concentration decreased. Engorged females treated with NiO NPs at a concentration ranging from 32 to 4 mg/mL yielded an EPI ranging from 0.377 ± 0.023 to 0.575 ± 0.020 compared with 0.15 ± 0.017 for deltamethrin treatment and 0.600 ± 0.054 for the control group. The egg number for the engorged females treated with the NiO NPs ranged from 3627.4 ± 157.2 to 4960.5 ± 325.3 compared with 623.7 ± 226.3 following deltamethrin treatment and 5380.26 ± 96.68 for the control group (Table 2). The eggs laid by females exposed to the NiO NPs exhibited a lower hatchability, ranging from 58.8 to 91.9% (Table 2).
Toxicity of nickel oxide nanoparticles on Swiss albino mice
Hematological changes
Changes in hematological parameters in mice after an oral administration of NiO NPs suspension are shown in Table 3. The level of WBCs (12.5 × 109/L), RBCs (8.7 × 1012/L), hematocrit (HCT) (44.6%), and hemoglobin (Hb) (14.6 g/dL) were elevated, whereas the platelet count (986.3 × 109/L) was decreased compared with the negative control group [WBCs (5.13 × 109/L), HCT (39.9%), Hb (11.93 g/dL), and platelets (1112 × 109/L)]. Increased level of WBC and Hb was statistically significant but increase or decrease in other parameters were not significant compared with that in the control group. The vehicle control group exhibited a normal blood profile compared with the negative control group.
Biochemical changes
Changes in biochemical parameters in the serum of the mice after oral administration of NiO NP suspension are shown in Table 4. The effect of the NiO NPs was evaluated using various hepatic and renal parameters including ALT and ALP for liver function and CR as a surrogate for kidney function. The level of ALP (53 U/L) and ALT (46 U/L) was decreased compared with the negative control group (85 U/L for ALP and 67 U/L for ALT), but it was statistically insignificant. The vehicle control group also showed decreased ALP (74 U/L) and ALT (41.6 U/L) levels that were similar to the NiO NPs-treated group. With respect to renal function, the creatinine level was 0.43 and 0.40 mg/dL for the NiO NPs and vehicle control groups, respectively, and was not significantly increased compared with the negative control group (0.33 mg/dL).
Histopathological examination of the liver and kidney of mice
The typical histopathological changes in liver and kidney after an oral administration of NiO NPs are shown in (Figs. 4 and 5). The liver of the mice treated with NiO NPs exhibited dilation and congestion of the central vein and the presence of inflammatory cells (Fig. 4c). Furthermore, the portal area showed aggregation of inflammatory cells, primarily lymphocytes, and macrophages (white head arrow, Fig. 4d). The liver of the vehicle control group showed primarily a focal aggregation of lymphocytes in the hepatic parenchyma (white arrow, Fig. 4b). The kidneys of the mice treated with NiO NPs exhibited hemolysis as well as glomerular segmentation (white head arrow, Fig. 5c). The vehicle control group showed mainly focal aggregation of lymphocytes in the kidney tissue (white arrow, Fig. 5b).
Histopathological changes in the liver of mice treated with NiO NPs. a Control negative group showing normal architecture of the liver. b Vehicle control showing aggregation of lymphocytes (white arrow). c NiO NPs treated group showing dilated and congested central vein. d Aggregation of inflammatory cells in the portal area (white arrow heads). Magnification ×200
Histopathological changes in the kidney of mice treated with NiO NPs. a Control negative group showing normal features of the kidney. b Vehicle control group showing aggregation of lymphocytes (white arrows). c NiO NPs treated group showing hemolysis and glomerular segmentation (white arrow heads). Magnification ×100
Discussion
Nanoparticles are present everywhere in the environment, particularly in the air, medical devices, nanotechnology structures, and even food (Kong et al. 2011). Several trials have been done to evaluate nanomaterials for diagnostics and treatment in veterinary medicine (Kumar 2010). Green synthesis of nanoparticles is considered safe and nontoxic and this approach eliminates problems associated with classic chemical and physical methods. Secondary metabolites of plants responsible for metallic ion reduction result in faster synthesis of nanoparticles compared with synthesis using chemicals and microbes (Sathishkumar et al. 2009). In previous studies, green synthesized nanoparticles exhibited acaricidal activity against the ixodid tick species. The acaricidal activity of green synthesized silver nanoparticles, prepared from an aqueous extract of Mimosa pudica leaves against R. (B.) microplus larvae, was evaluated by Marimuthu et al. (2011) and exhibited an LC50 of 8.98 mg/L. Titanium dioxide nanoparticles synthesized using Calotropis gigantea exhibited acaricidal activity against R. (B.) microplus larvae with an LC50 of 5.43 mg/L (Marimuthu et al. 2013). A study conducted by Gandhi et al. (2017) revealed larvicidal activity of green synthesized ZnO NPs prepared using Momordica charantia leaf extract against R. (B.) microplus larvae with an LC50 value of 6.87 mg/L. Rajakumar et al. (2015) evaluated green synthesized titanium dioxide nanoparticles using Mangifera indica leaf extract against larvae of R. (B.) microplus, H. a. anatolicum, and Haemaphysalis bispinosa which yielded LC50 values of 28.56, 33.17, and 23.81 mg/L, respectively. Green synthesized Ag NPs using aqueous leaf extracts of Ocimum canum against H. a. anatolicum and Hyalomma marginatum isaaci was investigated by Jayaseelan and Rahuman (2012) resulted in LC50 values of 0.78 and 1.00 mg/L, respectively. These studies were done mainly with a single stage of ixodid tick species using various types of synthesized nanoparticles.
