Abstract
The establishment of biocontrol agents is critical for success of biological control strategies. Predator-in-First (PIF) is a prophylactic control strategy that aims to establish predators before the appearance of pests in an agro-ecosystem. PIF uses the ability of generalist phytoseiid mites to survive, develop and reproduce on pollen and thus establish in the absence of prey. The early establishment of populations of natural enemies helps control the pests at their incipient stage of infestation. The current study was undertaken to screen pepper cultivars for their ability to support populations of the predatory mite Amblyseius swirskii Athias–Henriot in the absence of prey. Twenty-nine pepper cultivars (11 hot and 18 sweet) were tested through a series of experiments, and four cultivars (7141, 992-7141, FPP7039 and FPP9048) were found to sustain A. swirskii populations throughout the study period. The initial application of pollen was important for establishment and maintenance of the predatory mites within the greenhouse system. Among the three screening experiments, high densities of mites were obtained in the experiment where 20 mites were released per plant, even reaching densities of >100 mites/plant. Recovery of predatory mites was significantly higher (ca. 2–3 fold) on the four pepper cultivars when predatory mites were mass released using an indirect method (banker plants) than when they were released directly on the seedlings, suggesting an advantage of passive continuous release. Future work will evaluate the selected pepper cultivars with the PIF strategy under greenhouse and field production conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the early 1900s, when the first successful biological control result was reported against greenhouse whitefly (Speyer 1927), pest management professionals have worked continually to develop new ways to deliver consistent results with commercially produced natural enemies. Some of the strategies aiding establishment of beneficials in agro-ecosystems are: ‘Pest-in-First’—the deliberate initial release of a selective pest (Luckmann and Metcalf 1975; Gonzalez and Wilson 1982; Messelink et al. 2008); ‘Slow Release’—sachets consisting of beneficials along with a food source (Sampson 1998); ‘Banker Plants’—alternative host plants providing food and shelter (Stary 1970; Parr and Stacey 1976; Bennison 1992; Frank 2010; Huang et al. 2011); and the use of artificial liquid food sprays as dietary supplements for predators (Wade et al. 2008). Although these conservation biological control strategies can support beneficial populations under certain circumstances, each has limitations that discourage widespread adoption. These may include, but are not limited to the: (1) release of non-pest insect species-growers are often reluctant to do so, (2) slow dispersal of biocontrol agents from the release point (sachets), (3) complexity in screening of non-host banker plants and their integration into multiple cropping systems, and (4) identification of chemical pesticides with minimal non-target effects (Stary 1993; Huang et al. 2011). Furthermore, conservation of natural enemies does not always result in effective suppression of pests under field conditions (Wade et al. 2008).
To overcome some of the aforementioned limitations of conservation biological control and to provide a tool for sustainable pest management, Ramakers (1990) suggested the concept of ‘Predator-in-First’ (PIF). The PIF approach aims to establish biological control agents in the critical seedling stage and/or early post-transplanting period when pest populations are absent. It uses the characteristics of generalist predators, which can survive and reproduce on plant-provisioned food sources in the absence of pest organisms (Ramakers 1995; McMurtry and Croft 1997; Nomikou et al. 2003, 2010; Park et al. 2010; Kumar et al. 2014a). Thus, PIF brings together the inundative and conservational biological control strategies where generalist phytoseiid mites released on vegetable seedlings or transplants are initially supported with plants with nutritional supplements in the early plant stages and then by flowers with pollen and/or pests in the later stages. The early establishment of natural enemy populations helps target the pests at their incipient stage of infestation.
Within the US, Florida is the second largest producer of fresh market vegetables after California, with 218,000 acres under production, valued at $1.9 billion (FDACS 2007). However, in recent years the Florida vegetable industry has been facing great challenges because of establishment of numerous invasive pest species. According to a report by the Florida Department of Agriculture and Consumer Services (2000), 150 species of exotic arthropods established in Florida between 1986 and 2000 (ca. 1 species/month), and some of these have become serious agricultural pests. Silverleaf whitefly [Bemisia tabaci (Gennadius)], western flower thrips [Frankliniella occidentalis Pergande], common blossom thrips [Frankliniella schultzei Trybom], melon thrips [Thrips palmi Karny] and chilli thrips [Scirtothrips dorsalis Hood] are of particular concern for the Florida vegetable industry (Demirozer et al. 2012; Kakkar et al. 2012; Seal et al. 2013; McKenzie et al. 2014; Kumar et al. 2013, 2014b). Apart from causing feeding damage to their hosts, these pest species can vector plant-damaging viruses and several are developing resistance against various chemical insecticides. Thus, to maintain an adequate supply of fresh produce and prevent the loss of agricultural jobs, it is important to address key issues affecting growers.
