Abstract
This brief review aims to draw attention to the biotechnological potential of actinomycetes. Their main uses as sources of antibiotics and in agriculture would be enough not to neglect them; however, as we will see, their biotechnological application is much broader. Far from intending to exhaust this issue, we present a short survey of the research involving actinomycetes and their applications published in the last 23 years. We highlight a perspective for the discovery of new active ingredients or new applications for the known metabolites of these microorganisms that, for approximately 80 years, since the discovery of streptomycin, have been the main source of antibiotics. Based on the collected data, we organize the text to show how the cosmopolitanism of actinomycetes and the evolutionary biotic and abiotic ecological relationships of actinomycetes translate into the expression of metabolites in the environment and the richness of biosynthetic gene clusters, many of which remain silenced in traditional laboratory cultures. We also present the main strategies used in the twenty-first century to promote the expression of these silenced genes and obtain new secondary metabolites from known or new strains. Many of these metabolites have biological activities relevant to medicine, agriculture, and biotechnology industries, including candidates for new drugs or drug models against infectious and non-infectious diseases. Below, we present significant examples of the antimicrobial spectrum of actinomycetes, which is the most commonly investigated and best known, as well as their non-antimicrobial spectrum, which is becoming better known and increasingly explored.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
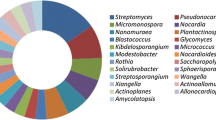
In the last century, the production of antibiotics provided exponential human and socioeconomic growth that had never been seen before (Okeke et al. 1999). However, the side effects of the misuse of antimicrobials have become a big concern in the twenty-first century in the form of difficult-to-treat infectious diseases in humans and animals, as well as biocide-resistant plant pathogens (Murray et al. 2022; Raymaekers et al. 2020). Resistance, the main side effect, has become a reality that significantly affects people's lives with regard to health and food, and resistant pathogens have caused the death of millions of people around the world, in addition to harming the production of huge amounts of animal and plant food sources every year (World Health Organization 2016; World Health Organization 2022a). In view of this, in order to mitigate antimicrobial resistance, many researchers have looked into chemical synthesis and semisynthesis as alternatives for the biosynthesis of new antimicrobials, whether inspired by nature or not. However, these alternatives have been unable to meet the increased demand for new medical and agro-industrial antimicrobials (Wright et al. 2014), which has been aggravated by the emergence of multidrug-resistant microorganisms. This has increased the search for microorganisms that can produce new bioactive substances (Hoque et al. 2022; Van Der Meij et al. 2017). Besides this, together with the resistance of pathogenic microorganisms, the growth in the incidence of diseases such as cancer and diabetes, among others, as well as the need to promote more sustainable technological alternatives (Oliveira et al. 2022), challenges us to explore to a greater extent the biotechnological potential of the actinomycetes that are present in the most varied ecosystems and which have a broad biotechnological spectrum (Fig. 1) (Azman et al. 2015; Mahajan and Balachandran 2012; Pereira et al. 2020).
In fact, the biotechnological potential of actinomycetes is far from being completely known; however, as we will see in this brief review, this potential is not only limited to antibiotics and use in agriculture, there could be many other biotechnological applications. In contrast to the rich potential indicated by the data of this review, there are very few biotechnological investigations and applications related to these bacteria, which belong to the phylum Actinobacteria. This phylum has approximately 374 genera and thousands of described species (Donald et al. 2022). The genus Streptomyces can be highlighted for having the greatest number of species and considerable reports of microbial natural products (MNPs). In addition, from the 1940s, this genus caused the rise in research involving actinomycetes, which began with a triumphant entry into the group of genetic resources that produce MNPs. The person responsible for this feat was Dr. Selman Waksman, who, while studying soil microorganisms, found Streptomyces griseus, from which he isolated streptomycin, which is an efficient bactericide against one of the greatest executioners of humanity: Mycobacterium tuberculosis. This feat earned his group the Nobel Prize for Medicine in 1952 (Schatz et al. 1944; Waksman and Woodruff 1940; Woodruff 2014).
From the discovery of streptomycin up until 2010, actinomycetes were responsible for 42% of the more than 23,000 MNPs discovered, most of these being antimicrobial, thus equaling the proportion of MNPs produced by fungi (Kekuda et al. 2010), a much larger group in terms of species. Recently, more than 15,000 MNPs originating from strains of the phylum Actinobacteria have been estimated, many of which showed activities outside the antimicrobial spectrum (Table 1) (Lacey and Rutledge 2022; Quinn et al. 2020; Wang et al. 2022; Yan et al. 2022). This extraordinary ability of actinomycetes to produce MNPs is consistent with their cosmopolitan nature, and the fact that, through evolution, they have adapted to the most diverse environments, from the mildest to the most extreme. This originates, among other causes, in the ecological relationships that actinomycetes have with other organisms, notably the plants, colonizing them in practically all tissues in a harmonious or pathogenic way, as well as in competitive relationships in soils and aquatic environments, fighting battles with other microbes armed with the most diverse metabolites (Al-Ansari et al. 2020; Janardhan et al. 2014; Kokkini et al. 2022; Nalini and Prakash 2017; Van Bergeijk et al. 2020).
In the exploitation of this wealth of metabolites, in general, within the universe of actinomycetes, new isolates stand out for presenting new activities in in vitro and in situ bioassays (Akshatha et al. 2014; Martinet et al. 2019) In parallel, several genomic and metabolomic studies have revealed a wealth of MNPs hidden in the genomes of several well-known isolates, as well as in newly discovered isolates, thus leading to the emergence in the twenty-first century of a new generation of bioactive substances (Challis 2008, 2014). Among the examples of successful metabolomic approaches, one of the most used for putatively targeting those new metabolites is the Global Natural Products Social Molecular Networking (GNPS), with its numerous tools and public spectrometric databases (Atanasov et al. 2021; Baskiyar et al. 2022; Xu et al. 2019; Wang et al. 2016).
Widely portrayed as sources of bioactive metabolites for various medical and agro-industrial applications, actinomycetes are still chemically very little known. Most related works only report the activity of the actinobacterial extracts but are inconclusive whether their bioactive compounds would be known, similar to known, or new. Furthermore, for medicines, it is not enough to identify the substances. Several studies are still needed to confirm its therapeutic viability as a new drug, including preclinical and clinical tests with animals and humans. Among the trials, it is critical to determine the patterns of absorption, distribution, metabolism, excretion, and chemical toxicity (ADMET) of a potential new drug. Unfortunately, about 50% of bioactive natural metabolites do not achieve good results in ADMET, which makes them unfeasible as chemotherapies and generates considerable wear (Cai et al. 2023; Guan et al. 2019; Ouyang et al. 2021).
In this context, our main objective in this brief review is to provide the reader with a survey of the research that has been published in the last 23 years involving actinomycetes and their applications, highlighting a perspective for the discovery of new active ingredients or new applications for the known metabolites of these microorganisms. As criteria for the bibliographic survey, priority was given to scientific publications from the last 23 years reporting biological activities and isolation of substances from actinomycetes. The databases consulted were Web of Science, Springer Nature, Elsevier, PubMed, and Google Scholar. Keywords selected were actinomycetes and antibiotics; anti-bacterial; antifungal; antiparasitic; antimalarial; anti-inflammatories; diabetes; Alzheimers; antileishmaniasis; antitrypanosomal; antiviral; biocontrol; fertilizers; antiacetylcholinesterase; biofuel. Each keyword was individually combined with the word actinomycetes in all the databases accessed. Based on the data collected, we considered: the cosmopolitanism of actinomycetes in relation to the expression of metabolites in the environment and the limitations and alternatives for the synthesis of MNPs in the laboratory; the most commonly targeted antimicrobial spectrum; and the increasingly explored non-antimicrobial spectrum. In the conclusion, we point out some practical issues with the aim of drawing the attention of people and interested entities to the great biotechnological potential of actinomycetes in various fields and the need to explore and preserve the environments that are still little explored.
Cosmopolitanism and environmental expressions vs. laboratory expressions of MNPs from actinomycetes
Actinomycetes are mostly free-living and polynutritional (Djebaili et al. 2021; Van Bergeijk et al. 2020). Their cosmopolitanism shows their ability to establish complex ecological relationships in different environments and with other living beings, a presumable consequence of their evolution that gives them an extraordinary capacity to produce primary and secondary metabolites. This capacity seems more evident when one considers the genomes of these microorganisms since they code approximately 800 proteins involved in various ecological functions. Most are hydrolytic enzymes, cellulases, chitinases, and proteases, which give them an incomparable arsenal to exploit any ecosystem (Bentley et al. 2002; Van Bergeijk et al. 2020). The influence of the ecological relationships of actinomycetes on their metabolism can be understood through chemical and genetic studies, which allow us to interpret their interactive signals via expressions of primary and secondary metabolites and discover the groups of genes (or biosynthetic gene clusters – BGCs) involved in the synthesis of MNPs (Fig. 2) (Rutledge and Challis 2015; Van Bergeijk et al. 2020; Javed et al. 2021). Below are some examples of the adaptability of actinomycetes to different environments and their complex ecological relationships that cause the expression of MNPs that can be used for various purposes. There are also examples of successful laboratory cases of the use of silenced genes of actinomycetes for the expression of new metabolites.
Methodological strategies for prospecting MNPs. A The microorganism is isolated from any nature environment; B Its DNA sequences and metabolites are obtained under appropriate conditions; C Metabolites are prospected by genomic mining and confronting the mass spectra with databases (for example, GNPS). For more details see the main text
Examples of the production potential of natural products by actinomycetes in different ecological relationships
As with our own organism, through evolution, microorganisms have adapted to different environmental and food conditions by utilizing their genetic arsenal to produce appropriate enzymes and MNPs. Presumably, coevolution with other living beings under the most diverse and often adverse conditions has enhanced this arsenal and its metabolic expression. The known specificity of this metabolic expression to different environmental stimuli suggests the need to collect actinomycetes from different habitats. As we will see in the examples below, this potentiates the discovery of different strains with different productions of MNPs.
Rhizosphere – In a given area of soil, the rhizosphere is the most nutritious and most biodiverse region, as it is filled with a nutritious exudate, which is disputed by actinomycetes, fungi, protozoa, and eubacteria (Lugtenberg 2015). In this chemical warfare environment, when fed by exudate, actinomycetes compensate vegetables with protection through the secretion of antimicrobials, nitrogen fixation, production of growth hormones and enzymes capable of metabolizing complex carbohydrates (Djebaili et al. 2021; Ujváry 2010). Protection against phytopathogens (biocontrol) and stringency conditions may have induced evolution to the point that there is communication between actinomycetes and plants, which occurs through inorganic and organic signaling (Djebaili et al. 2021; Javed et al. 2021; Ujváry 2010; Van Der Meij et al. 2017; Van Bergeijk et al. 2020; Van der Ent et al. 2009). Thus, in the rhizospheric exudate, many chemical distress signals can be emitted, such as the plant-stress hormones jasmonic acid and salicylic acid, which stimulate the secretion of antibiotics by actinomycetes (Van Der Meij et al. 2018; Van Bergeijk et al. 2020).
Insects – The class Insecta Linnaeus, 1758, a rich source of biodiversity, may harbor an even greater richness in actinomycetes and their metabolites. This is what can be deduced from studies such as that of Matarrita-Carranza et al. (2017), who evaluated the influence of these bacteria within the order Hymenoptera, which has more than 150,000 species of insects (Aguiar et al. 2013). From the 29 tropical species studied, including those of the families Apidae (bees), Vespidae (wasps), and Formicidae (ants), 197 cultivable actinomycetes were isolated, whose bioassays indicated the potential antimicrobial activities of their metabolites. There is an apparent mutualistic association in which bacteria, being fed, establish a defense mechanism against microbial pathogens in favor of the host insects (Hanshew et al. 2015; Huang et al. 2020).
Marine sponges – Equally exciting are the studies in search of knowledge regarding actinomycetes that interact with organisms in marine environments. These studies have resulted in the discovery of new candidates for producers of MNPs of biotechnological interest. This is the case of the actinomycetes from sponges, which are organisms that filter and decompose particles, and are of extreme relevance to our planet. They are also natural hosts of various groups of recycling microorganisms (Balskus 2014). This relationship of high complexity and adversity should certainly stimulate the production of a varied range of bioactive compounds. An important example in this sense is given by Cheng et al. (2015), who studied the diversity of 12 species of sponges from the Mediterranean Sea and isolated 64 actinomycetes, eight of which showed antiprotozoal activity for Trypanosoma brucei (TC221). In other studies, with actinomycetes isolated from marine sponges, Nagarajan et al. (2015) and Santos et al. (2015) detected antibacterial, antifungal, and anticancer activities for the metabolites produced by the studied strains.
The previous examples reveal that actinomycetes in different origins can present varied biotechnological potential—antibiotics for plant defense, antipathogens for insect protection, and antibacterial, antifungal, and anticancer metabolites associated with marine organisms. In perspective, the most diverse environment on the planet can host the most different actinomycetes with inestimable wealth in bioactive metabolites. However, many metabolites with potent medicinal and agroindustrial properties are not produced in laboratory conditions because these do not provide the physical and chemical stimuli of the original accessed ecosystems. Thus, as we will briefly discuss in the following sections, several studies have sought to understand the molecular systems involved in the activation of synthesis pathways and have revealed the limiting factors for the discovery of new MNPs.
Limiting factors for the synthesis of MNPs via cultivation in the laboratory
Recent reviews highlight the limitations of the current culture media and isolation methods that are available for actinomycetes (Donald et al. 2022; Hemmerling and Piel 2022). In laboratories, culture media lack complexity and are devoid of the environmental biological, chemical and physical stimuli necessary to activate biosynthetic gene clusters (BGCs) and thus promote the synthesis of microbial natural products (MNPs). For example, in the laboratory, how can one reproduce the environmental conditions of the diversity of marine actinomycetes, which are subject to great nutritional variations, of symbionts (animals and algae) and of temperature and pressure, without counting their diverse origins, as in the reported case of spores of Streptomyces strains transported from soils via river runoff to the bottom of the oceans, where they are subject to evolutionary pressure distinct from terrestrial pressure (Moran et al. 1995). Equally difficult to reproduce are the conditions to which microbial strains from nutrient-poor sites such as hot springs and oligotrophic Antarctic and desert soils are subjected to, as well as endophytic strains that lose their original phyto-communication and interaction with the host plant microbiome. All these factors are evolutionary agents that regulate the secondary metabolism and chemodiversity of actinomycetes and, although laboratory conditions are very different from environmental ones, strategies have been developed to increase the amount of MNPs produced by these bacteria. Strategies such as thermal and electric shocks, nutritional adaptations of culture media with the addition of environmental substances that have a stimulating effect and the heterologous expression of BGCs from genomes of non-cultivable strains, among others, have favored discoveries of new MNPs (Donald et al. 2022; Hemmerling and Piel 2022), mainly in marine prospections (Tenebro et al. (2021).
Actinomycetes and MNPs eliciting agents
The proposal of nutritional, physical, chemical, and biological agents for stimulation has become a promising strategy for the synthesis and discovery of MNPs, especially when combined with the knowledge of the genomic diversity of the biosynthetic gene clusters (BGCs) of a strain, as will be seen in the next section. An excellent example of the influence of nutritional stimulation on the synthesis of MNPs occurred via the limitation of carbon and nitrogen in cultures of Saccharopolyspora erythraea and Sc. hygroscopicus (Wilson and Bushell 1995), which resulted in increased production of the macrolide Erythromycin and coincided with the accumulation of tRNA and amino acids and the attenuation of protein synthesis. These results seem to reveal a typical situation of competition for nutrients, with a consequent need for territory protection that must occur in natural conditions.
An interesting stimulation strategy consists in disrupting the metabolism of strains with sub-inhibitory concentrations (SICs) of antibiotics, which leads to the stimulation of higher concentrations of other antibiotics and awakens cryptic BGCs involved with MNPs syntheses. For example, the SIC (of 5 µg/mL) of chloramphenicol – inhibitor of protein synthesis in prokaryotes – increased the synthesis of the antibiotic actinomycin (calcium-dependent synthesis) and piperidamycin in S. albus, while the SIC of the synthetic ARC2 (an analogue of triclosan) – which partially inhibits the synthesis of fatty acids – also increased the synthesis of polyketide antibiotics in S. albus (Tanaka et al. 2017). In another example, the marine strain Streptomyces sp. HB202 (Halichondria panicea), under the effects of SICs of tetracycline or bacitracin, produced several compounds from the group of phenazines named streptophenazines A-H. Streptophenazines C and H showed activities against Bacillus subtilis, while C was also active against Staphylococcus lentus (Mitova et al. 2008).
