Abstract
In humans, emerging infectious diseases are mostly zoonoses with ticks playing an important role as vectors. Tick-borne relapsing fever Borrelia and spotted fever Rickettsia occur in endemic foci along tropical and subtropical regions of the globe. However, both are widely neglected etiologic agents. In this study, we performed molecular analyses in order to assess the presence of Borrelia and Rickettsia DNA in ticks infesting small-mammals within a National Reserve located in the Andes Mountains, central Chile. While hard ticks were negative for the presence of both agents, sequences of four rickettsial (gltA, htrA, ompA, ompB) and two borrelial (16S rRNA and flaB) genes were obtained from larvae of an Ornithodoros sp. morphologically related with Ornithodoros atacamensis. Phylogenetic analyses indicated that the detected Borrelia and Rickettsia spp. belong to the relapsing fever and spotted fever groups, respectively. Moreover, the agents formed monophyletic clades with Rickettsia amblyommatis and “Candidatus Borrelia johnsonii.” As positive ticks parasitize rodents within a highly visited National Reserve where outdoor activities are of common practice, the risk for human parasitism should not be discarded.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ticks are common parasites of terrestrial vertebrates (Sonenshine and Roe 2014) and harbor the greatest plethora of microorganisms in terms of diversity (Jongejan and Uilenberg 2004). As bloodsucking arthropods, ticks can transmit bacteria to their hosts and eventually to humans (Jongejan and Uilenberg 2004). Current research on tick-borne diseases has led to the discovery of pathogenic agents previously unrecognized for a determined region (Pritt et al. 2016; Muñoz-Leal et al. 2018; Kingry et al. 2018). As a result, an on-going increase in the specific diversity of pathogens associated with ticks is now emerging (Parola et al. 2013; Cutler et al. 2017), and new areas of infection risk in nature have been identified.
Relapsing fever borreliae (RFB) are actively motile spirochetes that maintain chronic infections in ticks and vertebrates parasitized by these arthropods (Cutler 2015). RFB have distinct lineages: one associated with the Ixodidae (i.e. genera Amblyomma, Bothriocroton, Hyalomma, Ixodes, Rhipicephalus), another to the Argasidae (i.e. Argas, Ornithodoros), and a single species to the human body louse (Barbour and Schwan 2018). Particularly in South America, only one species, Borrelia venezuelensis, has been associated with human relapsing fever (Faccini-Martínez et al. 2018). However, RFB of unknown pathogenic roles have been detected in further South American Ornithodoros (Davis 1952; Marinkelle and Grose 1968; Parola et al. 2011; Muñoz-Leal et al. 2019), suggesting that these ticks constitute understudied reservoirs.
Bacteria of the genus Rickettsia are obligatory intracellular coccobacilli that infect soft ticks (Argasidae), hard ticks (Ixodidae), and other terrestrial and aquatic invertebrates (Weinert et al. 2009). Human-pathogenic rickettsiae belong to the spotted fever group and have been detected almost exclusively in hard ticks (Parola et al. 2013). Notwithstanding, Rickettsia rickettsii, the most pathogenic species of the group (Parola et al. 2013), was once isolated from Otobius lagophilus and Ornithodoros nicollei (Silva-Goytia and Elizondo 1952), showing that soft ticks do harbor spotted fever rickettsiae (SFR) in nature.
RFB and SFR are recognized as emerging agents globally (Parola et al. 2013; Cutler et al. 2017). While some tick species are well-known reservoirs and hold endemic foci of pathogenic strains in certain ecosystems (Parola et al. 2013; Cutler et al. 2017), RFB and SFR are still widely neglected (Chikeka and Dumler 2015). A risk to acquire both diseases relies on the exposure to the milieu where infected ticks occur in nature.
Along its territory, Chile holds large preserved areas proper for outdoor activities, yet the role of autochthonous ticks as vectors of RFB or SFR remains almost unstudied. The purpose of this study was to assess the presence of Borrelia and Rickettsia in ticks collected on wild vertebrates in a National Reserve emplaced along an Andean valley of central Chile.
Materials and methods
Site of study, capture of vertebrates and collection of ticks
Between 19 and 26 February 2018 (austral summer), we captured small-mammals and birds in order to collect ticks engorging on them in three areas emplaced at an average altitude of 1072 m within “Río Los Cipreses National Reserve” (RCNR), located in the higher basin of the Cachapoal River, Andes Mountains, central Chile (Fig. 1). We chose RCNR because it represents a highly visited protected area: ~ 28,000 visitors annually between the years 2015–2017 (http://www.conaf.cl/parques-nacionales/visitanos/estadisticas-de-visitacion/). RCNR was created to preserve a typical valley of central Chilean Andes, in which ecosystems are shaped by a temperate climate with dry and hot summers (December to March), and rainy winters (June to September) (Millán and Peña 2000). Small-mammals and birds were captured using 80 Sherman-like traps during five nights and three mist nets for 6 days, respectively. Traps were baited with oat and vanilla extract. While birds were manually restrained, small-mammals were weighed using a 300 g Pesola scale, and then anesthetized with an intraperitoneal injection of a ketamine (60 mg/kg)-xylazine (3 mg/kg) solution (Carpenter and Marion 2017). Ticks were visually screened upon hosts’ skins, manually detached with tweezers and stored in 100% ethanol vials. Animals were identified based on morphology using taxonomic guides and then released in the site of capture.
