Abstract
Biopesticides such as essential oils (EOs) are considered an improvement for integrated pest control as they appear to be less toxic to the environment than chemical acaricides. The current study aimed to evaluate the acaricidal activity of Artemisia herba-alba and Melia azedarach oil loaded nano-emulsion as alternatives for chemical acaricides against the camel tick Hyalomma dromedarii, besides evaluating their toxic effect on Swiss albino mice. Transmission electron microscopy (TEM) and Fourier transform infrared spectroscopy (FTIR) were used for the characterization of loaded nano-emulsions.The immersion test was used for the bioassay of both loaded nanoemulsions on tick stages (egg, nymph, larva, and adult). Mortality percentages and LC50 values of each tick stage were calculated. Reproductive performance for the survived engorged females after treatment was monitored. The toxicity of both loaded nano-emulsions was evaluated on Swiss albino mice by an oral dose of 1500 mg/kg/day for five successive days. The hematological, biochemical, and histopathological changes were evaluated. TEM characterization revealed spherical droplets for A. herba-alba and M. azedarach oil loaded nano-emulsion with droplet size ranging from 62 to 69 nm and 52–91 nm, respectively. FTIR revealed the absence of extra peaks in the loaded nano-emulsions that confirmed no chemical changes existed by ultrasonication. The LC50 values of A. herba-alba and M. azedarach oil loaded nano-emulsion on embryonated eggs, larvae, engorged nymphs, and unfed adults were 0.3 and 1.1%, 0.7 and 1.7%, 0.3 and 0.4%, 4.4 and 22.2%, respectively. The egg productive index (EPI), egg number, and hatchability percentage were lower in the treated females compared with Butox 5% (deltamethrin) and control. The hematological picture and biochemical analysis revealed insignificant changes in the treatment group compared with the negative control group. The liver of the A. herba-alba and M. azedarach oil loaded nano-emulsion treated group exhibited vacuolar degeneration and infiltration of lymphocytic cells. The kidney of mice treated with A. herba-alba and M. azedarach oil loaded nano-emulsion showed hemolysis and slight degeneration of epithelial cells of tubules. It is concluded that A. herba-alba and M. azedarach oil loaded nano-emulsion have good acaricidal activity against camel tick H. dromedarii.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In developing countries, farmers face several diseases that affect the productivity of their animals, many of which are caused by tick infestations. Tick-borne diseases such as theilerioses, babesiosis, borreliosis, and rickettsiosis are the most common diseases of large and small ruminants in Asia, Africa, and Latin America (Jongejan and Uilenberg 2004; Elhelw et al. 2021). In addition to disease transmission, ticks can cause a reduction in weight gain, losses of meat and milk production in domestic animals, severe blood loss, anemia, and damage to hides at the site of tick bites (Rajput et al. 2006). Hyalomma dromedarii (Acari: Ixodidae), is the predominant tick species affecting dromedary camels. This tick may have a three-host or a two-host life cycle; however, the two-host cycle is the most dominant. It was found during the year and increased in the period from March to September (Elghali and Hassan 2009).
Chemical acaricides are the most widely used intervention method for controlling ectoparasites. Overuse of these acaricides has resulted in resistance development, environmental pollution, and residues in meat and milk (Parizi et al. 2009; Chagas et al. 2012). These issues have motivated the researcher to seek an alternative such as new formulations from plant extracts to minimize the impact caused by chemical acaricides (Benelli 2016).
Artemisia herba-alba (Shih-balady) (Asteraceae) is a wild plant grown in Sinai and used in the treatment of various diseases in the Middle East. It has been used in folk medicine since ancient times as vermifuge, diuretic, tonic, and in skin troubles (Di Stasi et al. 2002). It exhibited acaricidal activity against larvae of H. dromedarii (Abdel-Shafy et al. 2007) and its essential oil presented a repulsive effect against Ixodes ricinus ticks (El-Seedi et al. 2017). Melia azedarach L. (Meliaceae) is naturalized in tropical and subtropical countries (Rubae 2009) and it has anti-cancer, anti-inflammatory, analgesic, and diuretic properties (Khan et al. 2018). It also possesses antiparasitic activity (Borges et al. 2003; Sousa et al. 2008, 2011; Sariosseiri et al. 2018). Biopesticides such as essential oils (EOs) may be used as an alternative for pest control (Tripathi et al. 2009; Athanassiou et al. 2013). These EOs contain mixtures of bioactive constituents, such as alcohols, aldehydes, ketones, aromatic phenols, lactones, esters as well as monoterpenes and sesquiterpenes (Regnault-Roger et al. 2012). Although, EOs have promising properties to be an alternative, some problems related to EOs have been reported such as volatility, poor solubility in water, and tendency to oxidation (Turek and Stintzing 2013). Therefore, the development of a nano-formulation system may be helpful to overcome these problems. Nano-emulsions can be formulated through the dispersion of the oil phase in an aqueous phase or an aqueous phase in the oil phase in the presence of surfactant (Solans et al. 2005). Various studies have notified the use of nano-emulsions as suitable carriers for active EOs protecting them from degradation and losses by evaporation, controlled release, and easy handling (Martin et al. 2010). In addition, encapsulation of EOs in nano-emulsions enhances their stability, utilization, and efficacy (Moghimi et al. 2016).
Nano-emulsions of EOs are promising tools for controlling arthropod vectors and infectious diseases (Wang et al. 2007). Some studies evaluated the acaricidal activity of nano-emulsion against hard ticks such as nano-structured Cinnamon oil and Eucalyptus globulus oil against Rhipicephalus microplus females (Dos Santos et al. 2017a, b; Baldissera et al. 2018), and Pilocarpus spicatus oil against R. microplus larvae (Nogueira et al. 2020). Other studies evaluated the larvicidal activity of nano-emulsion against different types of mosquito (Ghosh et al. 2013a; Duarte et al. 2015; Botas et al. 2017). To avoid negative impacts on human health and the environment, it is necessary to assess nanoformulation toxicity on non-target species such as Swiss albino mice.
This study aimed to evaluate the acaricidal activity of A. herba-alba and M. azedarach oil loaded nano-emulsion against all the developmental stages of H. dromedarii and estimate their toxicity in healthy adult mice.