In another approach using exposure to natural or synthetic chemical compounds, the strain Micromonospora kermanensis DSM 45485 was subjected to alkaline pH and the individual influence of valproic acid, dimethyl sulfoxide (DMSO), lanthanum chloride, triclosan, and of the culture supernatant of Pseudomonas aeruginosa UTMC 1404, and showed gram-negative biological activity (Mohammadipanah et al. 2020). Considering that in these tests the culture medium (ISP2) was the same in the presence and absence of stimulants and that, in the absence of these, biological activity was not observed, this example reveals possible space defense behavior in response to chemical attacks, which in the environment may mean the presence of hostile organisms. In another case, when trying to stimulate S. hygroscopicus to increase the production of the macrolide ascomycin (FK520), a potent antifungal and immunosuppressant, various chemicals at low concentration levels were used as stimulants. Among them, the chemical compound DMSO, used as a carbon source, stood out for doubling the production of ascomycin (FK520) (Wang et al. 2019b).
As actinomycetes are ubiquitous, they carry in their genomes the evolutionary marks of their experiences in communities and diverse environments of the Earth recorded in their DNA, especially in clusters of active or cryptic biosynthetic genes (BGCs). A good biological mechanism for stimulating cryptic BGCs is co-cultivation of two strains from the same environment. This was demonstrated in experiments of co-culture of S. luteireticuli NIIST-D31 with S. luteoverticillatus NIIST-D47 and resulted in the synthesis of new stereochemical variants of streptophenazine (S1 and S2) and 1-N-methylalbonoursin, and with S. thioluteus NIIST-D63, resulting in new streptophenazines and again in 1-N-methylalbonoursin (Induja et al. 2023). Moreover, in co-cultivation of Streptomyces venezuelae and Saccharomyces cerevisiae with an abundance of glucose, S. venezuelae produced the volatile trimethylamine (Jones et al. 2017).
Finally, there are also examples of metallic chemical elements, including rare earths and heavy metals, as stimulants of the synthesis of MNPs by actinomycetes. Thus, the syntheses of dactinomycin, actinomycin and streptomycin by S. antibioticus, S. parvulus and S. griseus were increased when the culture media were supplemented with scandium (Sc3+) (Zong et al. 2022). In another case, when supplemented with nickel, a marine strain of Streptomyces produced angucycline (Zong et al. 2022). All of the examples above seem to reveal that, in their evolution, the strains of actinomycetes were storing a powerful arsenal of chemical weapons in the form of genes or BGCs. This arsenal has made them uniquely prepared to survive numerous environmental adversities, including competition for space and food, attacks by other organisms, exposure to toxic chemical substances or elements, and abiotic environmental changes. This same arsenal could be the solution to numerous problems involving human health and agriculture, among other biotechnological possibilities. In this perspective, we will see below that the uses of stimulants have been improving and more robust techniques are being used in approaches that aim to improve the discovery of MNPs.
Genomic and metabolic mining for production of MNPs
Despite understanding some of the mechanisms of activation of BGCs, it is still a challenge to overcome their complexities, since many pathways share several enzymes (Craney et al. 2013). As we have seen, when isolating a microorganism from a given ecosystem, we limit them to laboratory conditions, and these do not provide the same extracellular signals of its former environment with all the abiotic and biotic challenges it had to overcome to survive, possibly causing it to lose its genuine production of active ingredients. However, through genomic studies, it is possible to quantify the BGCs in the DNA of a microorganism and estimateits potential ability to produce known and unknown MNPs. Let’s look at three examples: in the first, in a study that analyzed 1,110 genomes of Streptomyces, 34 main classes of BGCs were found among the strains, which presented a variety of 8 to 83 BGCs, with the predominance of NRPS (1,062 genomes), PKS1 (981 genomes), terpenes (697 genomes), lantipeptides (540 genomes), butyrolactone (503 genomes), pks2 (499 genomes), bacteriocin (419 genomes), and Pks3 (366 genomes) (Belknap et al. 2020). According to the authors, assessing whether the quantities of important copies of BGCs (e.g., NRPS and PKS) and their distributions in genomes are associated with variations in the syntheses and biological activities of MNPs is fundamental. In the second example, the distributions of BGCs linked to the metabolic pathways PKS and NRPS were investigated in the genomes of 75 strains of Salinispora arenicola (37), Salinispora tropica (7), and Salinispora pacifica (31), in which 1,924 KS domains and 1,693 C domains were found that represent enzymes related to these pathways (PKS and NRPS) in this exclusively marine genus (Ziemert et al. 2014). The authors estimated that this high diversity was acquired through horizontal gene transfer and noted that it focuses on strains in what they called genomic islands of BGCs that can change position. This dynamic of position change is still poorly understood but it has a great influence on the synthesis of MNPs. Finally, in the third example, in strains of Amycolatopsis spp. from different geographical regions, it is observed that they carry between 14 and 45 BGCs, with a predominance of PKS, NRPS, hybrid, RiPP and terpene (Adamek et al. 2018).
All of the above examples reveal only part of the operation of an approach aimed at rationally combining genomic mining with metabolomics. Figure 2 shows the main steps of these two fruitful combined methodologies utilized in recent years to prospect MNPs. After isolating the microorganism from nature (Fig. 2A), its DNA sequences and metabolic extracts are obtained. The production of bioactive metabolites can be induced, for example, by exposition to antimicrobial substances or by competitive cultivation with target pathogens (Fig. 2B). Finally, the MNPs are prospected by genomic mining and comparison of the mass spectra of components of the microorganism extracts with databases (for example, GNPS) (Fig. 2C).
Gene mining is widely used and fundamental for genetic engineering, which has numerous ways of encoding the products of silenced pathways. In a recent study, Cheng et al. (2023) identified a gene cluster (mich BGC) relative to benzoxazole alkaloids in the strain of Micromonospora sp. SCSIO 07395. The heterologous expression of the mich BGC gene in S. albus Del14 resulted in five new alkaloids, the microechmycins A–E, among which the microecmycin A demonstrated moderate antibacterial activity. On the other hand, the analysis of data obtained via LC-MS1/MS2, using the GNPS platform, has also been a powerful tool for the dereplication of extracts and the discovery of new metabolites. (Atanasov et al. 2021; Baskiyar et al. 2022; Xu et al. 2019; Wang et al. 2016). Although the chemical study of an extract by conventional metabolite identification and purification techniques can result in bioactive metabolites, the genomic quantification of BGCs, combined with the bio-guided and rapid characterization of metabolomic profiles, allows us to evaluate and optimize the acquisition of active ingredients and other promising substances from microorganisms (Gohain et al. 2015; Moon et al. 2019), as can be seen in the following examples.
Gohain et al. (2015) studied the microbial diversity of six Indian medicinal plants and obtained 76 actinomycetes, with a prevalence of the genus Streptomyces. According to the authors, 21 of the isolates presented activities in biological assays against fungal and bacterial pathogens. In addition, 85% were detected producing bands for the BGCs polyketide synthase (PKS) type-II and 14% for PKS-I. The characterization and quantification of silenced BGCs allows them to be cloned and expressed in model organisms. For example, Qian et al. (2019) sequenced the genome of Streptomyces sp. (Tü 6314) and found a cryptic BGC PKS type II (skt), then cloned it using the Streptomyces pSET152 vector. Via heterologous expression in S. coelicolor, they managed to produce six polyketides, of which four showed activities against the HIV1 virus.
With the same purpose of taking advantage of the potential of silenced genes, among the other methodologies used to activate these genes, the methodology called HiTES (high performance eliciting screens) has been used. In summary, it combines the use of a reporter gene that is integrated in the vicinity of the silenced BGCs and analyzes the effect of hundreds of substances, which are candidates for eliciting the metabolites associated with these cryptic genes. Any substance added individually to the culture in wells of 96-well plates that has the desired effect, signaled by the significantly increased response of the reporter gene in the modified actinomycetes, relative to the control (a well without any testing substance added), is then used as an inducer in a larger scale culture. This methodology ideally leads to the production of new metabolites from the genes that were silenced and were awakened by it (Xu et al. 2017). Using this methodology, these authors achieved the expression of 14 new products from S. albus (J1074), which included a novel antifungal and a cancer cell multiplication inhibitor.
The same group led by Professor Mohammad R. Seyedsayamdost, using the modified HiTES methodology they called “Bioactivity-HiTES” (Moon et al. 2019), detected cryptic antibiotics in three lineages of actinomycetes. The bioactivity-HiTES methodology was described as similar to the previous one, in that actinomycetes are grown in 96-well plates in the presence of a library of tens or hundreds of natural substances. Subsequently, the media cultured by each bacterium in the individual presence of each candidate elicitor substance were tested for bioactivity. In this case, they were tested for the inhibition of Gram-negative bacteria. As a result, the authors discovered two cryptic antibiotics against Escherichia coli and Acinetobacter baumannii, as well as a new naphthoquinone epoxy. The advantage of this new approach is that it dispenses any genetic manipulation of the strains of actinomycetes, thus saving resources and time.
Since the first decade of the twenty-first century, ever-increasing genome mining approaches seem to have overcome the most optimistic previsions that could be made about the incalculable natural products to be discovered from actinomycetes. In such approaches, the analyses of gene sequences frequently result in the discovery of many “orphan” biosynthetic pathways (Challis 2008). In addition to the aforementioned papers, in recent years, there have been a number of reviews on innovations and studies regarding the biosynthesis of new MNPs, among which those of Craney et al. (2013), De Simeis and Serra (2021), Gomez-Escribano et al. (2021), Gong et al. (2021), Jiang et al. (2018), Li et al. (2021), Wu et al. (2021) and Zhang et al. (2022) stand out. In general, all the above reports reveal the gene mining approaches as the most promising and powerful tools to address the continuous challenge of finding new bioactive metabolites, especially antimicrobials. Table 1 portrays some of the most recent studies and discoveries of new groups of bioactive natural products. Many of these discoveries were made using metabolomics via the GNPS platform, genomic mining of BGCs, or both approaches together (Le Loarer et al. 2023).
Antimicrobial spectrum
Cultivable and non-cultivable actinomycete strains can be found in soils or other environments ranging from oligotrophic ones to copiotrophic ones, from acidic to basic pH, from dry to flooded locations, and from high to low temperatures, among other factors. In their genomes, these strains carry BGCs and the epigenetic marks of ecological pressures that permeate their primary and secondary metabolic activities. Many of these ecological pressures come from forced coexistence with other microorganisms, often in hostile situations. This is not without reason since, historically, the main application of actinomycete metabolites has been within the antimicrobial spectrum, where it still has its greatest biotechnological importance. Below, we will see several examples from the last decades that consolidate the potential of actinomycetes and project them as fundamental sources of metabolites for the continuous need to combat human pathogens and agriculture, especially drug-resistant or multidrug-resistant strains.
Antibacterials
Microbial resistance to antibiotics (MRA) has claimed the lives of thousands of people in recent decades (Stephens et al. 2017; Alvarez-Uria et al. 2018). According to annual surveys by the World Health Organization that began in 2015, such a public health problem is a major threat to humanity because of the emergence of multi-drug resistant (MDR) strains (Exner et al. 2017; World Health Organization 2016; World Health Organization 2021). As examples, there are reports of multidrug-resistant strains of E. coli, the main causes of infant deaths from diarrhea and septicemia (Stephens et al. 2017). There are also records of multidrug-resistant strains of Pseudomonas aeruginosa, Proteus mirabilis, Klebsiella pneumoniae, and Enterobacter sp., which are the most frequently responsible for infections in people in intensive care units (ICU), and these may progress to septicemia (World Health Organization 2022a). In summary, resistance to antibacterials can be represented by the three most successful and understood mechanisms: (I) uses of efflux pumps, plasma membrane structures that expel them into the extracellular environment; (II) by the actions of cytosolic enzymes that metabolize them; (III) and through target alterations, which is another way to circumvent the actions of these drugs (Fisher et al. 2022).
The appeal is an urgent one since the global scenario resembles a pandemic condition because of the rapid spread of MDR strains. There have been several studies that have sought to explore the biotechnological potential of actinomycetes and present new candidate isolates for bioactive producers of MNPs via screening using the method of co-cultures with MDR strains. In one of these studies, isolates from Egyptian soils (identified as S. griseus, S. flaveolus, and other actinomycetes) showed themselves to have the potential to be effective against MDR bacterial and fungal pathogens (Elbendary et al. 2018). In another study, among 100 strains of endophytic actinomycetes from seaweed (Caulerpa racemosa) that were tested, five showed antagonist activities for gram-negative MDR bacteria, which are considered to be among the most virulent pathogens because they have a thicker cell wall (Rajivgandhi et al. 2018). While many examples like these remain to be confirmed or have had the active ingredients identified, they signalize substances capable of circumventing the resistance mechanisms of MDR microorganisms and reveal that new classes of antibiotics with different mechanisms of action may be possible.
As an example of the potential of the marine environment to be a source of new antibiotics, a new antibiotic, namely desertomycin G, was produced by S. althioticus MSM3 isolated from the macroalgae Ulva sp. (Braña et al. 2019). This substance stands out for its spectrum that is extended beyond bactericidal activity against M. tuberculosis, and presents activity against gram-positive and negative pathogens, and acts on cancer cells of the human breast adenocarcinoma (MCF-7) and colon carcinoma (DLD-1) lineages. Active ingredients that present these characteristics are of great relevance, since people who perform cancer treatments are more prone to infections and such substances can help prevent infections.
The studies above are just some of the examples of screenings carried out and published in several papers in recent years and emphasize the antibacterial and biotechnological potential of actinomycetes isolated from marine sponges (Almaary et al. 2021), soils (Rajivgandhi et al. 2018), vegetables (Tanvir et al. 2016), and mangroves (Dasgupta et al. 2015). Although reports like these reveal only a small fraction of an entire universe that is still little explored, it is exciting to find isolates that are so promising and capable of knocking out numerous pathogens with MDR strains and MRA, which give us hope that it will be possible to minimize the numerous deaths in hospital settings (Ahmad et al. 2017; Elsayed et al. 2020). On the other hand, the urgency and continued need for new drugs to face resistant-to-drug microorganisms seem to require more and improved research on the promising Phylum of actinomycetes, among other potential solutions.
Antifungals
As with pathogenic bacteria, in recent decades, fungal resistance and the emergence of MDR strains has been increasing alarmingly worldwide (Benedict et al. 2022). Pathogenic fungi are opportunists and the deadly enemies of immunosuppressed people; every year since 2012, at least 1.4 million deaths of people who are victims of fungal infections have been recorded (Benedict et al. 2022; Brown et al. 2012). Undoubtedly, Candida albicans, C. auris, C. glabrata, and C. tropicalis are the main culprits for these infections, a fact aggravated by the emergence of strains that resist at least two or more antifungals of the azole and polyene classes, and one of the echinocandins (Benedict et al. 2022; Chowdhary et al. 2017; Pristov and Ghannoum 2019). It is not without reason that one of the main disease control bodies, CDC (Centers for Disease Control and Prevention), emphasizes the urgent need for new antifungals in its recent warning to the scientific community about the increased lethality of fungal infections due to the rapid emergence of MDR strains (Zhang et al. 2022).
This global scenario has driven the prospection of new genetic resources that produce MNPs that can increase the arsenal of available antifungals (Pristov and Ghannoum 2019). In one of the first prospections at the beginning of the twenty-first century, in a screening of 320 actinomycetes from Moroccan soils for antifungal activities, 23 showed strong activities against C. tropicalis R2 and Pythium irregulare (the latter resistant to amphotericin B and nystatin) without interference in ergosterol synthesis, i.e., with non-polyenic and azoe mechanisms of action (Ouhdouch et al. 2001). Resistance to polyenes and azoes occurs when drugs of these classes do not inhibit the synthesis of ergosterol or do not have affinities for it in the cell membrane after modification (Pristov and Ghannoum 2019). Furthermore, ergosterol is very similar to human cell cholesterol and this is a limiting factor for these classes (Bhattacharya et al. 2020). As an alternative to ergosterol synthesis inhibitors, synthetic substances have been used, such as echinocandins, a class of antifungals that inhibit the synthesis of the fungal cell wall, a structure that is not seen in human cells. Another lesser-known class that does not inhibit ergosterol synthesis is that of flucytosines, among them 5-flucytosine (5FC), which acts by inhibiting nucleic acid synthesis in fungi (Bhattacharya et al. 2020; Pristov and Ghannoum 2019).