The present study evaluated the acaricidal activity of green synthesized NiO NPs using an aqueous extract from M. azedarach ripened fruits against various developmental stages of the camel tick, H. dromedarii. There have been no studies thus far evaluating the acaricidal activity of these green synthesized NiO NPs using aqueous extracts of M. azedarach against the camel tick H. dromedarii. However, there is one study of Ni NPs synthesized by chemical methods showing acaricidal activity against the larvae of R. (B.) microplus (Rajakumar et al. 2013). Our study evaluated NiO NP activity against active stages (on host) including larvae, unfed adults, engorged females, and dormant stages including embryonated eggs and engorged nymphs of H. dromedarii, as sustainable tick control must be on and off the host as previously mentioned by Abdel-Ghany et al. (2019).
Nickel oxide NPs exhibited high acaricidal activity against embryonated eggs, larvae, engorged nymphs, and engorged females, however, no mortality was observed in unfed adults. This may be attributed to its highly chitinized cuticles. The green synthesized NiO NPs showed high ovicidal activity against H. dromedarii eggs which reached 100% at a concentration of 32 mg/mL. Engorged nymphs were more sensitive compared with other developmental stages with mortality percentage of 92% at 8 mg/mL. Furthermore, NiO NPs exerted significant larvicidal activity with a mortality percentage of 100% at a concentration of 32 mg/mL. The LC50 values confirmed that the toxicity of NiO NPs was high against H. dromedarii nymphs followed consecutively by eggs and larvae. When compared with a reference acaricide (deltamethrin), the NiO NPs exhibited a higher effect against eggs and nymphs and a similar effect against larvae. The effect of NiO NPs on larvae was consistent with that of Rajakumar et al. (2013) who found that Ni NPs synthesized by a chemical method had larvicidal activity against R. (B.) microplus and H. a. anatolicum (100% mortality at 25 mg/L). The chemically synthesized ZnO NPs had significant acaricidal activity against R. (B.) microplus larvae with an LC50 of 13.41 mg/L (Kirthi et al. 2011). Copper nanoparticles synthesized by a polyol process exhibited an LC50 of 1.06 mg/L against the larvae of R. (B.) microplus (Ramyadevi et al. 2011).
In the present study, the green synthesized NiO NPs did not cause mortality for engorged females, but mainly exhibited activity on their fecundity. The EPI, egg number, and hatchability percentage of the full engorged females treated with NiO NPs and the reference acaricide (deltamethrin) were somewhat lower compared with that of the control group. These results were consistent with the study by Arafa et al. (2019) on the acaricidal activity of Ag NPs synthesized by a chemical reduction method and ZnO NPs synthesized by a hydrothermal method on adult of R. annulatus ticks. A low mortality percentage was observed for the engorged females as well as a low reduction in the percentage of eggs laid by treated females. Furthermore, they showed that treatment with both NPs resulted in 20% mortality at a concentration of 400 mg/L for Ag NPs and 8 g/L for ZnO NPs, as well as a reduction of the egg number by 20.3%. In contrast, Banumathi et al. (2016) evaluated the acaricidal activity of green synthesized ZnO NPs using Lobelia leschenaultiana leaf extract against R. (B.) microplus engorged adults, which resulted in 100% mortality at a concentration of 0.008 mg/L. Another study by Norouzi et al. (2019) evaluated Hyalomma spp. using Zn NPs produced by an evaporation process and resulted in 100% mortality by a spraying method and 87.7% by a contact method at a concentration of 250 mg/mL.