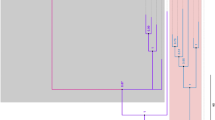
Map showing the points of small-mammal and bird captures within Río Los Cipreses National Reserve (RCNR): El Litral, 34°16′19″S, 70°27′35″W, elevation 963 m; Camping Ranchillo 34°18′09″S, 70°26′56″W, elevation 1124 m; Camping Maitenes 34°20′10″S, 70°24′16″W, elevation 1130 m. The right-hand photograph was taken from the point and orientation given in the map (camera draw), and exemplifies the typical vegetation and topography of the ecosystem at sampling sites
Identification of ticks, DNA extraction and sequencing
All ticks were identified to the genus level using morphological keys (Nava et al. 2017) complemented with original descriptions of Chilean species (Keirans et al. 1976; Muñoz-Leal et al. 2016). For hard ticks, taxonomically relevant characters were photographed with the software ZEN Pro 2 implemented in a Stereo Discovery V12 stereomicroscope (Carl Zeiss, Munich, Germany). On the other hand, collected soft ticks were clarified in 30% KOH (w/v), hydrated in distilled water and mounted on slides using Hoyer’s medium. A detailed observation of slide-mounted specimens was performed through optical microscopy (BX40 microscope, Olympus, Tokyo, Japan), and photographs were taken with an Olympus DP70A camera. The edition of micrographs was made with the software Image-Plus Pro v5.1.
After morphological identification, pools and individual tick specimens were submitted to DNA extraction using the guanidine isothiocyanate and phenol/chloroform protocol (Sangioni et al. 2005). Depending on the size of each tick, the final pelleted DNA was eluted in 10–30 µl of Tris EDTA buffer solution (TE) and stored at − 20 °C until tested. To check a successful DNA extraction and genetically identify collected specimens, a conventional polymerase chain reaction (PCR) using primers 5′-CCGGTCTGAACTCAGATCAAGT-3′ (forward) and 5′-GCTCAATGATTTTTTAAATTGCTG-3′ (reverse) targeting a ≈ 460-bp fragment of the tick mitochondrial 16S rRNA gene was implemented following Mangold et al. (1998). PCR products were resolved in 1.5% agarose gels stained with SybrSafe (Life Technologies/Thermo Fisher Scientific, Carlsbad, CA, USA) and results captured by imaging (AlphaImager HP, ProteinSimple). Amplicons of expected size were treated with ExoSAP-IT (Affimetryx/Thermo Fisher Scientific, Santa Clara, CA), prepared for sequencing with the Big Dye Terminator Cycle Sequencing kit (Applied Biosystems, Foster City, CA), and sequenced in an ABI 3500 automatic device (Applied Biosystems/Thermo Fisher Scientific, Foster City, CA). Obtained sequences were de novo assembled and trimmed with Geneious R9 (Kearse et al. 2012). Edited sequences were submitted to BLASTn analyses (www.ncbi.nlm.nih.gov/blast) in order to infer genetic similarities with congeneric organisms available in GenBank (Altschul et al. 1990).
Borrelia and Rickettsia PCR
For determining the presence of Borrelia and Rickettsia DNA, two initial PCRs were performed: (1) a nested reaction targeting the Borrelia flaB gene, and (2) a conventional reaction for amplifying a fragment of the Rickettsia gltA gene. Positive samples were then submitted to a battery of conventional and semi-nested protocols to amplify Borrelia flaB and 16S rRNA, and Rickettsia gltA, htrA, ompA, and ompB genes. References for thermal conditions and primers employed in each protocol are listed in Table 1. Conventional PCRs were performed using a mix of 25 µl, composed by 12.5 µl of DreamTaq Green PCR master mix (2X, Thermo Scientific, Baltics UAB, Vilnius, Lithuania), 1 µl of each primer (10 pmol/µl), 8 µl of ultrapure water and 2.5 µl of template DNA. For nested and semi-nested rounds, we employed 1 µl of the first reaction product and 9.5 µl of ultrapure water. Borrelia anserina strain PB (Ataliba et al. 2007) and Rickettsia vini strain Breclav (Nováková et al. 2016). Ultrapure water was employed in negative controls for each reaction. Mix preparation, pipetting of nested reactions, and electrophoresis were performed in separated rooms. Sequencing and further analyses of the obtained sequences were performed as above.