Materials and methods
Oil extraction from Artemisia herba-alba and Melia azedarach
Whole aerial parts of A. herba-alba and the ripened fruits of M. azedarach were kindly donated from Genetics and Cytology Department, Biotechnology Division, National Research Centre. The two plants were ground using stainless steel knife mill then subjected to Soxhlet extraction by adding 150 g powder of each plant to one litter of petroleum ether (grade 40–60 ℃) for 72 h. The solvent was evaporated using a vacuum rotary evaporator (Rotavap, China) at 40 ℃, at a rotation speed of 20 rpm.The obtained oil was transferred to dark glass bottles and stored at 4 ℃ until use (Benyacoub et al. 2019).
Preparation of Artemisia herba-alba and Melia azedarach oil loaded nano-emulsion
The oil-in-water nano-emulsion was formulated using A. herba-alba and M. azedarach oil, non-ionic surfactant (Tween 80), and distilled water. Polyethylene glycol (MW 6000, Sigma-Aldrich) was used in the PEGylation process of the nano-emulsion. The non-ionic surfactant (Tween 80, 2% v/v) was dissolved in distilled water at room temperature, then the mixture was homogenized using a magnetic stirrer (Thermo Scientific, CIMAREC) for 10 min, then 0.25% w/v SDS was added. Determining the concentration of the two oils under investigation was (10% v/v).
Artemisia herba-alba and M. azedarach nano-emulsions were prepared by slowly mixing the two oils to the last prepared non-ionic surfactant (in ratio 1:1) then well mixed using a magnetic stirrer for 30 min. The resulting emulsions were subjected to ultrasonic emulsification [using a 20 kHz Sonicator (Sonics, Vibra cell—Ultra sonicator, USA) with a power out put of 750 W] for 20 min that generates intensive and disruptive forces to minimize the nano-emulsion droplets (Moradi and Barati 2019). Polyethylene glycol (3% w/v; PEG) was prepared by dissolving in distilled water with the aid of a magnetic stirrer for 10 min till complete dissolving to be used in the encapsulation process according to the method of Zhang et al. (2008) with modification. The polyethylene glycol nano-capsule was made by drop-wise dispersion of diluted oil into an appropriate volume of polyethylene glycol under continuous mechanical stirring for 30 min, at ambient temperature then subjected to ultrasonication for 20 min. Avoiding temperature rising during the ultrasonication process, an ice bath was used to maintain the temperature difference (before and after sonication) less than 5 ℃. The obtained loaded nano-capsule suspension was equilibrated overnight for producing dispersed nano-capsules in an aqueous solution.
Characterization of loaded nano-emulsion
Transmission electron microscopy (TEM)
The morphology of the loaded nano-emulsion was characterized using TEM (JEOL, JEM-2100, NRC). One drop of the nano-capsule suspensions was deposited onto a carbon-coated copper grid and stained with phosphotungstic acid then examined and photographed (Sugumar et al. 2014).
Fourier transform infrared spectroscopy (FTIR)
FTIR (JASCO 6100-FTIR, NRC) spectrum of the pure oil and prepared loaded nano-emulsion were studied in the scan range of 4000–400 cm−1. FTIR analysis was used to examine the chemical changes that occurred in the oil molecules as a result of exposure to ultrasonication (Carpenter and Saharan 2017).
Tick colony
For the establishment of tick colony in the laboratory, fully engorged females were collected from camels from the Birqash village (30°09′58.4″N, 31°02′13.2″E) in Giza, Egypt then identified using stereomicroscope (LEICA DM 750, Russia) according to Walker et al. (2003). For oviposition, these engorged females incubated at 25 ± 1 ℃ and 75–80% relative humidity (RH) in an incubator (Friocell, MMM, Germany) in a plastic cup. Daily collection of the laid eggs in separate cups to obtain eggs of the same age and incubated under the same previous conditions. Some of these collected eggs were used in the egg immersion test (EIT) and the others were incubated for hatching. Part of the hatched larvae was used for larval immersion test (LIT) and the other part was fed on healthy rabbits using a capsule technique (Abdel-Shafy 2008) to obtain engorged nymphs. Part of the engorged nymphs was used in the nymphal immersion test (NIT) and the other part was incubated for molting to unfed adults. A group of molted adults was used in the unfed adult immersion test and the other group was fed on healthy rabbits (equal number of unfed male and female) to obtain fully engorged females that used in the immersion test.
Effect of Artemisia herba-alba and Melia azedarach oil loaded nano-emulsion
The effect of A. herba-alba and M. azedarach oil loadednano-emulsion on the developmental stages of H. dromedarii were evaluated. For the selection of suitable concentrations, a pilot test was performed for each tick stage. Butox 5% (deltamethrin, 1 ml/L) was used as reference acaricide, whereas Tween 80, SDS 0.25%, and PEG were considered as solvent control.
Egg immersion test (EIT)
To assess the effect of A. herba-alba and M. azedarach oil loadednano-emulsion on the embryonated eggs, nearly, 300 embryonated eggs were immersed in a plastic cup containing one ml of the tested concentrations 2.5, 1.25, 0.625, and 0.312% for A. herba-alba oil loaded nano-emulsion and 5, 2.5, 1.25, and 0.0625% for M. azedarach oil loaded nano-emulsion for 1 min (Abdel-Ghany et al. 2019). Subsequently, the solutions were decanted, and the cups closed with muslin clothe then incubated for 14 days. Each concentration was replicated 3 × . To calculate the mortality percentage of eggs, dead eggs, and hatched larvae were counted using a binocular dissecting microscope (LEICA DM 750, Russia).
Larval immersion test (LIT)
Approximately, 300 larvae of H. dromedarii larvae (7–14 day) were immersed in 1 ml of A. herba-alba oil loaded nano-emulsion 2.5, 1.25, 0.625, and 0.312%, M. azedarach oil nano-emulsion 10, 5, 2.5, 1.25% for 1 min. Each concentration was replicated 3 × . Dead larvae were counted after 24 h to calculate the mortality percentage. The larvae were considered dead when failed to move after stimulation by breathing or with ataxia.
Nymphal immersion test (NIT)
To evaluate the efficacy of A. herba-alba and M. azedarach oil loaded nano-emulsion on H. dromedarii nymphs, 30 engorged nymphs were immersed for 1 min in 5 ml of each concentration of A. herba-alba and M. azedarach oil loaded nano-emulsion 1.25, 0.625, 0.312, and 0.156%. Each concentration was used in triplicate. The treated nymphs were incubated at 25 ± 1 ℃ and 75–80% RH. Engorged nymphs that failed to molt were counted and their mortality percentages were calculated.
