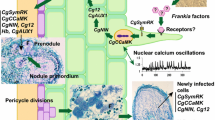
Abstract
Plants able to establish a nitrogen-fixing root nodule symbiosis with the actinobacterium Frankia are called actinorhizal. These interactions lead to the formation of new root organs, called actinorhizal nodules, where the bacteria are hosted intracellularly and fix atmospheric nitrogen thus providing the plant with an almost unlimited source of nitrogen for its nutrition. Like other symbiotic interactions, actinorhizal nodulation involves elaborate signalling between both partners of the symbiosis, leading to specific recognition between the plant and its compatible microbial partner, its accommodation inside plant cells and the development of functional root nodules. Actinorhizal nodulation shares many features with rhizobial nodulation but our knowledge on the molecular mechanisms involved in actinorhizal nodulation remains very scarce. However recent technical achievements for several actinorhizal species are allowing major discoveries in this field. In this review, we provide an outline on signalling molecules involved at different stages of actinorhizal nodule formation and the corresponding signalling pathways and gene networks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To cope with the rarity of assimilable nitrogen, a relatively small group of plants is able to form efficient symbiotic associations with soil bacteria capable of converting atmospheric dinitrogen into ammonium which can be used by plants thus covering their nitrogen needs. N2-fixing associations are restricted to four closely related orders: Fabales, Fagales, Cucurbitales and Rosales, all belonging to the clade of Fabids. These plants have developed highly sophisticated systems for housing bacterial diazotrophs in specialized root organs, called nodules. Two kinds of associations lead to the formation of root nodule symbiosis (RNS): those involving most legumes and Parasponia with proteobacteria collectively called rhizobia, and those involving a phylogenetically diverse group called actinorhizal plants able to interact with the actinobacteria Frankia. If the legumes/rhizobia symbiosis is largely known, the symbiotic association between Frankia and actinorhizal plants still remains poorly understood. However, phylogenomic studies confirmed that legumes and actinorhizal plants evolved from a common ancestor characterized by a “predisposition” to form symbiotic root nodules (Soltis et al. 1995; Doyle 2011; Griesmann et al. 2018) and actinorhizal symbioses emerged thus recently as original systems to explore developmental strategies to form nitrogen-fixing nodules.
Actinorhizal symbioses involve over 200 perennial species, mainly trees or shrubs with long generation times (Wall 2000) and only few of them are cultivated. In contrast to most rhizobia, the nitrogen-fixing symbiotic partners Frankia spp. are able to fix atmospheric nitrogen in soils or in symbiosis (in planta). Molecular phylogenetic approaches have identified four major Frankia lineages that have distinct plant host ranges (Tisa et al. 2016). However Frankia remains recalcitrant to stable genetic transformation making actinorhizal symbiosis difficult to study. The recent development of genetic transformation protocols for several actinorhizal species and huge amount of data generated with omics approaches have greatly expanded our knowledge on the interaction between Frankia and actinorhizal plants (Svistoonoff et al. 2014; Tisa et al. 2016; Gherbi et al. 2018). In this review, we report an inventory of the main recent results related to signalling molecules and gene networks involved in actinorhizal signalling.
Pre-infection signalling
The establishment of symbiotic relationships requires communication between the partners leading to a specific interaction. This recognition is a crucial step that is necessary for the selection of compatible microbes and to avoid entry of the numerous pathogens present in the soil. In most legumes the early signalling events involve the secretion of flavonoids by the host plant which act as chemotactic signals to rhizobia and induce the synthesis of lipo-chito-oligosaccharides called the Nod factors (NFs) that signal back to the plant (Oldroyd 2013). Chemoattraction and proliferation of Frankia bacteria was also reported in the rhizosphere of different actinorhizal species (Vessey et al. 2004) but direct evidence of flavonoids as early plant signals is still lacking. The presence of host plant root exudates increases the growth of Frankia and favours the infection and nodulation process (Gabbarini and Wall 2008; Gabbarini and Wall 2011; Beauchemin et al. 2012; Ktari et al. 2017a). Among the molecules found in root exudates from actinorhizal plants are flavonoids, which were shown to have strong impact on nodulation of Casuarina glauca (Auguy et al. 2011; Abdel-Lateif et al. 2013). Moreover, flavonoids extracted from seeds of Myrica gale (Fagales) were shown to promote Frankia growth and nitrogen fixation only in compatible strains (Popovici et al. 2010, 2011).
