Abstract
Stigmaeopsis celarius Banks (hereafter Sc) is a spider mite living and feeding on the leaves of various bamboo species such as Moso bamboo [Phyllostachys edulis (=P. pubescens)] and Pleioblastus spp. (Poaceae). A previous phylogenetic study revealed a cryptic, phylogenetic sister species to Sc (hereafter Ss). Although its life type appears to be similar to that of Sc, individuals of Ss make much smaller nests compared with Sc, and the nests have been found mostly on Nezasa bamboo (Pleioblastus argenteostriatus). To investigate whether Sc and Ss are reproductively isolated, we explored their populations in southwestern Japan, and crossed them to examine mating behaviors and fertilization success. Field surveys revealed that the nests of these two species occur on the same leaves and, thus, the individuals of these species may make frequent contact. Reciprocal crosses suggested that the two species are reproductively isolated. Though Sc males have tried to mate with Ss females, copulation seldom occurred because of their long opisthosoma (hind body), which prevented the insertion of the aedeagus into the genitalia of Ss females. In contrast, most Ss males ignored Sc females, and eggs were not fertilized even in the few cases where copulation appeared to occur. These results suggest that strong selection pressure is imposed on body length to prevent interspecific hybridization in the contact area of these species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many organisms, multiple species that differentiated from a common ancestor can be redistributed in the same geographic area, forming a secondary contact zone. These species can mate with other species and even form hybrids (Futuyma 1998; Coyne and Orr 2004). This is a form of reproductive interference, i.e., interspecific interaction that adversely affects the fitness of at least one species involved in mate acquisition (Gröning and Hochkirch 2008). One ecological consequence of the reproductive interference is that one species can be excluded from the contact zone through reproductive interference without resource allocation or niche partitioning (Bull 1991; Kuno 1992; Takafuji et al. 1996; Kishi et al. 2009; Kyogoku and Nishida 2013). Another consequence is that various kinds of mating traits change in the contact zone. In the reinforcement theory, selection against hybridization favors the evolution of premating isolating mechanisms (Gröning and Hochkirch 2008). Behavioral, life history, and morphological traits would be evolutionarily changed to prevent hybridization because hybrids usually suffer from lower fitness than their parental species (Gröning and Hochkirch 2008). Clarifying the process of mating interaction between proximately redistributed species is important to realize selection pressure on behavioral and morphological traits of each species, and to infer the possibility of sympatric coexistence of these species.
Stigmaeopsis spider mites (Acari: Tetranychidae) feed on bamboo species, e.g. Sasa spp. and Pleioblastus spp., or Chinese silver grass Miscanthus sinensis (Poaceae). They are suitable materials to investigate the consequences of a secondary contact zone on reproductive characters, because different species live on the same host-plant shoots across a wide range in Japan, i.e., they are sympatrically or parapatrically distributed (Mori and Saito 2004; Sato et al. 2008). The life type of Stigmaeopsis species is characterized by woven nests (Saito 1985). On the undersurface of host leaves, individuals construct densely-woven silken nests along the midrib, in the depressions of leaves, or on the curly parts of leaf edges. Offspring grow up and reproduce inside the nest, and in species making large nests, offspring of the following generations often live together in the same nest. Colony members are engaged in enlarging and repairing nests as well as sanitary behavior in several cooperative social species (Saito 1983, 1997, 2010; Sato et al. 2003; Sato and Saito 2006).
Both precopulatory and postcopulatory reproductive isolations are reported in the Miscanthus-inhabitor, Stigmaeopsis miscanthi (Saitō 1990). Two genetically differentiated forms (HG and LW) are parapatrically distributed and form the boundary of the distribution area in the mountainous regions of Japan (Sato et al. 2008). Crossing experiments suggest that interform copulation is partly successful (as inferred from the increase in fecundity compared with virgin females), so that prezygotic reproductive isolation is incomplete (Sato et al. 2000b). In addition, the eggs fertilized by the other form did not develop fully, though a few developed into adult females in a particular combination of populations (Sato et al. 2000a, b, 2008). Thus, reproductive isolation occurs at both prezygotic and postzygotic stages in the two S. miscanthi forms (Sato et al. 2000b). Precopulatory isolation exists between certain pairs of bamboo-inhabiting species (Saitô and Takahashi 1982; Mori 2000). The nest made by mites functions as an obstacle against intrusion of different species. For example, Stigmaeopsis longus (Saitō 1990), who has a large body size, could not enter the nest of Stigmaeopsis saharai (Mori 2000), who makes the smallest nests in this group (Mori and Saito 2004). These studies suggest that the mechanisms of reproductive isolation among Stigmaeopsis species are diverse.
