Abstract
Hospital environmental conditions, human occupancy, and the characteristics of the equipment influence the survival of microbial communities and raise a concern with regard to nosocomial infections. The objective of the present work was to use the monitoring of Pseudomonas aeruginosa, Klebsiella spp. and non-tuberculous mycobacteria as a strategy to improve knowledge on microbial colonization of non-critical equipment and surfaces, in a tertiary hospital from Central Portugal. A 3-month microbiological survey was performed in a district teaching hospital. A total of 173 samples were obtained from the wards Hematology, Urology, Medicine, and Renal Transplants, and 102 presumptive strains recovered. Per sampling, Pseudomonas Isolation agar showed 42.8 to 73.3% of presumptive P. aeruginosa colonies and MacConkey agar recovered mostly Staphylococcus. Most of the colonies recovered in Middlebrook 7H10-PANTA belonged to the genus Methylobacterium. Taps and WC shower curtains carry high bacterial species diversity. The Redundancy Analysis grouped the samples in those mostly handled by patients, and those mostly handled by healthcare staff or of mixed use. This study shows that the preferential users of the space and equipment seem to be important contributors to the microbial community. The most recovered genus was Methylobacterium, known as colonizer of the water distribution system therefore, it is possible that the water points and biofilms in taps also contribute as dispersion hotspots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hospital acquired infections (HAI) are an urgent and current problem worldwide, with the rate of hospital infections acting as an indicator for the quality of health care service. The costs associated with health care can be measured directly and indirectly. The latter costs include infections acquired at a health care facility that either prolong the patient stay, leading to a readmission, or hamper treatment (Umscheid et al. 2011). Incorrect disinfection by patients, staff and/or visitors, patient transfer between hospitals, poor disinfection of non-critical equipment and surfaces may contribute to dissemination of bacteria in a hospital (Tan et al. 2013; Leverstein-Van Hall et al. 2006).
The group of organisms ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) represent a substantial percentage of nosocomial infections in modern hospitals and constitute the majority of the isolates whose resistance to antimicrobial agents presents serious therapeutic dilemmas for healthcare workers (Pendleton et al. 2013).
P. aeruginosa, is implicated in various cases of HAI, with particular relevance in patients with cystic fibrosis (Høiby 2011), occurring mostly in patients from intensive care units (Falkinham et al. 2015). This species was present in 18% of the samples recovered from a hospital environment in Portugal (de Abreu et al. 2014). Strains from the genus Klebsiella have also been related to HAI mainly infecting immunocompromised patients (Struve and Krogfelt 2004). They are commonly found as part of the gut microbiome of several animals, including humans, and can be recovered from health care plastic surfaces and fabrics including health care workers attire (Neely 2000; Wiener-Well et al. 2011).
In recent years, non-tuberculous mycobacteria (NTM) have emerged as a major cause of opportunistic infections and, without evidence of person-to-person transmission of NTM, it is proposed that humans are infected from environmental sources (Fernandez-Rendon et al. 2012). Their importance has grown in recent years with the recovery of numerous isolates from aquatic environments, urban waste discharges, and recreational pools/fountains as well as industrial and health care waters (Falkinham et al. 2015). In consequence, it is important to know the prevalence of NTM in the hospital environment a study that, as far as known, was never attempted in Portugal.
The dynamics of indoor environmental conditions, building design, human occupancy, and operational characteristics influence indoor environmental quality and survival and progression of microbial communities (Bessonneau et al. 2013; Ramos et al. 2015) and, therefore, the progression, survival, and transmission of microbial pathogens (Richardson et al. 2014; Tang 2009).
Considering the published data on the species more frequently involved in human infections (Pendleton et al. 2013), and in order to contribute to the information needed to make decision in epidemiology, the objective of the present work was to use the monitoring of P. aeruginosa, Klebsiella spp. and non-tuberculous mycobacteria in a tertiary hospital from Central Portugal as a strategy to improve knowledge on microbial non-critical equipment and surfaces colonization. The selection of the species to develop the research was related to the existence, at the hospital, of clinical cases involving these species.
