Abstract
The tick Haemaphysalis tibetensis Hoogstraal is found uniquely in the Qinghai-Tibet plateau of Tibet and Gansu of China. Not much is known of this tick. Therefore, in this study we investigated the life cycle of H. tibetensis under field conditions from March 2014 to March 2015 in Damxung County, north Lhasa City in Tibet (Autonomous Region in China). The results of the study demonstrated that the tick H. tibetensis requires an average of 177.8 days (range 129–202 days) to complete a life cycle, with rabbits supplied as hosts in the field plot. Under natural lighting and climate conditions, the feeding period of females was an average of 7.7 days, and the pre-oviposition period was 9.4 days, followed by 28.2 days for oviposition. The premolting period of nymphs lasted 52.7 days, which was the longest life cycle phase. The average weight ratio of engorged to unfed females was 58.2. Additionally, there was a highly positive correlation between the weight of engorged and the number of the eggs that were laid (r = 0.83, P < 0.05). The reproductive efficiency index and reproductive fitness index in females were 5.1 and 4.7, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ticks are notorious chelicerate arthropods, blood-sucking ectoparasites of a wide range of vertebrate hosts, and are causes of a variety of severe human and animal diseases (Estrada-Peña and de la Fuente 2014). The tick Haemaphysalis tibetensis Hoogstraal is only distributed in Qinghai-Tibet plateau in Tibet and Gansu of China, and mainly parasitizes on dogs, sheep, and yaks (Deng and Jiang 1991). Although a newly GRD spirochete (Zhang et al. 1996) and an orbivirus (Ti3010) (Huang et al. 1999) have been isolated from this tick species, its vector potential has not been fully understood. Additionally, further understanding of the biology and life cycle of H. tibetensis, limited in distribution to the Qinghai-Tibet plateau, is needed. Earlier studies have described the morphology of the adults and nymphs of H. tibetensis (Hoogstraal 1965; Deng and Jiang 1991). However, it remains a challenge to further characterize this tick and tick-borne pathogens.
The Qinghai-Tibet plateau is regarded as the roof of the world with extremely hostile living environments, including poor oxygen, drought conditions, intense ultraviolet radiation, and low temperature (Dai et al. 2009). The annual mean temperature is between −2.8 and 1.6 °C, and it has an annual precipitation between 247.3 and 513.6 mm, which allows the growth and survival of selected organisms that are adapted to these conditions (Gao et al. 2006). The tick H. tibetensis is often found on the high plateau and on mountains above 4000 meters in altitude (Deng and Jiang 1991). In this study, we investigated the life cycle of H. tibetensis under field conditions from March 2014 to March 2015 in Damxung County, north Lhasa City in the Tibet Autonomous Region of China. The results of this study will provide basic knowledge on the adaptation of this tick species and suggest constructive measures for integrated control of this tick.
Materials and methods
Study site
The study was conducted in Damxung County (90°45′–91°31′E, 29°31′–31°04′N), north of Lhasa City (situated in the center of the Tibet Autonomous Region, southwest of China), from March 2014 to March 2015. To study the life cycle and biological characteristics of H. tibetensis, a 150 × 150 cm field plot (90°51′737″E, 30°25′325″N, and altitude 4353 m) (Fig. 1) was chosen in a natural tick habitat. The plot was defined by a 10 × 10 cm water-filled drain to prevent tick escape, and covered with scattered clusters of Kobresia schoenoides and Sabina pingii.
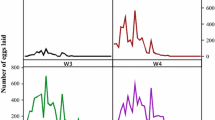
Monthly minimum, maximum, and mean ambient temperatures, as well as relative humidity were obtained during the study period using hygrothermographs located 50 cm above the ground that were planted in the plot (Fig. 2) (Qingsheng Electronic Technology, China).