On the bacterial side, several studies have investigated the presence of functional equivalents of rhizobial NFs in Frankia. In rhizobia, NF biosynthesis is dependent on nodABC genes (Masson-Boivin et al. 2009). Regarding Frankia, it was shown more than two decades ago that Frankia alni DNA cannot complement rhizobial nod mutants (Cérémonie et al. 1998). The absence of close homologs of nodABC in this Frankia strain was demonstrated when the first Frankia genomes became available (Normand et al. 2007). Similar results were found for 35 other sequenced genomes (Tisa et al. 2016). Distant homologs of nodB and nodC were often detected in those genomes but at least in F. alni, the expression of these genes is not induced under symbiotic conditions (Alloisio et al. 2010). Chitin oligomers similar to rhizobial NFs are not detected in F. alni culture supernatant and purified rhizobial NFs fail to elicit the symbiotic responses in Alnus glutinosa and C. glauca (Ghelue et al. 1997; Cérémonie et al. 1999; Svistoonoff et al. 2010; Chabaud et al. 2016). Taken together these observations suggest that at least in the case of C. glauca and A. glutinosa, early actinorhizal signalling is not dependant on canonical nodABC genes or molecules closely related to rhizobial NFs. Canonical nodABC genes have however recently been found in the genome of one isolated strain, Frankia sp. NRRL B-16219 (Ktari et al. 2017b) and two uncultured Frankia strains: Candidatus Frankia datiscae Dg1 and Candidatus Frankia californiensisDg2 (Persson et al. 2015; Nguyen et al. 2016). As in rhizobia, nodABC genes from Candidatus F. datiscae Dg1 are arranged in operons and are expressed in Datisca glomerata nodules (Persson et al. 2015). Unfortunately the presence of lipo-chito-oligosaccharides in Frankia exudates or in D. glomerata nodules has not been yet demonstrated and questions still remain regarding the hypothetical role of Frankia nodABC genes in actinorhizal symbiotic signalling.
Molecules potentially involved in symbiotic signalling have however been described in several Frankia strains. Root Hair Deformation Factors (RHDF) have been characterized in F. alni and Frankia casuarinae using the characteristic symbiotic deformation of plant root hairs observed few hours after the inoculation of Alnus sp. or Casuarina sp. respectively (Prin and Rougier 1987; Ghelue et al. 1997; Cérémonie et al. 1999; Gabbarini and Wall 2011; Cissoko et al. 2018). RHDF is a relatively small (< 3 kDa), hydrophilic molecule that is resistant to heat and to a chitinase treatment, but its precise chemical structure remains unknown (Cérémonie et al. 1999; Cissoko et al. 2018). Diffusible factors potentially involved in the interaction between Frankia discariae and Ochetophila trinervis (=Discaria trinervis) were also partially purified and interestingly these factors also act as RHDF on the non-host plant Alnus acuminata (Gabbarini and Wall 2008; Gabbarini and Wall 2011). In C. glauca, factors able to induce the expression of the symbiotic gene NIN (=NINA factors) were recently described. NIN encodes a transcription factor playing a crucial role in nodulation and importantly, it is expressed at pre-infection stages in legumes and actinorhizal plants in response to bacterial signalling molecules (Schauser et al. 1999; Radutoiu et al. 2003; Hocher et al. 2011; Clavijo et al. 2015). While rhizobial NFs are amphiphilic chitin-based molecules, NINA, like RHDF, is hydrophilic and resistant to chitinase (Chabaud et al. 2016). NINA shares many properties with RHDF but has apparently a different size (Cissoko et al. 2018).