UV–vis spectroscopy was initially used to evaluate the production and stability of metal oxide nanoparticles in aqueous solution. The formation of NiO NPs was confirmed by the presence of an absorption peak at 307 nm, which is in good agreement with Mamuru and Jaji (2015). FTIR spectroscopy detects biomolecules that are responsible for the reduction of metal salts to metal NPs and the capping effect of these biomolecules. The peak obtained at 1626.6 cm−1 was attributed to the C=O stretching vibration of primary amines. The sharp peak at 1421.2 cm−1 indicated a C–C stretch (in ring) in aromatics. The peak at 1334.5 cm−1 indicated C-H stretching vibrations of the alkene group (Rastogi and Arunachalam 2011). The peak at 1016 cm−1 resulted from a stretching amine and the five peaks at 958.4, 891.9, 767.5, 643.1, and 534.1 cm−1 resulted from a C-N stretching amine group (Litvin and Minaev 2013). The FTIR analysis revealed the presence of C=O, C–N, C–H, and O–H groups, which corresponds to the presence of metabolites and proteins that are responsible for the reduction and stabilization of the synthesized nanoparticles. Furthermore, the morphology and size of the NiO NPs was determined by SEM. The shape of NiO NPs appeared spherical at 500 °C and a size ranging from 21 to 35 nm. The EDS analysis showed that both nickel and oxygen were present in the sample, whereas no other elements confirmed the single crystalline nature of NiO NPs. These obtained results were in accordance with Abbasi et al. (2019).
The actual mode of action of the synthesized nanoparticles remains unknown. Speculation about the acaricidal effects includes their small size which may facilitate penetration through the nuclear membrane, followed by interaction with nucleic acids and proteins (Benelli et al. 2017). Furthermore, the adhesion of the green synthesized nanoparticles to the exoskeleton of the ectoparasites may reduce their physical movements (Baun et al. 2008). Accordingly, metal and metal oxide nanoparticles should be further studied as potential acaricides and further research should focus on their mechanism of action. Furthermore, the extensive use of synthesized nanoparticles warrants a safety evaluation by toxicologists. Different studies conducting in vitro tests of NiO NPs on mammalian cells documented a toxicological response (Horie et al. 2009, 2011; Siddiqui et al. 2012; Ahamed et al. 2013). When the nanoparticles are ingested, they are distributed to various regions because of their small size. They pass through the intestine and further disseminate into the blood, brain, heart, lung, spleen, liver, and kidney (Hillyer and Albrecht 2001). The toxicity of NPs may result from the production of intracellular reactive oxygen species (ROS) causing leakage of the plasma membrane, mitochondrial dysfunction, and cell death. The superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) are three primary ROS scavengers. A study conducted by Adeel et al. (2019) evaluated the effect of NiO NPs on the earthworm (Eisenia fetida) after exposure to 0, 5, 50, 200, 500, and 1000 mg kg−1of soil. Exposure to 500 and 1000 mg kg−1 NiO NPs resulted in lower SOD and CAT activity in earthworm tissues, thus the cellular antioxidant defense systems failed to reduce excessive ROS and oxidative stress in the earthworm.
In the present study, we have conducted an experiment on mice to detect the ability of NiO NPs to induce changes in the biochemistry and histopathology of liver and kidney tissues following their uptake by the gastrointestinal tract. Hematological indices revealed increased levels of WBCs, RBCs, Hb, and HCT, whereas the platelet count was decreased. Our results were in accordance with Dumala et al. (2019) who reported increased levels of WBC in albino Wister rats after repeated oral administration of NiO NPs for 28 days. Magaye et al. (2014) showed that NiO NPs increased the WBC count significantly in rats following intravenous administration. Increased levels of WBCs confirmed that there was activation of the innate immune system (Gui et al. 2011). In addition to increased levels of WBCs, RBC, and HCT levels were also increased which was concurrent with a significant increase in Hb concentration. The increase in RBC count (polycythemia) in the circulation occurs in cases of decreased oxygen levels and triggers increased erythropoietin secretion in the kidney. The polycythemia may have been a consequence of low oxygen reaching the alveoli because of pulmonary disease (Kohler and Dellweg 2010). These results are in agreement with a study by Magaye et al. (2014) in which the effect of NiO NPs on rats after an intravenous administration resulted in increased levels of WBCs, RBCs, and Hb. The biochemical analysis of serum from the mice treated with NiO NPs revealed decreased levels of ALP and ALT. The current findings are in contrast with the study of Dumala et al. (2019) who reported increased levels of ALP and ALT after repeated oral administration of NiO NPs to mice for 28 days. This difference may result from a long period of NiO NP administration in the previously mentioned study.