Kutuk and Yigit (2011) found in their greenhouse study in Turkey that pre-establishment of Amblyseius swirskii Athias–Henriot on sweet pepper using Pinus brutia (Ten.) pollen provided effective reduction of F. occidentalis populations. However, except for a few greenhouse studies (Ramakers 1990; Kutuk and Yigit 2011), application of PIF has never been tested under field crop production conditions on pepper against its pests including F. occidentalis. In this study, we conducted a series of initial experiments to determine the effectiveness of the PIF for supporting phytoseiid mites under greenhouse conditions. The results of this study will provide a baseline for further tests of the PIF approach in supporting phytoseiids and regulating pest populations in commercial vegetable fields. We theorized that the PIF strategy for vegetable production systems could be sustainable because, following an initial release of mites, the vegetable seedlings themselves would harbor viable mite populations and multiple releases of mites would not be necessary. In addition, labor and chemical insecticide costs would be reduced, and the generalist predatory mites would attack multiple pests (thrips, whiteflies, broad mites, etc.) of vegetable crops (Nomikou et al. 2002; Messelink et al. 2008; Arthurs et al. 2009; Dogramaci et al. 2011; Calvo et al. 2011; Xiao et al. 2012).
As a primary step of the PIF approach, we screened several commercial pepper cultivars for their ability to sustain the phytoseiid mite A. swirskii in the absence of prey. The specific objectives of this study were to: (1) assess the effect of supplemental pollen on establishment of phytoseiid mites on pepper seedlings, (2) screen commercial pepper cultivars for their ability to sustain predatory mites in the absence of prey, and (3) evaluate rates and methods of mass inoculation of transplants with A. swirskii under greenhouse conditions.
Materials and methods
Pepper plants
All studies were conducted between 2011 and 2013 at the University of Florida’s Mid-Florida Research and Education Center, Apopka (28.63N, 81.55W). The seeds of 29 commonly grown (11 hot and 18 sweet) pepper cultivars (Table 1) were sown on seedling trays containing Fafard 2-Mix growing medium (Conrad Fafard, Agawam, MA, USA) and when required, seedlings were transplanted into 10-cm-diameter plastic pots filled with the same growing medium in insect proof screen cages. Plants were watered as needed (ca. 3× a week) and fertilized with Peter’s Professional 20-10-20 (325 ppm) (Scotts, Marysville, OH, USA) once a week. All seedlings were maintained in air-conditioned greenhouses (27 ± 5 °C, 60 ± 10 % RH, L16:D8 h photoperiod). Plants selected for the studies were healthy, young, vigorous, and free of pests.
Laboratory rearing of predatory mites
Colonies of A. swirskii (Koppert Biological Systems, Howell, MI, USA), were reared on mixed pollens (peach and cattail) in 14 × 14 cm plastic trays isolated by water following a modified protocol of Carrillo et al. (2010). The culture arena consisted of a piece of black 5.5 × 5.5 cm cardstock dipped 2–3× in wax, and covered by a 1-mm2 wire mesh square. Card stocks were placed on the top of three stacked 75-mm-diameter cotton pads with a few threads of cotton simulating leaf trichomes to facilitate oviposition. A. swirskii colonies were maintained this way for several generations before use in the bioassays. All rearing was conducted under laboratory conditions as mentioned above.
Effect of supplemental pollen on establishment of Amblyseius swirskii on pepper seedlings
To determine the importance of an initial application of pollen on the establishment of A. swirskii during the early stage of pepper growth, mite abundances on pepper seedlings were assessed in the presence and absence of supplemental pollen. Six seeds of a pepper cultivar were sown in small (3 × 2 cell) plastic trays as mentioned above. At 30 days post germination, two small cell trays containing four seedlings of uniform size, growth and vigor were selected and placed in separate cages (120 × 120 × 120 cm). One of the two cages was provided with a small amount (ca. 10–12 mg/seedling) of cattail pollen on the top leaves. Approximately 2 h after pollen application, five female A. swirskii adults were released on each seedling using a camel hair brush. All life stages of A. swirskii were recorded weekly for 4 weeks post-release on five leaves per plant. The experiment was conducted on 17 sweet pepper cultivars.