Signalling related to infection and organogenesis
In actinorhizal plants two modes of infection have been described: intracellular (root hair) infection and intercellular infection. Intracellular infection is preceded by root hair deformation induced by RHDF secreted by Frankia and triggers limited cell-division in the cortex leading to the formation of the so-called prenodules. At this step nitrogen fixation already occurs but prenodules are not apparent anymore once the mature nodule develops (Laplaze et al. 2000a). Intercellular infection does not involve root hair deformation or prenodules and Frankia filaments penetrate inside the root intercellularly via the apoplastic interface between epidermal cells. Frankia filaments are internalized at later stages inside nodule cortical cells. Intracellular infection is found in actinorhizal Fagales and intercellular infection in actinorhizal Rosales and Cucurbitales (Wall 2000; Svistoonoff et al. 2014). Infection is a crucial step in symbiotic interactions and genes expressed during infection are regulated by mechanisms conserved between actinorhizal and rhizobial symbioses. This is the case for example for Cg12, a symbiotic gene encoding a subtilase specifically expressed during the intracellular infection of C. glauca by Frankia (Laplaze et al. 2000b; Svistoonoff et al. 2003). Specific activation of the Cg12 promoter (=ProCg12) was detected during the intracellular infection of Medicago truncatula by Sinorhizobium meliloti (Svistoonoff et al. 2004) and the intercellular infection of O. trinervis by Frankia (Fournier et al. 2018). Similar results were obtained with MtEnod11, a gene from M. truncatula expressed at pre-infection stages during rhizobial infection (Journet et al. 2001): activation of ProMtEnod11 was detected in C. glauca and O. trinervis during infection but interestingly no activation occurred at pre-infection stages (Svistoonoff et al. 2010; Imanishi et al. 2011).
As for other developmental processes plant hormones play an important role during actinorhizal infection and nodule organogenesis. The inhibition of auxin influx was shown to delay nodule formation in C. glauca and O. trinervis and auxins were detected in Frankia-infected cells in both species (Péret et al. 2007; Perrine-Walker et al. 2010; Imanishi et al. 2014).Treatments with exogenous auxins lead to the formation of thick lateral roots resembling nodules in actinorhizal Fagales (Hammad et al. 2003; Svistoonoff et al. 2003) and several Frankia strains are able to synthesize auxin (Wheeler et al. 1984; Berry et al. 1989; Hammad et al. 2003; Perrine-Walker et al. 2010; Solans et al. 2011). In C. glauca, an auxin influx carrier (CgAUX1) is expressed in infected cells whereas a PIN1-like auxin efflux carrier is present in surrounding uninfected cells probably leading to auxin accumulation in infected cells, where auxins could induce changes in gene expression, and cell wall properties (Péret et al. 2007; Perrine-Walker et al. 2010). Specific inhibition of auxin signalling in infected cells using a dominant-negative version of the endogenous auxin-signalling regulator IAA7 leads to increased nodulation suggesting that in C. glauca auxin is a negative regulator of symbiosis establishment (Champion et al. 2015). ProCgAux1 is not activated during AM formation (Péret et al. 2008) and DtAUX1, the putative orthologue of CgAUX1 in O. trinervis, showed a different activation pattern in O. trinervis nodules compared to CgAUX1 in C. glauca suggesting divergent roles of auxin and its transporters in those symbioses (Imanishi et al. 2014).
In addition to auxins other plant hormones might also be involved in actinorhizal nodulation. Ethylene and jasmonate are small organic molecules that are involved in plant response to a wide range of biotic and abiotic stresses (Bari and Jones 2009; Dar et al. 2015). In legumes, ethylene and jasmonate have been shown to inhibit plant nodulation, symbiotic calcium spiking in root hairs spiking and the expression of two early symbiotic genes RIP1 and MtEnod11 (Oldroyd et al. 2001; Sun et al. 2006). Little information is available regarding the potential involvement of plant stress hormones in actinorhizal nodulation. Ethylene is involved in modulating the susceptibility for nodulation of the basal portion of O. trinervis seedling roots (Valverde and Wall 2005). In C. glauca and Datisca glomerata higher levels of jasmonate were detected in nodules compared to roots. Immunolocalization experiments showed that the allene oxide cyclase enzyme which catalyses a committed step in JA biosynthesis is present in nodules but only in uninfected cells (Zdyb et al. 2011). We recently analyzed the combined effect of jasmonate and ethylene in C. glauca and found that both hormones negatively affect nodulation and deformation of root hairs (Ngom M., Cssoko M., Gray K. Hocher V., Svistoonoff S. and Champion A. unpublished results).