The histopathological study of liver showed some alterations including congestion and dilation of the central vein and aggregation of inflammatory cells in the portal area. Furthermore, the kidneys exhibited alterations including hemolysis and glomerular segmentation. The histopathological results of this study are consistent with a study conducted on NiO NPs toxicity after repeated oral administration for 14 days that resulted in hepatocyte damage, lymphocytic infiltration, dilation of blood sinusoids, enhanced Kupffer cells, and extensive vacuolization in the cytoplasm of hepatocytes in liver tissue (Saquib et al. 2017). Another study conducted by Dumala et al. (2019) on NiO NPs toxicity in albino Wister rats showed adverse effects on liver tissue including sinusoidal dilation and inflammatory cell infiltration. In addition, alterations in kidney tissues including glomerular sclerosis and degeneration of tubular cells were observed. A similar study evaluated the toxic effects of ZnO NPs after an oral administration for 5 days. The liver tissue exhibited cellular necrosis and congestion and the kidneys suffered glomerular segmentation (Esmaeillou et al. 2013).
The toxicological studies of NiO NPs in laboratory animals demonstrated variable toxic effects on hematological and biochemical parameters as well as variable histopathological lesions in different organs. This may depend on whether the route of NPs administration was oral or intravenous which results in the absorption of large amounts of the nanoparticles. In contrast, the external route of administration, such spraying the body of the animals, results in the absorption of small amounts of NPs, so its toxic effect is lower compared with other routes of administration. The NiO NPs used in this study exhibit acaricidal activity in vitro against various developmental stages of the camel tick, H. dromedarii. Further studies are needed to evaluate their acaricidal activity in vivo using external routes of administration, such as spraying.
Conclusion
Green synthesis of NiO NPs using aqueous extract of M. azedarach ripened fruits exhibited good acaricidal activity against various developmental stages of the camel tick H. dromedarii. NiO NPs were more toxic on nymph followed by eggs, larvae, and engorged females. This study provides valuable insight on the toxicity of NiO NPs after oral administration for five consecutive days. There was increase in some hematological parameters as WBC, RBC, HCT and Hb. Furthermore, ALP and ALT was decreased. In addition to histopathological lesions observed in liver as congestion and dilation of central vein as well as aggregation of inflammatory cells in the portal area. This study concluded that the green synthesized NiO NPs using an aqueous extract of M. azedarach could be considered as a promising alternative for the current chemical acaricides in the control of H. dromedarii ticks.
References
Abbasi BA, Iqbal J, Mahmood T, Ahmad R, Kanwal S, Afridi S (2019) Plant-mediated synthesis of nickel oxide nanoparticles (NiO) via Geranium wallichianum: characterization and different biological applications. Mater Res Express 6:0850a7
Abdel-Ghany HS, Fahmy MM, Abuowarda MM, Abdel-Shafy S, El-Khateeb RM, Hoballah EM (2019) In vitro acaricidal effect of Melia azedarach and Artemisia herba-alba extracts on Hyalomma dromedarii (Acari: Ixodidae): embryonated eggs and engorged nymphs. J Parasitic Dis 43:696–710
Abdel-Shafy S (2000). Microbiological and control studies on ticks infesting farm animals and poultry. PhD Thesis, Faculty of Agriculture, Cairo University
Abdel-Shafy S (2008) Scanning electron microscopy and comparative morphology of Hyalomma anatolicum excavatum, H. dromedarii and H. marginatum marginatum (Acari: Ixodidae) based on larvae. Acarologia 48:19–31
Abdel-Shafy S, Soliman MM, Habeeb SM (2007) In vitro acaricidal effect of some crude extracts and essential oils of wild plants against certain tick species. Acarologia 47:33–42