Based on the phylogenetic analysis of the cytochrome oxidase subunit I (COI) region, Ito and Fukuda (2009) discovered a cryptic strain (clade no. 4) closely related to Stigmaeopsis celarius (hereafter Sc) (clade no. 3). Although this cryptic strain has not been taxonomically described, we treat this strain as a species (hereafter Ss) in terms of the phylogenetic species concept (Coyne and Orr 2004), and regard the term species as a taxon corresponding to a monophyletic clade in Ito and Fukuda (2009). Note that this usage differs from Ito and Fukuda (2009), in which a single clade contained morphologically different variants, and each variant was assigned a species name according to the key provided by Saito et al. (2004) based on the morphological species concept (Coyne and Orr 2004).
Ss makes much smaller nests than Sc along major leaf veins or in the depression adjacent to leaf edges of Pleioblastus spp., but seldom inhabits the leaves of Sasa or Phyllostachys spp., on which other Stigmaeopsis species frequently live (K. Ito, personal observation). Occasionally, Sc and Ss live on the same Pleioblastus leaves. Thus, the opportunity for secondary contact of individuals of these species may exist in the wild, though the frequency of their encounters is unknown.
In this study, we first surveyed the parapatric and/or sympatric occurrence of the Sc and Ss species in the field to measure the frequency of their encounter. Second, we conducted crossing experiments to investigate whether they are reproductively isolated. In addition, we studied the mating behavior of males using video recording to seek for factors explaining the results. Finally, we investigated the lengths of idiosoma and opisthosoma (hind body) in males, which are closely linked with posture during copulation.
Materials and methods
Field survey of co-occurrence
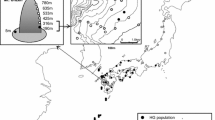
We surveyed the field occurrence of Sc and Ss on Pleioblastus spp. in southwestern Japan from 2007 to 2014. The co-occurrence of two species was determined on a discrete size of web nests on the same or adjacent plants. Infested leaves were collected and kept in plastic bags in a cooler box. In the laboratory, adult mites were prepared into slide specimens using Hoyer’s medium and observed under a phase-contrast microscope (BX50, Olympus, Tokyo, Japan) for identification purposes. When identification was difficult, the sequence of a partial COI region was used as supplementary information (Ito and Fukuda 2009; Ito et al. 2011).
The morphology of Ss closely resembles Stigmaeopsis takahashii (Saito et al. 2004) in that the length of dorsal seta d1 exceeds the base of the next posterior hair (e1), but does not reach to the base of the following hair (f1) (Saito et al. 2004; Ito and Fukuda 2009). However, the host plants and distributions of the two species differ: S. takahashii feeds mainly on Sasa spp. and is distributed in cooler regions such as the Hokkaido district. Furthermore, a phylogenetic study showed that “SC Tsukuba” and “SC Suita” specimens, which are Ss and Sc, respectively, in the present study, formed a single clade distinct from that of S. takahashii (Ito et al. 2011).
Host plants were morphologically identified according to Suzuki (1996) and Uchimura (2005). The scientific names followed the database index of the Botanical Gardens (YList [http://bean.bio.chiba-u.jp/bgplants/ylist_main.html]).
Mites
An experimental population of each species was collected from a field of the Yokonami peninsula, Tosa, Kochi, Japan (N33.429° E133.452°, WGS84). Though the contact zone of the two species was at this location, the experimental population of each species was collected 40–70 m away from the contact zone. Though the dispersal ranges of these species are unknown, microsatellite analyses in the two-spotted spider mite Tetranychus urticae showed a positive autocorrelation only between the subpopulations within 2.4–3.6 m for a rose greenhouse (Uesugi et al. 2009a) or 10–24 m for apple orchards (Uesugi et al. 2009b), so that the gene flow of T. urticae by crawling or short-distance aerial dispersal may be limited to a short range. Assuming that Sc and Ss have similar dispersal ability as T. urticae, we considered that the present collection sites were sufficiently distant to hamper short-scale gene flow.