Symbiotic signal transduction pathways
In the past 20 years, much knowledge regarding the signalling pathways activated during RNS has been gathered in model legumes and many crucial components are now identified, particularly those involved in NF signalling (Oldroyd 2013). Many components of the NF signalling pathway are also needed to form arbuscular mycorhizae (AM) and constitute a Common Symbiotic Signalling Pathway (CSSP). Most actinorhizal plants are able to form AM and genes involved in the CSSP are also present in actinorhizal plants and are expressed during nodulation (Hocher et al. 2006, 2011; Demina et al. 2013; Griesmann et al. 2018). For two genes, SymRK and CCaMK functional studies using RNAi knockdown and gain of function approaches combined with complementation of legume mutants have proven their crucial role in actinorhizal nodulation (Gherbi et al. 2008; Markmann et al. 2008; Svistoonoff et al. 2013). High frequency calcium oscillations which are a central feature of the CSSP have also recently been described in actinorhizal Fagales in response to Frankia signals (Granqvist et al. 2015; Chabaud et al. 2016). Components of the NF signalling pathway that are not involved in AM are less well characterized in actinorhizal plants with the exception of NIN, a transcription factor crucial for Legume nodulation (Schauser et al. 1999; Marsh et al. 2007) which is also essential for actinorhizal nodulation in C. glauca (Clavijo et al. 2015). Recently genome-wide comparative analysis of 37 plant genomes including legumes, several actinorhizal plants and related species unable to nodulate revealed that most genes involved in legume nodulation are present in all the analysed species regardless of their ability to form nodules with the exception of two genes: NIN and RPG (Griesmann et al. 2018). Loss or fragmentation of NIN and RPG correlates with the absence of nodules in several lineages of the nitrogen fixing clade pointing to a central role for those two genes in all root nodule symbioses (van Velzen et al. 2018; Griesmann et al. 2018). Importantly, losses of NIN and RPG occurred independently suggesting that the most recent common ancestor of the nitrogen fixing clade was able to form nitrogen fixing nodules but this ability was lost in several lineages probably because natural selection often counter selects nodulation (van Velzen et al. 2018; Griesmann et al. 2018).
Concluding remarks
Compared to model legumes, our knowledge of actinorhizal plants is still sparse and limited to a few species. However, thanks to progress in molecular research on actinorhizal, many obstacles have fallen in the past 20 years and knowledge on this original nitrogen fixing symbiosis has taken a leap forward.
Actinorhizal nodulation shares many features with rhizobial nodulation. Until recently it was believed that the common ancestor of all nodulators evolved a predisposition for nodulation but was not a nodulator itself; nodulation would have evolved independently at least 11 times and conserved mechanisms would be the result deep homology (Doyle 2011). However recent broad scale phylogenomics favours an alternative hypothesis where the ancestor was a nodulator and nodulation was lost multiple times, probably because of a strong selection pressure against nodulation in certain environmental conditions (Streeter and Wong 1988; Kiers et al. 2003; Griesmann et al. 2018). One intriguing question then arises: if there is one single ancestor how can nodulation functional diversity be explained? One hypothesis is convergent evolution at different rates. The ancestor probably used the simplest mechanism i.e. intercellular infection together with a poor selection of bacterial partners which is still observed in actinorhizal Rosales, some legumes (Arachis/Aeschynomene; (Sprent 2007) or in lotus mutants (Madsen et al. 2010). The availability of full-genome sequences for several actinorhizal trees also offers the possibility to develop deeper phylogenomic analyses and thus to confirm or deny these evolution hypotheses.