Phylogenetic analyses
Independent alignments using obtained and GenBank-retrieved sequences were constructed for each sequenced gene using CLUSTAL W (Thompson et al. 1994) implemented MEGA 5 (Tamura et al. 2011), and manually adjusted with GeneDoc (Nicholas et al. 1997). Two phylogenetic analyses were inferred with obtained sequences of tick 16S mitochondrial rDNA, one for soft ticks, joining 65 sequences, and another for hard ticks including 48 sequences. Ixodes uriae (AB030017) and Ixodes holocyclus (AB051084) were used as outgroups for the Argasidae tree, and Argas persicus (AF001402) rooted the Ixodidae tree. Nucleotide substitution models for all phylogenetic analyses were calculated with MEGA 5 (Tamura et al. 2011). Each phylogeny was inferred by two methods. A maximum parsimony (MP) analysis was implemented in PAUP 4.0b10 (Swofford 2002) with 500 bootstrap replicates, random stepwise addition of starting trees (with random addition of sequences), and TBR branch swapping. Then, a bayesian analysis was performed using MrBayes v3.1.2 (Huelsenbeck and Ronquist 2001) with four independent Markov chain runs for 5,000,000 metropolis-coupled MCMC generations, sampling a tree every 100 generations. The first 25% of the trees represented burn-in, and the remaining trees were used to calculate bayesian posterior probability values. GenBank accession numbers of sequences used for tick phylogeny are embedded in the tree.
Alignments of Borrelia 16S rRNA and flaB genes were constructed including sequences for 29 strains of the genus. Alignments of gltA, htrA, ompA, and ompB genes joined other 35 homologous sequences belonging to 28 Rickettsia spp. Borrelial and rickettsial genes were manually concatenated in the order 16S rRNA-flaB and gltA-htrA-ompA-ompB, respectively. Resulting matrixes were submitted to a bayesian and maximum likelihood (ML) phylogenetic analyses using the general time reversible (GTR) substitution model and a gamma variation rate between sites. The bayesian phylogeny for both agents was implemented as above stated. The ML tree was constructed with PhyML (Guindon and Gascuel 2003), with five substitution rate categories and 1000 bootstrap replicates. The nearest neighbour interchange (NNI) distance was used to improve the tree topology (Li et al. 1996). Borrelia coriaceae Co53 (CP005745) and Rickettsia canadensis McKiel (CP000409) rooted each tree, respectively. GenBank accession numbers for Borrelia and Rickettsia sequences used to construct both phylogenies are specified in Suppl. 1.
Results
Capture of vertebrates, collection and identification of ticks
A total of 46 animals (23 birds and 23 small-mammals) were captured totalizing a capture effort of 49 h for birds and 84 h for small-mammals. The numbers of captured specimens (birds–small-mammals) per each site were as follows: 0–5 at El Litral, 9–3 at Camping Ranchillo and 14–15 at Camping Maitenes. All birds were negative to tick infestation. A total of seven larvae and 12 nymphs of the Ixodidae family and eight larvae of the Argasidae family were collected upon rodents (Table 2).
All nymphs and larvae of the Ixodidae family were initially identified as Ixodes by the presence of an anal groove curved anteriorly to the anus. Nymphal characters observed through optical microscopy unveiled that these ticks matched the morphology of Ixodes sigelos (Keirans et al. 1976; Guglielmone et al. 2005) (Fig. 2a–d). All four slide-mounted argasid larvae were determined as an unidentified Ornithodoros sp. by sharing the following traits: dorsal surface provided with 18 pairs of setae, seven anterolateral, five central (one specimen with six central) and six posterolateral pairs; dorsal plate pyriform in shape; hypostome dental formula: 3/3 from the apex until one-fourth of its total length, then 2/2 towards the base; first, second and third files provided with 22, 20 and 8 denticles respectively (Fig. 2e–h). One nymph collected on Octodon bridgesi at Camping Ranchillo, one larva collected on Abrothrix olivaceus and four slide-mounted larvae collected on Phyllotis darwini at Camping Maitenes were deposited in the tick collection “Coleção Nacional de Carrapatos Danilo Gonçalves Saraiva” under CNC-3838 and -3839.
Micrographs of the Ixodes sp. nymph and the larva of Ornithodoros sp. collected in this study. Nymph of Ixodes sp.: a ventral view; b dorsal capitulum: wing shaped lateral protuberances (arrowed) and scutum; c ventral capitulum: posterior processes in palpal article I (arrowed); d coxae I, II, III and IV: note the number of spurs (arrowed) in each coxa. Larva of Ornithodoros sp.: e dorsal view (number of setal pairs indicated); f ventral view (number of setal pairs indicated); g hypostome; h dorsal plate. Al anterolateral, C central, Ca circumanal, Pl posterolateral, Pm posteromedian, Pl posterolateral
All 20 ticks submitted to DNA extraction were positive to mitochondrial 16S rRNA gene PCR. Obtained sequences confirmed morphological diagnoses of hard ticks partially since a single haplotype of 407 bp was only 97.05% (395/407 bp) identical with I. sigelos from Argentina (HM014413, Sanchez et al. 2010). Due to this percentage of similarity, we opted to treat our species as an Ixodes sp. belonging to the I. sigelos group. On the other hand, Ornithodoros larvae yielded a trimmed sequence 94.96% (377/397 bp) identical with Ornithodoros atacamensis, a parasite of lizards in the Atacama Desert of northern Chile (Muñoz-Leal et al. 2016). Sequences of ticks obtained in this study were deposited in GenBank under accession numbers MK110659 (Ornithodoros sp. Cachapoal) and MK110660 (Ixodes sp. sigelos group Cachapoal).
