Abstract
Two types of symbioses are known where nitrogen-fixing soil bacteria induce the formation of special organs, i.e. nodules, on the roots of their dicotyledonous host plants; legume-rhizobia symbioses and actinorhizal symbioses. The later are the symbioses between actinobacteria of the genus Frankia and a group of mostly woody plant species from eight families and three different orders (Fagales, Rosales, Cucurbitales). While so far, research has mostly focused on legume-rhizobia symbioses, actinorhizal symbioses with their wider phylogenetic range are more likely to hold the key to understanding the common principles underlying the evolution of an intracellular plant-bacterial symbiosis. In contrast with the unique stem-like structure of legume nodules, actinorhizal nodules are composed of modified lateral roots with infected cells in the expanded cortex. In contrast with rhizobia, Frankia strains can protect the oxygen-sensitive nitrogenase enzyme complex, and thus nitrogen fixation, from oxygen. Therefore, oxygen protection systems established in actinorhizal nodules from different host plants involve contributions of both symbiotic partners. In this chapter, structural and developmental features of actinorhizal symbioses are described.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
12.1 Introduction
Nitrogen is the element that most often limits plant growth. Biosphere nitrogen is continuously lost to the atmosphere by denitrification and can only be repleted by nitrogen fixation. Only some prokaryotes can form the enzyme complex nitrogenase that catalyzes the reduction of air dinitrogen to ammonia and thus moves nitrogen from the atmosphere to the biosphere. Several plant species can enter symbioses with nitrogen-fixing soil bacteria. The most efficient of those are root nodule symbioses. Rhizobial symbioses are entered between a polyphyletic group of Gram-negative soil proteobacteria, rhizobia, and species from the legume family as well as from one non-legume genus, Parasponia (Cannabaceae). Actinorhizal symbioses are between Gram-positive soil actinobacteria of the genus Frankia and 25 genera of dicotyledonous plants, from eight families belonging to three different orders, collectively called actinorhizal plants. All plants able to form root nodule symbioses go back to a common ancestor which is supposed to have acquired a unique predisposition based on which a root nodule could evolve (Soltis et al. 1995). How often root nodule symbioses evolved, and how often the symbiotic capacity was lost, is controversial (Werner et al. 2014).
In these symbioses, the host plants form special organs, the root nodules, upon signal exchange with the microsymbionts. In root nodules, the microsymbionts fix nitrogen while stably internally accommodated within nodule cells and export the products of nitrogen fixation to the host plant, thereby rendering it independent of soil nitrogen sources. With one exception, Datisca glomerata, actinorhizal plants are woody shrubs or trees, and actinorhizal nodules are perennial organs. These nodules consist of multiple lobes, each of which represents a modified lateral root without root cap and with infected cells in the expanded cortex.
Actinorhizal nodules were first described by Meyen (1829), but in 1895 it could be shown that these nodules contributed to the plants’ nitrogen nutrition (Hiltner 1895). Due to their symbiosis, actinorhizal plants mostly represent pioneer plants and are often used in reforestation or soil reclamation (Diem and Dommergues 1990).
Legume symbioses, in particular those involving the two model species Medicago truncatula and Lotus japonicus, are the best examined root nodule symbioses (Oldroyd 2013). However, legume root nodules have features that distinguish them from all other root nodule symbioses including that of Parasponia species; legume nodules represent stem-like organs with peripheral vascular systems and infected cells in the expanded cortex (Mylona et al. 1995). For identifying the common principles of nitrogen-fixing symbioses, these principles have to be distinguished from plant family-specific characteristics. In this chapter, legume symbioses will be mentioned for comparative purposes.
12.2 The Microsymbionts: Frankia Strains
In 1886, J. Brunchorst used the name Frankia for the microsymbiont of alder trees to honor his mentor, Swiss biologist A.B. Frank (Quispel 1990). But at that time, both of them thought the microorganism was a fungus. The genus was later reclassified as member of a new family Frankiaceae, of the order Actinomycetales. Based on their host plants, ten species, i.e., Frankia alni, F. elaeagni, F. brunchorstii, F. discariae, F. casuarinae, F. ceanothi, F. coriariae, F. dryadis, F. purshiae, and F. cercocarpi, were assigned (Becking 1970).
Members of the genus Frankia have been found on all continents except Antarctica (Dawson 2008). These strains infect host plants in a wide range of climates from glacial bays (Lawrence et al. 1967) to volcanic soils (Burleigh and Dawson 1994). Many studies have shown that Frankia can be found not only under host plants (Huss-Danell 1997), Frankia strains also occur in the soils with no recent presence (Burleigh and Dawson 1994; Huss-Danell and Frej 1986; Zitzer and Dawson 1992) or devoid of actinorhizal plants (Smolander and Sundman 1987; Maunuksela et al. 1999; Gauthier et al. 2000; Jeong 2001).
Phylogenetically, symbiotic Frankia strains can be divided into three main clusters (Normand et al. 1996; Clawson et al. 2004). Strains from Frankia cluster I nodulate members of the actinorhizal plant families Betulaceae, Casuarinaceae (with the exception of the genus Gymnostoma), and Myricaceae (Normand et al. 1996). Frankia cluster III strains nodulate plants from two families of order Rosales, i.e., Rhamnaceae (with the exception of the genus Ceanothus) and Elaeagnaceae, and two genera from the order Fagales (Gymnostoma and Morella) (Huguet et al. 2004). Strains from the cluster II nodulate the broadest range of host plants which belong to four families from two different orders, including Rosaceae and the rhamnaceous genus Ceanothus from the Rosales, and Datiscaceae and Coriariaceae from the order Curcubitales (Normand et al. 1996; Vanden Heuvel et al. 2004). This cluster also forms the basal group of the symbiotic Frankia clusters (Normand et al. 1996). With one exception, no member of the cluster II could be cultured; the only cultured strain, Frankia sp. BMG5.1, in contrast with all other known Frankia strains, is alkaliphilic (Gtari et al. 2015).
Cluster IV Frankia strains were isolated from nodules but cannot induce nodules or fix nitrogen on their own (Fix−/Nod., Ramírez-Saad et al. 1998). Presumably, these Fix−/Nod− strains colonize the nodule periderm and occasionally escape surface sterilization. Such strains have to be distinguished from Fix−/Nod+ strains which can induce ineffective, i.e., non-nitrogen-fixing nodules on certain host plants but cannot fix nitrogen (Baker et al. 1980; Wolters et al. 1997).
Frankia can form three cell types: hyphae, sporangia, and vesicles (Torrey and Callaham 1982). The width of septate hyphae of free-living Frankia cells ranges from 0.5 to 1.5 μm. In culture, the hyphae form multiple branches and produce multilocular sporangia (Schwintzer 1990). Under aerobic conditions and nitrogen limitation, vesicles are produced at the tips of growing vegetative hyphae or short side hyphae (Tjepkema et al. 1980; Fontaine et al. 1984). In these vesicles, the oxygen-sensitive nitrogenase enzyme complex is formed and nitrogen fixation takes place (Lechevalier 1994). Under microaerobic conditions and nitrogen limitation, Frankia expresses nitrogenase in hyphae (Murry et al. 1985).
All isolated strains of Frankia can produce sporangia in culture. Sporangia can be terminal or intercalary. Depending on the strain, the number of spores per sporangium can range from a few to several hundreds. Some strains can form sporangia within nodules; none of those strains could be cultured to date (VandenBosch and Torrey 1985). It was reported that inoculant from nodules that contain spores was much more infective than inoculant from nodules in which Frankia does not form spores (Burleight and Torrey 1990).
Analysis of Frankia strains was always impeded by the fact that so far, these strains cannot be transformed. Only when genome sequences started to become available in 2007 (Normand et al. 2007), Frankia’s full biochemical capacities could be assessed. Some features and the references of the currently available genomes of Frankia strains are summarized in Table 12.1. The sizes of Frankia genomes show an unusual variation; these range from 5.0 Mbp for a Casuarina-infective strain (CeD) to 10.45 Mbp for a strain that infects Elaeagnaceae (R43). Phylogenetic analysis has shown that cluster II is basal in the genus (Fig. 12.1; Sen et al. 2014; Gtari et al. 2015; Persson et al. 2015). These strains have genome sizes between 5 and 6 Mbp. Strains belonging to cluster IV, which neither can enter a root nodule symbiosis nor fix nitrogen, have genome sizes between 6.9 and 10 Mbp. Strains in the most derived clusters I and III show different genome sizes: cluster III genomes range between 9 and 10.45 Mbp, while in cluster I, one subgroup (Ic), the Casuarina-infective strains, shows strong genome reduction with 5–6 MB while the other subgroup, strains infecting Alnus sp. and the Myricaceae (Ia), shows less genome reduction with 7–8 Mbp.
The three cell types of Frankia. The photograph shows Frankia alni ACN14a grown under normal oxygen tension and nitrogen-limiting conditions, stained with trypan blue. h hypha, v vesicle, s sporangium (The photograph was kindly provided by Anke Sirrenberg (University of Göttingen, Göttingen, Germany))
Keeping in mind that the only cultured cluster II strain is alkaliphilic (Gtari et al. 2015), a feature that is unlikely to have evolved after the strain became a root symbiont, this raises the question of whether the precursors of the Frankia genus were extremophiles with genomes in the range of 5–6 Mbp, and genome size was extended when a subgroup of them adapted to moderate environments (cluster IV) or whether the ancestors of Frankia had genomes in the 10 Mbp range and cluster II strains underwent genome reduction. Given that genome reduction in symbiosis is also observed in cluster I, the second hypothesis seems more likely.