Observations on the biological characteristics of adults
The unfed adult ticks of H. tibetensis (50 females and 50 males) were collected by flag-dragging from vegetation (K. schoenoides, S. pingii and Caragana spinifera Kom), weighed individually, and then fed on the ears of white rabbits maintained in iron cages (40 × 40 × 50 cm) in the field plot. For collection, ticks were placed into cloth bags attached to rabbit ears by adhesive tape, and were monitored daily (9:00, 14:00 and 18:00). The rabbits were fed rabbit pellets and provided water ad libitum under natural lighting and climate conditions. The fully engorged female ticks were individually collected, weighed, and placed in glass tubes (1.5 × 10 cm) which were closed using gauze netting with proper ventilation. The tubes were then placed in the plot, covered with an approximately 2–3 cm thick leaf layer, and examined twice daily. The pre-oviposition and oviposition periods, as well as the egg mass laid were daily recorded. To determine the incubation period, 1000 eggs were counted and placed in separate tube. The reproductive efficiency index (REI) was calculated as the ratio of the number of eggs laid to the weight of the engorged female. The reproductive fitness index (RFI) was calculated as the ratio of the number of eggs hatched to larvae to the weight of engorged females (Drummond and Whetstone 1970; Chilton 1992).
Observations on the biology of immature stages
To determine the feeding periods of the larvae and nymphs of H. tibetensis, the unfed larvae (n = 300) and nymphs (n = 200) were released into cloth bags that were glued on the ears of rabbits in the plot during May and June 2014. To evaluate molting periods, engorged larvae and nymphs were collected daily, weighed individually, and placed in glass tubes closed with gauze netting. The glass tubes were then placed in the plot that allowed the engorged larvae and nymphs to molt under natural lighting and climate conditions. Furthermore, to assess the prefeeding periods, freshly hatched larvae (n = 200) and nymphs (n = 100) were placed on the ears of the rabbits, and checked daily. Prefeeding periods were defined as the number of days from hatching or molting to the beginning of attachment.
Survival of free-living H. tibetensis in the field plot
To determine survival ability without feeding, 100 newly hatched larvae in June 2014 and 100 newly emerged nymphs in July 2014 were placed in separate glass tubes and placed in the plot covered with an approximately 2–3 cm thick leaf layer. To record the overwintering survival rate, 100 newly molted adults confined in large glass tubes with organdie were placed directly into the plot in August 2014. Survival was determined by placing these tubes upon return to the laboratory and incubating them for 24 h [27 ± 1 °C, 70 % RH, 6-h light and 18-h dark] in March 2015.
Statistical analysis
The relationship between the number of eggs laid and the engorged weight of females was assessed by linear regression analysis using SPSS 19.0 (IBM, USA). All charts were drawn using Origin 9.0 (Origin Lab, USA).
Results
Biological characteristics and oviposition of adult H. tibetensis in field plot
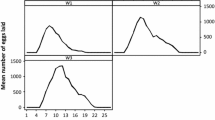
All engorged females were weighed and collected separately in glass tubes for oviposition under natural lighting and climate conditions in the confined field plot. On average, the oviposition period of females lasted for up to 28.2 days (range 14–33 days), and the number of eggs reached a peak on the 3rd days after onset of oviposition, which then declined gradually (Fig. 3). The total number of eggs averaged 983.7 (range of 235–1440), and the REI and RFI was 5.1 and 4.7, respectively (Table 1). There was a significant positive correlation (r = 0.8273, P < 0.05) between the number of eggs laid and the engorged weight of females (Fig. 4).
The life cycle of H. tibetensis under field conditions
Under natural lighting and climate conditions, the tick H. tibetensis requires an average of 177.8 days (range of 129–202 days) to complete its entire life cycle from unfed adult to next generation adult (between March and November 2014) (Table 2). The average duration of feeding, pre-oviposition, and oviposition of adults were 7.7, 9.4, and 28.2 days, respectively.