Borrelia and Rickettsia PCR
While ten nymphs and six larvae of an Ixodes sp. of the I. sigelos group, and four larvae of an Ornithodoros sp. collected from different hosts were screened to detect Borrelia and Rickettsia DNA, only soft ticks yielded positive amplicons (Table 3). While trimmed sequences for Borrelia 16S rRNA and flaB genes resulted into fragments of 1272 bp and 1005 bp each, Rickettsia gtlA, htrA, ompA, ompB genes yielded partial fragments of 992 bp, 479 bp, 587 bp, and 811 bp, respectively.
After BLASTn comparisons, sequences of Borrelia obtained in the current study were the most identical with “Candidatus Borrelia johnsonii” strains IA-1 and 15-3581 as follows: 16S rRNA gene 99.92% identical (1271/1272 bp, 0 gaps) to both strains (EU492388, MF062083); flaB gene 99.80% identical (1000/1002 bp, 0 gaps) to strain IA-1 (EU492387), and 100% identical (980/980 bp, 0 gaps) to strain 15-3581 (MF062084). Sequences of Rickettsia obtained in this study showed the highest identity percentages with two rickettsiae of the spotted fever group. On the one hand, gltA and htrA genes were 99.59% (988/992 bp, 0 gaps) and 99.37% (476/479 bp, 0 gaps) identical with homologous sequences of Rickettsia raoultii strain IM16 (CP019435). On the other hand, ompA and ompB sequences were 98.12% (576/587 bp, 0 gaps) and 97.79% (799/817 bp, 6 gaps) identical with Rickettsia amblyommatis strain Ac37 (CP012420). We tentatively named both detected agents as Borrelia sp. Cachapoal and Rickettsia sp. Cachapoal. Their respective sequences were deposited in GenBank (Borrelia: MK106060, MK112520; Rickettsia: MK112516, MK112517, MK112518, MK112519).
Phylogenies
The morphological identity of the collected Ixodes specimens was considered inconclusive when compared with sequences of other congeneric species available in GenBank. In line with the previous statement, the Ixodes sp. herein collected clusters within a monophyletic group composed by sequences of other morphologically closely related species from Argentina and Chile, and also with Ixodes abrocomae and Ixodes nuttalli. The sequence of an Ixodes sp. from Mocha Island (Chile) appears as its closest relative (Fig. 3). However, support values for this group of Ixodes spp. is low. On the other hand, with consistent bayesian and MP support values, the phylogeny for soft ticks indicates that the undetermined Ornithodoros sp. collected in RCNR corresponds to a sister taxon of O. atacamensis (Fig. 4). Additionally, another yet-to-be-described species recently reported in association with small-mammals from northeastern Brazil (Maia et al. 2018) appears closely related to both Ornithodoros sp. Cachapoal and O. atacamensis (Fig. 4).
Maximum parsimony (MP) and bayesian inferred phylogenetic tree constructed for ticks of the genus Ixodes. The tree is drawn to scale and scale bar indicates nucleotide substitutions per site. Otherwise omitted, numbers above and below branches represent bayesian posterior probability and MP bootstrap values ≥ 0.70 and ≥ 70%, respectively. The position of the Ixodes sp. collected in this study is highlighted in bold as “Ixodes sp. sigelos group Cachapoal.”
Maximum parsimony (MP) and bayesian inferred phylogenetic tree for Argasidae family. The tree is drawn to scale and scale bar indicates nucleotide substitutions per site. Otherwise omitted, numbers above and below branches represent bayesian posterior probability and MP bootstrap values ≥ 0.70 and ≥ 70%, respectively. The position of the Ornithodoros sp. collected in this study is highlighted in bold as “Ornithodoros sp. Cachapoal.”
Supported by high bayesian posterior probability and ML bootstrap values, Borrelia sp. Cachapoal formed a monophyletic group with “Ca. B. johnsonii.” Moreover, both species clustered as a sister group to a clade composed by Borrelia parkeri, Borrelia turicatae, and Borrelia venezuelensis (Fig. 5). On the other hand, Rickettsia sp. Cachapoal clustered between a group of R. amblyommatis strains and a clade formed by Rickettsia sp. ARANHA and Rickettsia sp. AL, two putative new rickettsial agents detected in Amblyomma longirostre from Brazil (Labruna et al. 2004a; Ogrzewalska et al. 2008) (Fig. 6).