12.3 Actinorhizal Plants
More than 200 species of dicotyledonous plants, distributed in 24 genera belonging to eight families from three different orders, can enter a root nodule symbiosis with Frankia (Fig. 12.2). Generally, all species of an actinorhizal genus can form root nodules. The only exception is Dryas, the ancestral genus in the tribe Dryadoideae, the actinorhizal subgroup of the Rosaceae family, where the species Dryas octopetala has never been found nodulated (Uemura 1971; Bond 1976).
Phylogeny and host specificity of Frankia. Comparison of core genomes of 14 sequenced Frankia strains (Normand et al. 2007; Persson et al. 2011; Ghodhbane-Gtari et al. 2013; Nouioui et al. 2013; Sen et al. 2013; Wall et al. 2013; Ghodbhane-Gtari et al. 2014; Hurst IV et al. 2014; Mansour et al. 2014; Gtari et al. 2015; Pujic et al. 2015; Tisa et al. 2015) using EDGAR (Blom et al. 2009). Outgroups were two actinobacterial genomes: Nocardia farcinica (Ishikawa et al. 2004) and Mycobacterium gilvum Spyr1 (Kallimanis et al. 2011). The phylogenetic tree was deduced from concatenated core gene alignments using PHYLIP (Felsenstein 2005). The bar below the phylogenetic tree represents the scale of sequence divergence. The phylogenetic tree was kindly provided by Daniel Wibberg (University of Bielefeld, Germany) and Jochen Blom (Justus Liebig University, Gießen, Germany). Host specificity is indicated in the table. Genera the members of which can enter symbioses with cluster I strains are depicted in blue. Hosts of cluster II strains are depicted in red, and hosts of cluster III strains are given in green. Strains of cluster IV are not able to induce root nodules
Thanks to their symbiosis, actinorhizal plants can grow on marginal soils and have been used in soil reclamation, erosion control, agroforestry, and dune stabilization (Diem and Dommergues 1990). Hippophae rhamnoides is currently being domesticated since its fruits are very nutritious, rich in vitamin C and carotenes, and the seed oil is highly unsaturated and has properties that make it a promising ingredient in cosmetics and phytopharmaceuticals (Suryakumar and Gupta 2011).
12.4 Nodule Structure
Root nodules of actinorhizal plants and legumes show some similarities but differ significantly in many respects (Fig. 12.3). Legume nodules represent stem-like organs with peripheral vascular system and infected cells in the central tissue. Legume nodules can be indeterminate or determinate. Indeterminate nodules have a persistent apical meristem, so the infected cells are arranged in a spatial developmental gradient. In determinate legume nodules, no persistent meristem exists, and the spatial developmental gradient is replaced by a temporal one. In contrast, all nodules of non-legumes – actinorhizal nodules and nodules of Parasponia sp. – are coralloid organs consisting of multiple lobes, each lobe representing a modified lateral root with central vascular system and infected cells in the expanded cortex (Pawlowski and Bisseling 1996). Due to their apical meristem of each lobe, the infected cortical cells are arranged in a developmental gradient. Right below the meristem is the infection zone where the cells are becoming filled with branching Frankia hyphae in infection thread-like structures. The next zone is the nitrogen fixation zone where in most host plants, Frankia vesicles have differentiated and nitrogen is fixed. In mature nodules, a senescence zone is present which contains infected cells with inactive Frankia bacteria which are degraded by the plant (Pawlowski and Demchenko 2012).
Comparison of longitudinal sections of different lateral root organs. Panel (a) shows a standard lateral root with root hairs (rh), apical meristem (m), and calyptra (c). The vascular system is depicted in black. Panel (b) shows an indeterminate legume nodule with peripheral vascular system and infected cells in the inner tissue. Due to the activity of the apical meristem (I), the cells of the inner tissue (shaded in gray) are arranged in a spatial developmental gradient (Vasse et al. 1990): The prefixation zone (II) is followed by the interzone (II–III) where nitrogen fixation commences. Zone III is the nitrogen fixation zone and Zone IV is the zone of senescence. Panel (c) shows a lobe of an actinorhizal nodule formed by a member of the Fagales or Rosales. Like a lateral root, the lobe has a central vascular bundle, but it is surrounded by a superficial periderm given in dark gray. The infected cells are located in the expanded cortex (shaded in gray), interspersed with uninfected cells. Due to the activity of the apical meristem (m), they are arranged in a developmental gradient: (2) zone of infection, (3) zone of nitrogen fixation, (4) zone of senescence. Panel (d) shows a nodule formed by a member of the Cucurbitales. The infected cells (shaded in orange) form a continuous section in the cortex, kidney shaped in cross section, on one side of the acentric stele, and are not interspersed with uninfected cells. Again, the activity of the apical meristem (m) leads to a spatial developmental gradient of infected cells: (2) zone of infection, (3) zone of nitrogen fixation, and (4) zone of senescence. The periderm can be interrupted by lenticels (le) which are always located opposite to the side of the infected cells
Actinorhizal nodules show a remarkable anatomic diversity. The distribution of infected cells in the cortex depends on the host plant. In Fagales and Rosales, the infected cortical cells are interspersed with uninfected cortical cells. In nodules of actinorhizal Cucurbitales, however, the infected cells make up an uninterrupted region, kidney shaped in cross section, on one side of the acentric vascular bundle (Newcomb and Pankhurst 1982; Berg et al. 1999).
Frankia morphology in nodules varies significantly, depending on the host. With the exception of nodules of Casuarina and Allocasuarina, Frankia strains fix nitrogen in vesicles within infected cells. The shape, septation, and subcellular position of the vesicles depend on the host plant species, i.e., the same strain can form different types of vesicles in different host plants (Huss-Danell 1997). The alder vesicle style – a septate sphere with a stalk – appears in Alnus, the family Elaeagnaceae and some members of the family Rhamnaceae (Berg 1994). This vesicle type has the closest similarity to vesicles formed in the free-living state. In alder nodules, vesicles are located at the periphery of the infected cortical cells (Lalonde and Knowles 1975). Vesicles in Ceanothus sp. are nonseptate, pear shaped, and have no stalk (Berg 1994). Like the alder-type, these vesicles are also formed at the periphery of the host cell. In Morella cerifera, the vesicles are septate, elongated, and club shaped (Berg 1994). In nodules of actinorhizal Cucurbitales, the vesicles are rod shaped, arranged in radial orientation, and form a sphere around the central vacuole of the infected cell (Newcomb and Pankhurst 1982; Berg et al. 1999). Casuarina sp. and Allocasuarina sp. are unique among host plant genera in that Frankia does not form vesicles in root nodules even though the corresponding Frankia strains are capable of doing so when grown in culture (Berg and McDowell 1987).
12.5 Nodule Physiology
On the whole-plant level, nodules represent carbon sinks and nitrogen sources. Nodules need assimilated carbon for growth and maintenance, for supporting bacterial N2 fixation, for ammonium assimilation and transport of nitrogenous solutes, and for starch biosynthesis. Uninfected cortical cells of actinorhizal nodules tend to contain large starch grains, but the function of nodule starch is not known. Nodules are supplied with sucrose via the phloem.
Legume nodule metabolism, and the exchange of metabolites between host plants and microsymbionts, has been analyzed in detail (reviewed by Udvardi and Poole 2013). In legume nodules, sucrose from the phloem is cleaved by sucrose synthases and metabolized further via the glycolytic pathway. The role of sucrose synthase could be confirmed for Alnus glutinosa (Van Ghelue et al. 1996), Casuarina glauca (Schubert et al. 2013), and Datisca glomerata (Schubert et al. 2011). Rhizobial bacteroids are supplied with carbon sources in the form of dicarboxylates, especially malate. This also seems to be the case for Frankia in actinorhizal Fagales, since a dicarboxylate transporter has been identified in the perisymbiont membrane of nodules of A. glutinosa (Jeong et al. 2004).
In legume nodules, rhizobial bacteroids fix nitrogen but avoid assimilation of the resulting ammonia. Instead, ammonia is exported and assimilated in the cytosol of the infected cells, mostly by the glutamine synthetase (GS)/glutamate synthase (GOGAT) pathway and also by aspartate amino transferase (Udvardi and Poole 2013). The cytosolic assimilation of ammonia in infected cells could be confirmed for actinorhizal Fagales, i.e., A. glutinosa (Guan et al. 1996). However, the situation in actinorhizal Cucurbitales – i.e., D. glomerata – is different in that the ammonia resulting from nitrogen fixation is assimilated by Frankia, and an assimilated form of nitrogen – most likely arginine – is exported to the plant cytosol (Berry et al. 2004, 2011).
In all root nodule symbioses, assimilated ammonium is exported from the nodule via the xylem. In actinorhizal symbioses, the transport forms are amino acids (glutamate, glutamine, aspartate, asparagine) or ureides (citrulline, arginine) (Schubert 1986; Guan et al. 1996; Persson et al. 2016).
