Abstract
Predation risk is a strong selective force shaping prey morphology, physiology, life history and/or behavior. As a prime stressor, predation risk may even induce trans-generational alterations, called maternal effects. Accordingly, maternal predation risk during offspring production may influence offspring life history and anti-predator behavior. Here, we assessed whether different levels of predation risk, posed by the predatory mite Phytoseiulus persimilis, induce graded maternal effects in its prey, the herbivorous two-spotted spider mite Tetranychus urticae. First, we generated four types of predation risk-stressed spider mite mothers by exposing them to living predators, direct and indirect predator cue combinations or no predator cues, respectively. Then, we investigated the life history (offspring developmental time, sex) and anti-predator response (activity, position on the leaf) of their offspring on leaves with and without direct and indirect predator cues. Maternal stress, no matter of the predation risk level, prolonged the offspring developmental time, as compared to offspring from unstressed mothers. This pattern was more pronounced on leaves with than without predator cues. Offspring from stressed mothers resided more likely on the leaf blade than close to the leaf vein. Offspring sex ratio and activity were not influenced by maternal predation risk but activity was higher on leaves with than without predator cues. We argue that the prolonged developmental time is non-adaptive, yet the changed site preference is adaptive because reducing the encounter likelihood with predators. Our study represents a key example for predation risk-mediated maternal effects on developmental trajectories of offspring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maternal effects are phenotypic effects of the mother on the offspring phenotype that are unrelated to, and do not affect, the offspring genotype (Bernardo 1996; Mousseau and Fox 1998; Wolf and Wade 2009). For example, maternal effects can happen, when a gravid female experiences food shortage or predation risk during internal egg formation and consequently influences the phenotype of her offspring (e.g., Mommer and Bell 2013). Proximately, maternal effects are induced by environmental conditions influencing the maternal condition, behavior, or physiological state and hence affecting the offspring via the yolk amount and composition, mRNAs, gene methylation, chemical cues, and/or hormones etc. Ultimately, maternal effects can be mechanisms increasing maternal and offspring fitness if they render the offspring phenotype better suited to its future environment (Bernardo 1996; Mousseau and Fox 1998). However, maternal effects are not necessarily and not always adaptive (e.g., McGhee et al. 2012), which may be due to physiological constraints or when there is a mismatch between the maternal and offspring environments (e.g., Coslovsky and Richner 2012). Maternal effects are ubiquitous and documented across organisms: plants, insects, amphibians or mammals can have adaptive as well as non- or mal-adaptive impacts on their offspring (Bernardo 1996; Mousseau and Fox 1998; Wolf and Wade 2009).
Many maternal effects are nutritionally mediated, that is, the quality and quantity of available food resources has direct impacts on offspring production, likely affecting their survival, growth, development and/or behavior (Mousseau and Fox 1998). For example, in predatory mites the maternal diet during egg production may modulate the foraging preferences of offspring (Peralta-Quesada and Schausberger 2012; Ambichl 2013). However, maternal effects can also be non-nutritionally mediated or mediated by the interaction between nutritional and non-nutritional environmental factors. For example, mothers experiencing predation risk often reduce food intake or change hormonal provisioning, which in turn affects the offspring phenotype (Lima and Dill 1990). Quite a few studies documented that maternal predation risk may influence the mothers’ physiology and behavior (Lima 1998), which consequently influences the offspring produced while experiencing predation risk (Roche et al. 2012; Mommer and Bell 2013). For example, in risky environments mothers commonly produce offspring having smaller or larger body sizes than usual (Bernardo 1996). Female lizards Pseudemoia pagenstecheri adaptively produce highly predator-sensitive offspring after living under strong predation pressure by the white-lipped snake Drysdalia coronoides (Shine and Downes 1999). In contrast, barn swallows, Hirundo rustica, experiencing stress by cats negatively influence their offspring by a higher corticosterone concentration in the eggs, slowing down offspring growth and reducing body mass (Saino et al. 2005). Predation risk-mediated maternal effects are poorly documented and understood in arthropods, such as insects and mites.