Maximum likelihood (ML) and bayesian inferred phylogenetic tree for Borrelia spp. The tree is drawn to scale and scale bar indicates nucleotide substitutions per site. Otherwise omitted, numbers above and below the nodes represent bayesian posterior probability and ML bootstrap values ≥ 0.70 and ≥ 70%, respectively. The position of Borrelia sp. Cachapoal is highlighted in bold
Maximum likelihood (ML) and bayesian inferred phylogenetic tree for Rickettsia spp. The tree is drawn to scale and scale bar indicates nucleotide substitutions per site. Strain names are indicated immediately after each species. Otherwise omitted, numbers above and below the nodes represent bayesian posterior probability and ML bootstrap values ≥ 0.70 and ≥ 70%, respectively. The position of Rickettsia sp. Cachapoal is highlighted in bold
Discussion
Vertebrate hosts and tick identities
Although capture efforts allowed the examination of ten species of birds and one marsupial, ticks were found parasitizing rodents only. An Ixodes sp. of the I. sigelos group was the sole hard tick identified in the current study. Ixodes sigelos was originally described from specimens collected along Chile (i.e. Metropolitan, Maule, and Biobío regions) in association with abrocomid (Abrocomidae), cricetid (Cricetidae) and octodontid (Octodontidae) rodents (Keirans et al. 1976). Further collections identified this tick in the Patagonia region from Argentina and Chile (Guglielmone et al. 2005; Sebastian et al. 2016). Here, our results point that the Ixodes sp. collected from cricetid and octodontid rodents in the RCNR would correspond to a species morphologically and phylogenetically related with I. sigelos. Remarkably, phylogenetic analyses including several sequences identified as I. sigelos from Argentina and Chile (Fig. 3) indicate that this taxon is paraphyletic. This fact reinforces the hypothesis that ticks morphologically affinis to I. sigelos could correspond to a group of cryptic species (Sanchez et al. 2010).
Engorged larval stages of an unidentified Ornithodoros sp. were collected on P. darwini, which corresponds to the first time that soft tick larvae are found parasitizing this rodent species. Slide-mounted specimens revealed a phenotype highly similar to O. atacamensis, a tick described in association with reptiles from the barren ecosystems of the Atacama Desert in northern Chile (Muñoz-Leal et al. 2016). Slight but evident differences separate currently collected Ornithodoros sp. from O. atacamensis larvae: six instead of five posterolateral pairs of setae, and the third row of denticles reaching a fourth rather than a third of the hypostome, respectively. This morphological relatedness seems to be explained after assessing the phylogenetic positions of both tick species. Indeed, the tree topology suggests that genetic divergence observed between them (> 5%) would be sufficient enough to consider the collected Ornithodoros sp. as a putative new taxon. However, the sequencing of additional loci, the examination of a major number of larvae, collecting material from other localities, including adult forms, is now needed to assess this hypothesis accurately.
Borrelia
Recently, a phylogenetic analysis of 16S rRNA, flaB and glpQ genes unveiled a close relatedness between a Brazilian strain of B. venezuelensis and B. turicatae (Muñoz-Leal et al. 2018), a human-pathogenic species associated with Ornithodoros turicata in North America (Dworkin et al. 2008). This scenario, in which two genetically similar Borrelia spp. are associated with vectors with distanced distributions is impressive, yet it constitutes an unsolved question. Similarly, genetic identities and phylogenetic analyses of 16S rRNA and flaB sequences of Borrelia sp. Cachapoal indicate a close relatedness with “Ca. B. johnsonii”, another North American species harbored by the bat tick Ornithodoros kelleyi (Schwan et al. 2009). Although the sequencing of more loci of both South American borreliae is undoubtedly necessary before stating any conclusion, the occurrence of close genetic relationship between North and South American RFB suggests a common origin at some point in their evolutionary history. This fact warrants special interest since northern and southern parts of the American continent were once isolated and joined only nearly three Mya (Estrada-Peña et al. 2018).
Recently, a sound molecular survey for Borrelia spp. in patients with symptoms of tick-borne illness detected “Ca. B. johnsonii” DNA in human blood, introduced this species as a previously unrecognized pathogenic agent (Kingry et al. 2018). Even if the role of Borrelia sp. Cachapoal as an etiologic agent of relapsing fever in humans remains unsolved, its phylogenetic position as a sister clade of “Ca. B. johnsonii” suggests that a pathogenic nature cannot be discarded.
For the moment, the role of P. darwini as a vertebrate reservoir of Borrelia sp. Cachapoal remains unknown. However, considering that ticks were collected feeding upon and that rodents are recognized natural reservoirs of other RFB (Cutler 2015), it is highly possible that this Chilean species could play a role in the cycle of Borrelia sp. Cachapoal in RCNR.
Rickettsia
Rickettsiae are common microorganisms among the South American Ixodidae (Labruna 2009; Parola et al. 2013). The study of Rickettsia has been largely biased towards ixodid ticks because of the well-known vector roles these parasites hold. In turn, limited attention has been paid to rickettsiae associated with the Argasidae in South America (but see Tahir et al. 2016). Here, larvae of an undetermined Ornithodoros sp. were positive to Rickettsia detection, suggesting that this species naturally harbor bacteria of this genus. Though, a blood origin for this molecular detection cannot be discarded since all collected ticks were almost fully engorged.