12.6 Oxygen Protection Mechanisms
The process of nitrogen fixation is oxygen sensitive in that nitrogenase is rapidly irreversibly denatured by oxygen (Gallon 1981). However, the process of nitrogen fixation requires high amounts of energy in the form of ATP, preferably provided by aerobic respiration. This causes the so-called oxygen dilemma of nitrogen fixation. The microsymbionts of legume-rhizobia symbioses rely on their hosts to solve this problem, which is achieved by providing a microaerobic environment in the infected cells while providing an oxygen-binding protein, leghemoglobin, to achieve efficient supply of oxygen to the respiratory chain (Minchin 1997). On the other hand, Frankia can fix nitrogen under microaerobic conditions in the free-living state by expressing nitrogenase in vesicles (Parsons et al. 1987). Vesicles have been confirmed as the site of nitrogen fixation by (i) showing that acetylene reduction activity occurs within the vesicles developed in culture and effective nodules (Tjepkema et al. 1980) with the nitrogenase activity of vesicle fraction was found to be 100-fold higher than that of the hyphae (Noridge and Benson 1986) and (ii) immunolabeling of bacterial cryosections proved that the localization of nitrogenase is restricted to the vesicles (Meesters et al. 1987). Vesicles are surrounded by envelopes consisting of multiple layers of hopanoids, bacterial steroid lipids (Berry et al. 1993). Since the number of the layers of the vesicle envelope increases with the external oxygen tension, it was concluded that the vesicle envelop forms an oxygen diffusion barrier (Parsons et al. 1987).
In a nitrogen-free culture grown under aerobic conditions, the hyphal termini can swell and form structures called provesicles (Fontaine et al. 1984). Provesicles are spherical and do not show nitrogenase activity. Subsequently, provesicles become mature vesicles, where nitrogenase is formed and nitrogen fixation occurs. Nitrogenase performance peaks after 5–6 days. Then the vesicles lose their function and become ghosts with deformed structure and empty appearance (Fontaine et al. 1984).
In nodules of (Allo-)Casuarina sp., the plant provides an oxygen protection system for the bacterial nitrogenase complex similar to the situation in legume nodules. The infected cells in nodules of Casuarina glauca have walls impregnated with a special lignin which provides an oxygen diffusion barrier, leading to microaerobic conditions in the infected cells (Berg and McDowell 1988; Schubert et al. 2013). Infected cells contain large amounts of a class II hemoglobin just like legume nodules (Jacobsen-Lyon et al. 1995). The problem is that in legume nodules, vascular system which needs energy for loading and unloading process is peripheral, so it is possible to place an oxygen diffusion barrier between the vascular system and the central tissue containing the infected cells. Since actinorhizal nodules have a central vascular system oxygen access to which should not be impaired, each infected cell needs its own oxygen diffusion barrier. The lignin in the primary walls of infected cells C. glauca nodules causes the apoplastic isolation of infected cells and also affects plasmodesmata, thereby gradually interfering with symplastic transport (Schubert et al. 2013).
In nodules of Alnus sp., it could be shown that the number of vesicle layers increases with the external oxygen tension and that the hopanoid composition is dependent on the depth of soil where Frankia is growing (Kleemann et al. 1994; Nalin et al. 2000). Hence, it seems that here, the bacterial oxygen protection system of nitrogenase is used. In nodules of Datisca glomerata, vesicle envelopes are thin but the positioning of the vesicles in the infected cells insures minimal oxygen access (Berg et al. 1999). Furthermore, the nodule periderm forms an oxygen diffusion barrier, and lenticels are always present at the side of the acentric vascular bundle, not at the side of the infected cells (Fig. 12.3). The presence of a bacterial hemoglobin could also contribute to shuttle oxygen to the sites of respiration (Pawlowski et al. 2007). The thickness of the suberized periderm that surrounds Coriaria nodules increases at elevated O2 concentrations (Silvester and Harris 1989). A single, large lenticel on the uninfected side of the nodule lobe limits the gas diffusion pathway to the infected cells to the narrow gap between the inner periderm and the steel (Silvester and Harris 1989).
In summary, in actinorhizal systems, both symbiotic partners can contribute to oxygen protection of nitrogenase. In Alnus sp., the bacterial contribution dominates, while in (Allo-)Casuarina sp., the plant contribution dominates. Mixed contributions are used in actinorhizal Cucurbitales.
Gas (oxygen/nitrogen) access to nodules can become limiting when the host plants are growing in wetlands. In well-drained soils, nodules of Alnus sp. are well aerated since their periderm is interrupted by lenticels and their outer cortex contains large intercellular spaces (Wheeler et al. 1979). In waterlogged soil, gas is transported thermo-osmotically from the aerial parts to the roots (Schröder 1989). Other actinorhizal plants growing in wet or waterlogged soils have developed a special mechanism for gas transport to their nodules: species of Casuarina, Gymnostoma, Myrica, and Comptonia provide oxygen to nodules via air spaces in the so-called nodule roots (Silvester et al. 1990). Nodule roots are formed at the tips of nodule lobes (the nodule lobe meristem turns into a nodule root meristem) and grow upward; their length is negatively correlated with the aeration of the growth substrate (Tjepkema 1978). Nodules of Datisca cannabina form nodule roots (Silvester et al. 1990), while nodules of D. glomerata form nodule roots in hydroculture or waterlogged soil and lenticels in well-drained soil (Pawlowski and Demchenko 2012).
12.7 Nodule Induction
In actinorhizal as in legume symbioses, the infection pathway is determined by the host plant (Miller and Baker 1985; Racette and Torrey 1989). For actinorhizal symbioses, two ways have been described for Frankia to enter the plant roots, intracellular via root hairs in Fagales or intercellular by penetration between epidermal cells in Rosales (Table 12.2). The infection pathway of Cucurbitales has not been analyzed yet.
12.7.1 Intracellular Infection via Root Hairs in the Fagales
This process is quite similar to the root hair infection process described for the model legumes M. truncatula and L. japonicus (Oldroyd 2013). The first response of the plant to the presence of the microsymbiont is the deformation and branching of growing root hairs (Torrey 1976; Callaham and Torrey 1977; Callaham et al. 1979; Berry et al. 1986). Only in a few root hairs, a Frankia hypha is entrapped in a root hair curl, and an infection thread-like structure is formed by dissolution of the cell wall and invagination of the root hair plasma membrane. Within this infection thread-like structure, the hypha is embedded in a plant-derived cell wall-like pectin-rich matrix, the so-called encapsulation (Lalonde and Knowles 1975; Callaham et al. 1979; Berry and Torrey 1983; Berry et al. 1986; Berg 1990). These actinorhizal infection thread-like structures have a smaller diameter than infection threads in legume nodules since they contain only one hypha.
The transcellular growth of infection threads in actinorhizal Fagales resembles infection thread growth in legume nodules: in both cases, before an infection thread crosses a cortical cell, a so-called preinfection thread (PIT) is formed in that cell (Berg 1999a). During this process, the nucleus moves to the center of the cell, and microtubules and cytoplasm rearrange to form a phragmoplast-like structure (van Brussel et al. 1992). These structures are polarized; most of the cytoplasm as well as the endomembranes are located at the outer side. This polarization of the cytoplasm is required for tip growth; root hairs, pollen tubes, and infection threads are the only plant structures showing tip growth (Van Brussel et al. 1992).
Concomitantly with the formation of an infection thread-like structure in a root hair, the formation of the so-called prenodule is initiated by cell divisions in the root cortex close to the infected root hair. The infection thread-like structures grow toward the prenodule by cell-to-cell passage and infect some, but not all, prenodule cells by extensive branching within these cells, filling them from the center outward (Schwintzer et al. 1982). This process – the branching of infection threads – does not involve PITs. Infected prenodule cells become hypertrophic, while uninfected prenodule cells accumulate starch (Callaham and Torrey 1977). Frankia can fix nitrogen in infected prenodule cells (Angulo Carmona 1974; Laplaze et al. 2000). Studies on C. glauca prenodules have shown that these structures represent primitive symbiotic organs consisting of three cell types with unique differentiation features equivalent to their counterparts in mature nodule lobes. These cell types are (1) infected cells harboring Frankia, (2) uninfected cells showing the same features as uninfected cortical cells in the mature nodule lobe while differing from root cortical cells (Laplaze et al. 2000), and (3) polyphenol-containing cells with gene expression pattern of which resembles those of polyphenol-containing cortical cells of mature nodule lobes while differing from that of polyphenol-containing root cortical cells (Smouni et al. 2002).
Nevertheless, the prenodule is only an intermediate stage in Fagales nodule development. While the prenodule developing, the formation of the nodule lobe primordium is initiated in the root pericycle near the infection site, opposite to a protoxylem pole, and Frankia hyphae in infection thread-like structures grow from the prenodule to the nodule primordium, again by cell-to-cell passage (transcellularly) and infect primordium cells.
Infection does not always lead to an effective symbiosis. Some strains can induce nitrogen-fixing nodules on one plant species but only ineffective (i.e., non-nitrogen fixing) nodules on another: these strains are incompatible with the second species (VandenBosch and Torrey 1983).