Screening of pepper cultivars
In order to screen pepper cultivars for their ability to sustain A. swirskii populations in the absence of prey, three separate experiments were conducted, using different rates of phytoseiid mite application. In the first experiment, the abundance of predatory mites was determined on 11 hot pepper cultivars with a low rate of initial mite (five per plant) release. For each cultivar, one potted seedling (5–7 leaf stage, non-flowering) was placed on an inverted small saucer (10 cm diameter) positioned in a larger saucer (20 cm diameter) filled with soapy water to prevent mites from escaping. The plants did not touch each other. Two weeks later, five adult female A. swirskii were brushed on the top leaves of each seedling and a small amount (ca. 10–12 mg) of cattail pollen was provided as a nutrition supplement. Eleven cultivars or species were used, each replicated 3× in a randomized complete block design. Starting 7 days after the release of A. swirskii, 15 leaves of each cultivar (5 leaves per plant × 3 plants) were non-destructively sampled weekly for 6 weeks. Life stages of A. swirskii were counted using a head-mounted 10× magnifier (Donegan Optical Company, Lenexa, KS, USA).
In a second experiment, eight sweet pepper cultivars were screened for their ability to sustain A. swirskii population in the absence of prey. The method of plant propagation and spatial arrangement for the experiment was similar to the above study except that it was conducted with a medium release rate of phytoseiid mites (10 per plant). Because the plants were not flowering at the beginning of the experiment, cattail pollen was added as a source of nutrition on the leaves of each seedling for the mites. Two hours after pollen application, ten adult females were released on each seedling. All stages of A. swirskii were recorded weekly on five top leaves of each pepper plant using a head-mounted 10× magnifier for a period of 8 weeks post release. Treatments were replicated 6× in a randomized complete block design.
Based on the results obtained in the previous experiment, we further screened four sweet pepper cultivars (7141, 992-7141, FPP 7039, and FPP-9048) to assess their ability to sustain a higher densities of A. swirskii without prey. The objective of the experiment was to assess if these cultivars had sufficient resources to support high mite densities in the absence of prey. The method of plant propagation, spatial arrangement for the experiment, and method of evaluation was similar to those in the above experiments, except that it was conducted with a high release rate of mites (20 per plant). The experiment had four treatments, replicated 6× in a randomized complete block design.
Effect of two release methods (banker vs. direct release) on densities of Amblyseius swirskii
This experiment served to evaluate two modes of release of phytoseiid mites: (a) direct application—mites released directly on the host plant, and (b) indirect application—mites released using banker plants in the treatment plots. The experiment utilized four sweet pepper cultivars (7141, 992-7141, FPP 7039, FPP-9048), and an ornamental pepper cultivar (Explosive Ember) as banker plants. Selection, preparation, and use of these banker plants under greenhouse conditions was described by Xiao et al. (2012). Each cultivar received A. swirskii through two release methods in the greenhouse. Seedlings were prepared and managed as described for previous experiments. For the banker plant release method, six potted (10 cm diameter) sweet pepper seedlings were placed on an isolated platform and one potted banker plant with a high and well-established population of A. swirskii was positioned so that its leaves touched those of the six sweet pepper plants enabling the mites to move between plants. In the direct-release treatment, six seedling pots were placed on an isolated platform without a banker plant. A. swirskii was released directly on each plant at the density of 20 female adults per plant. In order to compare the two release methods without any bias towards the direct-release method, pollen was not applied to the pepper plants. The populations of A. swirskii were visually checked on a weekly basis post-release for 6 weeks. During each sampling, five top leaves of each plant were examined using a head-mounted magnifier (10×). Each release method had four replications per cultivar with six seedlings per replicate.
Data analysis
Amblyseius swirskii data from the various experiments were analyzed independently using linear mixed model with the SAS procedure GLIMMIX with an autoregressive correlation structure (SAS Institute 2009). The model was used to determine the effect of plant cultivar, pollen/no pollen, and their interaction (effect of pollen experiment); cultivar, sampling week, and their interaction (screening of pepper cultivars); and cultivar, release method, and their interaction (mite release method), on A. swirskii numbers. The autoregressive correlation structure was applied to observations that were repeatedly measured each week to account for the correlation in data generated by re-sampling in time. The data were normalized using square root transformation to stabilize heterogeneous variance before analysis. When the interaction of treatment and time was found to be significant, mean separations were run only for differences in treatments in the same time period. The Tukey adjustment method was used because, as sets of comparison for a given time are orthogonal to each other, the total number of comparisons is greatly reduced, increasing the power to detect differences in the means. The effect of pollen or no pollen, and the two methods (banker vs. direct) of mite release, on mite density on each cultivar was tested using Student’s t test. All tests were run at α = 0.05. The data presented are the untransformed means.