After sequencing partial fragments of gltA, htrA, ompA, and ompB loci observed genetic divergences of Rickettsia sp. Cachapoal exceeded the divergence limits considered when inferring identities for a determined species within the genus (Raoult et al. 2005). Moreover, phylogenetic analyses inferred by bayesian and ML methods emplaced Rickettsia sp. Cachapoal as a well-supported branch, sister to R. amblyommatis (Fig. 6). A monophyletic clade of R. amblyommatis-related rickettsiae appears composed by the other two species, Rickettsia sp. ARANHA and Rickettsia sp. AL, both characterized from A. longirostre ticks collected in Brazilian Amazon and Atlantic rainforest (Labruna et al. 2004a; Ogrzewalska et al. 2008). With the current evidence, to propose any explanation between phylogenetically related, yet biogeographically vastly distanced Rickettsia spp. would be rather speculative. However, examples of phylogenetically related strains of Rickettsia associated with soft ticks and hard ticks occurring in distanced regions of the world have been already demonstrated (Izzard et al. 2018). This fact suggests that the study of soft tick-associated rickettsiae would bring valuable information in order to unveil evolutionary pathways of these bacteria among the Ixodoidea.
Although the role of R. amblyommatis as an etiologic agent is still unclear, this species does correspond to an SFR, and therefore it should be considered as a potentially pathogenic organism (Parola et al. 2013). Consequently, a pathogenic role of phylogenetically closely related Rickettsia sp. Cachapoal should not be ignored.
Final remarks
At least two species of ticks were identified parasitizing rodents in the RNRC. Two putative new agents, a relapsing fever Borrelia and a spotted fever Rickettsia, were characterized upon DNA extracted from a yet-to-be-identified soft tick species of the genus Ornithodoros. Although to date human cases of tick-borne relapsing or spotted fever are inexistent in Chile, and records of ticks parasitizing humans lack in recent times for the country (Guglielmone and Robbins 2018), it must not been overlooked that both diseases are still neglected in regions where they are endemic worldwide (Chikeka and Dumler 2015). From this point of view, our results call for the attention into scenarios gathering epidemiological factors compatible for both maladies to occur, such as outdoor enthusiasts exposed to ticks while entering Andean environments in central Chile.
References
Altschul SF, Gish W, Miller W et al (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Ataliba AC, Resende JS, Yoshinari N, Labruna MB (2007) Isolation and molecular characterization of a Brazilian strain of Borrelia anserina, the agent of fowl spirochaetosis. Res Vet Sci 83:145–149. https://doi.org/10.1016/j.rvsc.2006.11.014
Barbour A, Schwan TG (2018) Borrelia. In: Wang G, Schwartz I (eds) Bergey’s manual of systematics of archaea and bacteria. Wiley, in association with Bergey’s Manual Trust, New York. https://doi.org/10.1002/9781118960608.gbm01246.pub2
Carpenter J, Marion CJ (2017) Exotic animal formulary, 5th edn. Elsevier, St. Louis, p 776
Chikeka I, Dumler JS (2015) Neglected bacterial zoonoses. Clin Microbiol Infect 21:404–415. https://doi.org/10.1016/j.cmi.2015.04.022
Cutler SJ (2015) Relapsing Fever borreliae, a global review. Clin Lab Med 35:847–865. https://doi.org/10.1016/j.cll.2015.07.001
Cutler SJ, Ruzic-Sabljic E, Potkonjak A (2017) Emerging borreliae—expanding beyond Lyme borreliosis. Mol Cell Probes 31:22–27. https://doi.org/10.1016/j.mcp.2016.08.003
Davis GE (1952) Observations on the biology of the argasid tick, Ornithodoros brasiliensis Aragão, 1923, with the recovery of a spirochete, Borrelia brasiliensis, n. sp. J Parasitol 38:473–476
Dworkin MS, Schwan TG, Anderson DE, Borchardt SM (2008) Tick-borne relapsing fever. Infect Dis Clin North Am 22:449–468. https://doi.org/10.1016/j.idc.2008.03.006
Estrada-Peña A, Álvarez-Jarreta J, Cabezas-Cruz A (2018) Reservoir and vector evolutionary pressures shaped the adaptation of Borrelia. Infect Genet Evol 66:308–318. https://doi.org/10.1016/j.meegid.2018.03.023
Faccini-Martínez Á, González Tous M, Mattar Velilla S (2018) Fiebre recurrente transmitida por garrapatas: ¿otra etiología subdiagnosticada en Latinoamérica tropical? Rev MVZ Córdoba 23:6399. https://doi.org/10.21897/rmvz.1230
Guglielmone AA, Robbins RG (2018) Hard Ticks (Acari: Ixodida: Ixodidae) PARASITIZING humans. Springer International Publishing, Cham
Guglielmone AA, Acuña DG, Autino AG et al (2005) Ixodes sigelos Keirans, Clifford & Corwin, 1976 (Acari : Ixodidae) in Argentina and southern Chile. Syst Appl Acarol 1976:37–40