12.7.2 Intercellular Infection in the Rosales
During intercellular infection, Frankia hyphae penetrate the middle lamella between adjacent cells of the root epidermis and progressively colonize the intercellular spaces of the root cortex (Miller and Baker 1985; Racette and Torrey 1989; Berry and Sunell 1990; Liu and Berry 1991a, 1991b; Valverde and Wall 1999). Epidermal and cortical cells secrete pectin-rich material into apoplast; this material is likely to represent the equivalent of the cell wall-like material encapsulating Frankia hyphae in infection thread-like structures formed during intracellular infection (Liu and Berry 1991b). Concomitantly, the formation of a nodule lobe primordium is initiated in the root pericycle, and Frankia hyphae infect primordium cells from the apoplast. During this process, the plasma membrane of the infected cells invaginates, and the hyphae are embedded in cell wall-like material as infection thread-like structures. In the Rosales, infection threads do not show transcellular growth, and no PIT formation is observed.
A comparison between the infection mechanisms in Fagales and Rosales shows that there are two types of infection thread-like structures: those that show transcellular growth connected with PIT formation and those that do not show transcellular growth and whose growth does not involve PIT formation. Berg (1999a, 1991b) coined the terms “invasive hyphae” for infection thread-like structures showing transcellular growth and “vegetative hyphae” for the others. Intracellular infection involves both types of infection thread-like structures, while intercellular infection involves only the “vegetative hyphae.”
12.7.3 Unknown Infection Mechanism in the Cucurbitales
As mentioned above, the infection mechanism of actinorhizal Cucurbitales has not yet been analyzed. However, detailed cytological studies of mature nodules have been performed (Newcomb and Pankhurst 1982; Hafeez et al. 1984; Mirza et al. 1994; Berg et al. 1999). The absence of prenodules would lead to the assumption that Cucurbitales are infected intercellularly. Yet, infection threads show transcellular growth (Berg et al. 1999); this transcellular growth, however, did not involve the formation of PITs (Berg et al. 1999). Furthermore, in actinorhizal Cucurbitales, infected cells are filled with branching infection thread-like structures from the periphery inward and a large central vacuole is retained, while in actinorhizal Fagales as well as Rosales, the central vacuole is fragmented during infection (Berg et al. 1999; Pawlowski and Demchenko 2012). Altogether, the infection thread growth mechanism in actinorhizal Cucurbitales is clearly different from that in actinorhizal Fagales, in legumes, and from that in actinorhizal Rosales.
12.8 Signal Exchange Between Microsymbiont and Host Plant
The signal exchange between microsymbionts and host plant has been studied extensively in legume-rhizobia symbioses. Flavonoids from the host plant root exudate bind the rhizobial NodD protein, a transcriptional activator. In consequence, NodD activates the transcription of a number of nodulation (nod, nol, noe) genes that are required for the synthesis of the bacterial signal molecules, lipochito-oligosaccharide (LCO) Nod factors, which when perceived by plant receptors cause changes in the roots. The basic structure of Nod factors consists of a backbone of β-1,4-linked N-acetyl glucosamines carrying a fatty acid on the nonreducing end (Mylona et al. 1995). This basic structure is synthesized by the canonical Nod proteins NodA, NodB, and NodC. NodC is a chitin synthase and NodB an oligosaccharide deacetylase; both represent subfamilies of bacterial chitin synthases and deacetylases, respectively. NodA represents an acyl transferase that attaches a fatty acid to the deacetylated sugar residue and was considered unique to rhizobia (Atkinson et al. 1994). Nod factors differ with regard to the polymerization degree of the chito-oligosaccharide, the fatty acid, and the type of substitutions at both ends of the chitin oligomer; many different Nod (Nol, Noe) proteins are responsible for these individual modifications (Mergaert et al. 1997).
It has long been known that Nod factor signal transduction had recruited modules from arbuscular mycorrhizal (AM) fungi signaling systems (Markmann and Parniske 2009). Signaling of both rhizobia and AM fungi occurs via the common symbiotic signaling pathway (CSSP; Fig. 12.4). Nod factors bind to heterodimeric LysM receptor kinases in the plant root plasma membrane, Nod factor receptor 1 (NFR1) and Nod factor receptor 5 (NFR5) in Lotus japonicus and LysM receptor kinase 3 (LYK3) and Nod factor perception (NFP) in Medicago truncatula (Limpens et al. 2003; Madsen et al. 2003). These receptors evolved from chitin receptors (Zhang et al. 2009). The recognition of the Nod factor also involves a plasma membrane receptor kinase (LjSYMRK/MtDMI2/MsNORK; Endre et al. 2002; Stracke et al. 2002) which is also required for the signal exchange between AM fungi and plant roots. The signal transduction pathway leads to nuclear calcium spiking read by a complex of calcium/calmodulin-dependent kinase (LjCCaMK/MtDMI3) (Lévy et al. 2004) and the transcription factor LjCyclops/MtIPD3 (Yano et al. 2008; Horváth et al. 2011). This complex activates the transcription of nodule inception (LjNIN/MtNIN; Marsh et al. 2007; Singh et al. 2014) which encodes a transcription factor which will activate the expression of further transcription factors (Oldroyd 2013).
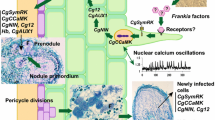
The common symbiotic signal transduction pathway. The left column shows the components known for legume-rhizobia symbioses, using the nomenclature for L. japonicus (top) and M. truncatula (bottom). All components which also participate in the Myc factor signal transduction pathway are highlighted in green. All components the function of which has been demonstrated for an actinorhizal symbiosis are given in red. Using L. japonica nomenclature, rhizobial LCO Nod factors bind to the LysM receptor kinases NFR1/NFR5 in the plasma membrane, which signal to SymRK. Signal transduction leads to nuclear Ca2+ spiking. The same happens during LCO Myc factor signaling, but the Ca2+ signature is different. In the nucleus, the Ca2+ signature is read by CCaMK. When the signature was caused by Nod factors, CCaMK phosphorylates Cyclops, and the CCaMK/Cyclops complex activates the transcription of NIN
All components between, and including, SymRK and Cyclops are shared between arbuscular mycorrhizal and rhizobial signaling: these components form the CSSP. The signal factors of AM fungi are LCOs (Maillet et al. 2011) or chito-oligosaccharides (COs; Sun et al. 2015) which, like rhizobial Nod factors, are also perceived by LysM receptor kinases. However, with the exception of Parasponia andersonii, no overlap was found between Nod factor-binding and Myc factor-binding LysM receptor kinases (Op den Camp et al. 2011).
Studies using RNAi in chimeric plants with transgenic root systems have shown for one actinorhizal member of the Cucurbitales (D. glomerata) and one of the Fagales (C. glauca) that this pathway is also involved in the communication between Frankia and their host plants (Gherbi et al. 2008; Markmann et al. 2008). RNAi studies have confirmed this further by showing that a CCaMK/DMI3 homolog is part of symbiotic signal transduction in C. glauca (Svistoonoff et al. 2013), and Granqvist et al. (2015) showed that culture supernatants of a homologous Frankia strain led to the induction of Ca2+ spiking in Alnus glutinosa root hairs. Furthermore, NIN has a similar function in C. glauca as in legumes (Clavijo et al. 2015). Hence, unsurprisingly, also Frankia signals via the CSSP.
12.8.1 Frankia Signal Factors
In the context of symbiotic signaling via the CSSP, one would expect Frankia signal molecules to represent LCOs since LCOs are used by rhizobia as well as by AM fungi. However, genomes of cluster I and cluster III Frankia strains do not contain the canonical nod genes nodABC.
The commonly used bioassay for rhizobial Nod factors is based on the induction of root hair deformation. During legume nodulation, a rhizobium attached to a growing root hair and producing Nod factors will cause the reorientation of root hair growth leading to the formation of a so-called shepherd’s crook which entraps the rhizobium. This will lead to the formation of an infection thread (Esseling et al. 2003). Purified Nod factors first block root hair extension and then re-induce it in a random position of the root hair, thereby leading to the formation of deformed root hairs (Heidstra et al. 1994). However, this bioassay could not be applied to Frankia signal factors. Knowlton et al. (1980) showed that also non-Frankia bacteria could induce root hair deformation on actinorhizal plants. Similarly, Van Ghelue et al. (1997) showed that Frankia strains that could induce root hair deformation on an actinorhizal plant could not necessarily nodulate this plant species. Cérémonie et al. (1999) tried to use a root hair deformation assay to isolate the signal factors of Frankia sp. ACoN24d Nod factors and could demonstrate that the compound(s) inducing A. glutinosa root hair deformation did not share the solubility features of rhizobial Nod factors. Recent data suggest that Frankia sp. CcI3 can produce hydrophilic and chitinase-resistant molecules that trigger Ca2+ spiking and activate the NIN promoter in its host plant C. glauca (Chabaud et al. 2016).
LysM receptor kinases not only recognize chitin and LCOs but also peptidoglycan (Willmann et al. 2011) and exopolysaccharides (Kawaharada et al. 2015). Hence, it seems plausible that Frankia cluster I and cluster III strains use signal molecules other than LCOs and that these signal molecules are recognized by LysM receptor kinases; however, the nature of these signal molecules remains to be examined.