Rearing cultures were maintained on a detached leaflet of P. argenteostriatus resting on water-soaked cotton pads in a Petri dish (internal dimensions of 91.3 × 38.2 mm, Insect Breeding Dish; SPL Life Sciences, Gyeonggi-do, Korea) at 25 °C, 60 % RH (relative humidity) and LD (light:dark) 16:8 h conditions in a plant growth chamber (LPH-240S, Nippon Medical & Chemical Instruments, Osaka, Japan). All experiments were conducted under the same conditions.
Nest size
The nest size of each species was measured as follows. A detached P. argenteostriatus leaf was securely fixed on the interior angle of a polyvinyl chloride (PVC) angle (1 × 1 cm) using double-faced adhesive tape to provide the mites with a constant physical structure for nest weaving (Mori and Saito 2006). This angle was placed on the water-soaked cotton pads; the leaf surface was raised and wet cotton was packed under the angle to provide support. This method is analogous to Mori and Saito (2006, 2013) except for the angle materials (acryl and PVC) and host plant.
A young mated female (3–10 days after molting to adult) randomly collected from the rearing stock was individually released on the leaflet. Forty-eight hours after introduction of the female, nests were photographed with a scale using a digital video camera recorder (NEX-VG20H, Sony, Tokyo, Japan) attached to a stereomicroscope (SZX7, Olympus). Images were analyzed using ImageJ ver. 1.46r software (NIH, http://imagej.nih.gov/ij/), and the maximum lengths of the major and minor axes were measured for each nest. The nest size was calculated as the product of these lengths assuming a rectangular shape (Mori and Saito 2006, 2013). The average nest size was compared by the Welch t test (this method led to a decimal degree of freedom).
Cross experiment
A teleiochrysalis (quiescent deutonymph) female of one species arbitrarily collected from the rearing culture was allowed to make a nest on a 1 × 1-cm leaf square. After 2 days, one matured male of the same or another species randomly collected from each culture was introduced onto the leaf square. The success of male intrusion into the nests was checked every day, and the number of eggs produced was recorded on day 14. After removing parental mites, all offspring were reared to adulthood to determine hatchability, egg-to-adult survival, and sex ratio. The number of replicates for each cross ranged between 15 and 18.
A one-way analysis of variance (ANOVA) and Tukey multiple comparisons were used to compare each subject between crosses. Sex ratio data were arcsine-root transformed before analysis.
Copulation behavior
Copulation consists of several elements (Cone 1985). Upon finding a matured virgin female, an adult male first tries to mount the female body, then slips himself under the female abdomen from behind, holds her legs with his front legs, and bends his opisthosoma (posterior part of the body behind the transverse crease) upward to insert his extruded aedeagus into the female genital opening (Cone 1985; Oku 2014; Oku and Saito 2014). In this study, the raising of the opisthosoma was defined as “copulation behavior” because preliminary observations revealed that most males completed copulation after this step in intraspecific copulation.
A teleiochrysalis female randomly sampled from the culture of each species was transferred onto a small leaf square (3 × 3 mm). After 24 h, when the adult female emerged and made a nest, an adult male of the same or different species was introduced onto the piece of leaf, and copulatory behavior was recorded for 6 h using a digital video camera recorder (NEX-VG20H) attached to a stereomicroscope (SZX7). Fifteen replicates were collected from each cross, though a few were accidentally lost. Based on the video data, the following four points were measured for each pair: (i) the proportion of males showing copulation behavior; (ii) the number of copulation attempts (i.e., raising the opisthosoma upward); (iii) copulation time: the period of deep contact of the male opisthosoma with the female genital opening. If contact occurred many times per replicate, the average values were used for analyses; and (iv) the time from the first encounter of a male with a female to the onset of mounting behavior.