Adult immersion test (AIT)
Unfed adults
In AIT, 30 unfed adults (equal number of males and females) of H. dromedarii (10 day old) were exposed to each concentration of A. herba-alba and M. azedarach oil loaded nano-emulsion 10, 5, 2.5, and 1.25% in 5 ml for 1 min. These concentrations were replicated 3 × . After that, the treated ticks were incubated at 25 ± 1 ℃ and 75–80% RH and daily checked for 7 days to record the mortality.
Engorged females
The acaricidal efficacy of A. herba-alba and M. azedarach oil loaded nano-emulsion on H. dromedarii engorged females were evaluated. The fully fed females were immersed in different concentrations of A. herba-alba and M. azedarach oil loaded nano-emulsion (5, 2.5, 1.25, and 0.625%) following the method described by Drummond et al. (1973) with slight modification. Each engorged female was weighed before treatment. For each concentration, nine engorged females were divided into three replicates. These females were immersed in 10 ml of the tested concentration for 1 min. After that, the solutions were removed then the ticks were dried by filter paper and kept in the incubator in separate cups at 25 ± 1 ℃ and 75–80% RH for 15 days. The laid eggs were collected and incubated at the same conditions until the hatching occurred to record the hatching rate. The egg productive index (EPI = [egg mass (mg)/initial weight of engorged female (mg)] × 100) and hatchability (%) of laid eggs were calculated (Abuowarda et al. 2020).
Toxicological effects of Artemisia herba-alba and Melia azedarach oil loaded nano-emulsion on Swiss albino mice
Forty adult male mice (2–3 months, 20–25 g) were obtained and housed in the animal house, National Research Centre in a ventilated room at 26 ± 2 ℃ and 44–56% RH, light and dark cycles of 14 and 10 h, respectively. Food and water were allowed ad libitum for all control and experimental group. These mice were divided into four groups with ten mice in each group. Both A. herba-alba and M. azedarach oil loaded nano-emulsion treated group received 1500 mg/kg/day. The negative control group received no treatment to measure basic parameters, the positive control group received (Tween 80, SDS 0.25% and PEG) for five successive days by oral gavage. Daily following of the mice for any behavior changes such as the water and food intake and the possible appearance of toxic symptoms. On day 10 the blood samples were collected from their retro-orbital plexus, some fresh blood collected in a sterile tube containing (EDTA) to determine the blood cell count (erythrocytes, hemoglobin, platelets, and total leukocytes, by an automatic hematology counter) (Medonic, NRC). The other blood samples were left at room temperature for 1 h followed by centrifugation at 3000 rpm for 15 min at 4 ℃ to obtain sera. Then, the sera were stored at −20 ℃ for clinical biochemistry. The sera were analyzed by an automatic biochemical analyzer for the determination of alanine aminotransferase (ALT), alkaline phosphatase (ALP), and creatinine (CR). A small piece of liver and kidney was fixed with 10% formalin then were dehydrated and embedded in paraffin wax. Sections with 5–8 µm thickness were prepared and stained with hematoxylin–eosin stain (Biodiagnostic, Giza, Egypt), and the sections were photographed under a light microscope (Leica DM 750, Switzerland).
Statistical analysis
Data were analyzed by a one-way ANOVA test followed by Duncan’s test using SPSS program v.20. The lethal concentration (LC50) values were calculated by applying regression equation analysis to the probit transformed data of mortality. The dose–response data were analyzed by the probit method (Finney 1962).
Results
Characterization of loaded nano-emulsion
Transmission electron microscope (TEM)
According to TEM characterization, A. herba-alba and M. azedarach oil loaded nano-emulsion droplets were spherical with droplet size ranged from 62 to 69 nm for A. herba-alba oil loaded nano-emulsion (Fig. 1a) and 52–91 nm for M. azedarach oil loaded nano-emulsion (Fig. 1b).
Fourier transforms infrared spectroscopy (FTIR)
FTIR analysis of loaded nano-emulsions was performed and compared with A. herba-alba and M. azedarach oils to detect the possible impact of ultrasonication to induce chemical effects on A. herba-alba and M. azedarach oils. FTIR of A. herba-alba and M. azedarach oils and loaded nano-emulsions are presented in (Figs. 2 and 3). The FTIR spectrum of A. herba-alba oil (Fig. 2a) revealed characteristic sharp peaks at 3440.39 cm−1 (O–H), 2923 cm−1 (aromatic and/or vinylic C-H), 2854 cm−1 (aliphatic C-H). These peaks were found with slight variation even after the formation of A. herba-alba oil loaded nano-emulsion (Fig. 2b). The FTIR spectrum of M. azedarach oil (Fig. 3a) revealed high absorption bands at 3437.49 cm−1 (O–H stretching vibration), 2926.45 cm−1 (aromatic and/or vinylic C–H), 2857.02 cm−1 (aliphatic C–H), 1745.27 cm−1 (C = O carbonyl groups of esters). These peaks were found with slight variation after the formation of M. azedarach loaded nano-emulsion (Fig. 3b). Therefore, the absence of extra peaks confirmed that no chemical changes existed by ultrasonication.
Acaricidal efficacy of Artemisia herba-alba and Melia azedarach loaded nano-emulsions
Effect of loaded nano-emulsions on embryonated eggs
Table 1 shows the acaricidal efficacy of A. herba-alba and M. azedarach oil loaded nano-emulsions on H. dromedarii embryonated eggs. Both formulations showed ovicidal activity higher than Butox 5% and control. At the highest concentrations of both A. herba-alba (2.5%) and M. azedarach (5%) oil loaded nano-emulsions, mortality was 100%. The calculated LC50 values of oil loaded nano-emulsion against embryonated eggs were 0.29 and 1.10% for A. herba-alba and M. azedarach, respectively (Table 1).
Effect of loaded nano-emulsionsons on larvae
The acaricidal activity of A. herba-alba and M. azedarach oil loaded nano-emulsions on H. dromedarii larvae are shown in Table 1. There was a significant effect of both formulations compared with reference acaricide (Butox 5%) and control. Artemisia herba-alba oil loaded nano-emulsion showed higher larvicidal activity especially at the highest concentration of 2.5%, where the mortality reached 100%, this result was similar with reference acaricide (Butox 5%). The larvicidal activity of M. azedarach oil loaded nano-emulsion was lower compared with A. herba-alba oil loaded nano-emulsion especially at the highest concentration of 10% the mortality rate was 90.4%. The calculated LC50 values of oil loaded nano-emulsion against larvae were 0.72 and 1.7% for A. herba-alba and M. azedarach, respectively (Table 1). The calculated LC50 confirmed that A. herba-alba oil loaded nano-emulsion had a higher effect against larvae than M. azedarach oil nano-emulsion (Table 1).