Results
Effect of supplemental pollen on establishment of Amblyseius swirskii on pepper seedling
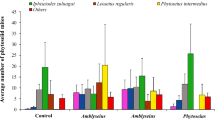
There was a significant effect of pollen, cultivars and their interaction on mite densities (Table 2). When mite abundance on 17 sweet pepper cultivars was compared, a significantly high number of A. swirskii was reported in pollen-treated plots, and on cultivar FPP9048 (Tukey’s test P < 0.05). Significant effects of the presence of pollen on predatory mite abundance were observed on nine pepper cultivars (Student’s t test P < 0.05), where the presence of pollen positively influenced the predatory mite population on eight cultivars (Fig. 1). When pollen was supplied as a supplement, the highest mean densities of A. swirskii were found on FPP9048 and FPP7039 cultivars, whereas the lowest numbers were recorded on Crusader and Hunter. In the absence of pollen, low mite abundances were observed on all of the pepper cultivars throughout the study period. The highest mean density of A. swirskii was observed on 992-7141 followed by Crusader and the lowest densities were observed on Intruder, Bayonet, Red Missile and SPP 6001 (Fig. 1). Mites on two cultivars, Aristotle and Crusader, performed better with no pollen although mite numbers were significantly higher only on Crusader (Fig. 1).
Screening of pepper cultivars
In all three screening experiments, both the main effects (pepper cultivar and time) had a significant effect on the abundance of A. swirskii (Table 2). The effect of cultivars on the abundance of predatory mites on host plants varied over time, explaining the cultivar × week effects (Table 2). Weekly samplings showed overlapping generations of A. swirskii on hot pepper cultivars throughout the study period. In the first experiment, predatory mite populations were low at the beginning of study, and increased rapidly and peaked during week 3–4, gradually decreased at week 5 and then maintained low-moderate levels towards the end at week 6 (Table 3). Nevertheless, each cultivar of pepper sustained the predatory mite populations from seedling to the matured fruiting stage. High densities of A. swirskii on all cultivars were observed during the flowering period. A significant increase in predatory mite abundance compared to previous weeks was reported during the third sampling on all the pepper cultivars except for Riot, Yellow Mushroom, and Anaheim TMR (Tukey’s test P < 0.05). Among hot pepper cultivars, a significant difference in predatory mite abundance was observed beginning at the second week (Table 3), where the highest density was observed on Fooled You Hybrid and the lowest on Anaheim TMR. From the third week onwards, a significantly higher abundance of predatory mites was reported on Chily Chili Hybrid than on Anaheim TMR, Numex Sunburst Orange (except week 3) and Riot on various sampling dates (Table 3). The highest mean number of predatory mites was recorded on Tam Jalapeno during week 4 followed by week 3, which were not significantly different from predatory mite densities on Chily Chili Hybrid and Explosive Ember on weeks 3 and 4, Yellow Mushroom on week 4, as well as from Super Chilli Hybrid and Fooled You Hybrid on week 3 (Tukey’s test P < 0.05).
In the second experiment, sweet pepper cultivars sustained a low-moderate mite population throughout the study period. During weekly surveys, mites performed the best on cultivar 992-7141 maintaining a moderate-high level of mites between weeks 2 and 7, followed by 7141, FPP9048 and FPP7039 (Table 4). Significantly higher numbers of predatory mites were reported on 992-7141 than on Hunter and Cutlass on all sampling dates between weeks 4–7, TomCat on weeks 2 and 4–7 and Bayonet on weeks 2–4 and 6, respectively (Table 4). No significant difference in predatory mite abundance was reported between the cultivars FPP9048 and FPP7039 on any of the sampling dates, and between the 992-7141 and 7141 cultivars on different sampling dates except for week 4. A significant increase in predatory mite abundance compared to previous weeks was reported during the third sampling on cultivars 7141, FPP9048 and Cutlass (Tukey’s test P < 0.05). A high density of A. swirskii was observed on all cultivars during the flowering period (weeks 3–6). The highest mean number of predatory mites was recorded on cultivar 992-7141 during week 5, which was not significantly different from densities on 7141 in week 5 and 992-7141 in week 4 (Tukey’s test P > 0.05). The lowest mite density was reported on Bayonet cultivar during week 8.
In the third experiment, where 20 mites were released per plant, cultivar 7141 outperformed the remaining three cultivars in supporting populations of A. swirskii. Low-moderate densities of A. swirskii were observed on 7141, FPP7039 and FPP9048 during the first few weeks after transplant, which peaked to the highest level after week 4 and then decreased to low levels after week 6 (Fig. 2). Low densities of mites were observed on 992-7141 during the entire study period. A. swirskii was highest on 7141 in week 5 and lowest on FPP7039 in week 1 (Tukey’s test P < 0.05).