These data were compared pairwise among crossing treatments using the nonparametric Steel–Dwass test because their distributions were highly non-normal. The proportion of pairs in which copulation occurred was compared between the crosses using the chi-square test adjusted using Ryan’s procedure for multiple comparisons (Ryan 1960).
Body length
To measure body length, each individual used in the experiments was individually mounted on glass slides (No. 2215, Matsunami Glass, Osaka, Japan) with a droplet of Hoyer’s solution and covered with a cover glass. A 10-g weight was placed on the cover glass to flatten the mite body (Saito et al. 1999). The specimens were kept at 50 °C in an incubator (SIB-35, SANSYO, Tokyo, Japan) for 3 days. A 100× image was recorded using a microscope camera (DP20, Olympus) and analyzed using ImageJ ver. 1.46r (NIH).
The opisthosoma length (OL) of male mites was inferred from the distance between the bases of the dorsal setae C1 and PA (Fig. 2) (Lindquist 1985; Saito et al. 2004) because the mite body is soft and collapses easily. We measured only male OL because this is the length of the body part erected in copulation, and is closely related with the successful contact of aedeagus at the tip of male opisthosoma with female abdominal genital opening. We also estimated the total body (idiosoma) length [IL, the distance between the base of setae P1 (anteriormost hair of the prodosum) and PA] for each sex. Because C1, P1, and PA were approximately on the same straight line (Fig. 2b), the length of the prosoma (PL), i.e., the distance between P1 and C1, was estimated simply by subtracting OL from IL. The body lengths of each sex were compared between species using the t test. Prior to the analyses, an F test was conducted to test the homogeneity of variances. The Welch t test was applied if variance was unequal between groups.
Dorsal view of a female and b male Stigmaeopsis sp. Setae P1, C1 and PA are indicated. The bases of these three hairs in male are almost on a straight line, and the base of C1 is located near the crevice between prosoma and opisthosoma. Thus, the distance between P1 and C1 is considered the prosoma length (PL), and the distance between C1 and PA is considered the opisthosoma length (OL, dashed arrow). The distance between P1 and PA denote the idiosoma length (IL, solid arrow)
To examine the effect of body size on copulation, we determined the correlation between female IL, male IL, the difference between them, and each copulation behavior (ii–iv) using Pearson’s product moment correlation. Analyses were conducted on data from each intra- or interspecific cross (ii: n = 6–14, iii: n = 6–13, iv: n = 3–10) and on data pooled from all crosses.
Results
Field survey of co-occurrence
The Sc and Ss populations co-occurred at seven locations in Japan (Fig. 1; Table 1). Their host plants were limited to several species of Pleioblastus except for Moso bamboo at one site. The species were found on the same shoots in four areas and on adjacent shoots (at intervals ≤5 m) in five areas of Japan.
Nest size
The nest areas of Sc and Ss were 6.99 (mean) ± 2.55 (SD) mm2 and 2.96 ± 1.02 mm2 (n = 10, respectively). The difference between the nest sizes was highly significant (t 11.805 = 4.647, P < 0.001, Welch t test).
Crossing experiment
The results of crossing experiments are shown in Table 2. All males of each species successfully entered the nests made by females of the same and other species during the 14 days. Females that mated with males of different species produced significantly fewer eggs than those resulting from intraspecific mating, and the Sc pair bore significantly more eggs than the Ss pair. The male ratios from intraspecific mating were 0.13 in Sc and 0.19 in Ss. No females offspring resulted from interspecific mating.
Copulation behavior
The copulation behavior of each cross is summarized in Table 3. The proportions of intraspecific copulation behavior (opisthosoma raising) were not significantly different between Sc♀ × Sc♂ and Ss♀ × Ss♂ crosses (Table 3[i]). For Ss males, the proportion in the interspecific pair (Sc♀ × Ss♂) was significantly smaller than in the intraspecific pair (Ss♀ × Ss♂). In Sc males, however, the proportion in the intraspecific pair (Sc♀ × Sc♂) was not significantly different from the interspecific pair (Ss♀ × Sc♂). Thus, the response of males to females of other species differed significantly between species.