Abdel-Shafy S, Allam NA, Mediannikov O, Parola P, Raoult D (2012) Molecular detection of spotted fever group rickettsiae associated with ixodid ticks in Egypt. Vector Borne Zoonotic Dis 12:346–359
Abdullah HH, El-Molla A, Salib FA, Allam NA, Ghazy AA, Abdel-Shafy S (2016) Morphological and molecular identification of the brown dog tick Rhipicephalus sanguineus and the camel tick Hyalomma dromedarii (Acari: Ixodidae) vectors of Rickettsioses in Egypt. Vet World 9:1087–1101
Abuowarda MM, Haleem MA, Elsayed M, Farag H, Magdy S (2020) Bio-pesticide control of the brown dog tick (Rhipicephalus sanguineus) in Egypt By Using Two Entomopathogenic Fungi (Beauveria bassiana and Metarhizium anisopliae). Int J Vet Sci 9:175–181
Adeel M, Ma C, Ullah S, Rizwan M, Hao Y, Chen C, Tsang DC (2019) Exposure to nickel oxide nanoparticles insinuates physiological, ultrastructural and oxidative damage: a life cycle study on Eisenia fetida. Environ Pollut 254:113032
Adeel M, Tingting J, Hussain T, He X, Ahmad MA, Irshad MK, Zhiyong Z (2020) Bioaccumulation of ytterbium oxide nanoparticles insinuate oxidative stress, inflammatory, and pathological lesions in ICR mice. Environ Sci Pollut Res 27:32944–32953
Ahamed M, Karns M, Goodson M, Rowe J, Hussain SM, Schlager JJ, Hong Y (2008) DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol Appl Pharmacol 233:404–410
Ahamed M, Ali D, Alhadlaq HA, Akhtar MJ (2013) Nickel oxide nanoparticles exert cytotoxicity viaoxidative stress and induce apoptotic response in human liver cells (HepG2). Chemosphere 93:2514–2522
Ajdari M, Ghahnavieh MZ (2014) Histopathology effects of nickel nanoparticles on lungs, liver, and spleen tissues in male mice. Int Nano Lett 4:113
Arafa WM, Mohammed AN, El-Ela FIA (2019) Acaricidal efficacy of deltamethrin-zinc oxide nanocomposite on Rhipicephalus (Boophilus) annulatus tick. Vet Parasitol 268:36–45
Aromal SA, Philip D (2012) Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity. Spectrochim Acta A Mol Biomol Spectrosc 97:1–5
Banumathi B, Malaikozhundan B, Vaseeharan B (2016) In vitro acaricidal activity of ethnoveterinary plants and green synthesis of zinc oxide nanoparticles against Rhipicephalus (Boophilus) microplus. Vet Parasitol 216:93–100
Baun A, Hartmann NB, Grieger K, Kusk KO (2008) Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology 17:387–395
Benelli G, Maggi F, Pavela R, Murugan K, Govindarajan M, Vaseeharan B, Petrelli R, Cappellacci L, Kumar S, Hofer A, Youssefi MR, Alarfaj AA, Hwang JS, Higuchi A (2017) Mosquito control with green nanopesticides: towards the One Health approach? A review of non-target effects. Environ Sci Pollut Res 25:10184–10206
Bhakyaraj K, Kumaraguru S, Gopinath K, Sabitha V, Kaleeswarran PR, Karthika V, Arumugam A (2017) Eco-friendly synthesis of palladium nanoparticles using Melia azedarach leaf extract and their evaluation for antimicrobial and larvicidal activities. J Cluster Sci 28:463–476
Chandler D, Davidson G, Pell JK, Ball BV, Shaw K, Sunderland KD (2000) Fungal biocontrol of acari. Biocontrol Sci Technol 10:357–384
Chandrasekaran R, Gnanasekar S, Seetharaman P, Keppanan R, Arockiaswamy W, Sivaperumal S (2016) Formulation of Carica papaya latex-functionalized silver nanoparticles for its improved antibacterial and anticancer applications. J Mol Liq 219:232–238
Chen HW, Su SF, Chien CT, Lin WH, Yu SL, Chou CC, Chen JJ, Yang PC (2006) Titanium dioxide nanoparticles induce emphysema-like lung injury in mice. FASEB J 20:2393–2395
Chinnasamy G, Chandrasekharan S, Bhatnagar S (2019) Biosynthesis of silver nanoparticles from Melia azedarach: Enhancement of antibacterial, wound healing, antidiabetic and antioxidant activities. Int J Nanomed 14:9823
Dhandapani P, Siddarth AS, Kamalasekaran S, Maruthamuthu S, Rajagopal G (2014) Bio-approach: ureolytic bacteria mediated synthesis of ZnO nanocrystals on cotton fabric and evaluation of their antibacterial properties. Carbohydr Polym 103:448–455
Drummond RO, Ernst SE, Trevino JL, Gladney WJ, Graham OH (1973) Boophilus annulatus and B. microplus: laboratory tests of insecticides. J Econ Entomol 66:130–133