Effect of loaded nano-emulsions on engorged nymphs
Table 1 shows the effect of A. herba-alba and M. azedarach oil loaded nano-emulsions on the molting of H. dromedarii engorged nymphs compared with Butox 5% and control. The effect of A. herba-alba oil loaded nano-emulsion was slightly higher than M. azedarach oil loaded nano-emulsion. The mortality rate was 100 and 90% at the concentration of 1.25% for A. herba-alba and M. azedarach oil loaded nano-emulsion, respectively. The calculated LC50 values were 0.33 and 0.38% for A. herba-alba and M. azedarach oil loaded nano-emulsion, respectively (Table 1).
Effect of loaded nano-emulsions on unfed adults
The acaricidal efficacy of A. herba-alba and M. azedarach oil loaded nano-emulsions against unfed adults of H. dromedarii are presented in Table 1. The assessment of both formulations was recorded 3 days post-treatment where some of the adults dead on the first day and the others were with the slow movement then died on the third day. The unfed adults were more sensitive to A. herba-alba oil loaded nano-emulsion than M. azedarach oil loaded nano-emulsion. At the highest concentration of 10%, the mortality was 86.6% for A. herba-alba oil loaded nano-emulsion which was insignificantly lower than that recorded for Butox 5% treatment, whereas M. azedarach loaded nano-emulsion recorded mortality 33.3% at the highest concentration (10%) which was significantly lower than recorded in Butox 5%. No mortality was recorded in the control group. The calculated LC50 values were 4.4 and 22.2% for A. herba-alba and M. azedarach oil loaded nano-emulsion, respectively (Table 1).
Effect of loaded nano-emulsions on the reproductive performance of engorged females
After treatment of H. dromedarii engorged females with A. herba-alba and M. azedarach oil loaded nano-emulsions, they were followed to evaluate their reproductive performance including egg productive index (EPI), egg number, and hatchability as shown in Table 2. As the concentrations of both formulations decreased, the EPI increased. The EPI recorded in the treatment of A. herba-alba oil loaded nano-emulsion ranged from 0.397 ± 0.03 to 0.543 ± 0.014 compared with Butox 5% treatment (0.106±0.023) and control 0.568 ± 0.008. For M. azedarach oil loaded nano-emulsion treatment, the EPI ranged from 0.373 ± 0.049 to 0.338 ± 0.043 compared with Butox 5% treatment and control. Egg numbers ranged from 2830.7 ± 263.0 to 4615.8 ± 233.3 and from 3277.2 ± 432.9 to 2010.6 ± 262.4 for A. herba-alba and M. azedarach oil loaded nano-emulsion, respectively, compared with 415.8±91.5 in Butox 5% treatment and 5354.4 ± 242.4 in control. The hatchability percentage for both formulations was lower compared with the control. The eggs laid by females exposed to A. herba-alba and M. azedarach oil loaded nano-emulsion exhibited lower hatchability, ranged from 55.1 to 91.7% and from 44.1 to 73%, respectively.
Toxicity of Artemisia herba-alba and Melia azedarach oil loaded nano-emulsions on Swiss albino mice
Hematological changes
The influence of A. herba-alba and M. azedarach oil loaded nano-emulsions was evaluated on various hematological parameters (Table 3). After treatment of mice with A. herba-alba oil loaded nano-emulsion, hematological parameters were close to those of the negative control group with a slight increase or decrease in some parameters which were not different from the negative control group. The level of WBCs (6.63 × 109/L) was slightly increased compared with the negative control (5.13 × 109/L), whereas the level of HCT (34%) was slightly decreased compared with the negative control (36.5%). Furthermore, the M. azedarach oil loaded nano-emulsion treated group showed normal WBCs, RBCs, HCT, and a slight decrease in the platelet level (872.0 × 109/L) and Hb (9.73 g/dL) compared with the negative control, whereas platelet and Hb levels were (1112.0 × 109/L) and (11.93 g/dL), respectively.
Biochemical changes
The influence of A. herba-alba and M. azedarach oil loaded nano-emulsions on some hepatic and renal parameters such as alanine aminotransferase (ALT) and alkaline phosphatase (ALP) for liver function and creatinine (CR) for kidney function was studied (Table 4). Concerning A. herba-alba and M. azedarach oil loaded nano-emulsion treatment, respectively, the level of ALP (72.6 and 70.6 U/L) and ALT (49.0 and 52.3U/L) were not different from the negative control group for ALP (85 U/L) or ALT (67 U/L). For renal parameter, the creatinine level was 0.40 mg/dl for A. herba-alba and M. azedarach oil loaded nano-emulsion which was not different from the negative control group (0.43 mg/dl).
Histopathological examination of the liver and kidney of mice
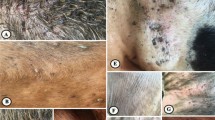
The histopathological changes of the liver and kidney after oral uptake of A. herba-alba and M. azedarach oil loaded nano-emulsion are shown in Figs. 4 and 5. The liver of the A. herba-alba oil nano-emulsion treated group exhibited vacuolar degeneration (black arrow) with an aggregation of lymphocytic cells (black arrow head) (Fig. 4c). In addition to vacuolar degeneration in the hepatic parenchyma, the central vein was congested (Fig. 4d). The liver of the M. azedarach oil loaded nano-emulsion treated group exhibited dilated central vein with inflammatory cells, hepatic parenchyma showed mild to moderate degenerative changes including cloudy swelling and vacuolar degeneration (black arrow head) in addition to mild infiltration of lymphocytes (Fig. 4e). Furthermore, individual cell necrosis with activation of Kupffer cells (white arrow) also occurred (Fig. 4f). The liver of the positive control group showed mainly activation of Kupffer cells (white arrow) (Fig. 4b).