There was no significant difference in the number of copulation attempts between any combination (Table 3[ii]). However, the period of copulation differed (Table 3[iii]). The copulation periods of Sc and Ss in intraspecific copulation were not significantly different. The copulation period of Ss♀ × Sc♂ was 0 (copulation failed) even though Sc males tried repeatedly to copulate (Table 3[ii]). In contrast, Ss males sometimes copulated with Sc females (Table 3[i]). The copulation period of Sc♀ × Ss♂ did not differ significantly from Ss♀ × Ss♂, though the average was almost half.
The time from an encounter of a male with a female to the onset of male mounting is shown in Table 3(iv). The time in Ss♀ × Sc♂ was significantly shorter than in Sc♀ × Sc♂, indicating that males tended to start copulation sooner in interspecific copulation compared with intraspecific copulation. No significant differences were observed in the other combinations.
Body length
The homogeneity of variances were preliminarily examined for the body length analyses. For IL, the variance between the two species was not significantly different in females (F 22, 23 = 1.385, P = 0.44) but significantly different in males (F 25, 17 = 2.976, P = 0.023). The variance between the two species for the male OL was significantly different (F 24, 17 = 3.279, P = 0.014). The variance in the male PL was also significantly different between species (F 24, 17 = 5.421, P < 0.001). Thus, male body lengths were analyzed using the Welch t test assuming unequal variance. Female size was analyzed by the ordinary t test.
The IL of Sc was significantly longer than that of Ss in both sexes (Fig. 3, female Sc: 308.3 ± 22.6 μm, female Ss: 291.2 ± 19.3 μm, t 45 = 2.791, P < 0.008; male Sc: 235.0 ± 14.3 μm, male Ss 214.4 ± 8.3 μm, t 40.965 = 6.063, P < 0.001). The male OL of Sc was significantly longer compared with Ss (Sc: 148.4 ± 10.7 μm, Ss: 130.6 ± 5.9 μm, t 38.808 = 6.969, P < 0.001), whereas the male PL did not differ significantly between species (Sc: 87.1 ± 10.0 μm, Ss: 83.8 ± 4.3 μm, t 34.662 = 1.446, P = 0.16). Therefore, interspecific differences in IL were attributed to the variation in the OL.
Body length measured as the distance between the bases of setae P1 and PA (see Fig. 2, IL). Unit: μm. a Females and b males. Boxes denote the interquartile ranges (IQR) between the first and third quartiles (25th and 75th percentiles, respectively), and the thick line inside denotes the median. Whiskers represent the lowest and highest values within 1.5 IQL from the first and third quartiles, respectively. Values outside these ranges are separately shown by open circles. Body length differed significantly between the sexes (P < 0.05, t test)
The IL of females, males, and the difference between them was not significantly correlated with any of the three copulatory behaviors in either cross (P > 0.05) except for the time for mounting (iv) in Ss♀ × Sc♂ (r 8 = −0.64, n = 10, P < 0.05). The correlations were also not significant when the data from all the crosses were pooled (n = 55 for copulation data, n = 33 for mounting data). Furthermore, the correlation between the OL and each copulation behavior was not significant for any cross (P > 0.05).
Discussion
Our field survey suggests that Sc and Ss inhabit the same shoots of Nezasa bamboo (Pleioblastus spp.) in various areas of southwestern Japan. Thus, individuals of these species may encounter one another in these areas. Interspecific mating at the boundary area has been reported in various spider mites, and male offspring are over-produced and sterile F1 females partly emerge in the cross of two forms of S. miscanthi (Sato et al. 2000b). In either case, the fitness of the parents should be decreased by a loss of reproductive opportunity to produce offspring with an appropriate sex ratio. Thus, the mechanisms to impede interspecific mating would be evolved in such contact zones.
The crossing experiments suggest that Sc and Ss are reproductively isolated. In the interspecific crosses, all developed offspring were males, suggesting that fertilization did not occur, though short-time copulation was observed only in a few pairs. Notably, significantly fewer eggs were produced in the interspecific crosses compared with intraspecific crossing. Because copulation itself, either with the same or different species, strongly increases the fecundity of Stigmaeopsis species (Saito 1987; Sato et al. 2000a, b), the decrease in egg numbers suggests that copulation itself was not successful in the interspecific crosses. Therefore, precopulatory reproductive isolation is the major factor inhibiting gene flow between these species. In contrast, because the males of each species could intrude the nests of the other species, the effects of the web nests to prevent the intrusion of males of different species (Mori 2000) were negligible. Thus, on the leaves or shoots that both species inhabit, males can move around the nests of both species and may encounter the females of the same or different species.