There is evidence that the signals of cluster I strains can be imitated by other organisms. The fungus Penicillium nodositatum can induce so-called myconodules on the alder roots (Sequerra et al. 1994, 1995). Structurally, myconodules resemble ineffective, i.e., non-nitrogen-fixing actinorhizal nodules which typically remain mostly single lobes and contain large amounts of polyphenols. As in ineffective nodules, the infection of the host plant by the fungus does not elicit a strong resistance response (Sequerra et al. 1994, 1995). The nodule induction process used by the fungus – intracellular infection via root hairs – resembles that employed by Frankia (Sequerra et al. 1994), indicating that P. nodositatum produces compounds that activate symbiotic signaling.
Interestingly, the first genome of a Frankia cluster II strain to be sequenced, Candidatus Frankia datiscae Dg1, contained the canonical nod genes nodABC which were expressed in symbiosis (Persson et al. 2011, 2015). A series of rather diverse nodA homologs was identified in the phylum Actinobacteria, while nodA genes are otherwise only present in rhizobia (alpha- and beta-Proteobacteria) where nodA displayed much less sequence diversity than in Actinobacteria. These new data suggest that nodA evolved in Actinobacteria and was laterally transferred to rhizobia (Persson et al. 2015). Yet, the question whether Frankia cluster II strains actually use LCO Nod factors to nodulate their host plants is still open.
12.9 Concluding Remarks
Over the last decade, evidence has emerged about the similarities between legume and actinorhizal symbioses in that both symbioses involve bacterial signaling via the CSSP which leads to the formation of the first dedicated transcription factor in root nodule organogenesis, NIN. Suzuki et al. (2013) have postulated that the duplication of transcription factors involved in the response to nitrate (NIN-like proteins, NLPs), which yielded NIN, and NIN’s subsequent loss of nitrate sensitivity was one of the events necessary for the evolution of symbiotic nitrogen fixation in legumes. Soyano et al. (2015) could confirm that in L. japonicus, NLPs and NIN indeed act antagonistically. Since NIN is also involved in the formation of actinorhizal nodules (C. glauca, Casuarinaceae, Fagales; Clavijo et al. 2015), its evolution must have preceded the separation of Fabales and Fagales and might represent the common predisposition acquired by the progenitor of the symbiotic clade (Soltis et al. 1995). Yet, NIN is not the only transcription factor specific to root nodule organogenesis, and much has to be learned about the network of transcription factors in both legumes and actinorhizal plants before the different steps in the evolution of both symbioses are understood.
Apart from the dissimilarity of the microsymbionts, there are two striking differences between actinorhizal and legume-rhizobia symbioses. The former have a much wider phylogenetic range, while the latter involve far more plant species – legumes are a very diverse family with ca. 20,000 species (Doyle and Luckow 2003), thanks to a burst of speciation ca. 60–50 million years ago (Lavin et al. 2005), i.e., probably after evolving a root nodule symbiosis. Why did no such burst of speciation occur in any actinorhizal symbiosis? One explanation could be that with one exception (Datisca glomerata), actinorhizal plants are woody, and net diversification rates are higher for herbaceous annuals than for woody perennials (Soltis et al. 2013). However, plant growth form can change in the course of evolution (Beaulieu et al. 2013). So the question remains why actinorhizal plants did not evolve to leave their ecological niches although this often seems to have happened in legume evolution.
References
Angulo Carmona AF (1974) La formation des nodules fixateurs d’azote chez Alnus glutinosa (L.) Acta Bot Neerl 23:257–303. doi:10.1111/j.1438-8677.1974.tb00944.x
Atkinson EM, Palcic MM, Hindsgaul O et al (1994) Biosynthesis of Rhizobium meliloti lipo oligosaccharide Nod factors: NodA is required for an N-acyltransferase activity. Proc Natl Acad Sci U S A 91:8418–8422
Baker D, Newcomb W, Torrey JG (1980) Characterization of an ineffective actinorhizal microsymbiont, Frankia sp. EuI1 (Actinomycetales). Can J Microbiol 26:1072–1089. doi:10.1139/m80-180
Beaulieu JM, O’Meara BC, Donoghue MJ (2013) Identifying hidden rate changes in the evolution of a binary morphological character: the evolution of plant habit in campanulid angiosperms. Syst Biol 62:725–737. doi:10.1093/sysbio/syt034
Becking JH (1970) Frankiaceae fam. nov. (Actinomycetales) with one new combination and six new species of the genus Frankia Brunchorst 1886, 174. Int J Syst Bacteriol 20:201–220. doi:10.1099/00207713-20-2-201
Berg RH (1990) Cellulose and xylans in the interface capsule in symbiotic cells of actinorhizae. Protoplasma 159:35–43. doi:10.1007/BF01326633
Berg RH (1994) Symbiotic vesicle ultrastructure in high pressure-frozen, freeze-substituted actinorhizae. Protoplasma 183:37–48. doi:10.1007/BF01276811
Berg RH (1999a) Cytoplasmic bridge formation in the nodule apex of actinorhizal root nodules. Can J Bot 77:1351–1357. doi:10.1139/b99-078
Berg RH (1999b) Frankia forms infection threads. Can J Bot 77:1327–1333
Berg RH, McDowell L (1987) Endophyte differentiation in Casuarina Actinorhizae. Protoplasma 136:104–117. doi:10.1007/BF01276359
Berg RH, McDowell L (1988) Cytochemistry of the wall of infected cells in Casuarina actinorhizae. Can J Bot 66:2038–2047. doi:10.1139/b88-279
Berg RH, Langenstein B, Silvester WB (1999) Development in the Datisca-Coriaria nodule type. Can J Bot 77:1334–1350. doi:10.1139/b99-076
Berry AM, Sunell LA (1990) The infection process and nodule development. In: Schwintzer CR, Tjepkema JD (eds) The biology of Frankia and actinorhizal plants. Academic, San Diego, pp 61–81
Berry AM, Torrey JG (1983) Root hair deformation in the infection process of Alnus rubra. Can J Bot 61:2863–2876. doi:10.1139/b83-319
Berry AM, McIntyre L, McCully ME (1986) Fine structure of root hair infection leading to nodulation in the Frankia-Alnus symbiosis. Can J Bot 64:292–305. doi:10.1139/b86-043
Berry AM, Harriott OT, Moreau RA et al (1993) Hopanoid lipids compose the Frankia vesicle envelop, presumptive barrier of oxygen diffusion to nitrogenase. Proc Natl Acad Sci USA 90:6091–6094. doi:10.1073/pnas.90.13.6091
Berry AM, Murphy TM, Okubara PA et al (2004) Novel expression pattern of cytosolic Gln synthetase in nitrogen-fixing root nodules of the actinorhizal host, Datisca glomerata. Plant Physiol 135:1849–1862. doi:10.1104/pp.103.031534
Berry AM, Mendoza-Herrera A, Guo Y-Y et al (2011) New perspectives on nodule nitrogen assimilation in actinorhizal symbioses. Funct Plant Biol 38:645–652. doi:10.1071/FP11095
Blom J, Albaum SP, Doppmeier D et al (2009) EDGAR: A software framework for the comparative analysis of prokaryotic genomes. BMC Bioinforma 10:154. doi:10.1186/1471-2105-10-154
Bond G (1976) The results of the IBP survey of root nodule formation in non-leguminous angiosperms. In: Nutman PS (ed) Symbiotic nitrogen fixation in plants. Cambridge University Press, London, pp 443–474
Burleigh SH, Dawson JO (1994) Occurrence of Myrica-nodulating Frankia in Hawaiian volcanic soils. Plant Soil 164:283–289. doi:10.1007/BF00010080
Burleight S, Torrey JG (1990) Effectiveness of different Frankia cell types as inocula for the actinorhizal plant Casuarina. Appl Environ Microbiol 8:2565–2567
Callaham D, Torrey JG (1977) Prenodule formation and primary nodule development in roots of Comptonia (Myricaceae). Can J Bot 51:2306–2318
Callaham D, Newcomb W, Torrey JG et al (1979) Root hair infection in actinomycete-induced root nodule initiation in Casuarina, Myrica, and Comptonia. Bot Gaz (Suppl) 140:S1–S9
Cérémonie H, Debelle F, Fernandez MP (1999) Structural and functional comparison of Frankia root hair deforming factor and rhizobia Nod factor. Can J Bot 77:1293–1301. doi:10.1139/b99-060
Chabaud M, Gherbi H, Pirolles E et al (2016) Chitinase-resistant hydrophilic symbiotic factors secreted by Frankia activate both Ca2+ spiking and NIN gene expression in the actinorhizal plant Casuarina glauca. New Phytol 209:86–93. doi:10.1111/nph.13732