Dumala N, Mangalampalli B, Kalyan Kamal SS, Grover P (2018) Biochemical alterations induced by nickel oxide nanoparticles in female Wistar albino rats after acute oral exposure. Biomarkers 23:33–43
Dumala N, Mangalampalli B, Kalyan Kamal SS, Grover P (2019) Repeated oral dose toxicity study of nickel oxide nanoparticles in Wistar rats: a histological and biochemical perspective. J Appl Toxicol 39:1012–1029
El-Hadidi MN, Boulos L (1988). The street trees of Egypt, no. revised edn. American University in Cairo Press, Cairo
Esmaeillou M, Moharamnejad M, Hsankhani R, Tehrani AA, Maadi H (2013) Toxicity of ZnO nanoparticles in healthy adult mice. Environ Toxicol Pharmacol 35:67–71
Ezhilarasi AA, Vijaya JJ, Kaviyarasu K, Maaza M, Ayeshamariam A, Kennedy LJ (2016) Cytotoxicity effect of nanoparticles against HT-29 cancer cells. J Photochem Photobiol B Biol 164:352–360
Finney DJ (1962) Probit analysis a statistical treatment of the response curve. Cambridge University Press, Cambridge
Gandhi PR, Jayaseelan C, Mary RR, Mathivanan D, Suseem SR (2017) Acaricidal, pediculicidal and larvicidal activity of synthesized ZnO nanoparticles using Momordica charantia leaf extract against blood feeding parasites. Exp Parasitol 181:47–56
Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada Pena A, Horak IG (2014) Hard ticks (Acari: Ixodida: Ixodidae) of the world. Springer, Heidelberg
Gui S, Zhang Z, Zheng L, Cui Y, Liu X, Li N, Sang X, Sun Q, Gao G, Cheng Z, Cheng J, Wang L, Tang M, Hong F (2011) Molecular mechanism of kidney injury of mice caused by exposure to titanium dioxide nanoparticles. J Hazard Mater 195:365–370
Handa SS, Rakesh DD, Vasisht K (2006). Compendium of medicinal and aromatic plants. United Nations Industrial Development Organization and the International Centre for Science and High Technology, pp 16–286
Hassan MI, Gabr HSM, Abdel-Shafy S, Hammad KM, Mokhtar MM (2017) Molecular detection of Borrelia sp. In Ornithodoros savignyi and Rhipicephalus annulatus by flab gene and Babesia bigemina in R. annulatus by 18s rRNA gene. J Egypt Soc Parasitol 47:403–414
Hillyer JF, Albrecht RM (2001) Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J Pharm Sci 90:1927–1936
Horie M, Nishio K, Fujita K, Kato H, Nakamura A, Kinugasa S, Endoh S, Miyauchi A, Yamamoto K, Murayama H (2009) Ultrafine NiO particles induce cytotoxicity in vitro by cellular uptake and subsequent Ni (II) release. Chem Res Toxicol 22:1415–1426
Horie M, Fukui H, Nishio K, Endoh S, Kato H, Fujita K, Miyauchi A, Nakamura A, Shichiri M, Ishida N (2011) Evaluation of acute oxidative stress induced by NiO nanoparticles in vivo and in vitro. J Occup Health 53(2):64–74
Iqbal J, Abbasi BA, Mahmood T, Hameed S, Munir A, Kanwal S (2019) Green synthesis and characterizations of Nickel oxide nanoparticles using leaf extract of Rhamnus virgata and their potential biological applications. Appl Organomet Chem 33:e4950
Irache JM, Esparza I, Gamazo C, Agueros M, Espuelas S (2011) Nanomedicine: novel approaches in human and veterinary therapeutics. Vet Parasitol 180:47–71
Jayaseelan C, Rahuman AA (2012) Acaricidal efficacy of synthesized silver nanoparticles using aqueous leaf extract of Ocimum canum against Hyalomma anatolicum anatolicum and Hyalomma marginatum isaaci (Acari: Ixodidae). Parasitol Res 111:1369–1378
Jebril S, Dridi C (2020) Green synthesis of silver nanoparticles using Melia azedarach leaf extract and their antifungal activities: in vitro and in vivo. Mater Chem Phys 248:122898
Khan TM, Mateen A (2018) Synthesis of CuO nanoparticles by using leaf extracts of Melia azedarach and Morusnigra and their antibacterial activity. J Innov Sci 4:120–129
Kirthi AV, Rahuman AA, Rajakumar G, Marimuthu S, Santhoshkumar T, Jayaseelan C, Velayutham K (2011) Acaricidal, pediculocidal and larvicidal activity of synthesized ZnO nanoparticles using wet chemical route against blood feeding parasites. Parasitol Res 109:461–472
Kohler D, Dellweg D (2010) Polycythemia. Dtsch Med Wochenschr 135:2300–2303
Kong B, Seog JH, Graham LM, Lee SP (2011) Experimental considerations on the cytotoxicity of nanoparticles. Nanomed J 6:929–941