Despite the relative efficacy of echinocandins and flucytosines, there are several other studies that have sought natural antifungals, preferably not inhibitors of ergosterol synthesis, as in the screening of actinomycetes by the Moroccan researchers mentioned in the previous paragraph. Another important finding was the discovery of turbinmycin, produced by the actinomycete strain Micromonospora sp. (WMMC-415), which was isolated from the sea sponge Ecteinascidia turbinata and is a promising active ingredient that suppresses one of the most resistant fungal pathogens, namely C. auris, as well as Aspergillus fumigatus (Zhang et al. 2020). In a differentiated mechanism of action, turbinmycin targets a cytosolic protein, Sec14, which is indispensable for fungi and is involved in the intracellular transport of substances produced in the endoplasmic reticulum.
In comparison to screening that seeks new antibacterials, the amount of prospection carried out for new antifungals is much lower; however, several studies and reviews have speculated on many candidate actinomycetes as potential producers of new fungicides against the MDR strains of C. albicans, C. auris, C. glabrata and C. Tropicalis, for example (Alkhalifah 2021; Liu et al. 2019b). The severity of fungal diseases, the continuous emergence of drug-resistant strains, the difficulty arising from fungal cells being eukaryotic, like ours, and the antifungal potential of actinomycetes, make them natural targets in the search for new antifungal models, ideally capable of attacking only fungal cells and not human ones.
Antivirals
Recently, for about three years, the world experienced a new pandemic triggered by the severe acute respiratory syndrome—Coronavirus 2 (SARS-CoV-2), which, up until 2022, claimed more than 6 million lives (Bharati et al. 2022). It is an exception, but respiratory syndromes and influenza are serious public health problems and are caused by the most prevalent viruses (World Health Organization 2022b)). One of these viral diseases, syncytial virus syndrome, affected about 33.8 million children under 5 years of age, with approximately 3.4 million severe cases and 199,000 deaths in 2005 (Nair et al. 2010). According to data from the World Health Organization, millions of people live with viral hepatitis (types: A, B and C), which causes millions of deaths every year. The development of recidivism of pathogenic viruses and the increase of emerging viruses due to lack of basic sanitation, malnutrition and climate change are expected (World Health Organization 2022b).
The development of antivirals is much slower compared to that of antibacterials and antifungals because immunization or vaccination is the preferred method for preventing or containing these types of pathologies. However, the pandemic caused by SARS-CoV-2 showed us the need for emergency drugs, and computational methods were very important in these circumstances since they enabled in silico assays using known substances. In one case using this method, 50 bioactive compounds isolated from mangrove actinomycetes were tested against the NSP10-methyltransferase of the etiological agent of SARS-CoV-2 (Muhammad et al. 2022). Among those substances, sespenine, xiamycin-C, xiamycin-D, xiamycin-E, xiamycin-methyl-ester, and xiamycin-A showed the greatest neutralization capabilities of this major enzyme for the replication cycle of this virus. In fact, the need for antivirals for widespread diseases predates COVID-19. In a recent example, in 2013, the American continent experienced the Zika virus (ZIKV) epidemic that affected millions of people, some of whom developed Guillain-Barré syndrome and microcephaly (Saiz and Martín-Acebes 2017). On this occasion, numerous bioactive isolates of actinomycetes were tested in silico and some, such as daptomycin and nanchangmycin, showed strong activities against the causative agent, though previously these had not been described for the treatment of this virus (Barrows et al. 2016; Rausch et al. 2017).
In this challenging context for the development of new antivirals, there are findings that have brought hope to people affected by HIV-1 viruses. Studies of the isolate S. albosporus resulted in the isolation of N,N,N-(trimethylated)-Tyr-L-Leu-L-Val-L-Leu-(dehydrated)-His, which is able to inhibit the protease-HIV-1 that is essential for the cycle of this pathogen (Liu et al. 2012b). Similarly, ahmpatinin-iBu from Streptomyces sp. (CPCC202950) was found to be a potent inhibitor of the same HIV-1 protease (Chen et al. 2018). On the other hand, 3-acetyl-5-methyl-2'-deoxyuridine, derived from S. microflavus, was active in bioassays against hepatitis B virus, herpes simplex type 1 and 2 (HSV-1 and HSV-2) and varicella zoster virus (VZV) (Li et al. 2011).
In the studies mentioned above, we see examples of the still little explored potential of actinomycetes for the production of antivirals, and in silico assays for the screening of substances with this potential. However, in silico assays allow us to only preliminarily observe the affinity of a substance for a target and this is not enough for emergency uses – since other experiments, including those involving the pharmacokinetics and pharmacodynamics of an active ingredient, are necessary in order to establish its real efficacy as well as the correct dosage, among other factors. As an example, while having been indicated by in silico assays for SARS-CoV-2, hydroxychloroquine and azithromycin were not effective against the COVID-19 desease (Braz et al. 2020). Viral diseases have already killed an incalculable number of people, and the most efficient weapon is vaccines; however, seeking new drugs that help in the treatment of viruses is essential in order to reduce mortality, and actinomycetes have already demonstrated their ability to provide MNPs that can help in the treatment of numerous viruses.
Antiparasitic
Of the parasitic diseases, malaria is one of the most devastating and deadly. The African continent is the most affected by this disease and approximately 96% or 627,000 deaths from the disease occurred here in 2020 (Chan et al. 2022). Around the world, the vectors of malaria are the mosquitoes from the genus Anopheles, including An. gambiae, An. coluzzii, and An. arabiensis, which transmit the protozoa Plasmodium vivax, P. falciparum, P. malariae, P. ovale, and P. knowlesi (Barney et al. 2022).
P. falciparum is the one that causes the greatest concern, since it is the most incident and responsible for the most aggressive form of this parasitosis, in addition to presenting strains that are resistant to current antiplasmodial drugs (Sissoko et al. 2017; World Health Organization et al. 2022b). In addition to malaria, which is an endemic parasitosis in tropical and subtropical regions, other protozoan-transmitted diseases, such as leishmaniasis and trypanosomiasis (Chagas disease), are public health problems in most of these regions because of antimonial resistance. When not treated correctly, they can progress to more serious conditions and lead to death (Davies-Bolorunduro et al. 2021). Similarly, and also very serious, Entamoeba histolytica infections affected approximately 50 million people in 2012 alone, of which 100,000 died. In a recent review, amoebiasis is already considered the third most deadly parasitosis worldwide, especially in underdeveloped countries in Central/South America, Africa, and Asia due to poor basic sanitation. With an average of 70,000 deaths in recent years, this parasitosis can affect several organs in our body, which can lead to a more serious condition (Jasni et al. 2022).
Tests of the crude extract of S. canus (N25) showed the best antiprotozoal activities for P. falciparum, in addition to also inhibiting the protozoan Toxoplasma gondii, the causative agent of toxoplasmosis. Phenazine-1-carboxylic acid, which is capable of inhibiting these pathogens, was identified in the fractions (Pagmadulam et al. 2020). Staurosporine (STS) and 7-oxostaurosporine (7OSTS), isolated from S. Sanyensis, showed excellent antiparasitic activities against Leishmania amazonensis, L. donovani, as well as T. cruzi and T. brucei (Cartuche et al. 2020), which are the causative agents of Chagas disease and sleeping sickness. In another study, these protozoa were neutralized in vitro by actinoallolide-A from Actinoallomurus fulvus (MK10-036) (Inahashi et al. 2015). Regarding infections caused by E. histolytica, noting the resistance and toxic effects of drugs, a study of extracts of marine actinomycetes yielded echinomycin-A and tyrandamycin-A, which showed strong antiamebiasis activities and reduced the growth of E. histolytica (HM1:IMSS) and E. histolytica (Col) by 84.2% and 64.8%, respectively (Espinosa et al. 2012). To date, several studies have highlighted numerous MNPs with the capacity to suppress numerous protozoa and their resistances, among which several isolates from actinomycetes, as shown in the review by Estrella-Parra et al. (2022). Since the beginning of the twenty-first century, many studies have been published reporting screening with isolates from actinomycetes, which, among other activities, showed antimicrobial activities. Although actinomycetes are natural targets in the search for new antimicrobial models, there is an urgent need for an investigation of the biological activities of recently isolated promising strains, since most studies end in preliminary stages without the substances responsible for the activities becoming known.
Non-antimicrobial spectrum
Anticancer
The field of medicine faces difficulties in treating various types of cancers. This disease has claimed millions of lives worldwide and is characterized by the emergence and proliferation of abnormal, aggressive, and invasive cells (Pimentel et al. 2011; Sung et al. 2021). Data from the beginning of the twenty-first century show that the global cancer burden in 2000 reached 10 million new cases and 6 million deaths, with 22 million people living with cancer in this period (Parkin 2001). Several projections of the time predicted that these numbers would increase or even double, with the need for more studies in search of new producers of anticancer drugs (Rahib et al. 2014; Sung et al. 2021). Recently, these projections were confirmed; for example, in a survey of data from 2018, a significant increase in annual incidence was observed, with general estimates of 18.1 million new cases and the occurrence of 9.6 million deaths that year (Ferlay et al. 2019). By 2020, these figures reached 19.3 million new cases, with female breast cancer (11.7%) surpassing lung cancer (11.4%), followed by colorectal cancer (10.0%), prostate (7.3%), and stomach (5.6%) cancer (Sung et al. 2021; World Health Organization 2022b).
Many isolates from actinomycetes are promising in producing active ingredients with antimicrobial and anticancer capabilities. As an example, among 41 endophytic isolates from Streptomyces spp., 31.7% showed cytotoxic activities against the cancer cells A549 (lung), 29.3% for HL-60 (blood), 85.4% for BEL-7404 (liver), and 90.2% for P388D1 (blood), with seven standing out as the most promising lineages (Li et al. 2008). It should be noted that lung and liver cancers are among the most common and the deadliest (Sung et al. 2021). In a more recent study, three isolates of Streptomyces spp. from soils and marine sediments showed antimicrobial and anticancer activities in their crude extracts (Abdel-Aziz et al. 2019). In its extract, the most notable isolate, Streptomyces sp. D-EGY, presented an IC50 of 0.85 µg/mL for the HepG2 lineage (human hepatocellular carcinoma). The authors reported forty isolated and identified compounds from this extract, whose correlations with anticancer activity should be investigated.
In two other studies carried out in recent years, expectations seem more favorable. Firstly, as reported above, the bactericidal desertomycin G against M. tuberculosis is also active against cancer cells of the human breast adenocarcinoma (MCF-7) and colon carcinoma (DLD-1) lineages (Braña et al. 2019). Next, caerulomycin A isolated from Actinoalloteichus cyanogriseus (DSM 43889) showed broad spectrum cytotoxic activities against cancer cell lines A375 (melanoma), A549 (lung), H1299 (lung), HepG2 (liver), HT29 (human colon), HL-60 (blood), and M624 (muscle) (Tong et al. 2022). The study of its mechanism of action indicated its ability to interfere in the formation of microtubes and DNA replication, acting specifically on the enzymes responsible for the polymerization of microtubes and on topoisomerase I, which is responsible for the relaxation of the DNA molecule during its replication. According to the authors, this is the second active ingredient isolated from microorganisms with anticancer activity that acts on two targets, unlike Taxol® (isolated from plants), which acts only on the synthesis of microtubes.
In comparison with the anticancer drugs available on the market, mostly derived from plants, these aspirants can be considered superior and have lower cost, thus revealing that screening for metabolites from actinomycetes can be very attractive. However, although numerous reviews and studies have consolidated the broad spectrum of action of the bioactive compounds of these bacteria (Aamir et al. 2020; Ek-Ramos et al. 2019; Law et al. 2020; Salam et al. 2017; Tanvir et al. 2019; Taechowisan et al. 2017), there is much more to discover regarding the anticancer potential of actinobacterial metabolites. Some of the anticancer metabolites are possibly the same ones that actinomycetes utilize against fungi in nature, since fungi are eukaryotic beings like us, substances that can fight fungi can also fight cancers that are made up of our own modified cells. While this hypothesis also signs a risk of toxicity for healthy human cells, it might be a good idea to look for both anticancer substances among antifungal compounds and antifungals among anticancer metabolites.
Antidiabetes
In the twenty-first century, among the chronic diseases, diabetes stands out, especially type 2 diabetes (on average 95% of cases), which is the deadliest. This is a metabolic dysfunction that results from low insulin availability or inadequate reception of target cells to insulin (Alharbi 2016; Cousin et al. 2022; Roper et al. 2002). According to one of the latest reports of the World Health Organization (2022b), this disease was one of the main factors that predisposes affected people to a more serious state of SARS-CoV-2 (World Health Organization 2022b). In the United States alone, one of the most obese populations in the world, it is estimated that 10% of the population has one of the types of diabetes (Hulett et al. 2022).
The endophytic isolate S. longisporoflavus stands out for producing an extract with inhibitory activity for the enzyme alpha-amylase (Akshatha et al. 2014)); this enzyme hinders the absorption of glucose in patients with type 2 diabetes mellitus. In fact, to treat type 2 diabetes, some bioactive isolates of actinomycetes, such as voglibose (Mahmud 2003) and acarbose (De Melo et al. 2006), obtained from S. hygroscopicus-limoneus and S. calvus, respectively, are already being used. Voglibose works by inhibiting alpha-glucosidase, thus lowering blood glucose levels in people with diabetes mellitus (De Melo et al. 2006), and acarbose is an inhibitor of alpha-glucosidase and alpha-amylase in the treatment of type 2 diabetes mellitus (Xu et al. 2009). Furthermore, more recently, in the study by Kawahara et al. (2023), a new candidate for the treatment of diabetes mellitus 2, the alkaloid amamine (1), isolated from Kitasatospora sp. HGTA304, was able to inhibit α-glucosidase with an IC50 value (56 µM) approximately ten times lower than acarbose (549 µM). Results such as these give us hope that we can find more promising isolates from actinomycetes that can be used to treat chronic diseases such as diabetes.
Anticholesterol
Cholesterol is an essential component of human cells, but its excess in the bloodstream can cause harm to human health, especially heart problems (Seenak et al. 2021). In an investigation of a BGC of S. lunaelactis MM109, which synthesizes distinct MNPs, it was observed that the availability of iron in mineral form is essential for the synthesis of p-vinylphenyl-3-nitroso-4-hydroxybenzoate, which is a precursor of trimeric ferroverdins that have anticholesterol activity (Martinet et al. 2019). The discovery of new ferroverdins may aid in the treatment of cardiac sarcoidosis, which is a heart inflammation related to high levels of cholesterol in the blood.
Alzheimer’s treatment
Alzheimer’s is a neurodegenerative disease that causes dementia and affects thousands of people every year (Almasi et al. 2018). Its physiological mechanisms are still being investigated; however, the accumulations of β-amyloid and acetylcholinesterase within the neocortex are determining factors. Indeed, acetylcholinesterase inhibitors help improve the availability of acetylcholine, a neurotransmitter that is indispensable for cognitive activities and memory. This activity helps to decrease the negative effects of β-amyloid accumulation in the neocortex (Barage and Sonawane 2015). It is estimated that by 2050 every 33 s a person will be affected by this disease (Alzheimer’s Association 2017; Almasi et al. 2018; Bush 2003; Calderon-Garcidueñas and Duyckaerts 2018). There are still no drugs made from actinomycetes that can be used in the treatment of this neurodegenerative disease, but some studies already report the possibility of actinomycetes being sources of active ingredients capable of assisting in its treatment. We can cite, as an example, a study of the endophytic community of Gynura cusimbua, a Chinese medicinal plant that, according to the traditional knowledge of this region, is used in the prevention of hypertension, coronary heart disease, Alzheimer’s and atherosclerosis. This study showed that this plant has a rich diversity of actinomycetes. The study also hypothesizes that some of the metabolites found in the plant related to biological activities may originate from actinomycetes (Zhang et al. 2016). Another study strengthens this hypothesis since, among more than 200 actinomycetes from sponges of the Caspian Sea and Persian Gulf, 50% presented extracts with anti-acetylcholinesterase activity (Almasi et al. 2018). The authors emphasize that the various compounds isolated and characterized with activities can assist in the treatment of neurodegenerative diseases that affect cognition and memory.
Stomach antiulcer and anti-inflammatory drugs
In studies of the microbiota of an endemic sponge in the Red Sea (Spheciospongia mastoidea), an actinomycete (RA2) was identified that is capable of producing two compounds, butylcycloheptylprodigiosin and undecylprodigiosin. These substances showed antiulcer and anti-inflammatory activities in experiments with rats that suffered stomach injuries induced by hydrochloric acid/ethanol. When these RA2 compounds were administered orally, decreased rates of lesions in areas of ulceration, histopathological abnormalities, and neutrophil infiltration were observed. These results are similar to those of omeprazole, the standard antiulcer drug (Abdelfattah et al. 2019). On the other hand, S. gramineus, associated with the lichen Leptogium trichophorum, together with three known actinofuranones produced six promising new actinofuranones (D to I) (Ma et al. 2018). Among them, two known and two new actinofuranones stood out as potential candidates for anti-inflammatory drugs due to their results in tests of attenuation of nitric oxide (NO) production and evasions of pro-inflammatory cytokines (IL-6) and tumor necrosis factor-α (TNF-α).