Effect of two release methods (banker vs. direct release) on population abundance of Amblyseius swirskii
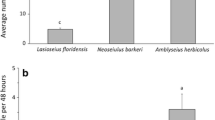
Abundance of A. swirskii was affected by the release method, but not by cultivars, nor by their interaction (Table 2). Except for a few occasions, consistently high mite numbers were found in the banker plant treatment compared to the direct-release treatment (Fig. 3). A significantly higher number of mites was found in the banker plant treatment than in the direct-release treatment for all the four cultivars (Student’s t test P < 0.05) (Fig. 3). The number of predatory mites sampled on the various cultivars fluctuated between 0 and 6 mites per five leaves for banker plants and 0–3 mites per five leaves for direct-release treatments. In the banker plant treatment and the direct-release method, cultivars 992-7141 and FPP7039 held the highest seasonal mean numbers of A. swirskii, respectively (Fig. 3).
The mean number (±SEM) of Amblyseius swirskii recorded weekly per five leaves in banker and direct-release treatments. Figure at the bottom shows seasonal means of A. swirskii recovered in two treatments. Asterisks indicate significant differences in A. swirskii abundance on a pepper cultivar between banker plant versus direct-release treatments
Discussion
Our study shows that A. swirskii could survive without prey on the most of screened pepper cultivars for at least 6 weeks in the presence of flowers, and 4 weeks when pre-planting-stage seedlings were provided with pollen. Amongst the 17 cultivars tested for the effect of pollen on mite establishment on pepper seedlings, a high mean density of predatory mites was recorded on 15 cultivars with pollen. This suggests that an initial pollen supply was important for mite survival and reproduction in the absence of their prey, flowers, or other plant-provisioned food sources. In a related study, Kutuk and Yigit (2011) reported that A. swirskii was able to feed and survive on pine pollen added to pepper seedlings for 2 weeks in the absence of prey and flowers. Ramakers (1995) also showed successful establishment of Iphiseius (Amblyseius) degenerans (Berlese) on cucumber plants using a bee-collected pollen suspension in the absence of prey or any other host. In a concurrent study, we tested the PIF approach on seedlings of various pepper cultivars supplied with an artificial diet, and found that predatory mite populations established during the seedling stage of the hosts with a single release of mites. This indicates that early establishment of a generalist phytoseiid can be ensured on seedlings in the absence of prey if an alternative food source is provided. Thus, we suggest that PIF has potential to serve as an important tool not only for field growers but also for nursery growers. This approach will reduce their input cost for pest management on vegetable seedlings and increase the value of their product.
While screening pepper cultivars, low mite densities were observed initially (during pre-flowering stage), and their population increased after week 2 of the study. Depending on the initiation of flowering, predatory mite populations peaked between weeks 3 and 5, and reached moderate-low levels towards the end of this study, coinciding with the end of the flowering stage. These results suggest that flowers played an important role in sustaining predatory mite populations on pepper cultivars. Our results are consistent with several other studies that report a positive effect of pollen on various generalist phytoseiid mite species. Van Rijn et al. (2002), Ragusa et al. (2009), Nomikou et al. (2010), Kutuk and Yigit (2011) demonstrated that the supply of pollen as food resulted in a population increase of the phytoseiid mite species I. (A.) degenerans, Cydnodromus californicus McGregor and A. swirskii, and subsequently resulted in decreased pest populations on the host plants.
High densities of mites were observed in the experiment where 20 mites were released per plant, even reaching densities of >100 mites/plant. This suggests that host plants offered sufficient resources to support high mite densities, and that 20 mites/plant would be an effective release rate for greenhouse applications. We speculate that in addition to plant pollen, the presence of domatia and their emergence at different growth stages of pepper also affected mite densities in our study. During the screening of the pepper cultivars, domatia appeared in week 2 after transplanting, along with the onset of flowering, coinciding with an increase of densities of A. swirskii. Although quantitative data on the number of domatia per pepper cultivar were not collected, weekly surveys on the presence or absence of domatia suggest that there was an apparent influence of its presence on the predatory mite populations. In the past, the role of domatia has been positively correlated with mite abundance by Romero and Benson (2005) and Loughner et al. (2008). It has been reported that domatia can help mite populations to increase by providing refugia for development and breeding; protecting against insecticides, natural enemies, intra-guild predation and adverse climatic condition; reducing the chance of dislodging from the host plant surface, as well as capturing plant pollen or fungal spores which might serve as sources of nutrition (Walter and O’Dowd 1992; Grostal and O’Dowd 1994; Walter 1996; Roda et al. 2000; Faraji et al. 2002; Ferreira et al. 2008, 2011; Avery et al. 2014). All these studies suggest that plant phenology plays an important role in supporting mite populations, and PIF can utilize these plant characteristics to establish mites during pre- and post-transplant stages of the crop prior to pest arrival. Nevertheless, more studies are needed to determine the potential role of domatia on the growth of mites in our system.