Kumar S (2010) Nanotechnology and animal health. Vet World 3:567–569
Litvin VA, Minaev BF (2013) Spectroscopy study of silver nanoparticles fabrication using synthetic humic substances and their antimicrobial activity. Spectrochim Acta Part A Mol Biomol Spectrosc 108:115–122
Magaye RR, Yue X, Zou B, Shi H, Yu H, Liu K, Zhao J (2014) Acute toxicity of nickel nanoparticles in rats after intravenous injection. Int J Nanomed 9:1393
Makarov VV, Love AJ, Sinitsyna OV, Makarova SS, Yaminsky IV, Taliansky ME, Kalinina NO (2014) Green nanotechnologies: synthesis of metal nanoparticles using plants. Acta Nat 6:20
Mamuru SA, Jaji N (2015) Voltammetric and impedimetric behaviour of phytosynthesized nickel nanoparticles. J Nanostruct Chem 5:347–356
Manokari M, Ravindran CP, Shekhawat MS (2016) Biosynthesis of zinc oxide nanoparticles using Melia azedarach L. extracts and their characterization. Int J Pharm Sci Res 1:31–36
Marimuthu S, Rahuman AA, Rajakumar G, Santhoshkumar T, Kirthi AV, Jayaseelan C, Bagavan A, Zahir AA, Elango G, Kamaraj C (2011) Evaluation of green synthesized silver nanoparticles against parasites. Parasitol Res 108:1541–1549
Marimuthu S, Rahuman AA, Jayaseelan C, Kirthi AV, Santhoshkumar T, Velayutham K, Bagavan A, Kamaraj C, Elango G, Iyappan M, Siva C, Karthik L, Rao KV (2013) Acaricidal activity of synthesized titanium dioxide nanoparticles using Calotropis gigantea against Rhipicephalus microplus and Haemaphysalis bispinosa. Asian Pac J Trop Med 6:682–688
Massoud AM, Kutkat MA, Abdel-Shafy S, El-Khateeb RM, Labib IM (2005) Acaricidal efficacy of Myrrh (Commiphora molmol) on the fowl tick Argas persicus (Acari: Argasidae). J Egypt Soc Parasitol 35:667–686
Mehmood A, Murtaza G, Bhatti TM, Kausar R (2017) Phyto-mediated synthesis of silver nanoparticles from Melia azedarach L. leaf extract: characterization and antibacterial activity. Arab J Chem 10:S3048–S3053
Nasseri MA, Ahrari F, Zakerinasab B (2016) Synthesis of nano-spherical nickel by templating hibiscus flower petals. J Nanosci Nanotechnol Appl Organomet Chem 30:978–984
Norouzi R, Ataei A, Hejazy M, Shahbazi P (2019) Acaricidal activity of zinc oxide nanoparticles against Hyalomma spp. in vitro. Nanomed Res J 4:234–238
Osuntokun J, Onwudiwe DC, Ebenso EE (2019) Green synthesis of ZnO nanoparticles using aqueous Brassica oleracea L. var. italica and the photocatalytic activity. Green Chem Lett Rev 12:444–457
Rai M, Ingle A (2012) Role of nanotechnology in agriculture with special reference to management of insect pests. Appl Microbiol Biotechnol 94:287–293
Rajakumar G, Rahuman AA, Velayutham K, Ramyadevi J, Jeyasubramanian K, Marikani A, Zahir AA (2013) Novel and simple approach using synthesized nickel nanoparticles to control blood-sucking parasites. Vet Parasitol 191:332–339
Rajakumar G, Rahuman AA, Roopan SM, Chung IM, Anbarasan K, Karthikeyan V (2015) Efficacy of larvicidal activity of green synthesized titanium dioxide nanoparticles using Mangifera indica extract against blood-feeding parasites. Parasitol Res 114:571–581
Ramyadevi J, Jeyasubramanian K, Marikani A, Rajakumar G, Rahuman AA, Santhoshkumar T, Kirthi AV, Jayaseelan C, Marimuthu S (2011) Copper nanoparticles synthesized by polyol process used to control hematophagous parasites. Parasitol Res 109:1403–1415
Rastogi L, Arunachalam J (2011) Sunlight based irradiation strategy for rapid green synthesis of highly stable silver nanoparticles using aqueous garlic (Allium sativum) extract and their antibacterial potential. Mat Chem Phys 129:558–563
Rubae AAY (2009) The potential uses of Melia azedarach L. as pesticidal and medicinal plant, review. Am Eurasian J Sustain Agric 3:185–195
Saquib Q, Attia SM, Ansari SM, Al-Salim A, Faisal M, Alatar AA, Al-Khedhairy AA (2017) p53, MAPKAPK-2 and caspases regulate nickel oxide nanoparticles induce cell death and cytogenetic anomalies in rats. Int J Biol Macromol 105:228–237
Sathishkumar M, Sneha K, Won WS, Cho CW, Kim S, Yun YS (2009) Cynamon zeylanicum bark extract and powder mediated green synthesis of nanocrystalline silver particles and its bactericidal activity. Colloids Surfaces B Biointerfaces 73:332–338