Here, we assessed predation risk-mediated maternal effects, induced by the predatory mite Phytoseiulus persimilis Athias-Henriot, on anti-predator behavior and life history of the two-spotted spider mite Tetranychus urticae Koch. Tetranychus urticae is a highly polyphagous herbivorous mite (Bolland et al. 1998) and an important pest wordwide (van Leeuwen et al. 2010). Tetranychus urticae predominantly feeds on the abaxial leaf surface and injures its host plants by piercing the mesophyll cells and extracting the cell contents (Helle and Sabelis 1985a). Predatory mites of the family Phytoseiidae are the most important natural enemies of spider mites (Helle and Sabelis 1985b; McMurtry and Croft 1997; Gerson et al. 2003; Hoy 2011). Within the family Phytoseiidae, some species, predominantly of the genus Phytoseiulus, evolved a prey preference for spider mites, particularly of the genus Tetranychus, and are thus highly dangerous for the spider mites (McMurtry and Croft 1997). Predation is a strong selective force shaping prey morphology, life history and behavior (e.g., Lima and Dill 1990) and this is also true for the interaction between P. persimilis and T. urticae. This is, among numerous others, documented in various studies on the anti-predator behavior of the spider mites towards their prime natural enemies, predatory mites (Grostal and Dicke 1999; Škaloudová et al. 2007; Fernández-Ferrari and Schausberger 2013; Hackl and Schausberger 2014). Behavioral anti-predator responses can be innate or learned or a combination of both (e.g., Walzer and Schausberger 2011). Grostal and Dicke (1999) were the first to reveal that the focal animals of our study, the spider mite T. urticae, innately recognize predatory mites and their cues, respectively, resulting in avoidance behaviors, fleeing and/or delayed and reduced oviposition. Hackl and Schausberger (2014) recently showed that the spider mites can modulate these anti-predator behaviors by learning. Both innate and learned anti-predator responses can principally be modified by maternal effects (Roche et al. 2012; Stratman and Taborsky 2014) but this has not yet been examined in spider mites.
Anti-predator behavior is every behavioral change an animal performs to avoid being detected and killed by a predator (e.g., Lima and Dill 1990). Prey animals often have to differentiate between various risk levels. Anti-predator behaviors like fleeing, avoiding, activity reduction or hiding, lower the risk of predation but also entail energy costs that could be used for other life activities such as feeding and/or reproduction. To find the optimal balance between energy investment for the appropriate behavioral response under predation risk and other life activities such as feeding and reproduction, animals should be sensitive to the level of predation risk (Sih 1982; Helfman 1989). To correctly recognize predators and interpret the associated level of risk, prey evolved sophisticated recognition abilities, using diverse sensory modalities such as vision, touch, audition and/or chemical senses (olfaction, taste). Chemical cues play an important role in enemy recognition in a broad diversity of animals including T. urticae (Kats and Dill 1998; Dicke and Grostal 2001). In general, information cues indicating predation risk, including chemical cues, can be direct or indirect (Kriesch and Dicke 1997; Grostal and Dicke 1999). Direct chemical cues are emitted by the predators themselves or their products, such as exuviae, faeces, eggs, or marking pheromones. Indirect chemical cues indicating predation risk emanate from the environment of the predator and may come from disturbed, injured or dead conspecifics and include, for example, prey alarm pheromones. Such indirect cues may affect the prey behavioral response similar to direct cues. However, due to the generality of indirect cues the behavioral changes brought about by their perception are largely unspecific. Our study animal, T. urticae, does not only respond to direct predator cues but also to indirect cues emanating from dead and injured conspecifics (e.g., Grostal and Dicke 1999; Dicke and Grostal 2001). Direct and indirect cues of predation risk may delay or reduce oviposition, induce escape behaviors, or reduce foraging activities of T. urticae in a risk-sensitive manner (Grostal and Dicke 1999; Škaloudová et al. 2007, Fernández-Ferrari and Schausberger 2013; Hackl and Schausberger 2014). Whether or not predation risk may induce maternal effects in T. urticae, and, if so, whether these effects are risk-sensitive and which offspring traits in life history and behavior are affected, is unknown.