Another intriguing question related to actinorhizal symbioses concerns signalling molecules. Recognition of RHDF by non-compatible host actinorhizal plants also points to a common origin suggesting that a common molecular backbone is recognized by all actinorhizal species but additional decorations or co-factors are needed to achieve full compatibility (Wall 2000; Gabbarini and Wall 2011; Cissoko et al. 2018). It is surprising not to find LCOs or molecules related to chitin at pre-infection stages as these are not only involved in rhizobial nodulation with legumes and Parasponia but also in the more ancient and widespread AM symbiosis (Oldroyd 2013). Like most components of CSSP, orthologs of legume genes involved in NFs recognition are also present in the genomes of actinorhizal plants (Griesmann et al. 2018) but functional evidence regarding their role in the perception of actinorhizal signalling molecules has to be provided. Nonetheless these observations will remain inconclusive until the molecular nature of actinorhizal signalling molecules like NINA or RHDF is determined. The availability of several bioassays developed to purify and characterize these signalling molecules (Chabaud et al. 2016; Cissoko et al. 2018) combined with promising results regarding the genetic transformation of Frankia reported by the UNH group (L. Tisa) during the 19th International Meeting on Frankia and Actinorhizal Plants will hopefully provide answers to these questions in the near future.
References
Abdel-Lateif K, Vaissayre V, Gherbi H et al (2013) Silencing of the chalcone synthase gene in Casuarina glauca highlights the important role of flavonoids during nodulation. New Phytol 199:1012–1021. https://doi.org/10.1111/nph.12326
Alloisio N, Queiroux C, Fournier P et al (2010) The Frankia alni symbiotic transcriptome. Mol Plant Microbe Interact 23:593–607
Auguy F, Abdel-Lateif K, Doumas P et al (2011) Activation of the isoflavonoid pathway in actinorhizal symbioses. Funct Plant Biol 38:690–696
Bari R, Jones JDG (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69:473–488. https://doi.org/10.1007/s11103-008-9435-0
Beauchemin NJ, Furnholm T, Lavenus J et al (2012) Casuarina root exudates alter the physiology, surface properties, and plant infectivity of Frankia sp. strain CcI3. Appl Environ Microbiol 78:575–580
Berry AM, Kahn RK, Booth MC (1989) Identification of indole compounds secreted by Frankia HFPArI3 in defined culture medium. Plant Soil 118:205–209
Cérémonie H, Cournoyer B, Maillet F et al (1998) Genetic complementation of rhizobial nod mutants with Frankia DNA: artifact or reality? Mol Gen Genet MGG 260:115–119
Cérémonie H, Debellé F, Fernandez MP (1999) Structural and functional comparison of Frankia root hair deforming factor and rhizobia Nod factor. Can J Bot 77:1293–1301
Chabaud M, Gherbi H, Pirolles E et al (2016) Chitinase-resistant hydrophilic symbiotic factors secreted by Frankia activate both Ca2+ spiking and NIN gene expression in the actinorhizal plant Casuarina glauca. New Phytol 209:86–93. https://doi.org/10.1111/nph.13732209:86-93
Champion A, Lucas M, Tromas A et al (2015) Inhibition of auxin signaling in Frankia species-infected cells in Casuarina glauca nodules leads to increased nodulation. Plant Physiol 167:1149–1157
Cissoko M, Hocher V, Gherbi H et al (2018) Actinorhizal signaling molecules: Frankia root hair deforming factor shares properties with NIN inducing factor. Front Plant Sci. https://doi.org/10.3389/fpls.2018.01494
Clavijo F, Diedhiou I, Vaissayre V et al (2015) The Casuarina NIN gene is transcriptionally activated throughout Frankia root infection as well as in response to bacterial diffusible signals. New Phytol 208:887–903. https://doi.org/10.1111/nph.13506