Protection, fertilization and improvement of plant production
High food yields in agricultural production depend on fertilizers, pesticides, and biocontrol agents. On this question, much remains to be explored within the fascinating actinomycetes group, as can be inferred from many studies. For example, a significant increase was observed in the fertility of the date palm (Phoenix dactylifera L.) when actinomycetes were inoculated into its rhizosphere, thus improving the life cycle of this vegetable of economic importance for Egypt. Improvements noted in the appearance of the fruits, which had higher levels of sugars, organic acids, essential amino acids, unsaturated fatty acids, phenolic acids, flavonoids, vitamins and minerals. In addition, improvements were observed in antitumor, antioxidant, antiprotozoal, and antimicrobial activities (fungi and bacteria) of fruits of date palms treated with actinomycetes (Abdelgawad et al. 2019). In another case, 11 isolates of the genera Norcadia, Streptomyces, and Janibacter from turmeric (Curcuma longa L.) and ginger (Zingiber officinale) presented activities against the phytopathogens Alternaria pimpriana and Colletotrichum coccodes (Osaro-Matthew et al. 2020).
The protection of plants and the improvement in the quality of their fruits exemplified above seem closely associated and this improvement is a possible consequence of the elimination of phytopathogens, rather than a direct advantage to the plant. This is also observed in the following two examples, in which, together with the biocontrol of phytopathogens, significant improvements in fruit quality were reported. In the first case, an endophytic community of actinomycetes from healthy cucumber palnts stood out due to the biocontrol of the phytopathogen Fusarium oxysporum f. sp. cucumerinum, which is the cause of wilting. The strain Streptomyces sp. NBRC 100767 showed the strongest activity and azalomycin B was responsible (Cao et al. 2020). In the other study, the strain Streptomyces sp. JKTJ-3 presented a broad spectrum of biocontrol and was capable of inhibiting 12 phytopathogens, including Pythium aphanidermatum, which is responsible for wilting in watermelon seedlings (Ge et al. 2023).
In addition to isolates from soils or endophytes, strains of actinomycetes from other ecosystems may show activity against phytopathogens. Recently, a consortium of actinomycetes isolated from insect intestines was reported as being promising strains for the biocontrol of Bipolaris maydis, which causes rust on wheat leaves (Wang et al. 2023). Streptomyces sp. SN5431 was the most successful in biological tests with extracts, and the substance tiuslactone B, from its fermented broth, was responsible for the fungicidal activity.
The fight against nematodes that affect vegetables can also be aided by actinomycetes. The strain Micromonospora sp. WH06 is reported as a potential biocontrol agent of the nematode Meloidogyne incognita, which causes lesions in the roots of Meloidogyne spp. Benzenepropanoic acid, isolated from the fermented broth of Micromonospora sp. WH06, causes 99% mortality with a dose of 200 µg/mL after 72 h and inhibition of egg hatching in M. incognita (Ran et al. 2022).
Volatile compounds (VOCs) from actinomycetes have also shown antimicrobial activities. The volatile 2-methyl-1-butanol, 3-methyl-1-butanol, pyridine and phenylethyl alcohol of Streptomyces sp. SPS-33 strain showed strong in vivo and in vitro activities against the phytopathogen Ceratocystis fimbriata, which causes charcoal rot in sweet potato plants (Li et al. 2020a, b). Decreased water loss and increased antioxidant activity were also observed.
The applicability of promising strains of actinomycetes in agriculture is becoming a more sustainable and economically viable strategy. In the harmonious relationship with plants, actinomycetes benefit from a nutritious and attractive phyto-exudate and produce several secondary metabolites with phyto-propagating and phyto-protective activities (Van der Meij et al. 2017; Olanrewaju and Babalola 2019; Trivedi et al. 2020). Hormones secreted by actinomycetes, or induced by them in plants, stimulate several important activities in plants. For example, the secretion of the phytohormone auxin (indole-acetic acid) promotes the elongation of the roots – which allows them to reach more nutrients – and also the activation of plant immunity against fungal phytopathogens. Cytokinin helps to delay plant deterioration and aging; gibberellin helps in root enlargement, resistance to salt stress and endosymbiotic interactions; ethylene stimulates root colonization and immune responses against microbial pathogens; and, finally, polyamines are involved in senescence, fruit maturation, flowering, organogenesis, morphogenesis and embryogenesis. These hormones, which are secreted by actinomycetes, act in all the physiological processes of plants, from seed germination to resilience to abiotic and biotic stresses and are synthesized mainly by strains of the genus Streptomyces and other rare genera (Nocardiopsis, Micromonospora and Amycolatopsis, among others) (Ebrahimi-Zarandi et al. 2023; Oyedoh et al. 2023a). Among the enzymes, nitrogenases are quite well known. They have the ability to convert N2 (not usable by plants) into NH3 (usable by plants) for the synthesis of proteins, secondary metabolites, DNA and RNA (AbdElgawad et al. 2020; Al-Rashdi et al. 2022; Rosenblueth et al. 2018).
As in these few examples, due to their ability to fix nitrogen in the rhizosphere and their potential to inhibit phytopathogens, actinomycetes are strong candidates that can be explored for improvements in the production of many plants. The ecological activities of actinomycetes in favor of plants are a model for more sustainable agriculture, with emphasis on the genus Streptomyces and some strains of rare genera of actinomycetes that have been gaining recognition (Oyedoh et. al. 2023a, 2023b).
Remediation, promotion of bioavailability and solubilization of minerals
The advantages of using actinomycetes as bioremediators consists in the fact that they do not present risks to the health of animals, humans, plants, or the soil and aquatic environments. Their biological activities are stimulated when the nutritional and ecological conditions of their niches are made available. Enzymes (chitinase, cellulase, glucanase, protease, lipases, and phospholipase, among others) secreted by actinomycetes exert important biological activities for the environment and plants. For example, chitinase, glucanase, lipases, and phospholipase degrade cell walls and plasma membranes of microbial phytopathogens, which are competing and neutral microbes (Selim et al. 2021). In fact, there are several examples of actinomycetes being environmental purifiers, among which we can highlight the genus Rhodococcus, which presents itself as a diverse remediator. Thus, strains of this genus are capable of producing 3-chlorobenzoate 1,2-dioxygenase (3CBDO), an enzyme capable of degrading the herbicide 3-chlorobenzoate (3CBA) (Emelyanova et al. 2023). Other strains, isolated from Arctic soils, are capable of oxidizing hydrocarbons (Semenova et al. 2022), and others are capable of degrading non-steroid anti-inflammatory pharmaceutical products, such as ibuprofen, meloxicam and naproxen (Ivshina et al. 2022). This genus is also promising in the promotion of mineral bioavailability, as in the case of a strain of Rhodococcus sp. that, through non-ribosomal peptide synthetases enzymes, synthesizes several siderophores (rhodochelin, rhequichelin, requibactin, rhodobactin and heterobactin A), which demonstrates that the strain can be used as an agricultural aid for iron bioavailability (Sarkar and Suthindhiran 2022). There are also reports of strains of Streptomyces and Promicromonospora isolated from Moroccan soils that are capable of solubilizing phosphate (Bousselham et al 2022). In fact, actinomycetes harbor a consortium of underexploited enzymes in their genomes, and are able to act in the decomposition of plant and animal organic matter, and solubilization of inorganic substances (manganese (Mn), cobalt (Co), lithium (Li), copper (Cu), zinc (Zn), cadmium (Cd), nickel (Ni), aluminum (Al), and magnesium (Mg)) in simpler states for biological uses (Imade and Babalola 2021; Schwabe et al. 2018).
Other biotechnological applications focused not only on medicine and agriculture
There is certainly still much to be revealed about the potential biotechnological application of actinomycetes, both in targeting products and processes, not only in medicine and agriculture. Two of these applications are related to the search for more sustainable alternatives for the production of fuels and biodegradable plastics. Recently, an innovative method for biodiesel production was created using the actinomycetes Piscicocus intestinalis (WA3) and the microalgae Tetradesmus obliquus (AARLG022) in co-culture for biomass enrichment using the biogas digestate effluent (BDE) method (Kumsiri et al. 2021). The authors observed the increase of long-chain lipids, indole-3-acetic acid, and siderophores by T. obliquus in co-culture with P. intestinalis-WA3 (fertilizer agent) when they compared the results with those of T. obliquus monoculture. It is an encouraging result that opens up a promising horizon to be explored, in this case, to improve biodiesel production using algae. In another relevant study, strains from three genera of actinomycetes: S. gougerotti, M. matsumotoense, and N. prasina demonstrated the ability to degrade low-density polyethylene (LDPE), polystyrene (PS), and polylactic acid (PLA) under varied conditions (Oliveira et al. 2022). Furthermore, mainly the S. gougerotti and M. matsumotoense strains were able to use those plastics as carbon sources to produce polyhydroxyalkanoate (PHA) bioplastics. In this sustainable context, actinomycetes gain yet another important applicability within their fascinating biotechnological spectrum.
Conclusion
The studies presented in this review are a small sample of the enormous biotechnological potential of actinomycetes, regarding which our knowledge and exploitation are rapidly expanding. As such, considering the data described here and the literature, the metabolites of actinomycetes can meet numerous medical, agricultural and industrial demands far beyond what is currently known and used. Using gene mining technologies further enhances the discovery of the immense metabolic wealth hidden in the genomes of known species and new isolates, as highlighted by several studies. Thus, with the help of genomics and metabolomics, a new generation of antibiotics and substances of biotechnological interest is being revealed. Considering the advances of the last century, since the discovery of streptomycin in 1944, the first 23 years of the twenty-first century represent a considerable leap forward in research into the potential of actinomycetes. Therefore, for the forthcoming decades, one can imagine the development of innovative products using actinomycetes as a source of metabolites that are capable of treating numerous pathologies and assisting in various therapies, among other biotechnological applications. However, it is necessary to improve many research approaches to go beyond preliminary results. It is required to face this question in its deep causes, among which we highlight the isolation of many research teams, low level of funding, and lack of strategic and effective planning. Everyone’s effort is essential: governments, funding bodies, research institutions, and the researchers themselves. We must also highlight the need to investigate the many little-known environments with a high potential for exploring the metabolic richness of actinomycetes and other microorganisms, among which we highlight the aquatic environments of fresh or saltwater and tropical forest environments, especially the Amazon. Unfortunately, many of these environments suffer constant degradation and much of their microbiota is being extinguished without us being able to discover and take advantage of their biotechnological potential.
Data availability
All data analysed during this study are included in this published article.
References
Aamir M, Rai KK, Zehra A, Dubey MK, Samal S, Yadav M, Upadhyay RS (2020) Endophytic actinomycetes in bioactive compounds production and plant defense system. In: Microbial endophytes, pp 189–229. Woodhead Publishing. https://doi.org/10.1016/B978-0-12-818734-0.00009-7
Abdel-Aziz MS, Hathou AS, El-Neleety AA, Hamed AA, Sabry BA, Aly SE, Abdel-Wahhab MA (2019) Molecular identification of actinomycetes with antimicrobial, antioxidant and anticancer properties. Comunicata Scientiae, 10(2), 218–231. https://doi.org/10.14295/cs.v10i2.2269
Abdelfattah MS, Elmallah MI, Ebrahim HY, Almeer RS, Eltanany RM, Abdel Moneim AE (2019) Prodigiosins from a marine sponge-associated actinomycete attenuate HCl/ethanol-induced gastric lesion via antioxidant and anti-inflammatory mechanisms. PLoS ONE 14(6):e0216737. https://doi.org/10.1371/journal.pone.0216737
AbdElgawad H, Saleh AM, Al Jaouni S, Selim S, Hassan MO, Wadaan MA, Hozzein WN (2019) Utilization of actinobacteria to enhance the production and quality of date palm (Phoenix dactylifera L.) fruits in a semi-arid environment. Sci Total Environ 665:690–697. https://doi.org/10.1016/j.scitotenv.2019.02.140
AbdElgawad H, Abuelsoud W, Madany MM, Selim S, Zinta G, Mousa AS, Hozzein WN (2020) Actinomycetes enrich soil rhizosphere and improve seed quality as well as productivity of legumes by boosting nitrogen availability and metabolism. Biomolecules 10(12):1675. https://doi.org/10.3390/biom10121675
Abdelmohsen UR, Szesny M, Othman EM, Schirmeister T, Grond S, Stopper H, Hentschel U (2012) Antioxidant and anti-protease activities of diazepinomicin from the sponge-associated Micromonospora strain RV115. Mar Drugs 10(10):2208–2221. https://doi.org/10.3390/md10102208
Adamek M, Alanjary M, Sales-Ortells H, Goodfellow M, Bull AT, Winkler A, Ziemert N (2018) Comparative genomics reveals phylogenetic distribution patterns of secondary metabolites in Amycolatopsis species. BMC Genomics 19(1):1–15. https://doi.org/10.1186/s12864-018-4809-4
Aguiar AP, Deans AR, Engel MS, Forshage M, Huber JT, Jennings JT, Yu DSK (2013) Order Hymenoptera. Zootaxa 3703(1):51–62. https://doi.org/10.1603/EN09221
Ahmad MS, El-Gendy AO, Ahmed RR, Hassan HM, El-Kabbany HM, Merdash AG (2017) Exploring the antimicrobial and antitumor potentials of Streptomyces sp. AGM12-1 isolated from Egyptian soil. Front Microbiol 8:438. https://doi.org/10.3389/fmicb.2017.00438