Clavijo F, Diedhiou I, Vaissayre V et al (2015) The Casuarina NIN gene is transcriptionally activated throughout Frankia root infection as well as in response to bacterial diffusible signals. New Phytol 208:887–903. doi:10.1111/nph.13506
Clawson ML, Bourret A, Benson DR (2004) Assessing the phylogeny of Frankia – actinorhizal plant nitrogen-fixing root nodule symbioses with Frankia 16S rRNA and glutamine synthetase gene sequences. Mol Phylogenet Evol 31:131–138. doi:10.1016/j.ympev.2003.08.001
Dawson JO (2008) Ecology of actinorhizal plants. In: Pawlowski K, Newton WE (eds) Nitrogen-fixing actinorhizal symbioses. Springer, New York, pp 199–234
Diem HG, Dommergues YR (1990) Current and potential uses and management of Casuarinaceae in tropics and subtropics. In: Schwintzer CR, Tjepkema JD (eds) The biology of Frankia and actinorhizal plants. Academic Press, San Diego, pp 317–342
Doyle LL, Luckow MA (2003) The rest of the iceberg. Legume diversity and evolution in a phylogenetic context. Plant Physiol 131:900–910. doi:10.1104/pp.102.018150
Endre G, Kereszt A, Kevei Z et al (2002) A receptor kinase gene regulating symbiotic nodule development. Nature 417:962–966. doi:10.1038/nature00842
Esseling JJ, Lhuissier FG, Emons AM (2003) Nod factor-induced root hair curling: continuous polar growth towards the point of Nod factor application. Plant Physiol 132:1982–1988. doi:10.1104/pp.103.021634
Felsenstein J (2005) PHYLIP (Phylogeny Inference Package). Available from: http://evolution.genetics.washington.edu/phylip.html
Fontaine MS, Lancelle SA, Torrey JG (1984) Initiation and ontogeny of vesicles in cultured Frankia sp. strain HFPArI3. J Bacteriol 160:921–927
Gallon JR (1981) The oxygen sensitivity of nitrogenase: a problem for biochemists and microorganisms. Trends Biol Sci 6:19–23. doi:10.1016/0968-0004(81)90008-6
Gauthier D, Jaffre T, Prin Y (2000) Abundance of Frankia from Gymnostoma spp. in the rhizosphere of Alphitonia neocaledonica, a non-nodulated Rhamnaceae endemic to New Caledonia. Eur J Soil Biol 36:169–175. doi:10.1016/S1164-5563(00)01061-X
Gherbi H, Markmann K, Svistoonoff S et al (2008) SymRK defines a common genetic basis for plant root endosymbioses with arbuscular mycorrhiza fungi, rhizobia, and Frankia bacteria. Proc Natl Acad Sci USA 105:4928–4932. doi:10.1073/pnas.0710618105
Ghodhbane-Gtari F, Beauchemin N, Bruce D et al (2013) Draft genome sequence of Frankia sp. strain CN3, an atypical, noninfective (Nod–) ineffective (Fix–) isolate from Coriaria nepalensis. Genome Announc 1:e00085–e00013. doi:10.1128/genomeA.00085-13
Ghodhbane-Gtari F, Hurst SG IV, Oshone R et al (2014) Draft genome sequence of Frankia sp. strain BMG5.23, a salt tolerant nitrogen-fixing actinobacterium isolated from the root nodules of Casuarina glauca grown in Tunisia. Genome Announc 2:e00520-14. doi:10.1128/genomeA.00520-14
Granqvist E, Sun J, Op den Camp R et al (2015) Bacterial-induced calcium oscillations are common to nitrogen-fixing associations of nodulating legumes and nonlegumes. New Phytol 207:551–558. doi:10.1111/nph.13464
Gtari M, Ghodhbane-Gtari F, Nouioui I et al (2015) Cultivating the uncultured: growing the recalcitrant cluster-2 Frankia strains. Sci Rep 5:13112. doi:10.1038/srep13112
Guan C, Ribeiro A, Akkermans AD et al (1996) Nitrogen metabolism in actinorhizal nodules of Alnus glutinosa: expression of glutamine synthetase and acetylornithine transaminase. Plant Mol Biol 32:1177–1184. doi:10.1007/BF00041403
Hafeez F, Akkermans ADL, Chaudhary AH (1984) Observations on the ultrastructure of Frankia sp. in root nodules of Datisca cannabina L. Plant Soil 79:383–402. doi:10.1007/BF02184330
Heidstra R, Geurts R, Franssen H et al (1994) Root hair deformation activity of nodulation factors and their fate on Vicia sativa. Plant Physiol 105:787–797
Hiltner L (1895) Über die Bedeutung der Wurzelknöllchen von Alnus glutinosa für die Stickstoffernährung dieser Pflanze. Landwirtschaftliche Verständnisstudien 46:153–161
Horváth B, Yeun LH, Domonkos A et al (2011) Medicago truncatula IPD3 is a member of the common symbiotic signaling pathway required for rhizobial and mycorrhizal symbioses. Mol Plant-Microbe Interact 24:1345–1358. doi:10.1094/MPMI-01-11-0015
Huguet V, Mergeay M, Cervantes E et al (2004) Diversity of Frankia strains associated to Myrica gale in Western Europe: impact of host plant (Myrica vs. Alnus) and of edaphic factors. Environ Microbiol 6:1032–1041. doi:10.1111/j.1462-2920.2004.00625.x
Hurst SG IV, Oshone R, Ghodhbane-Gtari F et al (2014) Draft genome sequence of Frankia sp. strain Thr, a nitrogen-fixing actinobacterium isolated from the root nodules of Casuarina cunninghamiana grown in Egypt. Genome Announc 2:e00493–14. doi:10.1128/genomeA.00493-14
Huss-Danell K (1997) Actinorhizal symbioses and their N2 fixation. Tansley review no. 93. New Phytol 136:375–405. doi:10.1046/j.1469-8137.1997.00755.x
Huss-Danell K, Frej AK (1986) Distribution of Frankia strains in forest and afforestation sites in Northern Sweden. Plant Soil 90:407–418. doi:10.1007/BF02277412
Ishikawa J, Yamashita A, Mikami Y et al (2004) The complete genomic sequence of Nocardia farcinica IFM 10152. Proc Natl Acad Sci USA 101:14925–14930. doi:10.1073/pnas.0406410101
Jacobsen-Lyon K, Jensen EO, Jørgensen JE et al (1995) Symbiotic and nonsymbiotic hemoglobin genes of Casuarina glauca. Plant Cell 7:213–223. doi:10.1105/tpc.7.2.213
Jeong SC (2001) Population size and diversity of Frankia in soils of Ceanothus velutinus and Douglas-fir stands. Soil Biol Biochem 33:931–941. doi:10.1016/S0038-0717(00)00241-8
Jeong J, Suh S, Guan C et al (2004) A nodule-specific dicarboxylate transporter from alder is a member of the peptide transporter family. Plant Physiol 134:969–978. doi:10.1104/pp.103.032102
Kallimanis A, Karabika E, Mavromatis K et al (2011) Complete genome sequence of Mycobacterium sp. strain (Spyr1) and reclassification to Mycobacterium gilvum Spyr1. Stand Genomic Sci 5:144–153. doi:10.4056/sigs.2265047
Kawaharada Y, Kelly S, Nielsen MW et al (2015) Receptor-mediated exopolysaccharide perception controls bacterial infection. Nature 523:308–312. doi:10.1038/nature14611
Kleemann G, Kellner R, Poralla K (1994) Purification and properties of the squalene-hopene cyclase from Rhodopseudomonas palustris, a purple non-sulfure bacterium producing hopanoids and tetrahymanol. Biochim Biophys Acta 1210:317–320. doi:10.1016/0005-2760(94)90235-6
Knowlton S, Berry A, Torrey JG (1980) Evidence that associated soil bacteria may influence root hair infection of actinorhizal plants by Frankia. Can J Microbiol 26:971–977. doi:10.1139/m80-228
Lalonde M, Knowles R (1975) Ultrastructure, composition and biogenesis of the encapsulation material surrounding the endophyte in Alnus crispa var. mollis root nodules. Can J Bot 53:1951–1971. doi:10.1139/b75-219
Laplaze L, Duhoux E, Franche C et al (2000) Casuarina glauca prenodule cells display the same differentiation as the corresponding nodule cells. Mol Plant-Microbe Interact 13:107–112. doi:10.1094/MPMI.2000.13.1.107
Lavin M, Herendeen P, Wojciechowski MF (2005) Evolutionary rates analysis of Leguminosae implicates a rapid diversification of lineages during the tertiary. Syst Biol 54:530–549. doi:10.1080/10635150590947131
Lawrence DB, Schoenike RE, Quispel A et al (1967) The role of Dryas drummondii in vegetation development following ice recession at Glacier Bay, Alaska, with special reference to its nitrogen fixation by root nodules. J Ecol 55:793–813. doi:10.2307/2258426
Lechevalier MP (1994) Taxonomy of the genus Frankia (Actinomycetales). Int J Syst Bacteriol 44:1–8. doi:10.1099/00207713-44-1-1
Lévy J, Bres C, Geurts R et al (2004) A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science 303:1361–1364. doi:10.1126/science.1093038
Limpens E, Franken C, Smit P et al (2003) LysM domain receptor kinases regulating rhizobial Nod factor-induced infection. Science 302:630–633. doi:10.1126/science.1090074
Liu Q, Berry AM (1991a) The infection process and nodule initiation in the Frankia-Ceanothus root nodule symbiosis: a structural and histochemical study. Protoplasma 163:82–92. doi:10.1007/BF01323332