Materials and methods
Sampling non-critical hospital objects and surfaces
The study was carried out in collaboration with a hospital from Central Portugal. The monitoring of the bacterial prevalence in the hospital environment was followed during a 3 month period, from November 2012 to January 2013. During this period, a total of 173 samples were recovered from the four different wards selected. The number of samples (59) per sampling was very similar in the different wards and covering the same type of non-critical equipment and surfaces.
The evaluation was performed in the standard chirurgical wards of Hematology, Urology, Renal Transplants and the medical ward of Medicine A (one of the three medical wards of this hospital). The majority of patients in the medical ward were elderly. The wards were non-adjacent with dedicated medical personnel in each one. In each one, an assortment of non-critical equipment and surfaces was evaluated (Table 1). Those included items mainly handled by health care staff, mainly handled by patients or handled by everyone. Samples were always taken from equivalent non-critical equipment and surfaces on all locations (different wards).
Samples were always collected at the end of the morning and during lunch time, after the medical visits and treatments. Swabs were used to sample an area of 10 × 10 cm of each surface. Taps were sampled by removing the biofilm. The swabs were first humidified in Peptone Water (Peptone 1%; Sodium chloride 0.5%) (PW) (Oxoid), then used to sample, and transported in 2 ml of PW tubes and then processed in the laboratory after 3 h shaking.
Determination of microbial load and bacterial isolation
Each volume of transporting broth (PW), containing a single swab, was vortexed for 1 min. Total microbial load was evaluated by plating 0.1 ml of the swab suspension in Reasoners 2 Agar (R2A), (Difco). Colony forming units (CFU) were determined after 48 h growth, at 22 °C. Results presented are the average counting per sample expressed in CFU/100 cm2, all counts over 200 CFU/100 cm2 were considered uncountable. All environmental samples were analyzed for bacterial content by inoculating 0.1 ml of the swab suspension in: 1) Pseudomonas Isolation Agar (Oxoid), 2) McConkey Agar (Difco), and 3) Middlebrook 7H10 medium supplemented with 10% OADC (Oleic Albumin Dextrose Catalase Growth Supplement) and 40 U/ml of polymyxin, 4 μg/ml of amphotericin B, 16 μg/ml of nalidixic acid, 4 μg/ml of trimethoprim, and 4 μg/ml of azlocillin (PANTA) (Radomski et al. 2010). The microbial load of the surfaces by cultivation on non-selective media involved the use of serial dilution to obtain countable plates. Therefore, the detection and isolation of microbial populations with low number of individuals as the target microorganisms were conditioned to the use of selective media.
Isolation of target bacterial taxa
The isolation of the target microorganisms was performed in the low selectivity and differentiative media mentioned above. Pseudomonas Isolation Agar (PIA) is a selective medium, containing the antibiotic Irgasan, for the isolation of Pseudomonas spp., differentiating P. aeruginosa, on the basis of fluorescent pigment formation. Samples were incubated for 24 h at 30 °C, and evaluated after this period for total counts and for the presence of colonies with fluorescence under UV light. All colonies showing fluorescence and therefore considered presumptively P. aeruginosa, were isolated and purified. From plates with fluorescent colonies, 47% of non-fluorescent colonies were also isolated.
For the isolation of Enterobacteriacea, the bacterial family that includes the Gram-negative and enteric bacilli Klebsiella spp., the complex medium MacConkey Agar without crystal violet was used. Samples were incubated at 37 °C during 48 h. Colonies with characteristics of Klebsiella, as described by the manufacturer (Difco), were isolated and cultivated in R2A medium up to purification. Since the isolation medium allows the growth of organisms different from Klebsiella, a first screen was performed by cultivating in Kligler’s iron Agar (Schau 1986). Where considered for further evaluation organisms able to produce acid during growth.