Histopathological changes in the liver of mice treated with Artemisia herba-alba and Melia azedarach oil loaded nano-emulsion. (a) Negative control group showing the normal architecture of liver. (b) Positive control group showing activation of Kupffer cells (white arrow). (c) Artemisia herba-alba oil loaded nano-emulsion treated group showing vacuolar degeneration (black arrow) with an aggregation of lymphocytic cells (blackhead arrow). (d) Central vein congested with vacuolar degeneration. (e) Melia azedarach oil loaded nano-emulsion treated group showing dilated central vein, degenerative changes cloudy swelling, and vacuolar degeneration (black arrow head). (f) Individual cell necrosis (white arrow) with activation of Kupffer cells. These micrographs were captured at a magnification of 200 ×
Histopathological changes in the kidney of mice treated with Artemisia herba-alba and Melia azedarach oil loaded nano-emulsion. (a) Negative control group showing normal features of the kidney. (b) Positive control group showing degeneration of epithelium of tubules as well as of lymphocytic infiltration (white arrow). (c) Artemisia herba-alba oil loaded nano-emulsion treated group showing an extensive area of hemolysis, lymphocytic infiltration (white arrow head), and degeneration of epithelial cells of tubules. (d) Melia azedarach oil loaded nano-emulsion treated group showing hemolysis and mild degeneration of epithelial cells of tubules. These micrographs were captured at a magnification of 100 ×
The kidney of mice treated with A. herba-alba oil loaded nano-emulsion showed an extensive area of hemolysis, slight degeneration of epithelial cells of tubules as well as lymphocytic aggregation in the kidney tissue (white arrow head) (Fig. 5c). Furthermore, the kidney tissue of M. azedarach oil nano-emulsion showed mainly hemolysis (Fig. 5d). The positive control group showed slight degeneration of epithelium of tubules as well as infiltration of lymphocytes in the kidney tissue (white arrow) (Fig. 5b).
Discussion
Tick control mostly depends on the use of chemical acaricides. Unfortunately, their continuous use results in resistance development and environmental pollution. Biopesticides such as EOs, are considered a more friendly way for integrated pest control as they appeared to be less toxic to the environment than chemical acaricides (Talbert and Wall 2012; Athanassiou et al. 2013). Nano formulation of these EOs is considered a promising tool for controlling parasites which may return to the physicochemical properties of the nanometric emulsion system. The efficacy of nano-emulsion was greater than bulk pesticide as their nano-size enhances specificity and delivery target (Wang et al. 2009). To decrease the losses resulted from tick infestations, it is necessary to make interventions during different developmental stages of their life cycle. The current study is targeting all developmental stages of H.dromedarii using A. herba-alba and M. azedarach oil loaded nano-emulsions. Few studies were conducted on the acaricidal activity of nano-emulsions against ixodid ticks. In terms of the acaricidal activity of A. herba-alba and M. azedarach oil nanoemulsions against arthropods, no previous studies were found. However, other species from the Meliaceae and Asteraceae families have been used: Carapa guianensis (Meliaceae) oil nano-emulsion exhibited high larvicidal activity against Aedes aegypti (Jesus et al. 2017), and Ayapana triplinervis (Asteraceae) oil nano-emulsion also revealed strong acaricidal activity against Ae. aegypti (Rodrigues et al. 2020).
The morphology and structure of A. herba-alba and M. azedarach oil loaded nano-emulsion were investigated by TEM. The droplets of both nano-emulsions were spherical and in a good dispersion. These findings were in agreement with other studies such as cinnamon oil nano-emulsion (Ghosh et al. 2013b) and neem oil nano-emulsion (Anjali et al. 2012). The droplet size A. herba-alba oil nano-emulsion ranged from 62 to 69 nm and from 52 to 91 nm for M. azedarach oil nano-emulsion. It has been reported that droplets of nano-emulsion have a size ranging from 20 to 200 nm (Sugumar et al. 2014). A smaller droplet of nano-emulsion can be achieved when the hydrophilic-lipophilic balance (HLB) of the surfactant is synchronized with the HLB of the oil (Fernandes et al. 2014).
FTIR analysis of A. herba-alba and M. azedarach oil loaded nano-emulsion were performed and compared with their pure oil. The FTIR spectrum of A. herba-alba oil and M. azedarach oil showed characteristic peaks that were appeared after the formation of A. herba-alba and M. azedarach oil loaded nano-emulsion with slight variation. These findings were similar to the study of (Carpenter and Saharan 2017) who found similar results for the preparation of mustard oil nano-emulsion using ultrasonication. All obtained peaks were the same as in pure mustard oil which confirmed no chemical changes induced by ultrasonication.
Results of the present study revealed good acaricidal efficacy of both loaded nano-emulsions against all developmental stages of H. dromedarii. Artemisia herba-alba oil loaded nano-emulsion had a slightly higher effect than M. azedarach oil loaded nano-emulsion. Artemisia herba-alba (2.5%) and M. azedarach (5%) oil loaded nano-emulsions exhibited ovicidal activity against H. dromedarii embryonated eggs reached 100% mortality that was higher than reference acaricide Butox 5%. Furthermore, A. herba-alba and M. azedarach oil loaded nano-emulsion revealed a high effect against H. dromedarii larvae that was similar to Butox 5%. Our results were following the study of Nogueira et al. (2020) who evaluated the repellent effect of Pilocarpus spicatus oil nano-emulsion against Rhipicephalus microplus larvae whereas the repellency at 50 mg/ml was greater than 97% at 6 and 10 h after treatment. Considering engorged nymphs, they were the most responded stage to both nano-emulsions at concentrations lower than the embryonated eggs, larvae, unfed adults, and engorged females. The calculated LC50 value of engorged nymphs treated with A. herba-alba and M. azedarach oil loaded nano-emulsions confirmed these results. The LC50 values for A. herba-alba and M. azedarach oil loaded nano-emulsion were 0.29 and 1.10%, 0.72 and 1.72%, 0.33 and 0.38%, and 4.38 and 22.24% for embryonated eggs, larvae, engorged nymphs, and unfed adults, respectively.
Artemisia herba-alba and M. azedarach oil loaded nano-emulsions exhibited a good effect on the reproductive performance of the fully engorged females whereas the EPI, egg number, and hatchability percentage were lower than those in the control group. These results contrasted with the result of Baldisser et al. (2018) who evaluated the acaricidal activity of Eucalyptus globulus oil in pure formand the nanostructured form (oil nano-emulsion and oil nano-capsule) against fully engorged females of R. microplus. The pure oil had a higher effect than the nanostructured forms.The pure oil at a concentration of 5 and 10% exhibited 85 and 97.8% efficacy whereas nano-emulsion and nano-capsule displayed 61.2 and 50% efficacy at 5%, respectively. An in vitro study conducted by Santos et al. (2017a, b) on the reproductive performance of R. microplus engorged females using nano-capsule and nano-emulsion containing 5% cinnamon oil revealed 95 and 97% efficacy, respectively. Moreover, an in vivo study using 0.5% of these formulations was conducted on infested cattle, where the animals were free from ticks at 20 days post-treatment. Nano-formulation containing essential oils can affect insects through a diverse mode of action, as deregulation of growth hormones and inhibition of enzymes which resulted in insect death (Hazra 2017; Mishra et al. 2018). It is necessary to assert that nanotechnology uses small-sized particles, facilitating oil penetration into ticks, so enhancing the biological activity of natural products. Therefore, improvement in nanotechnology may be advantageous in veterinary parasitology. This facilitates the use of plant materials to break the life cycle of the parasite and consequently prevent disease transmission (Chagas and Rabelo 2012).