Our primary objective was determining if different levels of predation risk posed by P. persimilis (no, low, moderate, severe) induce maternal effects in ovipositing T. urticae females and consequently change the life history and/or the anti-predator behavior of their offspring. In detail, we investigated the development, activity and site choice of juvenile spider mites, which emerged from mothers exposed to graded predation risk levels. Based on the knowledge that the spider mites are risk-sensitive and respond the strongest to the predator P. persimilis (Fernández-Ferrari and Schausberger 2013, Hackl and Schausberger 2014), we used direct and indirect cues of P. persimilis to create environments differing in predation risk for the spider mite mothers.
Materials and methods
Growing of bean plants, Phaseolus vulgaris
Whole common bean plants P. vulgaris were used as host plants for rearing T. urticae and detached trifoliate leaves of clean un-infested plants were used in experiments. Detached primary leaves were used for rearing the predatory mites P. persimilis. The potting substrate for all plants was a mixture consisting of 75 % commercial soil and 25 % expanded clay. Plants used to rear T. urticae were grown at 23 ± 2 °C, 50 ± 10 % relative humidity (RH) and a 16:8 h light/dark (L/D) ratio. Clean plants used for experiments were grown in a walk-in environmental chamber at 25 ± 1 °C, 60 ± 5 % RH and a 16:8 h L/D ratio.
Rearing of Tetranychus urticae
Tetranychus urticae used in the experiments derived from a population reared on whole common bean plants P. vulgaris in the laboratory. The population was originally founded with specimens obtained from BioHelp (Vienna, Austria) in 1999 and occasionally refreshed with individuals obtained from the same source. Adult females of T. urticae were used to generate experimental animals and mixed life stages were used as prey for rearing P. persimilis. To generate experimental animals, adult T. urticae females were randomly picked from the plants and transferred to detached leaf arenas varying in the level of risk (stress) posed by the predatory mite P. persimilis and its cues, respectively. Each leaf arena consisted of an un-infested trifoliate leaflet of P. vulgaris placed upside down on filter paper covering a moist foam cube (8 × 8 × 6 cm) kept in a plastic box (10 × 10 × 6 cm) half-filled with tap water. The leaf arenas were stored in an environmental chamber at 25 ± 1 °C, 60 ± 5 % RH and 16:8 h L/D.
Rearing of Phytoseiulus persimilis
Adult females of the predatory mite P. persimilis were used to create graded predation risk (stress) levels for the adult spider mite females during production of eggs, giving rise to the experimental animals, and for the experimental animals during the bioassay. To this end, a P. persimilis colony was established on a detached leaf arena consisting of a primary bean leaf resting upside down on a filter paper covering a moist foam cube kept in a plastic box (14 × 14 × 5 cm), half-filled with tap water. About 15 gravid predatory mite females were transferred from the base population of P. persimilis to the leaf arena and, every second day, fed with mixed spider mite stages brushed from infested leaves onto the arena. The base population of P. persimilis was originally founded with specimens collected on clementine trees in Valencia, Spain. The predatory mite arena was kept in an environmental chamber at 25 ± 1 °C, 60 ± 5 % RH, and 16:8 h L/D.