Our study also demonstrates that banker plants were a more effective mode to disperse A. swirskii to pepper transplants than direct releases of predators on the plants. The idea of using banker plants is related to conservation biological control, which provides ecological infrastructures required to sustain a reproducing population of natural enemies (Osborne and Barrett 2005; Frank 2010; Huang et al. 2011). We have demonstrated the role of banker plants in the establishment of biological control agents on host plants as well as in suppressing multiple pest populations previously (Xiao et al. 2011a, b, 2012; Avery et al. 2014). The results of our current study confirm those of our earlier studies, but they are novel in providing a comparison of the two methods. In the current study, the number of A. swirskii sampled every week in the screening experiments was several times higher than observed in the direct-release treatment in the banker versus direct-release experiment. An important difference between these experiments was that transplants in the direct-release treatment lacked an initial provisioning of pollens, unlike the pepper cultivar screening experiments. Thus, we suggest that the lack of food (pollen) at the time of mite release negatively affected mite establishment on the pepper transplants, resulting in a low recovery during the entire study period.
Conclusion
Various studies suggest that the initial application of pollen and host plant characteristics are important factors for predator establishment and should be taken into consideration before testing the PIF approach in commercial production. In the early growth stage of pepper (seedling or early transplant), pollen acts as a nutritional supplement and helps predatory mites to establish in the absence of their prey and, once established, mites can control the pest population in its incipient stage. Based on a series of screening tests, four pepper cultivars were found to warrant further testing. In future studies, two of these four varieties will be used to test the applicability of the PIF approach in protected and open pepper production units during various cropping seasons. The two release methods (direct vs. indirect) will also be evaluated under field conditions. If successful, this pest management method will increase the reliability of biological control strategies and reduce overall insecticide use.
References
Arthurs S, McKenzie C, Chen J, Dogramaci M, Brennan M, Houben K, Osborne L (2009) Evaluation of Neoseiulus cucumeris and Amblyseius swirskii (Acari: Phytoseiidae) as biological control agents of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) on pepper. Biol Control 49:91–96
Avery PB, Kumar V, Xiao YF, Powell CA, McKenzie CL, Osborne L (2014) Selecting an ornamental pepper banker plant for Amblyseius swirskii in floriculture crops. Arthropod Plant Interact 8:49–56
Bennison JA (1992) Biological control of aphids on cucumbers use of open rearing systems or ‘banker plants’ to aid establishment of Aphidius matricariae and Aphidoletes aphidimyza. Meded Fac Landbouwwet, Rijksuniv Gent 57:457–466
Calvo FJ, Bolckmans K, Belda JE (2011) Control of Bemisia tabaci and Frankliniella occidentalis in cucumber by Amblyseius swirskii. Biocontrol 56:185–192
Carrillo D, Peña JE, Hoy MA, Frank JH (2010) Development and reproduction of Amblyseius largoensis (Acari: Phytoseiidae) feeding on pollen, Raoiella indica (Acari: Tenuipalpidae), and other microarthropods inhabiting coconuts in Florida, USA. Exp Appl Acarol 52:119–129
Demirozer O, Tyler-Julian, K, Funderburk, J, Leppla, N, Reitz, S (2012) Frankliniella occidentalis (Pergande) integrated pest management programs for fruiting vegetables in Florida. Pest Manag Sci 68:1537–1545
Dogramaci M, Arthurs S, Chen J, Mckenzie CL, Irrizary F, Osborne LS (2011) Management of chilli thrips Scirtothrips dorsalis (Thysanoptera: Thripidae) on peppers by Amblyseius swirskii (Acari: Phytoseiidae) and Orius insidiosus (Hemiptera: Anthocoridae). Biol Control 59:340–347
Faraji F, Janssen A, Sabelis MW (2002) Oviposition patterns in a predatory mite reduce the risk of egg predation caused by prey. Ecol Entomol 27:660–664