Dar TA, Uddin M, Khan MMA et al (2015) Jasmonates counter plant stress: a review. Environ Exp Bot 115:49–57
Demina IV, Persson T, Santos P et al (2013) Comparison of the nodule vs. root transcriptome of the actinorhizal plant Datisca glomerata: actinorhizal nodules contain a specific class of defensins. PLoS ONE 8:e72442. https://doi.org/10.1371/journal.pone.0072442
Doyle JJ (2011) Phylogenetic perspectives on the origins of nodulation. Mol Plant Microbe Interact 24:1289–1295
Fournier J, Imanishi L, Chabaud M et al (2018) Cell remodeling and subtilase gene expression in the actinorhizal plant Discaria trinervis highlight host orchestration of intercellular Frankia colonization. New Phytol 219:1018–1030. https://doi.org/10.1111/nph.15216
Gabbarini L, Wall L (2008) Analysis of nodulation kinetics in Frankia–Discaria trinervis symbiosis reveals different factors involved in the nodulation process. Physiol Plant 133:776–785
Gabbarini L, Wall L (2011) Diffusible factors involved in early interactions of actinorhizal symbiosis are modulated by the host plant but are not enough to break the host range barrier. Funct Plant Biol 38:671–681
Ghelue MV, Løvaas E, Ringø E, Solheim B (1997) Early interactions between Alnus glutinosa and Frankia strain ArI3. Production and specificity of root hair deformation factor (s). Physiol Plant 99:579–587
Gherbi H, Markmann K, Svistoonoff S et al (2008) SymRK defines a common genetic basis for plant root endosymbioses with arbuscular mycorrhiza fungi, rhizobia, and Frankia bacteria. Proc Natl Acad Sci 105:4928–4932
Gherbi H, Hocher V, Ngom M et al (2018) Molecular methods for research on actinorhiza. In: Reinhardt D (ed) Rhizosphere biology research. Springer, Berlin
Granqvist E, Sun J, Op den Camp R et al (2015) Bacterial-induced calcium oscillations are common to nitrogen-fixing associations of nodulating legumes and nonlegumes. New Phytol 207:551–558. https://doi.org/10.1111/nph.13464
Griesmann M, Chang Y, Liu X et al (2018) Phylogenomics reveals multiple losses of nitrogen-fixing root nodule symbiosis. Science 361:eaat1743. https://doi.org/10.1126/science.aat1743
Hammad Y, Nalin R, Marechal J et al (2003) A possible role for phenyl acetic acid (PAA) on Alnus glutinosa nodulation by Frankia. Plant Soil 254:193–205
Hocher V, Auguy F, Argout X et al (2006) Expressed sequence-tag analysis in Casuarina glauca actinorhizal nodule and root. New Phytol 169:681–688
Hocher V, Alloisio N, Auguy F et al (2011) Transcriptomics of actinorhizal symbioses reveals homologs of the whole common symbiotic signaling cascade. Plant Physiol 156:700–711
Imanishi L, Vayssières A, Franche C et al (2011) Transformed hairy roots of Discaria trinervis: a valuable tool for studying actinorhizal symbiosis in the context of intercellular infection. Mol Plant Microbe Interact 24:1317–1324
Imanishi L, Perrine-Walker FM, Ndour A et al (2014) Role of auxin during intercellular infection of Discaria trinervis by Frankia. Front Plant Sci 5:399. https://doi.org/10.3389/fpls.2014.00399
Journet EP, El-Gachtouli N, Vernoud V et al (2001) Medicago truncatula ENOD11: a novel RPRP-encoding early nodulin gene expressed during mycorrhization in arbuscule-containing cells. Mol Plant Microbe Interact 14:737–748
Kiers ET, Rousseau RA, West SA, Denison RF (2003) Host sanctions and the legume-rhizobium mutualism. Nature 425:78–81. https://doi.org/10.1038/nature01931
Ktari A, Gueddou A, Nouioui I et al (2017a) Host plant compatibility shapes the proteogenome of Frankia coriariae. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00720
Ktari A, Nouioui I, Furnholm T et al (2017b) Permanent draft genome sequence of Frankia sp. NRRL B-16219 reveals the presence of canonical nod genes, which are highly homologous to those detected in Candidatus Frankia Dg1 genome. Stand Genomic Sci 12:51. https://doi.org/10.1186/s40793-017-0261-3