Pre-experimental procedures
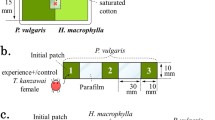
To assess how maternal stress during egg production affects the anti-predator behavior and life history of spider mite offspring during their juvenile phase, experimental animals were derived from four types of mothers exposed to graded predation risk (stress) levels. To this end, gravid spider mite females were held on one of four types of leaf arenas (~6 × 6 cm) representing differently stressful environments. Stress was defined by the risk associated with the predatory mite P. persimilis and its cues on the leaf arenas. The four graded stress levels were: (1) severe stress—P. persimilis traces, killed spider mites and live P. persimilis, (2) moderate stress—P. persimilis traces and killed spider mites, (3) low stress—only P. persimilis traces, and (4) no stress (control group)—blank leaf, that is, without any cues of P. persimilis and without any killed spider mites. Predator traces were metabolic waste products and possibly footprints left by the predators on the leaf surface (e.g., Walzer and Schausberger 2011). Leaf arenas type (1) and (2) first received mixed life stages of spider mites. Second, three gravid P. persimilis females were randomly picked from the stock colony and transferred to each leaf arena of type (1), (2) and (3). Leaf arena type (4) was left without any spider mites and predators (control). Third, after storing the leaf arenas for 20 h in an environmental chamber (25 ± 1 °C, 60 ± 5 % RH, 16:8 h L/D), the predatory mites were removed from arena types (2) and (3) and all arenas were ready to receive adult spider mite females. Six adult spider mite females, randomly chosen from the stock population, were transferred to each arena. In total, we had six to eight arenas for each of the four maternal stress environments. Before preparing arena type (1) we had determined the most adequate ratio (3:6) between predatory mite females and spider mite females: the adult spider mite females should experience stress, caused by physical presence of P. persimilis, but at the same time, the juvenile spider mite prey provided to the predators should be sufficiently high that the predators did not kill the adult spider mite females. Phytoseiulus persimilis have a life stage preference for eggs and juvenile spider mites (Blackwood et al. 2001).
After being placed on the arenas, the spider mites were exposed to the graded stress environments for 40–48 h. Arenas were stored in environmental chambers at 25 ± 1 °C, 60 ± 5 % RH, and 16:8 h L/D. Thereafter, the spider mite females were transferred to new clean leaf arenas for 3–4 h for oviposition of eggs giving rise to the experimental animals. Transfer to new clean arenas was necessary to avoid that the eggs used for experiments were externally contaminated by cues of the predators or cues of killed conspecifics.
Experimental procedures
We examined the developmental time (from egg to larva, protonymph, deutonymph, and adult), activity (moving or stationary), position of the leaf arena (close to the main vein, i.e. within leg touching distance, or on the leaf blade incl. side veins) and sex of T. urticae, emerging from the eggs of mothers exposed to graded stress levels, on leaf arenas with and without cues of the predatory mite P. persimilis. Eight different treatments, defined by the combination of maternal stress level and individual exposure to predator cues (on arena with/without predator traces), were distinguished: egg from heavily stressed mother (type 1) on arena without (1) or with (2) predator cues; egg from moderately stressed mother (type 2) on arena without (3) or with (4) predator cues; egg from little stressed mother (type 3) on arena without (5) or with (6) predator cues; egg from unstressed mother (type 4) on arena without (7) or with (8) predator cues. Each treatment was replicated 12 times, i.e. 24 eggs were randomly withdrawn from each of the four maternal environments and singly placed on arenas with or without predator cues.
The experiment was started by transferring the eggs at an age of about 4 d, that is shortly before eclosion, from the oviposition arenas to the experimental arenas with or without predator cues. Each spider mite egg singly placed on a small arena (~2 × 6 cm) represented a replicate. Each experimental arena consisted of a rectangular leaf arena harboring traces of the predator P. persimilis or not. To create such leaf arenas, trifoliate bean leaves were placed upside down on filter paper on moist foam cubes kept in plastic boxes (10 × 10 × 6 cm) half-filled with water. Arenas were delimited by strips of moist tissue paper. To create arenas with P. persimilis traces, single adult P. persimilis females were placed on the arena for 16 h before the experiment and then removed again.
Starting with the day of eclosion, the experimental animals were observed twice a day, in 8 and 16 h intervals, until reaching adulthood. After reaching the protonymphal stage all animals were transferred to fresh arenas with or without predator cues, because the predator cues are partially volatile and may thus decrease over time. Monitoring stopped when the spider mites had reached adulthood and their sex was determined. The experimental arenas were stored in an environmental chamber at 25 ± 1 °C, 60 ± 5 % relative humidity, and 16:8 h L/D.