FDACS (2000) The exotic invasion of Florida. A report on arthropod immigration into the sunshine state. Florida department of agriculture and consumer services. http://www.freshfromflorida.com/Divisions-Offices/Plant-Industry/Science/The-Exotic-Invasion-of-Florida
FDACS (2007) Florida Agricultural Statistical Directory. Florida Department of Agriculture and Consumer Services, Florida
Ferreira JAM, Eshuis B, Janssen A, Sabelis MW (2008) Domatia reduce larval cannibalism in predatory mites. Ecol Entomol 33:374–379
Ferreira JAM, Cunha DS, Pallini A, Sabelis MW, Janssen A (2011) Leaf domatia reduce intraguild predation among predatory mites. Ecol Entomol 36:435–441
Frank SD (2010) Biological control of arthropod pests using banker plants systems: past progress and future directions. Biol Control 52:8–16
Loughner R, Goldman, K, Loeb, G, Nyrop, J (2008) Influence of leaf trichomes on predatory mite (Typhlodromus pyri) abundance in grape varieties. Exp Appl Acarol 45:111–122
Gonzalez D, Wilson LT (1982) A food-web approach to economic thresholds: a sequence of pests/predaceous arthropods on California cotton. Entomophaga 27:31–43
Grostal P, O’Dowd DJ (1994) Plants, mites and mutualism: leaf domatia and the abundance and reproduction of mites on Viburnum tinus (Caprifoliaceae). Oecologia 97:308–315
Huang N, Enkegaard A, Osborne LS, Ramakers PMJ, Messelink GJ, Pijnakker J, Murphy G (2011) The banker plant method in biological control. Crit Rev Pla Sci 30:259–278
Kakkar G, Seal DR, Kumar V (2012) Assessing abundance and distribution of an invasive thrips Frankliniella schultzei (Trybom) (Thysanoptera: Thripidae) in South Florida. Bull Entomol Res 102:249–259
Kumar V, Kakkar G, Seal DR, McKenzie CL, Osborne L (2013) An overview of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) biology, distribution and management. In: Solenski S, Larramendy M (eds) Weed and pest control—conventional and new challenges. Intech, Rijeka, pp 53–77
Kumar V, Wekesa V, Avery PB, Powell CA, McKenzie CL, Osborne LS (2014a) Effect of pollens of various ornamental pepper cultivars on the development and reproduction of Amblyseius swirskii (Acari: Phytoseiidae). Fla Entomol 97:367–373
Kumar V, Kakkar G, Seal DR, McKenzie CL, Colee J, Osborne L (2014b) Temporal and spatial distribution of an invasive thrips species Scirtothrips dorsalis (Thysanoptera: Thripidae). Crop Prot 55:80–90
Kutuk H, Yigit A (2011) Pre-establishment of Amblyseius swirskii (Athias–Henriot) (Acari: Phytoseiidae) using Pinus brutia (Ten.) (Pinales: Pinaceae) pollen for thrips (Thysanoptera: Thripidae) control in greenhouse peppers. Intern J Acarol 37:95–101
Luckmann WH, Metcalf RL (1975) The pest-management concept. In: Metcalf RL, Luckmann WH (eds) Introduction to insect pest management. Wiley, New York, pp 3–35
McAvoy G, Ozores-Hampton M (2007) Common pepper cultivars for Florida production. University of Florida, IFAS extension publication #IPM204. http://edis.ifas.ufl.edu/in757. Accessed 15 July 2014
McKenzie CL, Kumar V, Palmer CL, Oetting RD, Osborne LS (2014) Chemical class rotations for control of Bemisia tabaci (Hemiptera: Aleyrodidae) on poinsettia and their effect on cryptic species population composition. Pest Manag Sci 70:1573–1587
McMurtry JA, Croft BA (1997) Life-styles of phytoseiid mites and their roles in biological control. Annu Rev Entomol 42:291–321
Messelink GJ, van Maanen R, van Steenpaal SEF, Janssen A (2008) Biological control of thrips and whiteflies by a shared predator: two pests are better than one. Biol Control 44:372–379
Nomikou M, Janssen A, Schraag R, Sabelis MW (2002) Phytoseiid predators suppress populations of Bemisia tabaci on cucumber plants with alternative food. Exp Appl Acarol 27:57–68
Nomikou M, Janssen A, Sabelis MW (2003) Phytoseiid predators of whiteflies feed and reproduce on non-prey food sources. Exp Appl Acarol 31:15–26
Nomikou M, Sabelis MW, Janssen A (2010) Pollen subsidies promote whitefly control through the numerical response of predatory mites. Biocontrol 55:253–260
Osborne LS, Barrett JE (2005) You can bank on it, banker plants can be used to rear natural enemies to help control greenhouse pests. Ornam Outlook, Sept, pp 26–27
Park HH, Shipp L, Buttenhuis R (2010) Predation, development, and oviposition by the predatory mite Amblyseius swirskii (Acari: Phytoseiidae) on tomato russet mite (Acari: Eriophyidae). J Econ Entomol 103:563–569