Liu Q, Berry AM (1991b) Localization and characterization of pectic polysaccharides in roots and root nodules of Ceanothus spp. during intercellular infection by Frankia. Protoplasma 164:93–101. doi:10.1007/BF01323333
Madsen EB, Madsen LH, Radutoiu S et al (2003) A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature 425:637–640. doi:10.1038/nature02045
Maillet F, Poinsot V, André O et al (2011) Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 469:58–63. doi:10.1038/nature09622
Mansour SR, Oshone R, Hurst SG et al (2014) Draft genome sequence of Frankia sp. strain CcI6, a salt-tolerant nitrogen-fixing actinobacterium isolated from the root nodule of Casuarina cunninghamiana. Genome Announc 2:e01205–e01213. doi:10.1128/genomeA.01205-13
Markmann K, Parniske M (2009) Evolution of root endosymbiosis with bacteria: how novel are nodules? Trends Plant Sci 14:77–86. doi:10.1016/j.tplants.2008.11.009
Markmann K, Giczey G, Parniske M (2008) Functional adaptation of a plant receptor-kinase paved the way for the evolution of intracellular root symbioses with bacteria. PLoS Biol 6:e68. doi:10.1371/journal.pbio.0060068
Marsh JF, Rakocevic A, Mitra RM et al (2007) Medicago truncatula NIN is essential for rhizobial-independent nodule organogenesis induced by autoactive calcium/calmodulin-dependent protein kinase. Plant Physiol 144:324–335. doi:10.1104/pp.106.093021
Maunuksela L, Zepp K, Koivula T et al (1999) Analysis of Frankia populations in three soils devoid of actinorhizal plants. FEMS Microbiol Ecol 28:11–21. doi:10.1111/j.1574-6941.1999.tb00556.x
Meesters TM, Van Vliet WM, Akkermans ADL (1987) Nitrogenase is restricted to the vesicles in Frankia strain EANlpec. Physiol Plant 70:267–271
Mergaert P, Van Montagu M, Holsters M (1997) Molecular mechanisms of Nod factor diversity. Mol Microbiol 25:811–817. doi:10.1111/j.1365-2958.1997.mmi526.x
Meyen J (1829) Über das Hervorwachsen parasitischer Gebilde aus den Wurzeln anderer Pflanzen. Flora (Jena) 12:49–64
Miller IM, Baker DD (1985) The initiation development and structure of root nodules in Elaeagnus angustifolia (Elaeagnaceae). Protoplasma 128:107–119. doi:10.1007/BF01276333
Minchin FR (1997) Regulation of oxygen diffusion in legume nodules. Soil Biol Biochem 29:881–888. doi:10.1016/S0038-0717(96)00204-0
Mirza S, Pawlowski K, Hafeez FY et al (1994) Ultrastructure of the endophyte and localization of nifH transcripts in root nodules of Coriaria nepalensis Wall. by in situ hybridization. New Phytol 126:131–136. doi:10.1111/j.1469-8137.1994.tb07538.x
Murry MA, Zhang Z, Torrey JG (1985) Effect of O2 on vesicle formation, acetylene reduction, and O2-uptake kinetics in Frankia sp. HFPCcI3 isolated from Casuarina cunninghamiana. Can J Microbiol 31:804–809. doi:10.1139/m85-151
Mylona P, Pawlowski K, Bisseling T (1995) Symbiotic nitrogen fixation. Plant Cell 7:869–885. doi:10.1105/tpc.7.7.869
Nalin R, Putra SR, Domenach A-M, Rohmer M, Gourbiere F, Berry AM (2000) High hopanoid/total lipids ratio in Frankia mycelia is not related to the nitrogen status. Microbiology 146:3013–3019. doi:10.1099/00221287-146-11-3013
Newcomb W, Pankhurst CE (1982) Fine structure of actinorhizal nodules of Coriaria arborea (Coriariaceae). New Zeal J Bot 20:93–103. doi:10.1080/0028825X.1982.10426409
Noridge NA, Benson DR (1986) Isolation and nitrogen-fixing activity of Frankia sp. strain CpI1 vesicles. J Bacteriol 166:301–305. doi:10.1155/2014/568549
Normand P, Orso S, Cournoyer B et al (1996) Molecular phylogeny of the genus Frankia and related genera and emendation of the family Frankiaceae. Int J Syst Bacteriol 46:1–9
Normand P, Lapierre P, Tisa LS et al (2007) Genome characteristics of facultatively symbiotic Frankia sp. strains reflect host range and host plant biogeography. Genome Res 17:7–15. doi:10.1101/gr.5798407
Nouioui I, Beauchemin N, Cantor MN et al (2013) Draft genome sequence of Frankia sp. strain BMG5.12, a nitrogen-fixing actinobacterium isolated from Tunisian soils. Genome Announc 1:e00468–13. doi:10.1128/genomeA.00468-13
Oldroyd GE (2013) Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nat Rev Microbiol 11:252–263. doi:10.1038/nrmicro2990
Op den Camp R, Streng A, De Mita S et al (2011) LysM-type mycorrhizal receptor recruited for rhizobium symbiosis in nonlegume Parasponia. Science 331:909–912. doi:10.1126/science.1198181
Parsons R, Silvester WB, Harris S et al (1987) Frankia vesicles provide inducible and absolute oxygen protection for nitrogenase. Plant Physiol 83:728–731. doi:10.1104/pp.83.4.728
Pawlowski K, Bisseling T (1996) Rhizobial and actinorhizal symbioses: what are the shared features? Plant Cell 8:1899–1913. doi:10.1105/tpc.8.10.1899
Pawlowski K, Demchenko KN (2012) The diversity of actinorhizal symbiosis. Protoplasma 249:967–979. doi:10.1007/s00709-012-0388-4
Pawlowski K, Jacobsen KR, Alloisio N et al (2007) Truncated hemoglobins in actinorhizal nodules of Datisca glomerata. Plant Biol 9:776–785. doi:10.1055/s-2007-965258
Persson T, Benson DR, Normand P et al (2011) Genome sequence of “Candidatus Frankia datiscae” Dg1, the uncultured microsymbiont from nitrogen-fixing root nodules of the dicot Datisca glomerata. J Bacteriol 193:7017–7018. doi:10.1128/JB.06208-11
Persson T, Battenberg K, Demina IV et al (2015) Candidatus Frankia datiscae Dg1, the actinobacterial microsymbiont of Datisca glomerata, expresses the canonical nod genes nodABC in symbiosis with its host plant. PLoS One 10:e0127630. doi:10.1371/journal.pone.0127630
Persson T, Nguyen TV, Alloisio N et al (2016) The N-metabolites of roots and actinorhizal nodules from Alnus glutinosa and Datisca glomerata: can D. glomerata change N-transport forms when nodulated? Symbiosis. doi:10.1007/s13199-016-0407-x
Pujic P, Bolotin A, Fournier P et al (2015) Genome sequence of the atypical symbiotic Frankia R43 strain, a nitrogen-fixing and hydrogen-producing actinobacterium. Genome Announc 3:e01387–e01315. doi:10.1128/genomeA.01387-15
Quispel A (1990) Discoveries, discussions and trends in research on actinorhizal root nodule symbioses before 1978. In: Schwintzer CR, Tjepkema JD (eds) The biology of Frankia and actinorhizal plants. Academic, San Diego, pp 15–33
Racette S, Torrey JG (1989) Root nodule initiation in Gymnostoma (Casuarinaceae) and Shephardia (Elaeagnaceae) induced by Frankia strain HFPGpI1. Can J Bot 67:2873–2879. doi:10.1139/b89-368
Ramírez-Saad H, Janse JD, Akkermans ADL (1998) Root nodules of Ceanothus caeruleus contain both the N2-fixing Frankia endophyte and a phylogenetically related Nod−/Fix− actinomycete. Can J Microbiol 44:140–148. doi:10.1139/cjm-44-2-140
Schröder P (1989) Aeration of the root system in Alnus glutinosa L. Gaertn. Ann Sci Forest 46:310–314. doi:10.1051/forest:19890571
Schubert KR (1986) Products of biological nitrogen fixation in higher plants: synthesis, transport and metabolism. Annu Rev Plant Physiol 37:539–574. doi:10.1146/annurev.pp.37.060186.002543
Schubert M, Koteeva NK, Wabnitz PW et al (2011) Plasmodesmata distribution and sugar partitioning in nitrogen-fixing root nodules of Datisca glomerata. Planta 233:139–152. doi: 0.1007/s00425-010-1285-8
Schubert M, Koteyeva NK, Zdyb A et al (2013) Lignification of cell walls of infected cells in Casuarina glauca nodules that depend on symplastic sugar supply is accompanied by reduction of plasmodesmata number and narrowing of plasmodesmata. Physiol Plant 147:524–540. doi:10.1111/j.1399-3054.2012.01685.x
Schwintzer CR (1990) Spore-positive and spore-negative nodules. In: Schwintzer CR, Tjepkema JD (eds) The biology of Frankia and actinorhizal plants. Academic Press, San Diego, pp 177–193
Schwintzer CR, Berry AM, Disney LD (1982) Seasonal patterns of root nodule growth, endophyte morphology, nitrogenase activity and shoot development in Myrica gale. Can J Bot 60:746–757
Sen R, Lahudkar S, Durairaj G, Bahumik SR (2013) Functional analysis of Bre1p, an E3 ligase for histone H2B ubiquitylation, in regulation of RNA polymerase II association with active genes and transcription in vivo. J Biol Chem 288(14):9619–9633