Our study revealed asymmetrical copulation behavior of the two species in interspecific crosses. In Sc♀ × Ss♂, Ss males made little attempt to copulate with Sc females. The contact of genitalia (apparent copulation) only occurred in a few pairs, but we observed no significant depression in hatchability from the death of hybrid eggs as in S. miscanthi (Sato et al. 2000b) and no female progeny (evidence of fertilization) in any pair. Therefore, we conclude that sperm transfer was unsuccessful in this cross. At present, the factors that deter Ss males from copulating with Sc females are unclear, but pheromone differences between the species might be a plausible explanation because males of various mite species use female pheromones for mate recognition (Cone 1985; Shimizu et al. 2001).
In contrast, in Ss♀ × Sc♂ crosses, males approached females as often as conspecific females, but they never succeeded in copulation and could not fertilize eggs. This could be attributed to the difference in body length between the two species. Though the lengths of male prosoma were similar in the two species, male opisthosoma, and thus idiosoma, of Sc was much longer than that of Ss. During the experiments, we repeatedly observed that the opisthosoma of Sc was too long to appropriately insert the aedeagus into the genitalia of a Ss female body. This suggests that the mismatch in body size between the sexes affects reproductive isolation, and the long opisthosoma of Sc functions as a barrier to interspecific mating. Such effects of mismatches on copulation success are reported in millipidae (Tanabe and Sota 2008) and carabid beetles (Okuzaki et al. 2010), in which sexual differences in body size play an important role in reproductive isolation. In sticklebacks with external fertilization, the probability of interspecific mating is strongly correlated with body size (Schluter and Nagel 1995; Nagel and Schluter 1998; Schluter 1998). In the present study, however, relationships between body size and copulatory behavior were not significant, presumably because of small sample sizes. Further studies are needed to elucidate the effect of size difference on copulation success in spider mites.
The results from the Ss♀ × Sc♂ cross may be related to reproductive interference (Gröning and Hochkirch 2008). Interspecific hybridization leads to exclusion without resource competition, local extinction, and causes parapatry (Bull 1991; Kuno 1992). Several studies have reported the occurrence of interspecific hybridization in spider mites, such as Tetranychus (Ben-David et al. 2009) and Panonychus (Fujimoto et al. 1996; Takafuji et al. 1996). Recent studies on insects suggest that even without interspecific copulation, female fitness is decreased only by the harassment behavior of males of different species, eventually leading to the extinction of one species (Hochkirch et al. 2007; Kishi et al. 2009; Kyogoku and Nishida 2012, 2013). In the present case, even if Sc males could not copulate with Ss females, copulatory attempts were repeated many times, and, thus, fitness loss by Sc male harassment is possible in the contact zone. We hypothesize that if the body sizes of the mates of two species match, females suffer from fitness loss by producing excessive male offspring or sterile hybrids (Sato et al. 2000b), otherwise females incur harassment by males of different species. To test this hypothesis, we should estimate the fertilization status of interspecies crossing as well as the magnitude of harassment imposed by males of different species living in the same habitat.