Akshatha VJ, Nalini MS, D’souza C, Prakash HS (2014) Streptomycete endophytes from anti-diabetic medicinal plants of the Western Ghats inhibit alpha-amylase and promote glucose uptake. Lett Appl Microbiol 58(5):433–439. https://doi.org/10.1111/lam.12209
Al-Ansari M, Kalaiyarasi M, Almalki MA, Vijayaraghavan P (2020) Optimization of medium components for the production of antimicrobial and anticancer secondary metabolites from Streptomyces sp. AS11 isolated from the marine environment. J King Saud Univ-Sci 32(3):1993–1998. https://doi.org/10.1016/j.jksus.2020.02.005
Al-Rashdi A, Al-Hinai FS, Al-Harrasi MMA, Al-Sabahi JN, Al-Badi RS, Al-Mahmooli IH, Velazhahan R (2022) The potential of endophytic bacteria from Prosopis cineraria for the control of Pythium aphanidermatum-induced damping-off in cucumber under saline water irrigation. J Plant Pathol 105(1):39–56. https://doi.org/10.1007/s42161-022-01237-5
Alharbi NS (2016) Novel bioactive molecules from marine actinomycetes. Biosci Biotechnol Res Asia 13(4):1905–1927. https://doi.org/10.13005/bbra/2346
Ali A R, Bahrami Y, Kakaei E, Mohammadzadeh S, Bouk S, Jalilian N (2022) Isolation and identification of endophytic actinobacteria from Citrullus colocynthis (L.) Schrad and their antibacterial properties. Microbial Cell Factories 21(1):206. https://doi.org/10.1186/s12934-022-01936-9
Alkhalifah DHM (2021) Sponge-associated sp. RM66 metabolome induction with N-acetylglucosamine: antibacterial, antifungal and anti-trypanosomal activities. Saudi J Biol Sci 28(8):4691–4698. https://doi.org/10.1016/j.sjbs.2021.04.082
Almaary KS, Alharbi NS., Kadaikunnan S, Khaled JM, Rajivgandhi G, Ramachandran G, Manoharan N (2021) Anti-bacterial effect of marine sea grasses mediated endophytic actinomycetes against K. pneumoniae. J King Saud Univ-Sci 33(6):101528. https://doi.org/10.1016/j.jksus.2021.101528
Almasi F, Mohammadipanah F, Adhami HR, Hamedi J (2018) Introduction of marine‐derived Streptomyces sp. UTMC 1334 as a source of pyrrole derivatives with anti‐acetylcholinesterase activity. J Appl Microbiol 125(5):1370–1382. https://doi.org/10.1111/jam.14043
Alvarez-Uria G, Gandra S, Mandal S, Laxminarayan R (2018) Global forecast of antimicrobial resistance in invasive isolates of Escherichia coli and Klebsiella pneumoniae. Int J Infect Dis 68:50–53. https://doi.org/10.1016/j.ijid.2018.01.011
Alvariño R, Alonso E, Lacret R, Oves-Costales D, Genilloud O, Reyes F, Botana LM (2019) Caniferolide A, a macrolide from Streptomyces caniferus, attenuates neuroinflammation, oxidative stress, amyloid-beta, and tau pathology in vitro. Mol Pharm 16(4):1456–1466. https://doi.org/10.1021/acs.molpharmaceut.8b01090
Alzheimer’s Association (2017) Alzheimer’s disease facts and figures. Alzheimer’s Dementia 13(4):325-373. https://doi.org/10.1016/j.jalz.2017.02.001
An JS, Shin B, Kim TH, Hwang S, Shin YH, Cui J, Oh DC (2021) Dumulmycin, an antitubercular bicyclic macrolide from a riverine sediment-derived Streptomyces sp. Org Lett 23(9):3359–3363. https://doi.org/10.1021/acs.orglett.1c00847
Arens JC, Berrué F, Pearson JK, Kerr RG (2013) Isolation and structure elucidation of satosporin A and B: new polyketides from Kitasatospora griseola. Org Lett 15(15):3864–3867. https://doi.org/10.1021/ol401598f
Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT (2021) Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov 20(3):200–216. https://doi.org/10.1038/s41573-020-00114-z
Azman AS, Othman I, Velu SS, Chan KG, Lee LH (2015) Mangrove rare actinobacteria: taxonomy, natural compound, and discovery of bioactivity. Front Microbiol 6:856. https://doi.org/10.3389/fmicb.2015.00856
Babu A, Pandey AK, Deka B, Kumhar KC, Sarkar S, Bordoloi M, Mani S (2022) Molecular characterization and functional properties of deep-soil-inhabiting actinobacteria for combating Fusarium dieback disease in tea crop. Biol Control 174:105027. https://doi.org/10.1016/j.biocontrol.2022.105027
Balskus EP (2014) Sponge symbionts play defense. Nat Chem Biol 10(8):611–612. https://doi.org/10.1038/nchembio.1588
Bao Y, Li H, Dong Y, Duan H, Li H, Li W (2022) Genome-guided discovery of antifungal filipins from a deep-sea-derived Streptomyces antibioticus. J Nat Prod 85(2):365–374. https://doi.org/10.1021/acs.jnatprod.1c00952
Barage SH, Sonawane KD (2015) Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides 52:1–18. https://doi.org/10.1016/j.npep.2015.06.008
Barney R, Velasco M, Cooper CA, Rashid A, Kyle DE, Moon RW, Jang IK (2022) Diagnostic characteristics of lactate dehydrogenase on a multiplex assay for malaria detection including the zoonotic parasite Plasmodium knowlesi. Am J Trop Med Hyg 106(1):275. https://doi.org/10.4269/ajtmh.21-0532
Barrows NJ, Campos RK, Powell ST, Prasanth KR, Schott-Lerner G, Soto-Acosta R, Garcia-Blanco MA (2016) A screen of FDA-approved drugs for inhibitors of Zika virus infection. Cell Host Microbe 20(2):259–270. https://doi.org/10.1016/j.chom.2016.07.004
Baskiyar S, Ren C, Heck KL, Hall AM, Gulfam M, Packer S, Calderón AI (2022) Bioactive natural products identification using automation of molecular networking software. J Chem Inf Model 62(24):6378–6385. https://doi.org/10.1021/acs.jcim.2c00307
Belknap KC, Park CJ, Barth BM, Andam CP (2020) Genome mining of biosynthetic and chemotherapeutic gene clusters in Streptomyces bacteria. Sci Rep 10(1):2003. https://doi.org/10.1038/s41598-020-58904-9
Benedict K, Whitham HK, Jackson BR (2022) Economic burden of fungal diseases in the United States. In: Open Forum Infectious Diseases, vol 9, no 4, p. ofac097. US: Oxford University Press. https://doi.org/10.1093/ofid/ofac097
Bentley SD, Chater KF, Cerdeño-Tárraga AM, Challis GL, Thomson NR, James KD, Hopwood DA (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3 (2). Nature 417(6885):141–147. https://doi.org/10.1038/417141a
Bharati S, Podder P, Mondal MRH, Podder P, Kose U (2022) A review on epidemiology, genomic characteristics, spread, and treatments of COVID-19. Data Science for COVID-19, 487–505. https://doi.org/10.1016/B978-0-323-90769-9.00011-6
Bhattacharya S, Sae-Tia S, Fries BC (2020) Candidiasis and mechanisms of antifungal resistance. Antibiotics 9(6):312. https://doi.org/10.3390/antibiotics9060312
Bi Y, Yu Z (2016) Diterpenoids from Streptomyces sp. SN194 and their antifungal activity against Botrytis cinerea. J Agric Food Chem 64(45):8525–8529. https://doi.org/10.1021/acs.jafc.6b03645
Bousselham M, Lemriss S, Dhiba D, Aallam Y, Souiri, Abbas, Y, Hamdali H (2022) Streptomycetaceae and ProMicromonosporaceae: two actinomycetes families from Moroccan oat soils enhancing solubilization of natural phosphate. Microorganisms 10(6):1116. https://doi.org/10.3390/microorganisms10061116
Bracegirdle J, Hou P, Nowak VV, Ackerley DF, Keyzers RA, Owen JG (2021) Skyllamycins D and E, non-ribosomal cyclic depsipeptides from lichen-sourced Streptomyces anulatus. J Nat Prod 84(9):2536–2543. https://doi.org/10.1021/acs.jnatprod.1c00547
Braesel J, Crnkovic CM, Kunstman KJ, Green SJ, Maienschein-Cline M, Orjala J, Eustáquio AS (2018) Complete genome of Micromonospora sp. strain B006 reveals biosynthetic potential of a Lake Michigan actinomycete. J Nat Prod 81(9):2057–2068. https://doi.org/10.1021/acs.jnatprod.8b00394
Braña AF, Sarmiento-Vizcaíno A, Pérez-Victoria I, Martín J, Otero L, Palacios-Gutiérrez JJ, Blanco G (2019) Desertomycin G, a new antibiotic with activity against Mycobacterium tuberculosis and human breast tumor cell lines produced by Streptomyces althioticus MSM3, isolated from the Cantabrian Sea Intertidal macroalgae Ulva sp. Mar Drugs 17(2):114. https://doi.org/10.3390/md17020114
Braz HLB, De Moraes Silveira JA, Marinho AD, MoraesMEA De, De Moraes Filho MO, Monteiro HSA, Jorge RJB (2020) In silico study of azithromycin, chloroquine and hydroxychloroquine and their potential mechanisms of action against SARS-CoV-2 infection. Int J Antimicrob Agents 56(3):106119. https://doi.org/10.1016/j.ijantimicag.2020.106119
Brown GD, Denning DW, Levitz SM (2012) Tackling human fungal infections. Science 336(6082):647–647. https://doi.org/10.1126/science.1222236
Bush AI (2003) The metallobiology of Alzheimer’s disease. Trends Neurosci 26(4):207–214. https://doi.org/10.1016/S0166-2236(03)00067-5
Cai C, Lin H, Wang H, Xu Y, Ouyang Q, Lai L, Pei J (2023) miDruglikeness: subdivisional drug-likeness prediction models using active ensemble learning strategies. Biomolecules 13(1):29. https://doi.org/10.3390/biom13010029
Calderon-Garcidueñas AL, Duyckaerts C (2018) Alzheimer disease. Handb Clin Neurol 145:325–337. https://doi.org/10.1016/B978-0-12-802395-2.00023-7
Cao P, Li C, Wang H, Yu Z, Xu X, Wang X, Xiang W (2020) Community structures and antifungal activity of root-associated endophytic actinobacteria in healthy and diseased cucumber plants and Streptomyces sp. HAAG3-15 as a promising biocontrol agent. Microorganisms 8(2):236. https://doi.org/10.3390/microorganisms8020236
Carlson S, Marler L, Nam SJ, Santarsiero BD, Pezzuto JM, Murphy BT (2013) Potential chemopreventive activity of a new macrolide antibiotic from a marine-derived Micromonospora sp. Mar Drugs 11(4):1152–1161. https://doi.org/10.3390/md11041152
Cartuche L, Sifaoui I, López-Arencibia A, Bethencourt-Estrella CJ, San Nicolás-Hernández D, Lorenzo-Morales J, Fernández JJ (2020) Antikinetoplastid activity of indolocarbazoles from Streptomyces sanyensis. Biomolecules 10(4):657. https://doi.org/10.3390/biom10040657
Challis GL (2008) Mining microbial genomes for new natural products and biosynthetic pathways. Microbiology 154(6):1555–1569. https://doi.org/10.1099/mic.0.2008/018523-0
Challis GL (2014) Exploitation of the Streptomyces coelicolor A3 (2) genome sequence for discovery of new natural products and biosynthetic pathways. J Ind Microbiol Biotechnol 41(2):219–232. https://doi.org/10.1007/s10295-013-1383-2
Chan K, Tusting LS, Bottomley C, Saito K, Djouaka R, Lines J (2022) Malaria transmission and prevalence in rice-growing versus non-rice-growing villages in Africa: a systematic review and meta-analysis. Lancet Planet Health 6(3):e257–e269. https://doi.org/10.1016/S2542-5196(21)00349-1
Chen X, Hu LF, Huang XS, Zhao LX, Miao CP, Chen YW, Li YQ (2019) Isolation and characterization of new phenazine metabolites with antifungal activity against root-rot pathogens of Panax notoginseng from Streptomyces. J Agric Food Chem 67(41):11403–11407. https://doi.org/10.1021/acs.jafc.9b0419
Chen L, Chai W, Wang W, Song T, Lian XY, Zhang Z (2017) Cytotoxic bagremycins from mangrove-derived Streptomyces sp. Q22. J Nat Prod 80(5):1450–1456. https://doi.org/10.1021/acs.jnatprod.6b01136
Chen MH, Chang SS, Dong B, Yu LY, Wu YX, Wang RZ, Si SY (2018) Ahmpatinin i Bu, a new HIV-1 protease inhibitor, from Streptomyces sp. CPCC 202950. RSC Adv 8(10):5138–5144. https://doi.org/10.1039/C7RA13241G
Chen Y, Wei Y, Cai B, Zhou D, Qi D, Zhang M, Wang W (2022) Discovery of Niphimycin C from Streptomyces yongxingensis sp. nov. as a promising agrochemical fungicide for controlling banana fusarium wilt by destroying the mitochondrial structure and function. J Agric Food Chem 70(40):12784–12795. https://doi.org/10.1021/acs.jafc.2c02810
Cheng C, MacIntyre L, Abdelmohsen UR, Horn H, Polymenakou PN, Edrada-Ebel R, Hentschel U (2015) Biodiversity, anti-trypanosomal activity screening, and metabolomic profiling of actinomycetes isolated from Mediterranean sponges. PLoS ONE 10(9):e0138528. https://doi.org/10.1371/journal.pone.0138528
Cheng Z, Zhang Q, Peng J, Zhao X, Ma L, Zhang C, Zhu Y (2023) Genomics-driven discovery of benzoxazole alkaloids from the marine-derived Micromonospora sp. SCSIO 07395. Molecules 28(2):821. https://doi.org/10.3390/molecules28020821
Chowdhary A, Sharma C, Meis JF (2017) Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog 13(5):e1006290. https://doi.org/10.1371/journal.ppat.1006290
Cousin E, Duncan BB, Stein C, Ong KL, Vos T, Abbafati C, Haque S (2022) Diabetes mortality and trends before 25 years of age: an analysis of the Global Burden of Disease Study 2019. Lancet Diabetes Endocrinol 10(3):177–192. https://doi.org/10.1016/S2213-8587(21)00349-1
Craney A, Ahmed S, Nodwell J (2013) Towards a new science of secondary metabolism. J Antibiot 66(7):387–400. https://doi.org/10.1038/ja.2013.25
Cui J, Kim E, Moon DH, Kim TH, Kang I, Lim Y, Oh DC (2022) Taeanamides A and B, nonribosomal lipo-decapeptides isolated from an intertidal-mudflat-derived Streptomyces sp. Mar Drugs 20(6):400. https://doi.org/10.3390/md20060400
Dasgupta N, Nandy P, Sengupta C, Das S (2015) RAPD and ISSR marker mediated genetic polymorphism of two mangroves Bruguiera gymnorrhiza and Heritiera fomes from Indian Sundarbans in relation to their sustainability. Physiol Mol Biol Plants 21:375–384. https://doi.org/10.1007/s12298-015-0308-0
Davies-Bolorunduro OF, Osuolale O, Saibu S, Adeleye IA, Aminah NS (2021) Bioprospecting marine actinomycetes for antileishmanial drugs: current perspectives and future prospects. Heliyon 7(8). https://doi.org/10.1016/j.heliyon.2021.e07710
De Simeis D, Serra S (2021) Actinomycetes: A never-ending source of bioactive compounds—An overview on antibiotics production. Antibiotics 10(5):483. https://doi.org/10.3390/antibiotics10050483
De Melo EB, Da Silveira GA, Carvalho I (2006) α-and β-Glucosidase inhibitors: chemical structure and biological activity. Tetrahedron 62(44):10277–10302. https://doi.org/10.1016/j.tet.2006.08.055
Deng RX, Zhang Z, Li HL, Wang W, Hu HB, Zhang XH (2021) Identification of a novel bioactive phenazine derivative and regulation of phoP on its production in Streptomyces lomondensis S015. J Agric Food Chem 69(3):974–981. https://doi.org/10.1021/acs.jafc.0c06498
Ding N, Jiang Y, Han L, Chen X, Ma J, Qu X, Huang X (2016) Bafilomycins and odoriferous sesquiterpenoids from Streptomyces albolongus isolated from Elephas maximus feces. J Nat Prod 79(4):799–805. https://doi.org/10.1021/acs.jnatprod.5b00827
Djebaili R, Pellegrini M, Ercole C, Farda B, Kitouni M, Del Gallo M (2021) Biocontrol of soil-borne pathogens of Solanum lycopersicum L. and Daucus carota L. by plant growth-promoting actinomycetes: in vitro and in planta antagonistic activity. Pathogens 10(10):1305. https://doi.org/10.3390/pathogens10101305
Donald L, Pipit A, Subramani R, Owen J, Keyzers RA, Taufa T (2022) Streptomyces: Still the biggest producer of new natural secondary metabolites, a current perspective. Microbiol Res 13(3):418–465. https://doi.org/10.3390/microbiolres13030031
Ebrahimi-Zarandi M, Etesami H, Glick BR (2023) Fostering plant resilience to drought with Actinobacteria: unveiling perennial allies in drought stress tolerance. Plant Stress 100242. https://doi.org/10.1016/j.stress.2023.100242