Onset of oviposition occurred in early April 2014 when observed in the confined plot, and the average incubation period of eggs was 32.1 days (range 25–40 days). Larvae first appeared in the confined plot in early May 2014, and if the host is available, the nymphs could appear in late May 2014. The mean feeding period of larvae and nymphs was 3.2 and 6.4 days, respectively. The mean premolt period of larvae and nymphs was 18.3 and 52.7 days, respectively. Overall, these results indicate that the time spent during the development stage of nymphs was longest compared to other stages in the life cycle of H. tibetensis.
Observations on the feeding behavior and survival of H. tibetensis
Both the immature and adult H. tibetensis fed slowly during the first few days following attachment, then larvae and nymphs engorged quickly and dropped. For female ticks, copulation is the prerequisite for rapid engorgement, and the weight ratio of the engorged to unfed females was 58.20. In contrast, male ticks demonstrated a weight ratio of engorged to unfed of 1.95, which is less than the weight change compared with larvae (weight ratio, 10.00) and nymphs (weight ratio, 15.75) (Table 3).
From June 2014 to July 2014, the unfed larvae survived for 15–18 days in the confined plot, while unfed nymphs and adults were able to survive for about 5–8 months during the winter. The survival rates of the adults and nymphs upon return to the incubator in March 2015 were 79.7 and 72.3 %, respectively.
Discussion
In this study, the life cycle and biological characteristics of H. tibetensis were investigated for the first time under field conditions in Qinghai-Tibet plateau. As a three-host tick, H. tibetensis is able to complete its life cycle in rabbits in an average of 177.8 days (range of 129–202 days) upon immediate availability of the host and feeding ability of the ticks. The results from our study indicate that under field conditions, some of the ticks may require increased time to complete a life cycle without availability of a host; thus, there may be more than one cohort that could co-exist within the same population at a certain time of the year and within the same population at a certain time of the year. Similar observations were also made on H. longicornis (Zheng et al. 2011) and H. concinna (Meng et al. 2014) under natural conditions. Additionally, though this tick species was mostly found on dogs, sheep and yaks (Deng and Jiang 1991), we found that rabbits were also suitable hosts of this tick species.
Our study demonstrated that the nymphal H. tibetensis spends much of its time during the life cycle in the molting period (range of 41–55 days), and similar observations have been reported on nymphal H. longicornis (Zheng et al. 2011). This could be attributed to a number of factors, including the complex interactions among photoperiod, temperature, relative humidity, and other ecological factors (Frenot et al. 2001; Padgett and Lane 2001; Troughton and Levin 2007; Zheng et al. 2011). The long premolting period in nymphal H. tibetensis may combine with the host availability to ensure that this tick species overwinters in nymphal and adult stages.
The climate of northern Tibet is featured by coldness and dryness (Gao et al. 2006), and a mean relative humidity above 50 % in the study site appeared only from July to September (Fig. 2) due to the melting of snow and ice from high mountains. However, under the extremely harsh natural environment from March to May 2014 (all of the monthly average RH was around 30 %, and the monthly average temperature was below 5 °C) (Fig. 2), the tick H. tibetensis was able to complete oviposition and successfully hatch. It was observed that there is a highly positive correlation between the number of eggs laid and the weight of engorged females in H. tibetensis (r = 0.8273) (Fig. 3), which is higher than that of H. longicornis (r = 0.6229) (Zheng et al. 2011) and Dermacentor silvarum (r = 0.76) (Yu et al. 2010) under field conditions. Additionally, the life span of unfed nymphs and adults from July 2013 to next March 2014 were about 8 and 5 months, respectively. This demonstrated that the nymph and adult H. tibetensis have the ability to survive in cold temperatures, with snow potentially providing a relative stable microhabitat (Estrada-Peña and de la Fuente 2014). These results indicate that the tick H. tibetensis may have evolved special physiological adaptation mechanisms to coldness and dryness over time. The physiology and molecular mechanisms underlying this evolved adaptability the tick H. tibetensis requires further studies.
Although the investigation on the life cycle of H. tibetensis under field conditions in the confined plot may not accurately and comprehensively reflect the natural habitat, our results suggests that it is necessary and effective to treat sheep or yaks with acaricides in March and August, which will decrease the overwinter population of nymph and adult ticks.