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate Large phylogenies by Maximum Likelihood. Syst Biol 52:696–704. https://doi.org/10.1080/10635150390235520
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755. https://doi.org/10.1093/bioinformatics/17.8.754
Izzard L, Chung M, Dunning Hotopp J et al (2018) Isolation of a divergent strain of Rickettsia japonica from Dew’s Australian bat Argasid ticks (Argas (Carios) dewae) in Victoria, Australia. Ticks Tick Borne Dis 9:1484–1488. https://doi.org/10.1016/j.ttbdis.2018.07.007
Jongejan F, Uilenberg G (2004) The global importance of ticks. Parasitology 129:S3–S14. https://doi.org/10.1017/S0031182004005967
Kearse M, Moir R, Wilson A et al (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Keirans JE, Clifford CM, Corwin D (1976) Ixodes sigelos, n. sp. (Acarina: Ixodidae), a parasite of rodents in Chile, with a method for preparing ticks for examination by scanning electron microscopy. Acarologia 18:217–225
Kingry LC, Anacker M, Pritt B et al (2018) Surveillance for and discovery of Borrelia species in US patients suspected of tickborne illness. Clin Infect Dis 66:1864–1871. https://doi.org/10.1093/cid/cix1107
Labruna MB (2009) Ecology of Rickettsia in South America. Ann N Y Acad Sci 1166:156–166. https://doi.org/10.1111/j.1749-6632.2009.04516.x
Labruna MB, McBride JW, Bouyer DH et al (2004a) Molecular evidence for a spotted fever group Rickettsia species in the tick Amblyomma longirostre in Brazil. J Med Entomol 41:533–537. https://doi.org/10.1603/0022-2585-41.3.533
Labruna MB, Whitworth T, Horta MC, Bouyer DH, McBride JW, Pinter A et al (2004b) Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of São Paulo, Brazil, where Brazilian spotted fever is endemic. J Clin Microbiol 42(1):90–98. https://doi.org/10.1128/JCM.42.1.90-98.2004
Li M, Tromp J, Zhang L (1996) On the nearest neighbour interchange distance between evolutionary trees. J Theor Biol 182:463–467. https://doi.org/10.1006/jtbi.1996.0188
Maia MO, Koppe VC, Muñoz-Leal S et al (2018) Detection of Rickettsia spp. in ticks associated to wild mammals in Northeastern Brazil, with notes on an undetermined Ornithodoros sp. collected from marsupials. Exp Appl Acarol 76:523–535. https://doi.org/10.1007/s10493-018-0323-2
Mangold AJ, Bargues MD, Mas-Coma S (1998) Mitochondrial 16S rDNA sequences and phylogenetic relationships of species of Rhipicephalus and other tick genera among Metastriata (Acari: Ixodidae). Parasitol Res 84:478–484
Marinkelle CJ, Grose ES (1968) Species of Borrelia from a Colombian Bat (Natalus tumidirostris). Nature 218:487
Millán J, Peña E (2000) Plan de manejo Reserva Nacional Río de Los Cipreses periodo 2000–2004. Ministerio de Agricultura - CONAF, Santiago
Muñoz-Leal S, Venzal JM, González-Acuña D et al (2016) A new species of Ornithodoros (Acari: Argasidae) from desert areas of northern Chile. Ticks Tick Borne Dis 7:901–910. https://doi.org/10.1016/j.ttbdis.2016.04.008
Muñoz-Leal S, Faccini-Martínez ÁA, Costa FB et al (2018) Isolation and molecular characterization of a relapsing fever Borrelia recovered from Ornithodoros rudis in Brazil. Ticks Tick Borne Dis. https://doi.org/10.1016/j.ttbdis.2018.03.008
Muñoz-Leal S, Lopes MG, Marcili A et al (2019) Anaplasmataceae, Borrelia and Hepatozoon agents in ticks (Acari: Argasidae, Ixodidae) from Chile. Acta Trop 192:91–103. https://doi.org/10.1016/j.actatropica.2019.02.002
Nava S, Venzal JM, González-Acuña D et al (2017) Ticks of the Southern Cone of America: diagnosis, distribution and hosts with taxonomy, ecology and sanitary importance. Elsevier, Academic Press, New York
Nicholas KB, Nicholas HB, Deerfield D (1997) GeneDoc: analysis and visualization of genetic variation. Embnew News 4:14
Nováková M, Costa FB, Krause F et al (2016) Rickettsia vini n. sp. (Rickettsiaceae) infecting the tick Ixodes arboricola (Acari: Ixodidae). Parasit Vectors 9:1–8. https://doi.org/10.1186/s13071-016-1742-8
Ogrzewalska M, Pacheco RC, Uezu A et al (2008) Ticks (Acari: Ixodidae) infesting wild birds in an Atlantic forest area in the state of São Paulo, Brazil, with isolation of Rickettsia from the tick Amblyomma longirostre. J Med Entomol 45:770–774. https://doi.org/10.1093/jmedent/45.4.770
Parola P, Ryelandt J, Mangold AJ et al (2011) Relapsing fever Borrelia in Ornithodoros ticks from Bolivia. Ann Trop Med Parasitol 105:407–411. https://doi.org/10.1179/1364859411Y.0000000021
Parola P, Paddock CD, Socolovschi C et al (2013) Update on tick-borne Rickettsioses around the world: a Geographic approach. Clin Microbiol Rev 26:657–702. https://doi.org/10.1128/CMR.00032-13
Pritt BS, Respicio-Kingry LB, Sloan LM et al (2016) Borrelia mayonii sp. nov., a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper midwestern United States. Int J Syst Evol Microbiol 66:4878–4880. https://doi.org/10.1099/ijsem.0.001445