Ek-Ramos MJ, Gomez-Flores R, Orozco-Flores AA, Rodríguez-Padilla C, González-Ochoa G, Tamez-Guerra P (2019) Bioactive products from plant-endophytic Gram-positive bacteria. Front Microbiol 10:463. https://doi.org/10.3389/fmicb.2019.00463
Elbendary AA, Hessain AM, El-Hariri MD, Seida AA, Moussa IM, Mubarak AS, El Jakee JK (2018) Isolation of antimicrobial producing Actinobacteria from soil samples. Saudi J Biol Sci 25(1):44–46. https://doi.org/10.1016/j.sjbs.2017.05.003
Elsayed TR, Galil DF, Sedik MZ, Hassan HM, Sadik MW (2020) Antimicrobial and anticancer activities of actinomycetes isolated from egyptian soils. Int J Curr Microbiol Appl Sci 9(9):1689–1700. https://doi.org/10.20546/ijcmas.2020.909.209
Emelyanova EV, Ramanaiah SV, Prisyazhnaya NV, Shumkova ES, Plotnikova EG, Wu Y, Solyanikova IP (2023) The contribution of actinobacteria to the degradation of chlorinated compounds: variations in the activity of key degradation enzymes. Microorganisms 11(1):141. https://doi.org/10.3390/microorganisms11010141
Espinosa A, Soch AM, Ryke E, Rowley DC (2012) Antiamoebic properties of the actinomycete metabolites echinomycin A and tirandamycin A. Parasitol Res 111:2473–2477. https://doi.org/10.1007/s00436-012-3019-2
Estrella-Parra EA, Arreola R, Álvarez-Sánchez ME, Torres-Romero JC, Rojas-Espinosa O, De la Cruz-Santiago JA, Ramírez-Camacho MA (2022) Natural marine products as antiprotozoal agents against amitochondrial parasites. Int J Parasitol Drugs Drug Resist. https://doi.org/10.1016/j.ijpddr.2022.05.003
Exner M, Bhattacharya S, Christiansen B, Gebel J, Goroncy-Bermes P, Hartemann P, Trautmann M (2017) Antibiotic resistance: what is so special about multidrug-resistant Gram-negative bacteria? GMS Hyg Infect Control 12. https://doi.org/10.3205/dgkh000290
Fang Q, Maglangit F, Mugat M, Urwald C, Kyeremeh K, Deng H (2020) Targeted isolation of indole alkaloids from Streptomyces sp. CT37. Molecules 25(5):1108. https://doi.org/10.3390/molecules25051108
Feng Y, Yu Z, Zhang S, Xue Z, Huang J, Zhang H, Wang J (2019) Isolation and characterization of new 16-membered macrolides from the aveA3 gene replacement mutant strain Streptomyces avermitilis TM24 with acaricidal and nematicidal activities. J Agric Food Chem 67(17):4782–4792. https://doi.org/10.1021/acs.jafc.9b00079
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Bray F (2019) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144(8):1941–1953. https://doi.org/10.1002/ijc.31937
Fisher MC, Alastruey-Izquierdo A, Berman J, Bicanic T, Bignell EM, Bowyer P, Verweij PE (2022) Tackling the emerging threat of antifungal resistance to human health. Nat Rev Microbiol 20(9):557–571. https://doi.org/10.1038/41579-022-00720-1
Gärtner A, Ohlendorf B, Schulz D, Zinecker H, Wiese J, Imhoff JF (2011) Levantilides A and B, 20-membered macrolides from a Micromonospora strain isolated from the mediterranean deep sea sediment. Mar Drugs 9(1):98–108. https://doi.org/10.3390/md9010098
Ge M, Cai X, Wang D, Liang H, Zhu J, Li G, Shi X (2023) Efficacy of Streptomyces murinus JKTJ-3 in suppression of pythium damping-off of watermelon. Microorganisms 11(6):1360. https://doi.org/10.3390/microorganisms11061360
Gill KA, Berrué F, Arens JC, Carr G, Kerr RG (2015) Cystargolides, 20S proteasome inhibitors isolated from Kitasatospora cystarginea. J Nat Prod 78(4):822–826. https://doi.org/10.1021/np501060k
Gohain A, Gogoi A, Debnath R, Yadav A, Singh BP, Gupta VK, Saikia R (2015) Antimicrobial biosynthetic potential and genetic diversity of endophytic actinomycetes associated with medicinal plants. FEMS Microbiol Lett 362(19):fnv158. https://doi.org/10.1093/femsle/fnv158
Gomez-Escribano JP, Holmes NA, Schlimpert S, Bibb MJ, Chandra G, Wilkinson B, Bibb MJ 2021 Streptomyces venezuelae NRRL B-65442: genome sequence of a model strain used to study morphological differentiation in filamentous actinobacteria. J Ind Microbiol Biotechnol 48(9–10): kuab035. https://doi.org/10.1093/jimb/kuab035
Gong R, Yu L, Qin Y, Price NP, He X, Deng Z, Chen W (2021) Harnessing synthetic biology-based strategies for engineered biosynthesis of nucleoside natural products in actinobacteria. Biotechnol Adv 46:107673. https://doi.org/10.1016/j.biotechadv.2020.107673
Guan L, Yang H, Cai Y, Sun L, Di P, Li W, Tang Y (2019) ADMET-score–a comprehensive scoring function for evaluation of chemical drug-likeness. Medchemcomm 10(1):148–157. https://doi.org/10.1039/C8MD00472B
Hanshew AS, McDonald BR, Díaz Díaz C, Djiéto-Lordon C, Blatrix R, Currie CR (2015) Characterization of actinobacteria associated with three ant–plant mutualisms. Microb Ecol 69:192–203. https://doi.org/10.1007/s00248-014-0469-3
Hao X, Yu J, Wang Y, Connolly JA, Liu Y, Zhang Y, Gan M (2020) Zelkovamycins B-E, cyclic octapeptides containing rare amino acid residues from an endophytic Kitasatospora sp. Org Lett 22(23):9346–9350. https://doi.org/10.1021/acs.orglett.0c03565
Hao X, Li S, Wang G, Li J, Peng Z, Zhang Y, Gan M (2022) Zelkovamycins F and G, cyclopeptides with Cα-Methyl-threonine residues, from an endophytic Kitasatospora sp. J Nat Prod 85(7):1715–1722. https://doi.org/10.1021/acs.jnatprod.2c00174
Heine D, Martin K, Hertweck C (2014) Genomics-guided discovery of endophenazines from Kitasatospora sp. HKI 714. J Nat Prod 77(4):1083–1087. https://doi.org/10.1021/np400915p
Hemmerling F, Piel J (2022) Strategies to access biosynthetic novelty in bacterial genomes for drug discovery. Nat Rev Drug Discov 21(5):359–378. https://doi.org/10.1038/s41573-022-00414-6
Hoque MN, Jahan MI, Hossain MA, Sultana M (2022) Genomic diversity and molecular epidemiology of a multidrug-resistant Pseudomonas aeruginosa DMC30b isolated from a hospitalized burn patient in Bangladesh. J Glob Antimicrob Resist 31:110–118. https://doi.org/10.1016/j.jgar.2022.08.023
Hu C, Zhou SW, Chen F, Zheng XH, Shen HF, Lin BR, Zhou GX (2017) Neoantimycins A and B, two unusual benzamido nine-membered dilactones from marine-derived Streptomyces antibioticus H12–15. Molecules 22(4):557. https://doi.org/10.3390/molecules22040557
Huang H, Ren L, Li H, Schmidt A, Gershenzon J, Lu Y, Cheng D (2020) The nesting preference of an invasive ant is associated with the cues produced by actinobacteria in soil. PLoS Pathog 16(9):e1008800. https://doi.org/10.1371/journal.ppat.1008800
Hulett NA, Scalzo RL, Reusch JE (2022) Glucose uptake by skeletal muscle within the contexts of type 2 diabetes and exercise: an integrated approach. Nutrients 14(3):647. https://doi.org/10.3390/nu14030647
Igarashi Y, Ogura H, Furihata K, Oku N, Indananda C, Thamchaipenet A (2011) Maklamicin, an antibacterial polyketide from an endophytic Micromonospora sp. J Nat Prod 74(4):670–674. https://doi.org/10.1021/np100727h
Igarashi Y, Matsuyuki Y, Yamada M, Fujihara N, Harunari E, Oku N, Urabe D (2021) Structure determination, biosynthetic origin, and total synthesis of akazaoxime, an enteromycin-class metabolite from a marine-derived actinomycete of the genus Micromonospora. J Org Chem 86(9):6528–6537. https://doi.org/10.1021/acs.joc.1c00358
Imade EE, Babalola OO (2021) Biotechnological utilization: the role of Zea mays rhizospheric bacteria in ecosystem sustainability. Appl Microbiol Biotechnol 105(11):4487–4500. https://doi.org/10.1007/s00253-021-11351-6
Inahashi Y, Iwatsuki M, Ishiyama A, Matsumoto A, Hirose T, Oshita J, O̅mura S (2015) Actinoallolides A–E, new anti-trypanosomal macrolides, produced by an endophytic actinomycete, Actinoallomurus fulvus MK10-036. Org Lett 17(4):864-867. https://doi.org/10.1021/ol5037216
Induja DK, Jesmina ARS, Joseph MM, Shamjith S, Ingaladal N, Maiti KK, Lankalapalli RS (2023) Isolation of two new stereochemical variants of streptophenazine by cocultivation of Streptomyces NIIST-D31, Streptomyces NIIST-D47, and Streptomyces NIIST-D63 strains in 3 C 2 combinations. J Antibiot 1–12. https://doi.org/10.1038/s41429-023-00638-7
Ivshina I, Bazhutin G, Tyan S, Polygalov M, Subbotina M, Tyumina E (2022) Cellular modifications of Rhodococci exposed to separate and combined effects of pharmaceutical pollutants. Microorganisms 10(6):1101. https://doi.org/10.3390/microorganisms10061101
Janardhan A, Kumar AP, Viswanath B, Saigopal DVR, Narasimha G (2014) Production of bioactive compounds by actinomycetes and their antioxidant properties. Biotechnol Res Int 2014. https://doi.org/10.1155/2014/217030
Jasni N, Saidin S, Arifin N, Azman DK, Shin LN, Othman N (2022) A Review: natural and synthetic compounds targeting Entamoeba histolytica and its biological membrane. Membranes 12(4):396. https://doi.org/10.3390/membranes12040396
Javed Z, Tripathi GD, Mishra M, Dashora K (2021) Actinomycetes-The microbial machinery for the organic-cycling, plant growth, and sustainable soil health. Biocatal Agric Biotechnol 31:101893. https://doi.org/10.1016/j.bcab.2020.101893
Jiang YJ, Zhang DS, Zhang HJ, Li JQ, Ding WJ, Xu CD, Ma ZJ (2018) Medermycin-type naphthoquinones from the marine-derived Streptomyces sp. XMA39. J Nat Prod 81(9):2120–2124. https://doi.org/10.1021/acs.jnatprod.8b00544
Jones SE, Ho L, Rees CA, Hill JE, Nodwell JR, Elliot MA (2017) Streptomyces exploration is triggered by fungal interactions and volatile signals. Elife 6:e21738. https://doi.org/10.7554/eLife.21738
Kang S, Han J, Jang SC, An JS, Kang I, Kwon Y, Oh DC (2022) Epoxinnamide: an epoxy cinnamoyl-containing nonribosomal peptide from an intertidal mudflat-derived Streptomyces sp. Mar Drugs 20(7):455. https://doi.org/10.3390/md20070455
Karim MRU, In Y, Zhou T, Harunari E, Oku N, Igarashi Y (2021) Nyuzenamides A and B: bicyclic peptides with antifungal and cytotoxic activity from a marine-derived Streptomyces sp. Org Lett 23(6):2109–2113. https://doi.org/10.1021/acs.orglett.1c00210
Kawahara T, Ueda M, Kishimoto N, Yasutake T, Misumi S, Devkota HP, Wada M (2023) Amamine, an isoquinoline alkaloid from the Kitasatospora sp. HGTA304. J Antibiot 1–3. https://doi.org/10.1038/s41429-023-00641-y
Kekuda TP, Shobha KS, Onkarappa R (2010) Fascinating diversity and potent biological activities of Actinomycete metabolites. J Pharm Res 3(2):250–256
Kim DG, Moon K, Kim SH, Park SH, Park S, Lee SK, Oh DC (2012) Bahamaolides A and B, antifungal polyene polyol macrolides from the marine actinomycete Streptomyces sp. J Nat Prod 75(5):959–967. https://doi.org/10.1021/np3001915
Kim SJ, Cantrell CL, Avula B, Chen J, Schrader KK, Santo SN, Khan IA (2022) Streptomyces distallicus, a potential microbial biolarvicide. J Agric Food Chem 70(36):11274–11280. https://doi.org/10.1021/acs.jafc.2c03537
Kim HY, Kim JD, Hong JS, Ham JH, Kim BS (2013) Identification of antifungal niphimycin from Streptomyces sp. KP 6107 by screening based on adenylate kinase assay. J Basic Microbiol 53(7):581–589. https://doi.org/10.1002/jobm.201200045
Kim HJ, Bo AB, Kim JD, Kim YS, Khaitov B, Ko YK, Choi JS (2020) Herbicidal characteristics and structural identification of the potential active compounds from Streptomyces sp. KRA17-580. J Agric Food Chem 68(52):15373–15380. https://doi.org/10.1021/acs.jafc.0c01974
Kokkini M, González Heredia C, Oves-Costales D, De la Cruz M, Sánchez P, Martín J, Reyes F (2022) Exploring Micromonospora as phocoenamicins producers. Mar Drugs 20(12):769. https://doi.org/10.3390/md20120769
Komaki H (2023) Recent progress of reclassification of the genus streptomyces. Microorganisms 11(4):831. https://doi.org/10.3390/microorganisms11040831
Komaki H, Tamura T, Igarashi Y (2023) Taxonomic positions and secondary metabolite-biosynthetic gene clusters of akazaoxime-and levantilide-producers. Life 13(2):542. https://doi.org/10.3390/life13020542
Kumsiri B, Pekkoh J, Pathom-Aree W, Lumyong S, Phinyo K, Pumas C, Srinuanpan S (2021) Enhanced production of microalgal biomass and lipid as an environmentally friendly biodiesel feedstock through actinomycete co-culture in biogas digestate effluent. Bioresour Technol 337:125446. https://doi.org/10.1016/j.biortech.2021.125446
Lacey HJ, Rutledge PJ (2022) Recently discovered secondary metabolites from Streptomyces species. Molecules 27(3):887. https://doi.org/10.3390/molecules27030887
Law JW, Law LNS, Letchumanan V, Tan LTH, Wong SH, Chan KG, Lee LH (2020) Anticancer drug discovery from microbial sources: the unique mangrove streptomycetes. Molecules 25(22):5365. https://doi.org/10.3390/molecules25225365
Le Loarer A, Marcellin-Gros R, Dufossé L, Bignon J, Frédérich M, Ledoux A, Fouillaud M (2023) Prioritization of microorganisms isolated from the Indian Ocean Sponge Scopalina hapalia based on metabolomic diversity and biological activity for the discovery of natural products. Microorganisms 11(3):697. https://doi.org/10.3390/microorganisms11030697
Leetanasaksakul K, Koomsiri W, Suga T, Matsuo H, Hokari R, Wattana-Amorn P, Thamchaipenet A (2022) Sattahipmycin, a hexacyclic xanthone produced by a marine-derived Streptomyces. J Nat Prod 85(5):1211–1217. https://doi.org/10.1021/acs.jnatprod.1c00870
Leiros M, Alonso E, Sanchez JA, Rateb ME, EbelR HWE, Botana LM (2014) Mitigation of ROS insults by Streptomyces secondary metabolites in primary cortical neurons. ACS Chem Neurosci 5(1):71–80. https://doi.org/10.1021/cn4001878
Li J, Zhao GZ, Chen HH, Wang HB, Qin S, Zhu WY, Li WJ (2008) Antitumour and antimicrobial activities of endophytic streptomycetes from pharmaceutical plants in rainforest. Lett Appl Microbiol 47(6):574–580. https://doi.org/10.1111/j.1472-765X.2008.02470.x
Li K, Li QL, Ji NY, Liu B, Zhang W, Cao XP (2011) Deoxyuridines from the marine sponge associated actinomycete Streptomyces microflavus. Mar Drugs 9(5):690–695. https://doi.org/10.3390/md9050690
Li L, Maclntyre LW, Brady SF (2021) Refactoring biosynthetic gene clusters for heterologous production of microbial natural products. Curr Opin Biotechnol 69:145–152. https://doi.org/10.1016/j.copbio.2020.12.011
Li X, Li B, Cai S, Zhang Y, Xu M, Zhang C, Qin S (2020a) Identification of rhizospheric actinomycete Streptomyces lavendulae sps-33 and the inhibitory effect of its volatile organic compounds against Ceratocystis fimbriata in postharvest sweet potato (Ipomoea batatas (L.) Lam.). Microorganisms 8(3):319. https://doi.org/10.3390/microorganisms8030319
Li H, Zhang M. Li H, Yu H, Chen S, Wu W, Sun P (2020b) Discovery of venturicidin congeners and identification of the biosynthetic gene cluster from Streptomyces sp. NRRL S-4. J Nat Prod 84(1):110–119. https://doi.org/10.1021/acs.jnatprod.0c01177
Li W, Ding L, Li J, Wen H, Liu Y, Tan S, He S (2022) Novel antimycin analogues with agricultural antifungal activities from the sponge-associated actinomycete Streptomyces sp. NBU3104. J Agric Food Chem 70(27):8309–8316. https://doi.org/10.1021/acs.jafc.2c02626