Statistical analyses
IBM SPSS 21 (IBM, Armonk, NY, USA) was used for all statistical analyses. The influence of maternal stress (no, low, moderate, severe) and individual exposure to predator cues (on leaf with/without predator cues) on larval, protonymphal, deutonymphal and total developmental times of the spider mites was analyzed by separate generalized linear models (GLM; normal distribution, identity link function). For post hoc pairwise comparisons between maternal stress levels, least significant difference (LSD) tests were used. The activity (moving or stationary) of the mites on the leaf and their position preferences (close to the main vein or on the leaf blade incl. side veins) as affected by maternal stress and individual exposure to predator cues (on arena with/without predator cues) were analyzed by generalized linear models (GLM; binomial distribution—counts of events, logistic-link function) and post hoc Sidak test for pairwise comparisons among maternal stress levels. Both activity and position preference observations were aggregated before analyses, resulting in one value per individual. Similarly, the female proportion among mites reaching adulthood was assessed by GLM (binomial distribution, logistic link).
Results
Maternal stress level (severe, moderate, low or no stress) affected the total developmental time from egg to adult. Offspring produced by unstressed mothers developed more quickly than offspring produced by mothers experiencing stress (low, moderate, severe stress) (Fig. 1; Table 1). Growing up on leaves with or without predator cues did not affect offspring developmental times as a main effect but the significant interaction indicates that the difference in developmental time between offspring from stressed and unstressed mothers was much more pronounced in absence than presence of predator cues (Fig. 1; Table 1). Maternal stress retarding offspring development was evident in the larval and protonymphal but not deutonymphal stage (Fig. 2; Table 1). The female proportion among mites reaching adulthood ranged from 0.32 to 0.82 but was unaffected by maternal stress levels (GLM; Wald χ2 = 2.180, d.f. = 3, p = 0.54) and presence of predator cues on leaves (Wald χ2 = 1.322, d.f. = 1, p = 0.25) and their interaction (Wald χ2 = 4.326, d.f. = 3, p = 0.23).
Total developmental time (egg to adult) of spider mite offspring, Tetranychus urticae, from mothers exposed to graded stress levels, induced by predation risk, on bean leaves with and without cues of the predatory mite Phytoseiulus persimilis (N = 7–11 per treatment). Different letters on top of bars indicate significant differences between maternal stress levels (LSD, p < 0.05, following GLM)
a Larval (N = 11–12 per treatment), b protonymphal (N = 10–12 per treatment) and c deutonymphal (N = 8–11 per treatment) developmental times of spider mite offspring, Tetranychus urticae, from mothers exposed to graded stress levels, induced by predation risk, on bean leaves with and without cues of the predatory mite Phytoseiulus persimilis. Different letters on top of bars indicate significant differences between maternal stress levels (LSD, p < 0.05, following GLM)
The juvenile spider mites were more active on leaves with than without predator cues (GLM; Wald χ2 = 5.058, d.f. = 1, p = 0.025). In contrast, maternal stress levels (Wald χ2 = 1.313, d.f. = 3, p = 0.73) and the interaction of maternal stress level and presence of predator cues on leaves (Wald χ2 = 0.081, d.f. = 3, p = 0.99) did not influence offspring activity (Fig. 3).
Activity (moving/stationary) of spider mite offspring, Tetranychus urticae, from mothers exposed to graded stress levels, induced by predation risk, on bean leaves with and without cues of the predatory mite Phytoseiulus persimilis (N = 11–12 per treatment; GLM, p = 0.73 for maternal stress levels; p = 0.025 for presence/absence of predator cues)
The position preference of the juvenile spider mites—to sit close to the main vein or on the leaf blade incl. side veins—was influenced by maternal stress level (Wald χ2 = 13.131, d.f. = 3, p = 0.004) but not presence of predator cues (Wald χ2 = 0.168, d.f. = 3, p = 0.68). The marginally significant interaction (Wald χ2 = 6.830, d.f. = 3, p = 0.08) points at position preferences varying with predator cues within maternal stress levels (Fig. 4). Under no and moderate stress levels, the preference for the leaf blade was more pronounced on leaves with than without predator cues, whereas under light and severe stress levels the preference for the leaf blade was similar on leaves with and without predator cues (Fig. 4). Overall, offspring from mothers stressed by predation risk resided more likely on the leaf blade than offspring from unstressed mothers.