Laplaze L, Duhoux E, Franche C et al (2000a) Casuarina glauca prenodule cells display the same differentiation as the corresponding nodule cells. Mol Plant Microbe Interact 13:107–112
Laplaze L, Ribeiro A, Franche C et al (2000b) Characterization of a Casuarina glauca nodule-specific subtilisin-like protease gene, a homolog of Alnus glutinosa ag12. Mol Plant Microbe Interact 13:113–117
Madsen LH, Tirichine L, Jurkiewicz A et al (2010) The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat Commun 1:10. https://doi.org/10.1038/ncomms1009
Markmann K, Giczey G, Parniske M (2008) Functional adaptation of a plant receptor-kinase paved the way for the evolution of intracellular root symbioses with bacteria. PLoS Biol 6:e68
Marsh JF, Rakocevic A, Mitra RM et al (2007) Medicago truncatula NIN is essential for rhizobial-independent nodule organogenesis induced by autoactive calcium/calmodulin-dependent protein kinase. Plant Physiol 144:324–335. https://doi.org/10.1104/pp.106.093021
Masson-Boivin C, Giraud E, Perret X, Batut J (2009) Establishing nitrogen-fixing symbiosis with legumes: how many rhizobium recipes? Trends Microbiol 17:458–466
Nguyen TV, Wibberg D, Battenberg K et al (2016) An assemblage of Frankia Cluster II strains from California contains the canonical nod genes and also the sulfotransferase gene nodH. BMC Genom 17:796. https://doi.org/10.1186/s12864-016-3140-1
Normand P, Lapierre P, Tisa LS et al (2007) Genome characteristics of facultatively symbiotic Frankia sp. strains reflect host range and host plant biogeography. Genome Res 17:7–15
Oldroyd GED (2013) Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nat Rev Microbiol 11:252–263. https://doi.org/10.1038/nrmicro2990
Oldroyd GE, Engstrom EM, Long SR (2001) Ethylene inhibits the Nod factor signal transduction pathway of Medicago truncatula. Plant Cell 13:1835–1849
Péret B, Swarup R, Jansen L et al (2007) Auxin influx activity is associated with Frankia infection during actinorhizal nodule formation in Casuarina glauca. Plant Physiol 144:1852–1862
Péret B, Svistoonoff S, Lahouze B et al (2008) A role for auxin during actinorhizal symbioses formation? Plant Signal Behav 3:34–35
Perrine-Walker F, Doumas P, Lucas M et al (2010) Auxin carriers localization drives auxin accumulation in plant cells infected by Frankia in Casuarina glauca actinorhizal nodules. Plant Physiol 154:1372–1380
Persson T, Battenberg K, Demina IV et al (2015) Candidatus Frankia Datiscae Dg1, the actinobacterial microsymbiont of Datisca glomerata, expresses the canonical nod genes nodABC in symbiosis with its host plant. PLoS ONE 10:e0127630. https://doi.org/10.1371/journal.pone.0127630
Popovici J, Comte G, Bagnarol É et al (2010) Differential effects of rare specific flavonoids on compatible and incompatible strains in the Myrica gale-Frankia actinorhizal symbiosis. Appl Environ Microbiol 76:2451–2460
Popovici J, Walker V, Bertrand C et al (2011) Strain specificity in the Myricaceae–Frankia symbiosis is correlated to plant root phenolics. Funct Plant Biol 38:682–689
Prin Y, Rougier M (1987) Preinfection events in the establishment of Alnus–Frankia symbiosis: study of the root hair deformation step. Plant Physiol (Life Sci Adv) 6:96–106
Radutoiu S, Madsen LH, Madsen EB et al (2003) Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 425:585–592
Schauser L, Roussis A, Stiller J, Stougaard J (1999) A plant regulator controlling development of symbiotic root nodules. Nature 402:191–195. https://doi.org/10.1038/46058
Solans M, Vobis G, Cassán F et al (2011) Production of phytohormones by root-associated saprophytic actinomycetes isolated from the actinorhizal plant Ochetophila trinervis. World J Microbiol Biotechnol 27:2195–2202. https://doi.org/10.1007/s11274-011-0685-7