Li Y, Ding X, Du Y, Li Y, Ren W, Lu Y, Hong B (2023a) Genome-directed discovery of bicyclic cinnamoyl-containing nonribosomal peptides with anticoronaviral activity from Streptomyces griseus. Org Lett 25(26):4874–4879. https://doi.org/10.1021/acs.orglett.3c01683
Li Y, Xu Z, Chen P, Zuo C, Chen L, Yan W, Ye Y (2023b) Genome mining and heterologous expression guided the discovery of antimicrobial naphthocyclinones from Streptomyces eurocidicus CGMCC 4.1086. J Agric Food Chem 71(6):2914–2923. https://doi.org/10.1021/acs.jafc.2c06928
Liu CX, Zhang J, Wang XJ, Qian PT, Wang JD, Gao YM, Xiang WS (2012a) Antifungal activity of borrelidin produced by a Streptomyces strain isolated from soybean. J Agric Food Chem 60(5):1251–1257. https://doi.org/10.1021/jf2044982
Liu X, Gan M, Dong B, Zhang T, Li Y, Zhang Y, Si S (2012b) 4862F, a new inhibitor of HIV-1 protease, from the culture of Streptomyces I03A–04862. Molecules 18(1):236–243. https://doi.org/10.3390/molecules18010236
Liu Q, Liu Z, Sun C, Shao M, Ma J, Wei X, Ju J (2019a) Discovery and biosynthesis of atrovimycin, an antitubercular and antifungal cyclodepsipeptide featuring vicinal-dihydroxylated cinnamic acyl chain. Org Lett 21(8):2634–2638. https://doi.org/10.1021/acs.orglett.9b00618
Liu C, Zhuang X, Yu Z, Wang Z, Wang Y, Guo X, Huang S (2019b) Community structures and antifungal activity of root-associated endophytic actinobacteria of healthy and diseased soybean. Microorganisms 7(8):243. https://doi.org/10.3390/microorganisms7080243
Liu H, An M, Si H, Shan Y, Xu C, Hu G, Wu Y (2022a) Identification of cyclic dipeptides and a new compound (6-(5-Hydroxy-6-methylheptyl)-5, 6-dihydro-2 H-pyran-2-one) produced by Streptomyces fungicidicus against Alternaria solani. Molecules 27(17):5649. https://doi.org/10.3390/molecules27175649
Liu Z, Yashiroda Y, Sun P, Ma H, Wang Y, Li L, Sun Y (2022b) Argenteolides A and B, glycosylated polyketide-peptide hybrid macrolides from an actinomycete Streptomyces argenteolus. Org Lett 25(4):571–575. https://doi.org/10.1021/acs.orglett.2c03290
Lugtenberg B (2015) Life of microbes in the rhizosphere. Principles of plant-microbe interactions: microbes for sustainable agriculture. 7–15. https://doi.org/10.1007/978-3-319-08575-3_3
Ma J, Cao B, Liu C, Guan P, Mu Y, Jiang Y, Huang X (2018) Actinofuranones DI from a lichen-associated actinomycetes, streptomyces gramineus, and their anti-inflammatory effects. Molecules 23(9):2393. https://doi.org/10.3390/molecules23092393
Mahajan GB, Balachandran L (2012) Antibacterial agents from actinomycetes-a review. Front Biosci-Elite 4(1):240–253
Mahmud T (2003) The C 7 N aminocyclitol family of natural products. Nat Prod Rep 20(1):137–166
Martinet L, Naômé A, Deflandre B, Maciejewska M, Tellatin D, Tenconi E, Rigali S (2019) A single biosynthetic gene cluster is responsible for the production of bagremycin antibiotics and ferroverdin iron chelators. Mbio 10(4):10–1128. https://doi.org/10.1128/mbio.01230-19
Matarrita-Carranza B, Moreira-Soto RD, Murillo-Cruz C, Mora M, Currie CR, Pinto-Tomas AA (2017) Evidence for widespread associations between neotropical hymenopteran insects and Actinobacteria. Front Microbiol 2016. https://doi.org/10.3389/fmicb.2017.02016
Menegatti C, Lourenzon VB, Rodriguez-Hernandez D, Da Paixao Melo WG, Ferreira LLG, Andricopul AD, Pupo MT (2020) Meliponamycins: antimicrobials from stingless bee-associated Streptomyces sp. J Nat Prod 83(3):610–616. https://doi.org/10.1021/acs.jnatprod.9b01011
Mitova MI, Lang G, Wiese J, Imhoff JF (2008) Subinhibitory concentrations of antibiotics induce phenazine production in a marine Streptomyces sp. J Nat Prod 71(5):824–827. https://doi.org/10.1021/np800032a
Mohammadipanah F, Kermani F, Salimi F (2020) Awakening the secondary metabolite pathways of ProMicromonospora kermanensis using physicochemical and biological elicitors. Appl Biochem Biotechnol 192:1224–1237. https://doi.org/10.1007/s12010-020-03361-3
Moon K, Xu F, Zhang C, Seyedsayamdost MR (2019) Bioactivity-HiTES unveils cryptic antibiotics encoded in actinomycete bacteria. ACS Chem Biol 14(4):767–774. https://doi.org/10.1021/acschembio.9b00049
Moran MA, Rutherford LT, Hodson RE (1995) Evidence for indigenous Streptomyces populations in a marine environment determined with a 16S rRNA probe. Appl Environ Microbiol 61(10):3695–3700. https://doi.org/10.1128/aem.61.10.3695-3700.1995
Muhammad S, Qaisar M, Iqbal J, Khera RA, Al-Sehemi AG, Alarfaji SS, Adnan M (2022) Exploring the inhibitory potential of novel bioactive compounds from mangrove actinomycetes against nsp10 the major activator of SARS-CoV-2 replication. Chem Pap 76(5):3051–3064. https://doi.org/10.1007/s11696-021-01997-x
Mullowney et al, 2015Mullowney MW, Ó hAinmhire E, Tanouye U, Burdette JE, Pham VC, Murphy BT (2015) A pimarane diterpene and cytotoxic angucyclines from a marine-derived Micromonospora sp. in Vietnam’s east sea. Mar Drugs 13(9):5815-5827. https://doi.org/10.3390/md13095815
Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, Naghavi M (2022) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399(10325):629–655. https://doi.org/10.1016/S0140-6736(21)02724-0
Nagarajan M, Rajesh Kumar R, Meenakshi Sundaram K, Sundararaman M (2015) Marine biotechnology: Potentials of marine microbes and algae with reference to pharmacological and commercial values. Plant Biology and Biotechnology: Volume II: Plant Genomics and Biotechnology, 685–723. https://doi.org/10.1007/978-81-322-2283-5_35
Nair H, Nokes DJ, Gessner BD, Dherani M, Madh SA, Singleton RJ, Campbell H (2010) Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375(9725):1545–1555. https://doi.org/10.1016/S0140-6736(10)60206-1
Nalini MS, Prakash HS (2017) Diversity and bioprospecting of actinomycete endophytes from the medicinal plants. Lett Appl Microbiol 64(4):261–270. https://doi.org/10.1111/lam.12718
Okeke IN, Lamikanra A, Edelman R (1999) Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg Infect Dis 5(1):18. https://doi.org/10.3201/eid0501.990103
Olanrewaju OS, Babalola OO (2019) Streptomyces: implications and interactions in plant growth promotion. Appl Microbiol Biotechnol 103:1179–1188. https://doi.org/10.1007/s00253-018-09577-y
Oliveira J, Almeida PL, Sobral RG, Lourenço ND, Gaudêncio SP (2022) Marine-derived actinomycetes: biodegradation of plastics and formation of PHA bioplastics—a circular bioeconomy approach. Mar Drugs 20(12):760. https://doi.org/10.3390/md20120760
Osaro-Matthew RC, Ire FS, Frank-Peterside N (2020) Screening of actinomycetes from turmeric (Curcuma longa L.) and ginger (Zingiber officinale) rhizosphere for antifungal activity. J Adv Microbiol 20(2):18–28. https://doi.org/10.9734/JAMB/2020/v20i230214
Ouhdouch Y, Barakate M, Finance C (2001) Actinomycetes of Moroccan habitats: isolation and screening for antifungal activities. Eur J Soil Biol 37(2):69–74. https://doi.org/10.1016/S1164-5563(01)01069-X
Ouyang Y, Huang JJ, Wang YL, Zhong H, Song BA, Hao GF (2021) In Silico resources of drug-likeness as a mirror: what are we lacking in pesticide-likeness? J Agric Food Chem 69(37):10761–10773. https://doi.org/10.1021/acs.jafc.1c01460
Oyedoh OP, Yang W, Dhanasekaran D, Santoyo G, Glick BR, Babalola OO (2023a) Rare rhizo-Actinomycetes: a new source of agroactive metabolites. Biotechnol Adv 108205. https://doi.org/10.1016/j.biotechadv.2023.108205
Oyedoh OP, Yang W, Dhanasekaran D, Santoyo G, Glick BR, Babalola OO (2023b) Sustainable agriculture: rare-actinomycetes to the rescue. Agronomy 13(3):666. https://doi.org/10.3390/agronomy13030666
Pagmadulam B, Tserendulam D, Rentsenkhand T, Igarashi M, Sawa R, Nihei CI, Nishikawa Y (2020) Isolation and characterization of antiprotozoal compound-producing Streptomyces species from Mongolian soils. Parasitol Int 74:101961. https://doi.org/10.1016/j.parint.2019.101961
Parkin DM (2001) Global cancer statistics in the year 2000. Lancet Oncol 2(9):533–543. https://doi.org/10.1016/S1470-2045(01)00486-7
Pereira F, Almeida JR, Paulino M, Grilo IR, Macedo H, Cunha I, Gaudêncio SP (2020) Antifouling napyradiomycins from marine-derived actinomycetes Streptomyces aculeolatus. Mar Drugs 18(1):63. https://doi.org/10.3390/md18010063
Pettit G R, Tan R, Pettit RK, Smith TH, Feng S, Doubek DL, Chapuis JC (2007) Antineoplastic agents. 560. Isolation and structure of kitastatin 1 from an Alaskan Kitasatospora sp. J Nat Prod 70(7):1069–1072. https://doi.org/10.1021/np068072c
Pimentel MR, Molina G, Dionísio AP, Maróstica Junior MR, Pastore GM (2011) The use of endophytes to obtain bioactive compounds and their application in biotransformation process. Biotechnol Res Int 2011. https://doi.org/10.4061/2011/576286
Pristov KE, Ghannoum MA (2019) Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect 25(7):792–798. https://doi.org/10.1016/j.cmi.2019.03.028
Qian Z, Bruhn T, D’Agostino PM, Herrmann A, Haslbeck M, Antal N, Gulder TA (2019) Discovery of the streptoketides by direct cloning and rapid heterologous expression of a cryptic PKS II gene cluster from Streptomyces sp. Tu 6314. J Org Chem 85(2):664–673. https://doi.org/10.1021/acs.joc.9b02741
Quinn GA, Banat AM, Abdelhameed AM, Banat IM (2020) Streptomyces from traditional medicine: sources of new innovations in antibiotic discovery. J Med Microbiol 69(8):1040. https://doi.org/10.1099/jmm.0.001232
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Can Res 74(11):2913–2921. https://doi.org/10.1158/0008-5472.CAN-14-0155
Rajivgandhi G, Muneeswaran T, Maruthupandy M, Ramakritinan CM, Saravanan K, Ravikumar V, Manoharan N (2018) Antibacterial and anticancer potential of marine endophytic actinomycetes Streptomyces coeruleorubidus GRG 4 (KY457708) compound against colistin resistant uropathogens and A549 lung cancer cells. Microb Pathog 125:325–335. https://doi.org/10.1016/j.micpath.2018.09.025
Ran Y, Zhang Y, Wang X, Li G (2022) Nematicidal Metabolites from the Actinomycete Micromonospora sp. WH06. Microorganisms 10(11):2274. https://doi.org/10.3390/microorganisms10112274
Rausch K, Hackett BA, Weinbren NL, Reeder SM, Sadovsky Y, Hunter CA, Cherry S (2017) Screening bioactives reveals nanchangmycin as a broad spectrum antiviral active against Zika virus. Cell Rep 18(3):804–815. https://doi.org/10.1016/j.celrep.2016.12.068
Raymaekers K, Ponet L, Holtappels D, Berckmans B, Cammue BP (2020) Screening for novel biocontrol agents applicable in plant disease management–a review. Biol Control 144:104240. https://doi.org/10.1016/j.biocontrol.2020.104240
Roper NA, Bilous RW, Kelly WF, Unwin NC, Connolly VM (2002) Cause-specific mortality in a population with diabetes: South Tees Diabetes Mortality Study. Diabetes Care 25(1):43–48. https://doi.org/10.2337/diacare.25.1.43
Rosenblueth M, Ormeño-Orrillo E, López-López A, Rogel MA, Reyes-Hernández BJ, Martínez-Romero JC, Martínez-Romero E (2018) Nitrogen fixation in cereals. Front Microbiol 9:1794. https://doi.org/10.3389/fmicb.2018.01794
Rutledge PJ, Challis GL (2015) Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat Rev Microbiol 13(8):509–523. https://doi.org/10.1038/nrmicro3496
Saiz JC, Martín-Acebes MA (2017) The race to find antivirals for Zika virus. Antimicrob Agents Chemother 61(6):e00411-e417. https://doi.org/10.1128/AAC.00411-17
Salam N, Khieu TN, Liu MJ, Vu TT, Chu-Ky S, Quach NT, Li J (2017) Endophytic actinobacteria associated with Dracaena cochinchinensis Lour.: isolation, diversity, and their cytotoxic activities. BioMed Res Int 2017. https://doi.org/10.1155/2017/1308563
Santos OCS, Soares AR, Machado FLS, Romanos MTV, Muricy G, Giambiagi-deMarval M, Laport MS (2015) Investigation of biotechnological potential of sponge-associated bacteria collected in Brazilian coast. Lett Appl Microbiol 60(2):140–147. https://doi.org/10.1111/lam.12347
Sarkar G, Suthindhiran K (2022) Diversity and biotechnological potential of marine actinomycetes from India. Indian J Microbiol 62(4):475–493. https://doi.org/10.1007/s12088-022-01024-x
Sarmiento-Vizcaíno A, Braña AF, Pérez-Victoria I, Martín J, De Pedro N, Cruz MDL, Blanco G (2017) Paulomycin G, a new natural product with cytotoxic activity against tumor cell lines produced by deep-sea sediment derived Micromonospora matsumotoense M-412 from the Avilés Canyon in the Cantabrian Sea. Mar Drugs 15(9):271. https://doi.org/10.3390/md15090271
Schatz A, Bugle E, Waksman SA (1944) Streptomycin, a substance exhibiting antibiotic activity against gram-positive and gram-negative bacteria.∗. Proc Soc Exp Biol Med 55(1):66–69. https://doi.org/10.3181/00379727-55-14461
Schneider K, Rose I, Vikineswary S, Jones AL, Goodfellow M, Nicholson G, Fiedler HP (2007) Nocardichelins A and B, siderophores from Nocardia strain acta 3026. J Nat Prod 70(6):932–935. https://doi.org/10.1021/np060612i
Schwabe R, Anke MK, Szymańska K, Wiche O, Tischler D (2018) Analysis of desferrioxamine-like siderophores and their capability to selectively bind metals and metalloids: development of a robust analytical RP-HPLC method. Res Microbiol 169(10):598–607. https://doi.org/10.1016/j.resmic.2018.08.002
Seenak P, Kumphune S, Malakul W, Chotima R, Nernpermpisooth N (2021) Pineapple consumption reduced cardiac oxidative stress and inflammation in high cholesterol diet-fed rats. Nutr Metab 18:1–10. https://doi.org/10.1186/s12986-021-00566-z
Seidu S, Barrat J, Khunti K (2020) Clinical update: the important role of dual kidney function testing (ACR and eGFR) in primary care: identification of risk and management in type 2 diabetes. Prim Care Diabetes 14(4):370–375. https://doi.org/10.1016/j.pcd.2020.02.006
Selim MSM, Abdelhamid SA, Mohamed SS (2021) Secondary metabolites and biodiversity of actinomycetes. J Genet Eng Biotechnol 19(1):72. https://doi.org/10.1186/s43141-021-00156-9
Semenova EM, Babich TL, Sokolova DS, Ershov AP, Raievska YI, Bidzhieva SK, Nazina TN (2022) Microbial communities of seawater and coastal soil of Russian Arctic region and their potential for bioremediation from hydrocarbon pollutants. Microorganisms 10(8):1490. https://doi.org/10.3390/microorganisms10081490
Seo J, Shin YH, Jo SJ, Du YE, Um S, Kim Y R, Moon K (2022) Cystargamides C and D, new cyclic lipopeptides from a tidal mudflat-derived Streptomyces sp. JMS132. Front Microbiol 13:904954. https://doi.org/10.3389/fmicb.2022.904954
Shan W, Zhou Y, Liu H, Yu X (2018) Endophytic actinomycetes from tea plants (Camellia sinensis): isolation, abundance, antimicrobial, and plant-growth-promoting activities. BioMed Res Int 2018. https://doi.org/10.1155/2018/1470305
Shin D, Byun WS, Moon K, Kwon Y, Bae M, Um S, Oh DC (2018) Coculture of marine Streptomyces sp. with Bacillus sp. produces a new piperazic acid-bearing cyclic peptide. Front Chem 6:498. https://doi.org/10.3389/fchem.2018.00498