Position (close to the main vein, i.e. within leg touching distance, or on the leaf blade incl. side veins) of spider mite offspring, Tetranychus urticae, from mothers exposed to graded stress levels, induced by predation risk, on bean leaves with and without cues of the predatory mite Phytoseiulus persimilis (N = 11–12 per treatment). Different letters on top of bars indicate significant differences between maternal stress levels (Sidak, p < 0.05, following GLM)
Discussion
Predation risk-induced maternal effects can be adaptive (Storm and Lima 2010; Seiter and Schausberger 2015) but can also result in unfavorable adjustments of offspring (McGhee et al. 2012), particularly when there is a mismatch between maternal and offspring environments (Coslovsky and Richner 2012). Maternal effects, especially those induced by stress such as predation risk, may impair the allocation of nutritional resources for offspring production and/or result in hormonal imbalance, which in turn can influence offspring growth and developmental trajectories.
In our study, we found a significant difference in developmental time between the offspring from stressed mothers and those from unstressed mothers. Offspring of unstressed mothers developed more quickly, especially when growing up on leaves without predator cues. Most studies on predation risk-induced maternal effects on offspring life history focused on offspring weight and/or body size but not on developmental time. For example, Saino et al. (2005) examined the influence of maternal predation risk on egg quality of barn swallows Hirundo rustica. Due to stress exposure, egg-producing females had an elevated level of the hormone corticosterone. This hormone can be passed from the mother to her offspring and can have adverse effects on egg hatchability, juvenile growth and plumage development. Saino et al. (2005) observed that exposure to predatory cats raised the corticosterone concentration in the eggs, as compared to exposure to control animals (rabbit), and high corticosterone levels are known to negatively influence bird development. Similarly, McCormick (2009) revealed that female damselfish, Pomacentrus amboinensis, produce higher levels of the stress hormone cortisol when they experience predation risk posed by con- or hetero-specific individuals. Cortisol influenced the developmental rhythm of the fish embryos, allowed allocating less endogenous energy to growth and therefore resulted in larvae experiencing higher mortality and being smaller at time of hatching. Analogous to those findings on birds and fish, in our experiments, maternal predation risk likely influenced the hormonal and nutritional composition of T. urticae eggs (Feiertag-Koppen and Pijnacker 1985), which led to retarded juvenile development and thereby extended the length of time being in the highly vulnerable juvenile stages. However, individual experience of predator cues by the offspring themselves slightly counterbalanced this negative effect because the difference in developmental time was less evident on leaves with than without predator cues. Detailed separate examination of the different juvenile developmental stages of the spider mites (larvae, protonymphs, deutonymphs) showed that the difference in offspring developmental time was only true until the protonymphal stage but not in deutonymphs. In the later phases of development, the offspring might thus have compensated their initial disadvantages by increased food intake, suggesting that the maternally influenced differences in the early developmental stages may weaken or disappear with increasing age.
Maternal stress did not affect the offspring sex ratio of T. urticae, as indicated by the lacking difference in female proportions reaching adulthood. Spider mite females are principally able to adjust the offspring sex ratio and shift it towards more males in unfavorable environments (e.g., Jackson and Martin 2010), in response to both biotic (food quality, density of conspecifics) and/or abiotic (temperature, photoperiod, pH) factors. It may be that in our experiments the length of exposure of the mother mites to stressful conditions was too short to induce offspring sex-ratio adjustments.
The behavioral anti-predator strategies examined in our work were activity and position preference on the leaf, with only the latter influenced by maternal effects. The offspring of stressed mothers resided more likely on the leaf blade than offspring from unstressed mothers, independent of the presence of predator cues (with or without cues). Oku and Yano (2008) found that quiescent females of the spider mite T. kanzawai preferred staying near veins without predators but tended to reside more likely on the leaf blade if there were predators present. Such changes in site preference might be adaptive because predatory mites start their prey search primarily along the leaf edges and the veins (Sabelis and Dicke 1985; Oku and Yano 2008). Consequently, avoiding such areas should increase the survival chances of the spider mites. Presence of predator cues but not maternal predation risk changed offspring activity. Offspring activity was higher on leaves with than without predator cues, indicating that elevated activity levels represent an innate anti-predator response of the spider mites to chemical predator cues. This finding is in accordance with those of Škaloudová et al. (2007), Fernández-Ferrari and Schausberger (2013), and Hackl and Schausberger (2014), all of which observed that increased ambulation is a characteristic anti-predator response of the spider mites to chemical cues of their predator P. persimilis, representing attempts to escape and leave risky sites. The spider mites apparently linked the perceived chemical cues with the presence of predators and hence a dangerous environment. Analogous anti-predator behaviors, i.e. increased running speed or higher overall activity levels in response to chemical predator cues, are found in diverse animals (Kats and Dill 1998).