A toxicity experiment was conducted to evaluate the possible toxic effect of A. herba-alba and M. azedarach oil loaded nano-emulsion on the mice. The toxic effect of these formulations was investigated concerning some hematological and biochemical parameters as well as histopathological changes in the liver and kidney. Assessment of hematological parameters is valuable in determining the toxic effect of the drugs, as it gives information about the reaction of the body toward injury, deprivation, and stress (Raza et al. 2002; Rahman et al. 2001). The effect of A. herba-alba and M. azedarach oil loaded nano-emulsion on all hematological parameters (WBC, RBC, HCT, Hb, and platelet) was insignificant compared with the negative control group. These results were in the same line with the study of Ribeiro et al. (2015) who evaluated theoral administration of Eucalyptus staigeriana oil nano-emulsion for 30 days and did not exhibit changes in the hematological parameters. Another study conducted by Milhomem-Paixao et al. (2017) evaluating the possible toxicity of Carapa guianensis nano-emulsion after oral administration of (0.5, 1, and 2 g/kg) for 14 successive days which resulted insignificant alteration in the blood parameters with no genotoxicity or cytotoxicity.
The liver is the initial target organ responsible for the detoxification of pesticides and toxic compounds. The serum level of ALP, ALT, and AST is considered a bioindicator to evaluate the pesticide toxicity in humans and animals (Abbassy et al. 2014). Increased levels of liver enzymes or nitrogenous wastes excreted by the kidney might be an indicator for their spillage into circulation as a result of tissue necrosis (Prakash and Manavalan 2011).
In this study, the effect of A. herba-alba and M. azedarach oil nano-emulsion on serum biochemical parameters (ALP, ALT, and CR) also was insignificant compared with the negative control group. The liver of the A. herba-alba oil loaded nano-emulsion treated group exhibited vacuolar degeneration with congestion in the central vein. Furthermore, the liver of the M. azedarach oil nano-emulsion treated group presented dilated central vein with inflammatory cells and degenerative changes in the hepatic parenchyma. Despite abnormal changes appeared in the hepatocytes in the treated mice, the biochemical indicators not exhibited significant changes. These results may denote that these histopathological changes were minor or focal so, a functional reserve of the liver masks the clinical importance of early liver injury (Kumar et al. 2002). The study of Ragavan et al. (2017) evaluated the in vivo toxicity of garlic oil nano-emulsion (GNE) in Wistar rats after daily oral administration of 250, 100, and 50 mg for 45 successive days. Treated animals exhibited a significant change in the ALP, ALT, and AST levels compared with the control group. Furthermore, renal markers and hematological parameters showed insignificant changes compared with the negative control group. The liver exhibited inflammation with significant lymphocytic infiltration. The kidney presented mild acute tubular necrosis and brownish pigment in the glomerular regions.
Conclusion
Artemisia herba-alba and M. azedarach oil loaded nano-emulsions revealed high acaricidal activity against all the developmental stages of H. dromedarii. The calculated LC50 values showed that A. herba-alba and M. azedarach oil loaded nano-emulsion were more toxic for engorged nymphs followed by embryonated eggs, larvae, and unfed adults. The toxicity study of both loaded nano-emulsions revealed insignificant changes in the hematological and some biochemical parameters. This study concluded that A. herba-alba and M. azedarach oil loaded nano-emulsions may be considered as an alternative for controlling the camel ticks H. dromedarii.
References
Abbassy MA, Marei AESM, Al-Ashkar MAM, Mossa ATH (2014) Adverse biochemical effects of various pesticides on sprayers of cotton fields in El-Behira Governorate Egypt. Biomed Aging Pathol 4:251–256
Abdel-Ghany HS, Fahmy MM, Abuowarda MM, Abdel-Shafy S, El-Khateeb RM, Hoballah EM (2019) In vitro acaricidal effect of Melia azedarach and Artemisia herba-alba extracts on Hyalomma dromedarii (Acari: Ixodidae): embryonated eggs and engorged nymphs. J Parasitic Dis 43:696–710
Abdel-Shafy S (2008) Scanning electron microscopy and comparative morphology of Hyalomma anatolicum excavatum, H. dromedarii and H. marginatum marginatum (Acari: Ixodidae) based on nymphs. Acarologia 48:3–18
Abdel-Shafy S, Soliman MM, Habeeb SM (2007) In vitro acaricidal effect of some crude extracts and essential oils of wild plants against certain tick species. Acarologia 47:33–42
Abuowarda MM, Haleem MA, Elsayed M, Farag H, Magdy S (2020) Bio-pesticide control of the brown dog tick (Rhipicephalus sanguineus) in Egypt by using two entomopathogenic fungi (Beauveria bassiana and Metarhizium anisopliae). Int J Vet Sci 9:175–181
Anjali CH, Sharma Y, Mukherjee A, Chandrasekaran N (2012) Neem oil (Azadirachta indica) nanoemulsion a potent larvicidal agent against Culex quinquefasciatus. Pest manag sci 68:158–163
Athanassiou CG, Kavallieratos NG, Evergetis E, Katsoula AM, Haroutounian SA (2013) Insecticidal efficacy of silica gel with Juniperus oxycedrus spp. oxycedrus (Pinales: Upressaceae) essential oil against Sitophilus oryzae (Coleoptera: Curculionidae) and Tribolium confusum (Coleoptera: Tenebrionidae). J Econ Entomol 106:1902–1910
Baldissera MD, Stefani LM, Da Silva AS (2018) Effects of essential oil of Eucalyptus globulus loaded in nanoemulsions and in nanocapsules on reproduction of cattle tick (Rhipicephalus microplus). Arc De Zootec 67:494–498