References
Ben-David T, Gerson U, Morin S (2009) Asymmetric reproductive interference between two closely related spider mites: Tetranychus urticae and T. turkestani (Acari: Tetranychidae). Exp Appl Acarol 48:213–227
Bull CM (1991) Ecology of parapatric distributions. Annu Rev Ecol Syst 22:19–36
Cone WW (1985) Mating and chemical communication. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies, and control, vol 1A. Elsevier, Amsterdam, pp 243–251
Coyne JA, Orr HA (2004) Speciation. Sinauer Associates, Incorporated Publishers, Sunderland, MA
Fujimoto H, Hiramatsu T, Takafuji A (1996) Reproductive interference between Panonychus mori Yokoyama and P. citri (McGregor) (Acari: Tetranychidae) in peach orchards. Appl Entomol Zool 31:59–65
Futuyma DJ (1998) Evolutionary biology, 3rd edn. Sinauer Associates, Incorporated Publishers, Sunderland, MA
Gröning J, Hochkirch A (2008) Reproductive interference between animal species. Q Rev Biol 83:257–282
Hochkirch A, Gröning J, Bücker A (2007) Sympatry with the devil: reproductive interference could hamper species coexistence. J Anim Ecol 76:633–642
Ito K, Fukuda T (2009) Molecular phylogeny of Stigmaeopsis spider mites (Acari: Tetranychidae) based on the Cytochrome Oxidase subunit I (COI) region of mitochondrial DNA. Appl Entomol Zool 44:343–355
Ito K, Yokoyama N, Hayakawa H, Minamiya Y, Yokoyama J, Fukuda T (2011) Molecular phylogenetic relationship of Stigmaeopsis spider mites (Acari: Tetranychidae) collected from Yamagata Prefecture. Bull Yamagata Univ Nat Sci 17:19–29
Kishi S, Nishida T, Tsubaki Y (2009) Reproductive interference determines persistence and exclusion in species interactions. J Anim Ecol 78:1043–1049
Kuno E (1992) Competitive exclusion through reproductive interference. Res Popul Ecol 34:275–284
Kyogoku D, Nishida T (2012) The presence of heterospecific males causes an Allee effect. Popul Ecol 54:391–395
Kyogoku D, Nishida T (2013) The mechanism of the fecundity reduction in Callosobruchus maculatus caused by Callosobruchus chinensis males. Popul Ecol 55:87–93
Lindquist EE (1985) Anatomy, phylogeny and systematics. 1.1.1 External anatomy. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies, and control, vol 1A. Elsevier, Amsterdam, pp 3–28
Mori K (2000) Factors causing variation in social systems of spider mites, Schizotetranychus celarius species group (Acari: Tetranychidae), Ph.D. thesis, Hokkaido University, Sapporo
Mori K, Saito Y (2004) Variation in social behavior within a spider mite genus, Stigmaeopsis (Acari: Tetranychidae). Behav Ecol 16:232–238
Mori K, Saito Y (2006) Communal relationships in a social spider mite, Stigmaeopsis longus (Acari: Tetranychidae): an equal share of labor and reproduction between nest mates. Ethology 112:134–142
Mori K, Saito Y (2013) Genetic basis of woven nest size in subsocial spider mites. Exp Appl Acarol 60:463–469
Nagel L, Schluter D (1998) Body size, natural selection, and speciation in sticklebacks. Evolution 52:209–218
Oku K (2014) Sexual selection and mating behavior in spider mites of the genus Tetranychus (Acari: Tetranychidae). Appl Entomol Zool 49:1–9
Oku K, Saito Y (2014) Do males evaluate female age for precopulatory mate guarding in the two-spotted spider mite? J Ethol 32:1–6
Okuzaki Y, Takami Y, Sota T (2010) Resource partitioning or reproductive isolation: the ecological role of body size differences among closely related species in sympatry. J Anim Ecol 79:383–392
Ryan TA (1960) Significance tests for multiple comparison of proportions, variances and other statistics. Psychol Bull 57:318–328
Saito Y (1983) The concept of “life types” in Tetranychinae: an attempt to classify the spinning behaviour of Tetranychinae. Acarologia 24:377–391
Saito Y (1985) Life types of spider mites. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies, and control, vol 1A. Elsevier, Amsterdam, pp 253–264
Saito Y (1987) Extraordinary effects of fertilization status on the reproduction of an arrhenotokous and sub-social spider mite (Acari: Tetranychidae). Res Popul Ecol 29:57–71