To recover presumptive NTM strains, all samples were directly plated in Middlebrook 7H10-PANTA Agar medium. Samples were incubated at 30 °C and evaluated for the presence of colonies over time (1–6 weeks). Colonies with different morphologies were counted on each plate and purified on new Middlebrook 7H10-PANTA plates at 30 °C. All isolates were stored in glycerol-containing medium at −80 °C.
16S rRNA gene sequence identification of the isolates
DNA from each isolate was obtained using the protocol from Pitcher et al. (1989) or for NTM strains, the protocol adapted from Nielsen and colleagues (Nielsen et al. 1995) with initial incubation for 2 h in GTE buffer (50 mM glucose, 25 mM Tris–HCl at pH 8.0, and 10 mM EDTA) containing lysozyme (20 mg/ml), at 37 °C. Amplification of the nearly full-length 16S rRNA gene sequence was performed by PCR with universal primers 27F and 1525R. (Rainey et al. 1996). The 1500 bp PCR products were purified using the EZNA Gel purification Kit (OMEGA-VWR) according to the manufacturer’s instructions and send to sequence at Macrogen (Neaderlands).
All sequences were compared with sequences available in the NCBI database using BLAST network services (Altschul et al. 1997), aligned with the CLUSTAL X program (Thompson et al. 1997), visually examined, and relocated to allow maximal alignment. The method of Jukes and Cantor (1990) was used to calculate evolutionary distances. Phylogenetic dendrograms were than constructed by the neighbor-joining method using the MEGA6 package (Tamura et al. 2007).
Statistical analysis
Redundancy analysis (RDA) was used to analyze the relationships between the level of contamination of the non-critical equipment and surfaces observed using the different selective media, the type of non-critical equipment and surfaces (response variables), and the sampling location (explanatory variables) using the software package CANOCO (version 4.5). The covariance relation was determined based on the percentage of each isolate against the total number of isolates at the same sample. Environmental variables (explanatory variables) were attributed to discriminate samples according to their source ward, Renal Transplants (T), Medicine (M), Hematology (H) and Urology (U) and preferential user, patients (Patient), medical staff (Infirmary), and common usage (Mixed).
Nucleotide sequence accession numbers
The 16S rRNA gene sequences of the isolates reported in this study have been deposited in EMBL and GenBank databases under the accession numbers from GenBank KT168232 to KT168284 for the strains recovered on PIA and MacConkey Agar and from GenBank KT347459 to KT347502 and KT832812 to KT832816 for the NTM and others strains recovered on Middlebrook 7H10-PANTA.
Results
Identification of the isolates
The 16S rRNA gene sequences of the type strains of the closest relatives of all isolates obtained in this study were used to construct the phylogenetic tree where only representative strains (57) are included (Fig. 1). The strains grouped in different phylogenetic clusters each of them including a different type species. The exception was the group formed by Stenotrophomonas maltophilia, P. geniculata, and Pseudomonas beteli that grouped together as expected (Van den Mooter and Swings 1990; Anzai et al. 2000). From all isolates, one strain, (HUC3 30p) grouped with the type strain of the species P. aeruginosa. The closest phylogenetic group included the type species P. hunanensis and P. taiwanensis and 7 isolates that were identified by the 16S rRNA gene sequencing as belonging to one of the species.
Phylogenetic tree of 16S rRNA genes obtained from hospital’s non-critical equipment and surfaces isolates. Sequences were aligned using Mega 5 for construction of phylogenetic dendrograms using the Neighbor-Joining algorithm with the following parameters: Jukes–Cantor correction model for nucleotides and 1000 bootstraps. Organisms used for phylogenetic analyses include all PIA, and MacConkey isolates as well as all NTM. Isolates nomenclature for strains recovered from PIA and McConkey identifies the hospital (HUC), the sampling (1/2/3), the number of the isolate and the media of recovery (k/p). Isolates nomenclature for strains recovered from Middlebrook 7H10-PANTA identifies the number of the isolate (first number and letter) and the sampling (roman numerals I/II/III)
A large cluster included Klebsiella pneumonia, Raoultella planticola, and Citrobacter freundii separated in subclusters according to the species. All Staphylococcus were included in a large cluster separated in subclusters according to the species.