Benelli G (2016) Plant-mediated synthesis of nanoparticles: A newer and safer tool against mosquito-borne diseases? Asian Pac J Trop Biomed 6:353–354
Benyacoub A, Skender A, Boutemak K, Hadj-Ziane-Zafour A (2019) Inclusion complexes of Melia azedarach L. seed oil/β-cyclodextrin polymer: preparation and characterization. Chem Pap 73:525–553
Borges LMF, Ferri PH, Silva WJ, Silva WC, Silva JG (2003) In vitro efficacy of extracts of Melia azedarach against the tick Boophilus microplus. Med Vet Entomol 17:228–231
Botas GDS, Cruz RA, De Almeida FB, Duarte JL, Araujo RS, Souto RNP, Fernandes CP (2017) Baccharis reticularia DC. and limonene nanoemulsions: promising larvicidal agents for Aedes aegypti (Diptera: Culicidae) control. Mol 22:1990
Carpenter J, Saharan VK (2017) Ultrasonic assisted formation and stability of mustard oil in water nanoemulsion: effect of process parameters and their optimization. Ultrasonsonochem 35:422–430
Chagas ACS, Barros LD, Cotinguiba F, Furlan M, Giglioti R, Oliveira MCS, Bizzo HR (2012) In vitro efficacy of plant extracts and synthesized substances on Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Parasitol Res 110:295–303
Chagas ACS, Rabelo MD (2012) Metodo para deteccao de substancias com atividaderepelentesobrelarvas do carrapato Rhipicephalus (Boophilus) microplus: revisao e recomendacoes. Embrapa Pecuaria Sudeste Documentos 106:27
Drummond RO, Ernst SE, Trevino JL, Gladney WJ, Graham OH (1973) Boophilus annulatus and B. microplus: laboratory tests of insecticides. J Econ Entomol 66:130–133
Duarte JL, Amado JRR, Oliveira AEMFM, Cruz RAS, Ferreira AM, Souto RNP, Falcao DQ, Carvalho JCT, Fernandes CP (2015) Evaluation of larvicidal activity of a nanoemulsion of Rosmarinus officinalis essential oil. Rev Bras Farmacogn 25:189–192
Elghali A, Hassan SM (2009) Ticks (Acari: Ixodidae) infesting camels (Camelus dromedarius) in Northern Sudan. Onderstepoort J Vet Res 76:177–185
Elhelw R, Elhariri M, Hamza D, Abuowarda M, Ismael E, Farag H (2021) Evidence of the presence of Borrelia burgdorferi in dogs and associated ticks in Egypt. BMC Vet Res 17:1–9
El-Seedi HR, Azeem M, Khalil NS, Sakr HH, Khalifa SA, Awang K, Borg-Karlson AK (2017) Essential oils of aromatic Egyptian plants repel nymphs of the tick Ixodes ricinus (Acari: Ixodidae). Exp Appl Acarol 73:139–157
Fernandes CP, de Almeida FB, Silveira AN, Gonzalez MS, Mello CB, Feder D, Falcao DQ (2014) Development of an insecticidal nanoemulsion with Manilkara subsericea (Sapotaceae) extract. J Nanobiotechnol 12:1–9
Finney DJ (1962) Probit analysis a statistical treatment of the response curve. Cambridge University Press, Cambridge
Ghosh V, Mukherjee A, Chandrasekaran N (2013a) Formulation and characterization of plant essential oil based nanoemulsion: evaluation of its larvicidal activity against Aedes aegypti. Asian J Chem. 25:321–323
Ghosh V, Sugumar S, Mukherjee A, Chandrasekaran N (2013b) Cinnamon oil nanoemulsion formulation by ultrasonic emulsification: investigation of its bactericidal activity. J Nanosci Nanotechnol 13:114–122
Hazra DK (2017) Nano-formulations: High-definition liquid engineering of pesticides for nano-formulations: high-definition liquid engineering of pesticides for advanced crop protection in agriculture. Adv Plant Agric Res 6:1–2
Jesus FL, de Almeida FB, Duarte JL, Oliveira AE, Cruz RA, Souto RN, Fernandes CP (2017) Preparation of a nanoemulsion with Carapa guianensisaublet (Meliaceae) oil by a low-energy/solvent-free method and evaluation of its preliminary residual larvicidal activity. Evid Based Compl Alt Med. https://doi.org/10.1155/2017/6756793
Jongejan F, Uilenberg G (2004) The global importance of ticks. Parasitol 129. Cambridge University Press, Cambridge
Khan MF, Rawat AK, Khatoon S, Hussain MK, Mishra A, Negi DS (2018) In vitro and in vivo antidiabetic effect of extracts of Melia azedarach, Zanthoxylum alatum, and Tanacetum nubigenum. Integr Med Res 7:176–183
Kumar V, Cotran R, Robbins S (2002) Robbins Basic Pathology (7thedn.). Philadelphia: Elsevier Saunders
Martin A, Varona S, Navarrete A, Cocero MJ (2010) Encapsulation and co precipitation processes with supercritical fluids: applications with essential oil. Open Chem Eng J 4:31–41
Milhomem-Paixao SSR, Fascineli ML, Muehlmann LA, Melo KM, Salgado HLC, Joanitti GA, Grisolia CK (2017). Andiroba oil (Carapa guianensis Aublet) nano-emulsions: development and assessment of cytotoxicity, genotoxicity, and hematotoxicity. J Nanomater 1–11
Mishra P, Balaji APB, Mukherjee A, Chandrasekaran N (2018). Bio-based nanoemulsions: An eco-safe approach towards the eco-toxicity problem. In Handbook of Ecomaterials. Springer, Singapore, pp 1–23
Moghimi R, Ghaderi L, Rafati H, Aliahmadi A, McClements DJ (2016) Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chem 194:410–415
Moradi S, Barati A (2019) Essential oils nanoemulsions: preparation, characterization and study of antibacterial activity against Escherichia coli. Inter J Nanosci Nanotechnol 15:199–210
Nogueira JA, Figueiredo A, Duarte JL, de Almeida FB, Santos MG, Nascimento LM, Chagas ACS (2020) Repellency effect of Pilocarpus spicatus A. St.-Hil essential oil and nanoemulsion against Rhipicephalus microplus larvae. Exp Parasitol 215:107919
Parizi LF, Pohl PC, Masuda A, Vaz Junior IDS (2009) New approaches toward anti-Rhipicephalus (Boophilus) microplu stick vaccine. Rev Bras DE Parasitol Vet 18:1–7
Prakash SEL, Manavalan R (2011) Acute toxicity studies of Andrographolide. Res J Pharmac Biol Chem Sci 3:547–552
Ragavan G, Muralidaran Y, Sridharan B, Ganesh RN, Viswanathan P (2017) Evaluation of garlic oil in nano-emulsified form: optimization and its efficacy in high-fat diet induced dyslipidemia in Wistar rats. Food Chem Toxicol 105:203–213