Sharmila G, Thirumarimurugan M, Muthukumaran C (2019) Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem J 145:578–587
Shimizu S, Nojiri K, Matsunaga N, Yamanea I, Minamia T (2000) Reduction in tick numbers (Haemaphysalis longicornis), mortality and incidence of Theileria sergenti infection in field-grazed calves treated with flumethrin pour-on. Vet Parasitol 92:129–138
Siddiqui MA, Ahamed M, Ahmad J, Khan MM, Musarrat J, Al-Khedhairy AA, Alrokayan SA (2012) Nickel oxide nanoparticles induce cytotoxicity, oxidative stress and apoptosis in cultured human cells that is abrogated by the dietary antioxidant curcumin. Food Chem Toxicol 50:641–647
Singh N, Manshian B, Jenkins GJ, Griffiths SM, Williams PM, Maffeis TG, Wright CJ, Doak SH (2009) Nano Genotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials 30:3891–3914
Sone BT, Fuku XG, Maaza M (2016) Physical and electrochemical properties of green synthesized bunsenite NiO nanoparticles via Callistemon viminalis’ extracts. Int J Electrochem Sci 11:8204–8220
Sousa LA, Soares SF, Pires Junior HB, Ferri PH, Borges LMF (2008) Evaluation of efficacy of ripe and unripe fruit oil extracts of Melia azedarach against Rhipicephalus (Boophilus) microplus (Acari: ixodidae). Rev Bras DE Parasitol Vet 17:36–40
Sousa LAD, Junior HBP, Soares SF, Ferri PH, Ribas P, Lima EM, Borges LMF (2011) Potential synergistic effect of Melia azedarach fruit extract and Beauveria bassiana in the control of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) in cattle infestations. Vet Parasitol 175:320–324
Underwood C, Van Eps AW (2012) Nanomedicine and veterinary science: The reality and the practicality. Vet J 193:12–23
Walker AR, Bouattour A, Camicas JL, Estrada-Pena A, Horak IG, Latif AA, Pegram RG, Preston PM (2003). Ticks of domestic animals in Africa: a guide to identification of species. Bio Science Reports, Edinburgh, pp 3:210
Wandscheer CB, Duque JE, da Silva MA, Fukuyama Y, Wohlke JL, Adelmann J, Fontana JD (2004) Larvicidal action of ethanolic extracts from fruit endocarps of Melia azedarach and Azadirachta indica against the dengue mosquito Aedes aegypti. Toxicon 44:829–835
Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, Li Y (2007) Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett 168:176–185
Yuvakkumar R, Suresh J, Nathanael AJ, Sundrarajan M, Hong SI (2014) A green biosynthesis of NiO nanoparticles using aqueous extract of Tamarix serotina and their characterization and application. Mater Lett 128:170–174
Acknowledgements
This study is a part of a PhD thesis to be submitted to the Department of Parasitology, Faculty of Veterinary Medicine, Cairo University. The study was financially supported by the National Research Centre as a part of PhD Thesis No. 12/2/19.
Author information
Authors and Affiliations
Contributions
MMF, SA, MMA, RME, and HSMA designed the experiments. EMH and HSMA participated in the preparation of Nickel oxide nanoparticles. MMF, SA, MMA, RME, AMH and HSMA shared in bioassay of the NiO NPs against various developmental stages of H. dromedarii and evaluation the toxic effect of NiO NPs on Swiss albino mice. SA and HSMA analyzed and tabulated the data. MMF, SA, MMA, and HSMA wrote the draft of the manuscript. All authors revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This study was approved by Ethical Committee for Medical and Veterinary Research at the National Research Centre (NRC), Egypt in accordance with local laws and regulations (Approval Protocol No. 20148). Consent was obtained from the owners of camels included in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Ghany, H.S.M., Abdel-Shafy, S., Abuowarda, M.M. et al. In vitro acaricidal activity of green synthesized nickel oxide nanoparticles against the camel tick, Hyalomma dromedarii (Ixodidae), and its toxicity on Swiss albino mice. Exp Appl Acarol 83, 611–633 (2021). https://doi.org/10.1007/s10493-021-00596-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-021-00596-5