Saitō Y (1990) Two new spider mite species of the Schizotetranychus celarius complex (Acari: Tetranychidae): Study on variation of Schizotetranychus celarius (Banks) III. Appl Entomol Zool 25:389–396
Saito Y (1997) Sociality and kin selection in Acari. In: Choe JC, Crespi BJ (eds) The evolution of social behaviour in insects and arachnids. Cambridge University Press, London, pp 443–457
Saito Y (2010) Plant mites and sociality. Springer, Tokyo
Saitô Y, Takahashi K (1982) Study on variation of Schizotetranychus celarius (Banks) II. Comparison of mode of life between two sympatric forms (Acarina: Tetranychidae). Jpn J Ecol 32:69–78 (in Japanese)
Saito Y, Mori K, Chittenden AR (1999) Body characters reflecting the body size of spider mites in flattened specimens (Acari, Tetranychidae). Appl Entomol Zool 34:383–386
Saito Y, Mori K, Sakagami T, Lin J (2004) Reinstatement of the genus Stigmaeopsis Banks, with descriptions of two new species (Acari: Tetranychidae). Ann Entomol Soc Am 97:635–646
Sato Y, Saito Y (2006) Nest sanitation in social spider mites: interspecific differences in defecation behavior. Ethology 112:664–669
Sato Y, Saito Y, Mori K (2000a) Reproductive isolation between populations showing different aggression in a subsocial spider mite, Schizotetranychus miscanthi Saito (Acari: Tetranychidae). Appl Entomol Zool 35:605–610
Sato Y, Saito Y, Mori K (2000b) Patterns of reproductive isolation between two groups of Schizotetranychus miscanthi Saito (Acari: Tetranychidae) showing different male aggression traits. Appl Entomol Zool 35:611–618
Sato Y, Saito Y, Sakagami T (2003) Rules for nest sanitation in a social spider mite, Schizotetranychus miscanthi Saito (Acari: Tetranychidae). Ethology 109:713–724
Sato Y, Saito Y, Chittenden A (2008) The parapatric distribution and contact zone of two forms showing different male-to-male aggressiveness in a social spider mite, Stigmaeopsis miscanthi (Acari: Tetranychidae). Exp Appl Acarol 44:265–276
Schluter D (1998) Ecological cause of speciation. In: Howard DJ, Berlocher SH (eds) Endress forms. Oxford University Press, New York, pp 114–129
Schluter D, Nagel LM (1995) Parallel speciation by natural selection. Am Nat 146:292–301
Shimizu N, Mori N, Kuwahara Y (2001) Aggregation pheromone activity of the female sex pheromone, beta-acaridial, in Caloglyphus polyphyllae (Acari: Acaridae). Biosci Biotechnol Biochem 65:1724–1728
Suzuki S (1996) Illustrations of Japanese Bambusaceae. Jukai Shorin, Funabashi (in Japanese)
Takafuji A, Kuno E, Fujimoto H (1996) Reproductive interference and its consequences for the competitive interactions between two closely related Panonychus spider mites. Exp Appl Acarol 21:379–391
Tanabe T, Sota T (2008) Complex copulatory behavior and the proximate effect of genital and body size differences on mechanical reproductive isolation in the millipede genus Parafontaria. Am Nat 171:692–699
Uchimura E (2005) Bamboo, bamboo grass picture book: type, features and applications. Soshinsha, Tokyo (in Japanese)
Uesugi R, Kunimoto Y, Osakabe Mh (2009a) The fine-scale genetic structure of the two-spotted spider mite in a commercial greenhouse. Exp Appl Acarol 47:99–109
Uesugi R, Sasawaki T, Osakabe Mh (2009b) Evidence of a high level of gene flow among apple trees in Tetranychus urticae. Exp Appl Acarol 49:281–290
Acknowledgements
We thank Drs. Kotaro Mori, Takane Sakagami, Ken Sahara for their valuable suggestions. We are indebted to Mr. Y. Kumekawa and Ms. K. Tamura, Drs. H. Hayakawa and Y. Minamiya for providing sampling material. This study was supported by JSPS-KAKENHI 24570014, China Recruitment Program of Global Experts (Foreign Experts) (2012-323) and State Administration of Foreign Experts Affairs Key Project for Introduction of Foreign Experts (SZ2013003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chae, Y., Yokoyama, N., Ito, K. et al. Reproductive isolation between Stigmaeopsis celarius and its sibling species sympatrically inhabiting bamboo (Pleioblastus spp.) plants. Exp Appl Acarol 66, 11–23 (2015). https://doi.org/10.1007/s10493-014-9865-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-014-9865-0