Silva LJ, Crevelin EJ, Souza DT, Lacerda-Júnior GV, De Oliveira VM, Ruiz ALTG, Melo IS (2020) Actinobacteria from Antarctica as a source for anticancer discovery. Sci Rep 10(1):13870. https://doi.org/10.1038/s41598-020-69786-2
Sissoko MS, Healy SA, Katile A, Omaswa F, Zaidi I, Gabriel EE, Duffy PE (2017) Safety and efficacy of Pfspz Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis 17(5):498–509. https://doi.org/10.1016/S1473-3099(17)30104-4
Stephens CM, Adams-Sapper S, Sekhon M, Johnson JR, Riley LW (2017) Genomic analysis of factors associated with low prevalence of antibiotic resistance in extraintestinal pathogenic Escherichia coli sequence type 95 strains. Msphere 2(2):e00390-e416. https://doi.org/10.1128/mSphere.00390-16
Sun X, Wang G, Xiao H, Jiang J, Xiao D, Xing B, Ma M (2020) Strepimidazoles A–G from the plant endophytic Streptomyces sp. PKU-EA00015 with inhibitory activities against a plant pathogenic fungus. J Nat Prod 83(7):2246–2254. https://doi.org/10.1021/acs.jnatprod.0c00362
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Szczeblewski P, Laskowski T, Kubacki B, Dziergowska M, Liczmańska M, Grynda J, Borowski E (2017) Analytical studies on ascosin, candicidin and levorin multicomponent antifungal antibiotic complexes. The stereostructure of ascosin A2. Sci Rep 7(1):40158. https://doi.org/10.1038/srep40158
Taechowisan T, Chaisaeng S, Phutdhawong WS (2017) Antibacterial, antioxidant and anticancer activities of biphenyls from Streptomyces sp. BO-07: an endophyte in Boesenbergia rotunda (L.) Mansf A. Food Agric Immunol 28(6):1330–1346. https://doi.org/10.1080/09540105.2017.1339669
Takahashi Y (2017) Genus Kitasatospora, taxonomic features and diversity of secondary metabolites. J Antibiot 70(5):506–513. https://doi.org/10.1038/ja.2017.8
Tanaka Y, Izawa M, Hiraga Y, Misaki Y, Watanabe T, Ochi K (2017) Metabolic perturbation to enhance polyketide and nonribosomal peptide antibiotic production using triclosan and ribosome-targeting drugs. Appl Microbiol Biotechnol 101:4417–4431. https://doi.org/10.1007/s00253-017-8216-6
Tanvir R, Sajid I, Hasnain S, Kulik A, Grond S (2016) Rare actinomycetes Nocardia caishijiensis and Pseudonocardia carboxydivorans as endophytes, their bioactivity and metabolites evaluation. Microbiol Res 185:22–35. https://doi.org/10.1016/j.micres.2016.01.003
Tanvir R, Sheikh AA, Javeed A (2019) Endophytic actinomycetes in the biosynthesis of bioactive metabolites: chemical diversity and the role of medicinal plants. Stud Nat Prod Chem 60:399–424. https://doi.org/10.1016/B978-0-444-64181-6.00011-5
Tenebro CP, Trono DJVL, Vicera CVB, Sabido EM, Ysulat JA Jr, Macaspac AJM, Dalisay DS (2021) Multiple strain analysis of Streptomyces species from Philippine marine sediments reveals intraspecies heterogeneity in antibiotic activities. Sci Rep 11(1):17544. https://doi.org/10.1038/s41598-021-96886-4
Tong L, Sun W, Wu S, Han Y (2022) Characterization of Caerulomycin A as a dual-targeting anticancer agent. Eur J Pharmacol 922:174914. https://doi.org/10.1016/j.ejphar.2022.174914
Trivedi P, Leach JE, Tringe SG, Sa T, Singh BK (2020) Plant–microbiome interactions: from community assembly to plant health. Nat Rev Microbiol 18(11):607–621. https://doi.org/10.1038/s41579-020-0412-1
Ujváry I (2010) Pest control agents from natural products. In: Hayes' Handbook of Pesticide Toxicology, pp 119–229. Academic Press. https://doi.org/10.1016/B978-0-12-374367-1.00003-3
Um S, Guo H, Thiengmag S, Benndorf R, Murphy R, Rischer M, Beemelmanns C (2021) Comparative genomic and metabolic analysis of Streptomyces sp. RB110 morphotypes illuminates genomic rearrangements and formation of a new 46-membered antimicrobial macrolide. ACS Chem Biol 16(8): 1482–1492. https://doi.org/10.1021/acschembio.1c00357
Van Bergeijk DA, Terlouw BR, Medema MH, Van Wezel GP (2020) Ecology and genomics of Actinobacteria: new concepts for natural product discovery. Nat Rev Microbiol 18(10):546–558. https://doi.org/10.1038/s41579-020-0379-y
Van der Ent S, Van Wees SC, Pieterse M (2009) Jasmonate signaling in plant interactions with resistance-inducing beneficial microbes. Phytochemistry 70(13–14):1581–1588. https://doi.org/10.1016/j.phytochem.2009.06.009
Van der Meij A, Worsley SF, Hutchings MI, van Wezel GP (2017) Chemical ecology of antibiotic production by actinomycetes. FEMS Microbiol Rev 41(3):392–416. https://doi.org/10.1093/femsre/fux005
Van der Meij A, Willemse J, Schneijderberg MA, Geurts R, Raaijmakers JM, Van Wezel GP (2018) Inter-and intracellular colonization of Arabidopsis roots by endophytic actinobacteria and the impact of plant hormones on their antimicrobial activity. Antonie Van Leeuwenhoek 111(679–690):90. https://doi.org/10.1007/s10482-018-1014-z
Waksman SA, Woodruff HB (1940) The soil as a source of microorganisms antagonistic to disease-producing bacteria. J Bacteriol 40(4):581–600
Wang M, Carver JJ, Phelan VV, Sanchez LM, Garg N, Peng Y, Bandeira N (2016) Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat Biotechnol 34(8):828–837. https://doi.org/10.1038/nbt.3597
Wang J, Cong Z, Huang X, Hou C, Chen W, Tu Z, Liu Y (2018) Soliseptide A, a cyclic hexapeptide possessing piperazic acid groups from Streptomyces solisilvae HNM30702. Org Lett 20(5):1371–1374. https://doi.org/10.1021/acs.orglett.8b00142
Wang C, Wang J, Yuan J, Jiang L, Jiang X, Yang B, Huang D (2019b) Generation of Streptomyces hygroscopicus cell factories with enhanced ascomycin production by combined elicitation and pathway-engineering strategies. Biotechnol Bioeng 116(12):3382–3395. https://doi.org/10.1002/bit.27158
Wang Z, Wen Z, Liu L, Zhu X, Shen B, Yan X, Huang Y (2019c) Yangpumicins F and G, enediyne congeners from Micromonospora yangpuensis DSM 45577. J Nat Prod 82(9):2483–2488. https://doi.org/10.1021/acs.jnatprod.9b00229
Wang X, Elshahawi SI, Shaaban KA, Fang L, Ponomareva LV, Zhang Y, Thorson JS (2014) Ruthmycin, a new tetracyclic polyketide from Streptomyces sp. RM-4–15. Org Lett 16(2):456–459. https://doi.org/10.1021/ol4033418
Wang W, Song T, Chai W, Chen L, Chen L, Lian XY, Zhang Z (2017) Rare polyene-polyol macrolides from mangrove-derived Streptomyces sp. ZQ4BG. Sci Rep 7(1):1703. https://doi.org/10.1038/s41598-017-01912-z
Wang X, Elshahawi SI, Ponomareva LV, Ye Q, Liu Y, Copley GC Shaaban KA (2019a) Structure determination, functional characterization, and biosynthetic implications of nybomycin metabolites from a mining reclamation site-associated Streptomyces. J Nat Prod 82(12): 3469-3476. https://doi.org/10.1021/acs.jnatprod.9b01015
Wang K, Ke S, Fang W, Wu Z, Zhang Y (2022) Novel Agroactive secondary metabolites from actinomycetes in the past two decades with focus on screening strategies and discovery. Natural Products from Actinomycetes: Diversity, Ecology and Drug Discovery, 199–221. https://doi.org/10.1007/978-981-16-6132-7_9
Wang Y, Yang D, Yu Z (2023) New lactones produced by Streptomyces sp. SN5431 and their antifungal activity against Bipolaris maydis. Microorganisms 11(3):616. https://doi.org/10.3390/microorganisms11030616
Wibowo M, Gotfredsen CH, Sassetti E, Melchiorsen J, Clausen MH, Gram L, Ding L (2020) Azodyrecins A-C: azoxides from a soil-derived Streptomyces species. J Nat Prod 83(12):3519–3525. https://doi.org/10.1021/acs.jnatprod.0c00339
Wilson GC, Bushell ME (1995) The induction of antibiotic synthesis in Saccharopolyspora erythraea and Streptomyces hygroscopicus by growth rate decrease is accompanied by a down-regulation of protein synthesis rate. FEMS Microbiol Lett 129(1):89–96. https://doi.org/10.1111/j.1574-6968.1995.tb07562.x
Woodruff, 2014Woodruff HB (2014) Selman A. Waksman, winner of the 1952 Nobel Prize for physiology or medicine. Appl Environ Microbiol 80(1):2-8. https://doi.org/10.1128/AEM.01143-13
World Health Organization (2016) World Health Organization statistics 2016: monitoring health for the SDGs sustainable development goals. https://www.who.int/data/gho/data/themes/antimicrobial-resistance-(amr). Accessed 15 May 2022
World Health Organization (2021) World Health Organization: Antimicrobial resistance fact sheet. Geneva (2021) https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Accessed 30 May 2022
World Health Organization (2022a) Global values are median of country figures and are not population weighted averages. Global Antimicrobial Resistance Surveillance System (GLASS). Geneva. https://apps.who.int/iris/bitstream/handle/10665/277175/WHO-WSI-AMR-2018.4-eng.pdf. Accessed 28 May 2022
World Health Organization (2022b) World health statistics 2022: Monitoring health for the SDGs, sustainable development goals. https://www.who.int/publications/i/item/9789240051157. Accessed 20 May 2022
Wright PM, Seiple IB, Myers AG (2014) The evolving role of chemical synthesis in antibacterial drug discovery. Angew Chem Int Ed 53(34):8840–8869. https://doi.org/10.1002/anie.201310843
Wu C, Du C, Gubbens J, Choi YH, Van Wezel GP (2015a) Metabolomics-driven discovery of a prenylated isatin antibiotic produced by Streptomyces species MBT28. J Nat Prod 78(10):2355–2363. https://doi.org/10.1021/acs.jnatprod.5b00276
Wu C, van Wezel G, Hae Choi Y (2015b) Identification of novel endophenaside antibiotics produced by Kitasatospora sp. MBT66. J Antibiot 68:445–452. https://doi.org/10.1038/ja.2015.14
Wu P, Chen K, Li B, Zhang Y, Wu H, Chen Y, Zhang B (2021) Polyketide starter and extender units serve as regulatory ligands to coordinate the biosynthesis of antibiotics in actinomycetes. Mbio 12(5):e02298-21. https://doi.org/10.1128/mBio.02298-21
Wyche TP, Hou Y, Vazquez-Rivera E, Braun D, Bugni TS (2012) Peptidolipins B-F, antibacterial lipopeptides from an ascidian-derived Nocardia sp. J Nat Prod 75(4):735–740. https://doi.org/10.1021/np300016r
Xie X, Lu S, Pan X, Zou M, Li F, Lin H, He J (2021) Antiviral bafilomycins from a feces-inhabiting Streptomyces sp. J Nat Prod 84(2):537–543. https://doi.org/10.1021/acs.jnatprod.0c01243
Xu H, Yang J, Bai L, Deng Z, Mahmud T (2009) Genetically engineered production of 1, 1′-bis-valienamine and validienamycin in Streptomyces hygroscopicus and their conversion to valienamine. Appl Microbiol Biotechnol 81:895–902. https://doi.org/10.1007/s00253-008-1711-z
Xu F, Nazari B, Moon K, Bushin LB, Seyedsayamdost MR (2017) Discovery of a cryptic antifungal compound from Streptomyces albus J1074 using high-throughput elicitor screens. J Am Chem Soc 139(27):9203–9212. https://doi.org/10.1021/jacs.7b02716
Xu S, Wang JJ, Wei Y, Deng WW, Wan X, Bao GH, Ning J (2019) Metabolomics based on UHPLC-Orbitrap-MS and global natural product social molecular networking reveals effects of time scale and environment of storage on the metabolites and taste quality of raw Pu-erh tea. J Agric Food Chem 67(43):12084–12093. https://doi.org/10.1021/acs.jafc.9b05314
Yan S, Zeng M, Wang H, Zhang H (2022) Micromonospora: A prolific source of bioactive secondary metabolites with therapeutic potential. J Med Chem 65(13):8735–8771. https://doi.org/10.1021/acs.jmedchem.2c00626
Yu HL, Jiang SH, Bu XL, Wang JH, Weng JY, Yang XM, Xu MJ (2017) Structural diversity of anti-pancreatic cancer capsimycins identified in mangrove-derived Streptomyces xiamenensis 318 and post-modification via a novel cytochrome P450 monooxygenase. Sci Rep 7(1):40689. https://doi.org/10.1038/srep40689
Yuan G, Hong K, Lin H, She Z, Li J (2013) New azalomycin F analogs from mangrove Streptomyces sp. 211726 with activity against microbes and cancer cells. Mar Drugs 11(3):817–829. https://doi.org/10.3390/md11030817
Zhang YL, Li S, Jiang DH, Kong LC, Zhang PH, Xu JD (2013) Antifungal activities of metabolites produced by a termite-associated Streptomyces canus BYB02. J Agric Food Chem 61(7):1521–1524. https://doi.org/10.1021/jf2044982)
Zhang X, Gao Z, Zhang M, Jing F, Du J, Zhang L (2016) Analysis of endophytic actinobacteria species diversity in the stem of Gynura cusimbua by 16S rRNA gene clone library. Microbiology 85:379–385. https://doi.org/10.1134/S0026261716030176
Zhang F, Zhao M, Braun DR, Ericksen SS, Piotrowski JS, Nelson J, Bugni TS (2020) A marine microbiome antifungal targets urgent-threat drug-resistant fungi. Science 370(6519):974–978. https://doi.org/10.1126/science.abd6919
Zhang M, Kong L, Gong R, Iorio M, Donadio S, Deng Z, Chen W (2022) Biosynthesis of C-nucleoside antibiotics in actinobacteria: recent advances and future developments. Microbial Cell Factories 21(1):2. https://doi.org/10.1186/s12934-021-01722-z
Zhang D, Yi W, Ge H, Zhang Z, Wu B (2019) Bioactive streptoglutarimides A–J from the marine-derived Streptomyces sp. ZZ741. J Nat Prod 82(10): 2800–2808. https://doi.org/10.1021/acs.jnatprod.9b00481
Zhang Y, Cheema M T, Ponomareva LV, Ye Q, Liu T, Sajid I, Shaaban KA (2021) Himalaquinones A–G, Angucyclinone-Derived Metabolites Produced by the Himalayan Isolate Streptomyces sp. PU-MM59. J Nat Prod 84(7):1930–1940. https://doi.org/10.1021/acs.jnatprod.1c00192
Ziemert N, Lechner A, Wietz M, Millán-Aguiñaga N, Chavarria KL, Jensen PR (2014) Diversity and evolution of secondary metabolism in the marine actinomycete genus Salinispora. Proc Natl Acad Sci 111(12):E1130–E1139. https://doi.org/10.1073/pnas.132416111
Zong G, Fu J, Zhang P, Zhang W, Xu Y, Cao G, Zhang R (2022) Use of elicitors to enhance or activate the antibiotic production in Streptomyces. Crit Rev Biotechnol 42(8):1260–1283. https://doi.org/10.1080/07388551.2021.1987856
Acknowledgements
We would like to thank Universidade Federal do Amazonas (UFAM) and the Programa de Pós-graduação em Biotecnologia and Biodiversidade da Amazonia for their support. This work was funded by the Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM) via the call 007/2021—Programa Biodiversa and project POSGRAD 2021/2022, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES (Finance code 001). The authors also acknowledge the FAPEAM for the PhD scholarship awarded to Rafael de Souza Rodrigues and the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq for the research grant awarded to ADLS.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
RSR: Conceptualization, Methodology, Reviews and Literature review; AQLS: Curatorship and Supervision; MDOF: Reviews and Literature review; TCL A: Reviews and Literature review; ANB: Revision and Editing; SRSSS: Revisions; ADLS: Curatorship and Supervision. This study is part of the doctoral thesis of Rafael de Souza Rodrigues.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Souza Rodrigues, R., de Souza, A.Q.L., Feitoza, M.D.O. et al. Biotechnological potential of actinomycetes in the 21st century: a brief review. Antonie van Leeuwenhoek 117, 82 (2024). https://doi.org/10.1007/s10482-024-01964-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10482-024-01964-y