Soltis DE, Soltis PS, Morgan DR et al (1995) Chloroplast gene sequence data suggest a single origin of the predisposition for symbiotic nitrogen fixation in angiosperms. Proc Natl Acad Sci USA 92:2647–2651
Sprent JI (2007) Evolving ideas of legume evolution and diversity: a taxonomic perspective on the occurrence of nodulation. New Phytol 174:11–25
Streeter J, Wong PP (1988) Inhibition of legume nodule formation and N2 fixation by nitrate. Crit Rev Plant Sci 7:1–23. https://doi.org/10.1080/07352688809382257
Sun J, Cardoza V, Mitchell DM et al (2006) Crosstalk between jasmonic acid, ethylene and Nod factor signaling allows integration of diverse inputs for regulation of nodulation. Plant J Cell Mol Biol 46:961–970. https://doi.org/10.1111/j.1365-313X.2006.02751.x
Svistoonoff S, Laplaze L, Auguy F et al (2003) cg12 expression is specifically linked to infection of root hairs and cortical cells during Casuarina glauca and Allocasuarina verticillata actinorhizal nodule development. Mol Plant Microbe Interact 16:600–607
Svistoonoff S, Laplaze L, Liang J et al (2004) Infection-related activation of the cg12 promoter is conserved between actinorhizal and legume-rhizobia root nodule symbiosis. Plant Physiol 136:3191–3197
Svistoonoff S, Sy MO, Diagne N et al (2010) Infection-specific activation of the Medicago truncatula Enod11 early nodulin gene promoter during actinorhizal root nodulation. Mol Plant Microbe Interact 23:740–747
Svistoonoff S, Benabdoun FM, Nambiar-Veetil M et al (2013) The independent acquisition of plant root nitrogen-fixing symbiosis in fabids recruited the same genetic pathway for nodule organogenesis. PLoS ONE 8:e64515. https://doi.org/10.1371/journal.pone.0064515
Svistoonoff S, Hocher V, Gherbi H (2014) Actinorhizal root nodule symbioses: what is signalling telling on the origins of nodulation? Curr Opin Plant Biol 20C:11–18. https://doi.org/10.1016/j.pbi.2014.03.001
Tisa LS, Oshone R, Sarkar I et al (2016) Genomic approaches toward understanding the actinorhizal symbiosis: an update on the status of the Frankia genomes. Symbiosis 70:5–16
Valverde C, Wall LG (2005) Ethylene modulates the susceptibility of the root for nodulation in actinorhizal Discaria trinervis. Physiol Plant 124:121–131
van Velzen R, Holmer R, Bu F et al (2018) Comparative genomics of the nonlegume Parasponia reveals insights into evolution of nitrogen-fixing rhizobium symbioses. Proc Natl Acad Sci 115:E4700–E4709. https://doi.org/10.1073/pnas.1721395115
Vessey KJ, Pawlowski K, Bergman B (2004) Root-based N2-fixing symbioses: legumes, actinorhizal plants, Parasponia sp. and cycads. Plant Soil 266:205–230
Wall LG (2000) The actinorhizal symbiosis. J Plant Growth Regul 19:167–182
Wheeler CT, Crozier A, Sandberg G (1984) The biosynthesis of indole-3-acetic acid by Frankia. Plant Soil 78:99–104
Zdyb A, Demchenko K, Heumann J et al (2011) Jasmonate biosynthesis in legume and actinorhizal nodules. New Phytol 189:568–579
Acknowledgements
We gratefully acknowledge support from IRD, CNRS (Project EC2CO), Genoscope, Genopole of Montpellier, and Agence Nationale de la Recherche (AN-06-BLAN-0095, BLAN 1708 01, 12-BSV7-0007-02) and United States Department of Agriculture (USDA NIFA 2015-67014-22849) and ECOS-SUD (A07B02 and A13B03).
Author information
Authors and Affiliations
Contributions
VH, MN, ACM, PT, HG and SS wrote the manuscript. All the authors approved the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interest exists.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Hocher, V., Ngom, M., Carré-Mlouka, A. et al. Signalling in actinorhizal root nodule symbioses. Antonie van Leeuwenhoek 112, 23–29 (2019). https://doi.org/10.1007/s10482-018-1182-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-018-1182-x