Raoult D, Fournier PE, Eremeeva M et al (2005) Naming of rickettsiae and rickettsial diseases. Ann N Y Acad Sci 1063:1–12. https://doi.org/10.1196/annals.1355.002
Ras NM, Lascola B, Postic D, Cutler SJ, Rodhain F, Baranton G, Raoult D (1996) Phylogenesis of relapsing fever Borrelia spp. Int J Syst Bacteriol 46(4):859–865. https://doi.org/10.1099/00207713-46-4-859
Regnery RL, Spruill CL, Plikaytis BD (1991) Genotypic identification of Rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol 173(5):1576–1589
Roux V, Raoult D (2000) Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int J Syst Evol Microbiol 50:1449–1455
Roux V, Fournier PE, Raoult D (1996) Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol 34(9):2058–2065
Sanchez JP, Nava S, Lareschi M et al (2010) Host range and geographical distribution of Ixodes sigelos (Acari: Ixodidae). Exp Appl Acarol 52:199–205. https://doi.org/10.1007/s10493-010-9358-8
Sangioni LA, Horta MC, Vianna MCB et al (2005) Rickettsial infection in animals and Brazilian Spotted Fever endemicity. Emerg Infect Dis 11:265–270. https://doi.org/10.3201/eid1102.040656
Schwan TG, Raffel SJ, Schrumpf ME, Policastro PF, Rawlings JA, Lane RS et al (2005) Phylogenetic analysis of the spirochetes Borrelia parkeri and Borrelia turicatae and the potential for Tick-borne Relapsing Fever in Florida. J Clin Microbiol 43(8):3851–3859. https://doi.org/10.1128/JCM.43.8.3851-3859.2005
Schwan TG, Raffel SJ, Schrumpf ME et al (2009) Characterization of a novel relapsing fever spirochete in the midgut, coxal fluid, and salivary glands of the bat tick Carios kelleyi. Vector Borne Zoonot Dis 9:643–647. https://doi.org/10.1089/vbz.2008.0177
Sebastian PS, Bottero MNS, Carvalho L et al (2016) Borrelia burgdorferi sensu lato in Ixodes cf. neuquenensis and Ixodes sigelos ticks from the Patagonian region of Argentina. Acta Trop 162:218–221. https://doi.org/10.1016/j.actatropica.2016.06.030
Silva-Goytia R, Elizondo A (1952) Estudios sobre Fiebre Manchada en México. II. Parásitos hematófagos encontrados naturalmente infectados. Rev Med México 32:278–282
Sonenshine DE, Roe RM (2014) Biology of ticks. Volume I. Oxford University Press, Oxford
Stromdahl EY, Williamson PC, Kollars TMJ, Evans SR, Barry RK, Vince MA, Dobbs NA (2003) DNA evidence of Borrelia lonestari in Amblyomma americanum (Acari: Ixodidae) removed from humans. J Clin Microbiol 41(12):5557–5562. https://doi.org/10.1128/JCM.41.12.5557
Swofford LD (2002) PAUP*: phylogenetic analysis using parsimony (* and other methods). Sinauer Associates, Sunderland
Tahir D, Socolovschi C, Marié J-L et al (2016) New Rickettsia species in soft ticks Ornithodoros hasei collected from bats in French Guiana. Ticks Tick Borne Dis 7(6):1089–1096. https://doi.org/10.1016/j.ttbdis.2016.09.004
Tamura K, Peterson D, Peterson N et al (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. https://doi.org/10.1093/molbev/msr121
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. https://doi.org/10.1093/nar/22.22.4673
Webb L, Carl M, Malloy DC, Dasch GA (1990) Detection of murine typhus infection in fleas by using the polymerase chain reaction. J Clin Microbiol 28(3):530–534
Weinert L, Werren JH, Aebi A et al (2009) Evolution and diversity of Rickettsia bacteria. BMC Biol 7:6. https://doi.org/10.1186/1741-7007-7-6
Acknowledgements
We thank Hugo Durán for his valuable help during fieldwork and CONAF personnel for logistic support within the RCNR. Fieldwork and the collection of samples were funded by the “Fondo Científico del Alto Cachapoal versión 7 – Pacific Hydro Chile”. Laboratory work was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). SML was funded by FAPESP (Grant #2018/02521-1). DFC was funded by the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT, Grant BCH #72170436).
Author information
Authors and Affiliations
Contributions
SML and MBL conceived the study. SML, DFC, and MA made vertebrate capture and tick collection in the field. SML identified ticks, performed laboratory work, and drafted the manuscript. SML and AM performed phylogenetic analyses. All authors contributed to reviewing the manuscript, read, and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
Animal captures, handling, and the collection of biological samples have been approved by the “Corporación Nacional Forestal” (CONAF) and by permit 417/2018 given by the “Servicio Agrícola y Ganadero” (SAG), Chile. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muñoz-Leal, S., Marcili, A., Fuentes-Castillo, D. et al. A relapsing fever Borrelia and spotted fever Rickettsia in ticks from an Andean valley, central Chile. Exp Appl Acarol 78, 403–420 (2019). https://doi.org/10.1007/s10493-019-00389-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-019-00389-x