Sen A, Daubin V, Abrouk D et al (2014) Phylogeny of the class Actinobacteria revisited in the light of complete genomes. The orders ‘Frankiales’ and Micrococcales should be split into coherent entities: proposal of Frankiales ord. nov., Geodermatophilales ord. nov., Acidothermales ord. nov. and Nakamurellales ord. nov. Int J Syst Evol Microbiol 64:3821–3832. doi:10.1099/ijs.0.063966-0
Sequerra J, Capellano A, Faure-Raynaud M et al (1994) Root hair infection process and myconodule formation on Alnus incana by Penicillium nodositatum Valla. Can J Bot 72:955–975. doi:10.1139/b94-121
Sequerra J, Capellano A, Gianinazzi-Pearson V (1995) Ultrastructure of cortical root cells of Alnus incana infected by Penicillium nodositatum. New Phytol 130:545–555. doi:10.1111/j.1469-8137.1995.tb04331.x
Silvester WB, Harris SL (1989) Nodule structure and nitrogenase activity of Coriaria arborea in response to varying pO2. Plant Soil 118:97–109. doi:10.1007/BF02232794
Silvester WB, Harris SL, Tjepkema JD (1990) Oxygen regulation and hemoglobin. In: Schwintzer CR, Tjepkema JD (eds) The biology of Frankia and actinorhizal plants. Academic Press, San Diego, pp 157–176
Singh S, Katzer K, Lambert J et al (2014) CYCLOPS, a DNA-binding transcriptional activator, orchestrates symbiotic root nodule development. Cell Host Microbe 15:139–152. doi:10.1016/j.chom.2014.01.011
Smolander A, Sundman V (1987) Frankia in acid soils of forests devoid of actinorhizal plants. Physiol Plant 70:297–303. doi:10.1111/j.1399-3054.1987.tb06147.x
Smouni A, Laplaze L, Bogusz D et al (2002) The 35S promoter is not constitutively expressed in the transgenic tropical actinorhizal tree Casuarina glauca. Funct Plant Biol 29:649–656. doi:10.1071/PP01121
Soltis DE, Soltis PS, Morgan DR et al (1995) Chloroplast gene sequence data suggest a single origin of the predisposition for symbiotic nitrogen fixation in angiosperms. Proc Natl Acad Sci USA 92:2647–2651. doi:10.1073/pnas.92.7.2647
Soltis DE, Mort ME, Latvis M et al (2013) Phylogenetic relationships and character evolution analysis of Saxifragales using a supermatrix approach. Am J Bot 100:916–929. doi:10.3732/ajb.1300044
Soyano T, Shimoda Y, Hayashi M (2015) Nodule inception antagonistically regulates gene expression with nitrate in Lotus japonicus. Plant Cell Physiol 56:368–376. doi:10.1093/pcp/pcu168
Stracke S, Kistner C, Yoshida S et al (2002) A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature 417:959–962. doi:10.1038/nature00841
Sun J, Miller JB, Granqvist E, Wiley-Kalil A et al (2015) Activation of symbiosis signaling by arbuscular mycorrhizal fungi in legumes and rice. Plant Cell 27:823–838. doi:10.1105/tpc.114.131326
Suryakumar G, Gupta A (2011) Medicinal and therapeutic potential of sea buckthorn (Hippophae rhamnoides L.) J Ethnopharmacol 138:269–278. doi:10.1016/j.jep.2011.09.024
Suzuki W, Konishi M, Yanagisawa S (2013) The evolutionary events necessary for the emergence of symbiotic nitrogen fixation in legumes may involve a loss of nitrate responsiveness of the NIN transcription factor. Plant Signal Behav 8:e25975. doi:10.4161/psb.25975
Svistoonoff S, Benabdoun FM, Nambiar-Veetil M et al (2013) The independent acquisition of plant root nitrogen-fixing symbiosis in Fabids recruited the same genetic pathway for nodule organogenesis. PLoS One 8:e64515. doi:10.1371/journal.pone.0064515
Tisa LS, Beauchemin N, Cantor MN, Furnholm T et al (2015) Draft genome sequence of Frankia sp. strain DC12, an atypical, noninfective, ineffective isolate from Datisca cannabina. Genome Announc 3:e00889–e00815. doi:10.1128/genomeA.00889-15
Tjepkema JD (1978) The role of diffusion from the shoots and nodule roots in nitrogen fixation by root nodules of Myrica gale L. Am J Bot 70:59–63. doi:10.1139/b78-156
Tjepkema JD, Ormerod W, Torrey JG (1980) On vesicle formation and in vitro acetylene reduction by Frankia. Nature 287:633–635
Torrey JG (1976) Initiation and development of root nodules of Casuarina (Casuarinaceae). Am J Bot 63:335–344
Torrey JG, Callaham D (1982) Structural features of the vesicle of Frankia sp. cell in culture. Can J Microbiol 28:749–757. doi:10.1139/m82-114
Udvardi M, Poole PS (2013) Transport and metabolism in legume-rhizobia symbioses. Annu Rev Plant Biol 64:781–805. doi:10.1146/annurev-arplant-050312-120235
Uemura S (1971) Non-leguminous root nodules in Japan. Plant Soil 29:349–350. doi:10.1007/BF02661863
Valverde C, Wall LG (1999) Time course of nodule development in the Discaria trinervis (Rhamnaceae)–Frankia symbiosis. New Phytol 141:345–354. doi:10.1046/j.1469-8137.1999.00345.x
Van Brussel AAN, Bakhuizen R, van Spronsen PC et al (1992) Induction of pre-infection thread structures in the leguminous host plant by mitogenic lipooligosaccharides of Rhizobium. Science 257:70–72. doi:10.1126/science.257.5066.70
Van Ghelue M, Ribeiro A, Solheim B et al (1996) Sucrose synthase and enolase expression in actinorhizal nodules of Alnus glutinosa: comparison with legume nodules. Mol Gen Genet 250:437–446. doi:10.1007/s004380050096
Van Ghelue M, Løvaas E, Ringø E et al (1997) Early interactions between Alnus glutinosa and Frankia strain ArI3. Production and specificity of root hair deformation factor(s). Physiol Plant 99:579–587. doi:10.1111/j.1399-3054.1997.tb05360.x
Vanden Heuvel BD, Benson DR, Bortiri E et al (2004) Low genetic diversity among Frankia spp. strains nodulating sympatric populations of actinorhizal species of Rosaceae, Ceanothus (Rhamnaceae) and Datisca glomerata (Datiscaceae) west of the Sierra Nevada (California). Can J Microbiol 50:989–1000. doi:10.1139/W04-079
VandenBosch KA, Torrey JB (1983) Host-endophyte interactions in effective and ineffective nodules induced by the endophyte of Myrica gale. Can J Bot 61:2898–2909. doi:10.1139/b83-323
VandenBosch KA, Torrey JG (1985) Development of endophytic Frankia sporangia in field- and laboratory-grown nodules of Comptonia peregrina and Myrica gale. Am J Bot 72:99–108
Vasse J, de Billy F, Camut S et al (1990) Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J Bacteriol 172:4295–4306
Wall LG, Beauchemin N, Cantor MN et al (2013) Draft genome sequence of Frankia sp. strain BCU110501, a nitrogen-fixing actinobacterium isolated from nodules of Discaria trinervis. Genome Announc 1:e00503–e00513. doi:10.1128/genomeA.00503-13
Werner GD, Cornwell WK, Sprent JI et al (2014) A single evolutionary innovation drives the deep evolution of symbiotic N2-fixation in angiosperms. Nat Commun 5:4087. doi:10.1038/ncomms5087
Wheeler CT, Gordon JC, Ching TM (1979) Oxygen relations of the root nodules of Alnus rubra bong. New Phytol 82:449–457. doi:10.1111/j.1469-8137.1979.tb02671.x
Willmann R, Lajunen H, Erbs G et al (2011) Arabidopsis lysin-motif proteins LYM1/LYM3/CERK1 mediate bacterial peptidoglycan sensing and immunity to bacterial infection. Proc Natl Acad Sci USA 108:19824–19829. doi:10.1073/pnas.1112862108
Wolters DJ, Van Dijk C, Zoetendal EG, Akkermans AD (1997) Phylogenetic characterization of ineffective Frankia in Alnus glutinosa (L.) Gaertn. nodules from wetland soil inoculants. Mol Ecol 6:971–981. doi:10.1046/j.1365-294X.1997.00265.x
Yano K, Yoshida S, Müller J et al (2008) CYCLOPS, a mediator of symbiotic intracellular accommodation. Proc Natl Acad Sci USA 105:20540–20545. doi:10.1073/pnas.0806858105
Zhang XC, Cannon SB, Stacey G (2009) Evolutionary genomics of LysM genes in land plants. BMC Evol Biol 9:183. doi:10.1186/1471-2148-9-183
Zitzer SF, Dawson JO (1992) Soil properties and actinorhizal vegetation influence nodulation of Alnus glutinosa and Elaeagnus angustifolia by Frankia. Plant Soil 140:197–204. doi:10.1007/BF00010597
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Van Nguyen, T., Pawlowski, K. (2017). Frankia and Actinorhizal Plants: Symbiotic Nitrogen Fixation. In: Mehnaz, S. (eds) Rhizotrophs: Plant Growth Promotion to Bioremediation. Microorganisms for Sustainability, vol 2. Springer, Singapore. https://doi.org/10.1007/978-981-10-4862-3_12
Download citation
DOI: https://doi.org/10.1007/978-981-10-4862-3_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4861-6
Online ISBN: 978-981-10-4862-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)