References
Chilton NB (1992) An index to assess the reproductive fitness of female ticks. Int J Parasitol 22:109–111
Dai J, Wang Y, Zhang L, Tang YL, Luo XS, An H, Fang C (2009) Hymenobacter tibetensis sp. nov., a UV-resistant bacterium isolated from Qinghai-Tibet plateau. Syst Appl Microbiol 32:543–548
Deng GF, Jiang ZJ (1991) Economic insect fauna of China. Fasc. 39, Acarina, Ixodidae 127–128 (in Chinese)
Drummond RQ, Whetstone TM (1970) Oviposition of the Gulf Coast tick. J Econ Entomol 63:1548–1551
Estrada-Peña A, de la Fuente J (2014) The ecology of ticks and epidemiology of tick-borne viral diseases. Antiviral Res 108:104–128
Frenot Y, de Oliviera E, Gauthier-Clerc M, Deunff J, Bellido A, Vernon P (2001) Life cycle of the tick Ixodes uriae in penguin colonies: relationships with host breeding activity. Int J Parasitol 31:1040–1047
Gao QZ, Li YE, Wan YF, Lin E, Xiong W, JiangCun WZ, Wang Z, Wang BS, Li WF (2006) Grassland degradation in Northern Tibet based on remote sensing data. J Geogr Sci 16:165–173
Hoogstraal H (1965) Haemaphysalis tibetensis sp. n., and its significance in elucidating phylogenetic patterns in the genus (Ixodoidea, Ixodidae). J Parasitol 51(3):452–459
Huang XR, Chen J, Chen LY, Wu QL, Yang XX, Li XY, Li DR (1999) Studies on sedimentation coefficient and molecular weight of the orbivirus (Ti3010) isolated from Tibet China. Bull Acad Mil Med Sci 23(4):269–271 (in Chinese)
Meng H, Xu SQ, Yu ZJ, Liu Z, Liu JN, Yang XL, Liu JZ (2014) The life cycle and occurrence of Haemaphysalis concinna (Acari: Ixodidae) under field conditions. Ticks Tick Borne Dis 5:887–891
Padgett K, Lane RS (2001) Life cycle of Ixodes pacificus (Acari: Ixodidae): timing of developmental processes under field and laboratory conditions. J Med Entomol 38:684–693
Troughton DR, Levin ML (2007) Life cycle of seven ixodid tick species (Acari: Ixodidate) under standardized laboratory conditions. J Med Entomol 44:732–740
Yu ZJ, Zheng HY, Chen Z, Zheng B, Ma H, Liu JZ (2010) The life cycle and biological characteristics of Dermacentor silvarum Olenev (Acari: Ixodidate) under field conditions. Vet Parasitol 168:323–328
Zhang PH, Cao JT, Li YC, Zhang QE (1996) Ultrastructure observation of newly GRD spirochetes isolate in Tibet. In: Zhang QE (ed) Corpus of epidemiological investigation, 2nd edn. Publishing House of Academy of Military Medical Sciences of PLA, Beijing, pp 62–66 (in Chinese)
Zheng HY, Yu ZJ, Chen Z, Zhou L, Zheng B, Ma H, Liu JZ (2011) Development and biological characteristics of Haemaphysalis longicornis (Acari: Ixodidae) under field conditions. Exp Appl Acarol 53:377–388
Acknowledgments
This work was supported by National Natural Science Foundation of China (31272372), the Specialized Research Fund for the Doctoral Program of Higher Education of China (20131303130001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Ming Liu and Tuo Li have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, M., Li, T., Yu, ZJ. et al. Characterization of the life cycle of the tick Haemaphysalis tibetensis under field conditions in Qinghai-Tibet plateau. Exp Appl Acarol 69, 107–115 (2016). https://doi.org/10.1007/s10493-016-0020-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-016-0020-y