Rahman MF, Siddiqui MKJ, Jamil K (2001) Effects of vepacide (Azadirachta indica) on aspartate and alanine aminotransferase profiles in a sub-chronic study with rats. Hum Exp Toxicol 20:243–249
Rajput ZI, Hu SH, Chen WJ, Arijo AG, Xiao CW (2006) Importance of ticks and their chemical and immunological control in livestock. J Zhejiang Univ Sci 7:912–921
Raza M, Al-Shabanah OA, El-Hadiyah TM, Al-Majed AA (2002) Effect of prolonged vigabatrin treatment on haematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Sci Pharm 2:135–145
Regnault-Roger C, Vincent C, Arnason JT (2012) Essential oils in insect control: low risk products in a high-stakes world. Ann Rev Entomol 57:405–424
Ribeiro WLC, Camurca-Vasconcelos ALF, Macedo ITF, dos Santos JML, de Araújo-Filho JV, de Carvalho RJ, Bevilaqua CML (2015) In vitro effects of Eucalyptus staigeriana nanoemulsion on Haemonchus contortus and toxicity in rodents. Vet Parasitol 212:444–447
Rodrigues ABL, Lopes RM, Rabelo EM, Tomazi R, Santos LL, Brandao LB, Galardo AKR (2020). Development of nano-emulsions based on Ayapana triplinervis for the control of Aedes aegypti larvae. bioRxivdoi https://doi.org/https://doi.org/10.1101/2020.07.09.194985
Rubae AAY (2009) The potential uses of Melia azedarach L. as pesticidal and medicinal plant, review. Am Eurasian J Sustain Agric 3:185–195
Santos DS, Boito JP, Santos RCV, Quatrin PM, Ourique AF, dos Reis JH, Gebert RR, Glombowsky P, Klauck V, Boligon AA (2017b) Nanostructured cinnamon oil has the potential to control Rhipicephalus microplus ticks on cattle. Exp Appl Acarol 73:129–138
Dos Santos DS, Boito JP, Santos RC, Quatrin PM, Ourique AF, Dos Reis JH, Da Silva AS (2017a) Nanostructured cinnamon oil has the potential to control Rhipicephalus microplus ticks on cattle. Exp Appl Acarol 73:129–138
Sariosseiri A, Moshaverinia A, Khodaparast MHH, Kalidari GA (2018) In vitro acaricidal effect of Melia azedarach ripe fruit extract against Dermanyssus gallinae (Acari: Dermanyssidae). Persian J Acarol 7:203–208
Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ (2005) Nano-emulsions. CurrOpin Colloid Interface Sci 10:102–110
Sousa LA, Soares SF, Pires Junior HB, Ferri PH, Borges LMF (2008) Evaluation of efficacy of ripe and unripe fruit oil extracts of Melia azedarach against Rhipicephalus (Boophilus) microplus (Acari: ixodidae). Rev Bras DE Parasitol Vet 17:36–40
Sousa LAD, Junior HBP, Soares SF, Ferri PH, Ribas P, Lima EM, Borges LMF (2011) Potential synergistic effect of Melia azedarach fruit extract and Beauveria bassiana in the control of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) in cattle infestations. Vet Parasitol 175:320–324
Di Stasi LC, Oliveira GP, Carvalhaes MA, Queiroz MJR, Tien OS, Kakinami SH, Reis MS (2002) Medicinal plants popularly used in the Brazilian Tropical Atlantic Forest. Fitoterapia 73:69–91
Sugumar SKS, Clarke MJ, Nirmala BK, Tyagi AM, Chandrasekaran N (2014) Nanoemulsion of eucalyptus oil and its larvicidal activity against Culex quinquefasciatus. Bull Entomol Res 104:393–402
Talbert R, Wall R (2012) Toxicity of essential and non-essential oils against the chewing louse, Bovicola (Werneckiella) ocellatus. ResVet Sci 93:831–835
Tripathi AK, Upadhyay S, Bhuiyan M, Bhattacharya PR (2009) A review of essential oils as biopesticide in insect-pest management. J Pharmacogn Phytotherapy 1:52–63
Turek C, Stintzing FC (2013) Stability of essential oils: a review. Compr Rev Food Sci F 12(40):53
Walker AR, Bouattour A, Camicas JL, Estrada-Pena A, Horak IG, Latif AA, Pegram RG, Preston PM (2003) Ticks of domestic animals in Africa: a guide to identification of species. Bioscience Reports, Edinburgh
Wang L, Dong J, Chen J, Eastoe J, Li X (2009) Design and optimization of a new self-nanoemulsifying drug delivery system. J Colloid Interface Sci 330:443–448
Wang L, Li X, Zhang G, Dong J, Eastoe J (2007) Oil-in-water nanoemulsions for pesticide formulations. J Colloid Interface Sci 314:230–235
Zhang Y, Zhu S, Yin L, Qian F, Tang C, Yin C (2008) Preparation, characterization and biocompatibility of poly (ethylene glycol)-poly (n-butyl cyanoacrylate) nanocapsules with oil core via miniemulsion polymerization. Eur Polym J 44:1654–1661
Acknowledgments
This study is part of a Ph.D. thesis to be submitted to the Department of Parasitology, Faculty of Veterinary Medicine, Cairo University. The study was carried out by financial support of the National Research Centre as a part of Ph.D. Thesis No. 12/2/19.
Author information
Authors and Affiliations
Contributions
MMF, SA, MA, RME, and HSMA designed the experiments. EMH and HSMA participated in the preparation of A. herba-alba and M. azedarach oil nano-emulsions. MMF, SA, MA, RME, and HSMA shared in the bioassay of the A. herba-alba and M. azedarach oil nano-emulsion against different developmental stages of H. dromedarii and evaluated the toxic effect of A. herba-alba and M. azedarach oil nano-emulsion on Swiss albino mice. SA and HSMA analyzed and tabulated the data. MMF, SA, MA, and HSMA wrote the draft of the manuscript. All authors revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethical Approval
This study was approved by Ethical Committee for Medical and Veterinary Research at the National Research Centre (NRC), Egypt following local laws and regulations (approval protocol No 20148). Consent was obtained from the owners of camels included in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Ghany, H.S.M., Abdel-Shafy, S., Abuowarda, M. et al. Acaricidal activity of Artemisia herba-alba and Melia azedarach oil nanoemulsion against Hyalomma dromedarii and their toxicity on Swiss albino mice. Exp Appl Acarol 84, 241–262 (2021). https://doi.org/10.1007/s10493-021-00618-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-021-00618-2