Altogether, we observed maternal effects on the developmental trajectory of T. urticae offspring and on their site preference. Our experiments revealed that maternal predation risk influences the development of offspring in their early juvenile stages whereas there was little to no influence later on. A likely explanation is that in the course of development, the offspring’s own experiences and compensatory strategies during ontogeny counter-balanced the initially acting maternal effects. The extended developmental times of offspring produced by stressed mothers, as compared to unstressed mothers, are unfavorable for the offspring themselves, particularly if placed in risky environments because leaving them for longer time in the vulnerable juvenile phase. In contrast, the changed site preference is adaptive because reducing the likelihood of detection by the predators. The changed site preference may also alleviate or counter-balance the negative effects of extended developmental times in risky environments. Seiter and Schausberger (2015) recently suggested that maternal stress enhanced the risk-sensitivity of juvenile predatory mites allowing them to distinguish between high immediate and low latent intraguild predation risks. While our study clearly revealed predation risk-induced maternal effects in spider mites, web did not find any evidence for risk-sensitivity.
References
Ambichl A (2013) Maternale Effekte bei der Raubmilbe Neoseiulus californicus: Einfluss der Mutternahrung auf die Nahrungspräferenz der Nachkommen. Diplomarbeit, Universität für Bodenkultur Wien
Bernardo J (1996) Maternal effects in animal ecology. Am Zool 36:83–105
Blackwood JS, Schausberger P, Croft BA (2001) Prey stage preference in generalist and specialist phytoseiid mites (Acari: Phytoseiidae) when offered Tetranychus urticae (Acari: Tetranychidae) eggs and larvae. Environ Entomol 30:1103–1111
Bolland HR, Gutierrez J, Flechtmann CHW (1998) World catalogue of the spider mite family (Acari, Tetranychidae). Brill, Leiden
Coslovsky M, Richner H (2012) Preparing offspring for a dangerous world: potential costs of being wrong. PLoS One 7:e48840
Dicke M, Grostal P (2001) Chemical detection of natural enemies by arthropods: an ecological perspective. Annu Rev Ecol Syst 32:1–23
Feiertag-Koppen CCM, Pijnacker LP (1985) Oogenesis. In: Helle W, Sabelis MW (eds) Spider mites—their biology, natural enemies and control. World Crop Pests, vol 1A. Elsevier, Amsterdam, pp 117–127
Fernández-Ferrari MC, Schausberger P (2013) From repulsion to attraction: species- and spatial context-dependent threat sensitive response of the spider mite Tetranychus urticae to predatory mite cues. Naturwissenschaften 100:541–549
Gerson U, Smiley RL, Ochoa R (2003) Mites (Acari) for pest control. Wiley-Blackwell, Oxford
Grostal P, Dicke M (1999) Direct and indirect cues of predation risk influence behavior and reproduction of prey: a case for acarine interactions. Behav Ecol 10:422–427
Hackl T, Schausberger P (2014) Learned predation risk management by spider mites. Front Ecol Evol 2:58
Helfman GS (1989) Threat-sensitive predator avoidance in damselfish–trumpetfish interactions. Behav Ecol Sociobiol 24:47–58
Helle W, Sabelis MW (1985a) Spider mites—their biology, natural enemies and control. World Crop Pests, vol 1A. Elsevier, Amsterdam
Helle W, Sabelis MW (1985b) Spider mites—their biology, natural enemies and control. World Crop Pests, vol 1B. Elsevier, Amsterdam
Hoy MA (2011) Agricultural acarology: introduction to integrated mite management. CRC Press, Boca Raton
Jackson DE, Martin S (2010) Sex allocation: size matters for red spider mites. Curr Biol 20:R1080