Parr WJ, Stacey DL (1976) ‘Banker’-plant system of whitefly parasite release on tomatoes. Rep Glasshouse Crops Res Inst 1975:96
Ragusa E, Tsolakis H, Palomero RJ (2009) Effect of pollens and preys on various biological parameters of the generalist mite Cydnodromus californicus. Bull Insectol 62:153–158
Ramakers PMJ (1990). Manipulation of phytoseiid thrips predators in the absence of thrips. IOBC/WPRS Bull 13:169–172
Ramakers PMJ (1995) Biological control using oligophagous predators. In: Parker BL, Skinner M, Lewis T (eds) Thrips biology and management. Plenum Press, New York, pp 225–229
Roda A, Nyrop J, Dicke M, English-Loeb G (2000) Trichomes and spider-mite webbing protect predatory mite eggs from intraguild predation. Oecologia 125:428–435
Romero GQ, Benson WW (2005) Biotic interactions of mites, plants and leaf domatia. Curr Opin Plant Biol 8:436–440
Sampson C (1998) The commercial development of an Amblyseius cucumeris controlled release method for the control of Frankliniella occidentalis in protected crops. In: Brighton crop protection conference: pests and diseases—1998. Proceedings of an international conference, vol 2. Brighton, UK, 16–19 Nov 1998, pp 409–416
SAS Institute (2009) JMP statistics and graphics guide version 8.0.1. SAS Institute, Cary
Seal DR, Kumar V, Kakkar G, Mello SC (2013) Abundance of adventive Thrips palmi (Thysanoptera: Thripidae) populations in Florida during the first sixteen years. Fla Entomol 96:789–796
Speyer ER (1927) An important parasite of the greenhouse whitefly. Bull Entomol Res 17:301–308
Stary P (1970) Biology of aphid parasites (Hymenoptera: Aphidiidae) with respect to integrated control. Series Entomololgica 6. Dr.W. Junk, The Hague, p 641
Stary P (1993) Alternative host and parasitoid in first method in aphid pest management in glasshouses. J Appl Entomol 116:187–191
van Rijn PCJ, van Houten YM, Sabelis MW (2002) How plants benefit from providing food to predators even when it is also edible to herbivores. Ecology 83:2664–2679
Wade MR, Zalucki MP, Wratten SD, Robinson KA (2008) Conservation biological control of arthropods using artificial food sprays: current status and future challenges. Biol Control 45:185–199
Walter DE (1996) Living on leaves: mites, tomenta, and leaf domatia. Annu Rev Entomol 41(297):101–114
Walter DE, O’Dowd DJ (1992) Leaves with domatia have more mites. Ecology 73:1514–1518
Xiao YF, Chen JJ, Cantliffe D, McKenzie CL, Houben K, Osborne L (2011a) Establishment of a papaya (Carica papaya L.) banker plant system for parasitoid, Encarsia sophia (Hymenoptera: Aphilidae) against Bemisia tabaci (Hemiptera: Aleyrodidae) in greenhouse tomato production. Biol Control 58:239–247
Xiao YF, Osborne L, Chen JJ, McKenzie CL, Houben K, Irizarry F (2011b) Evaluation of corn plant as potential banker plants for predatory gall midge, Feltiella acarisuga (Diptera: Cecidomyiidae) against Tetranychus urticae (Acari: Tetranychidae) in greenhouse vegetable crops. Crop Prot 36:1635–1642
Xiao YF, Avery PB, Chen JJ, McKenzie CL, Osborne L (2012) Ornamental pepper as banker plants for establishment of Amblyseius swirskii (Acari: Phytoseiidae) for biological control of multiple pests in greenhouse vegetable production. Biol Control 63:279–286
Acknowledgments
The authors would like to thank Katherine Houben, John Prokop, Irma Herrera, Fabieli Irizarry, and Younes Belmourd for their technical assistance. We would also like to thank Dr. James Colee for assisting in the statistical analysis and the internal reviewers Drs. Joseph Patt, J. Howard Frank and Aaron Dickey for their constructive criticism and helpful suggestions. Funding for this study was supported by Grants from the EPA, USDA/FDACS—Specialty Crop Block Grant Program, the USDA-ARS Floriculture and Nursery Research Initiative, and IFAS/UF Line Item grants program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Vivek Kumar and Yingfang Xiao have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kumar, V., Xiao, Y., McKenzie, C.L. et al. Early establishment of the phytoseiid mite Amblyseius swirskii (Acari: Phytoseiidae) on pepper seedlings in a Predator-in-First approach. Exp Appl Acarol 65, 465–481 (2015). https://doi.org/10.1007/s10493-015-9895-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-015-9895-2