Kats L, Dill L (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394
Kriesch S, Dicke M (1997) Avoidance of predatory mites by the two-spotted spider mite Tetranychus urticae: the role of infochemicals. Proc Exp App Entomol 8:121–126
Lima SL (1998) Non-lethal effects in the ecology of predator-prey interactions—What are the ecological effects of anti-predator decision-making? Bioscience 48:25–34
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 98:619–659
McCormick MI (2009) Indirect effects of heterospecific interactions on progeny size through maternal stress. Oikos 118:744–752
McGhee KE, Pintor LM, Suhr EL, Bell AM (2012) Maternal exposure to predation risk decreases offspring antipredator behavior and survival in three-spined stickleback. Funct Ecol 26:932–940
McMurtry JA, Croft BA (1997) Life-styles of phytoseiid mites and their roles in biological control. Annu Rev Entomol 42:291–321
Mommer BC, Bell AM (2013) A test of maternal programming of offspring stress response to predation risk in threespine sticklebacks. Physiol Behav 122:222–227
Mousseau TA, Fox CW (1998) The adaptive significance of maternal effects. Trends Ecol Evol 13:403–407
Oku K, Yano S (2008) Effects of predation risk on mating behavior of the Kanzawa spider mite. J Ethol 26:251–266
Peralta-Quesada PC, Schausberger P (2012) Prenatal chemosensory learning by the predatory mite Neoseiulus californicus. PLoS One 7:e53229
Roche DP, McGhee KE, Bell AM (2012) Maternal predator-exposure has lifelong consequences for offspring learning in threespined sticklebacks. Proc R Soc B 8:932–935
Sabelis MW, Dicke M (1985) Long-range dispersal and searching behaviour. In: Helle W, Sabelis MW (eds) Spider mites—their biology, natural enemies and control. World Crop Pests, vol 1B. Elsevier, Amsterdam, pp 141–160
Saino N, Romano M, Ferrari RP, Martinelli R, Møller AP (2005) Stressed mothers lay eggs with high corticosterone levels which produce low quality offspring. J Exp Zool A Comp Exp Biol 303A:998–1006
Seiter M, Schausberger P (2015) Maternal intraguild predation risk affects offspring anti-predator behavior and learning in mites. Sci Rep 5:15046
Shine R, Downes SJ (1999) Can pregnant lizards adjust their offspring phenotypes to environmental conditions? Oecologia 119:1–8
Sih A (1982) Foraging strategies and the avoidance of predation by an aquatic insect, Notonecta hoffmanni. Ecology 63:786–796
Škaloudová B, Zemek R, Krivan V (2007) The effect of predation risk on an acarine system. Anim Behav 74:813–821
Storm J, Lima SL (2010) Mothers forewarn offspring about predators: a transgenerational maternal effect on behavior. Am Nat 175:382–390
Stratman A, Taborsky A (2014) Antipredator defences of young are independently determined by genetic inheritance, maternal effects and own early experience in mouthbrooding cichlids. Funct Ecol 28:944–953
Van Leeuwen T, Vontas J, Tsagkarakou A, Dermauw W, Tirry L (2010) Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: a review. Insect Biochem Mol Biol 40:563–572
Walzer A, Schausberger P (2011) Threat-sensitive anti-intraguild predation behaviour: maternal strategies to reduce offspring predation risk in mites. Anim Behav 88:177–184
Wolf JB, Wade MJ (2009) What are maternal effects (and what are they not)? Proc R Soc B 364:1107–1115
Acknowledgments
We thank I.C. Christiansen and M. Seiter for comments on a previous version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Freinschlag, J., Schausberger, P. Predation risk-mediated maternal effects in the two-spotted spider mite, Tetranychus urticae . Exp Appl Acarol 69, 35–47 (2016). https://doi.org/10.1007/s10493-016-0014-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-016-0014-9