Other distinct cluster included the NTM isolates grouped with the type strain of the species Mycobacterium gordonae (10AIII, 29AIII, and 35AIII), Mycobacterium obuence (22DIII), and Mycobacterium mucogenicum (24AIII).
Abundance and diversity of hospital isolates
During the whole period of the experiment, the number of isolates recovered was relatively constant, with an average of 34 colony forming units (CFU) per 100 cm2, per sampling (Table 1). High CFU/100 cm2 counts were observed mainly in water delivery hotspots as taps, showers, and drains. In total, the number of isolates (including typical and atypical colonies) was 23 in PIA, 30 in MacConkey and 49 in Middlebrook 7H10-PANTA medium.
In PIA medium, per sampling, between 42.8 and 73.3% of the samples showed presumptive P. aeruginosa colonies. Most of the isolates identified belong to the genus Pseudomonas (45.83%), represented by the species P. hunanensis (5 isolates), P. beteli (3 isolates), P. geniculata (2 isolates), P. taiwanensis, and P. aeruginosa (all with 1 isolate each), the last recovered from a tap in the Renal Transplants ward. The largest number of organisms was recovered in Hematology ward and the lowest in Urology ward (Table 2).
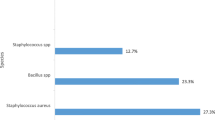
Species recovered in MacConkey agar belonged mostly to the genus Staphylococcus (72.4%), especially in Medicine A ward. The remaining isolates belonged to the classes beta-, and gammaproteobacteria, Table 2. A single strain of K. pneumoniae subsp. rhinoscleromatis was isolated in MacConkey agar.
The majority of the isolates recovered from Middlebrook 7H10-PANTA plates were identified, after 16S rRNA sequencing, as belonging to class alphaproteobacteria as representatives of genera Methylobacterium (22 isolates) and Sphingomonas (10 isolates) and to class gammaproteobacteria as Pseudomonas (7 isolates). Among the ten other isolates recovered in this medium were strains identified as closely related to M. gordonae M. obuense, and M. mucogenicum, (Table 2). These NTM were isolated from all wards sampled except from Medicine A.
Bacterial colonization of non-critical equipment and surfaces is diverse and site/equipment specific
The distribution of bacteria was unequal throughout the non-critical equipment and surfaces (Fig. 2). Moreover, in all three sampling campaigns, there were non-critical equipment and surfaces with high numbers of microorganism and non-critical equipment and surfaces with low numbers. A large number of strains, thirteen and nineteen strains, were isolated from taps and showers, respectively. The bacterial community in taps, although diverse, was mainly composed by phylogenetically related taxa Sphingobium yanoikuyae (4 strains), P. geniculata (3 strains), and P. beteli (2 strains). On the other hand, species isolated from showers included Staphylococcus, Pseudomonas, and Raoultella species. The strain P. aeruginosa was isolated from a tap in the Renal Transplants ward. In the used nursing tray, sampled in Renal Transplants ward, was isolated a K. pneumoniae subsp. rhinoscleromatis. Atop the list of the most widespread bacterial species were M. radiotolerans recovered in 13 different samples, Pseudomonas sp. from six different samples, and S. haemolyticus recovered from five samples (Fig. 2). MacConkey agar recovered a large number of isolates from the genus Staphylococcus. Staphylococcus (12.2%) were isolated from the area related to patient bedside area (lamp, support table) and from a clean nursing tray, a crash cart, and door handles. From the 47 non-critical equipment and surfaces surveyed, only four had a single microorganism species recovered.
Representation of the number of species per equipment. All strains isolated and identified by 16S rRNA were evaluated as to their presence in the different equipments. Equipments and surfaces listed did not contemplate the number of samplings or their source. Circumferences represent 1, 2, 3, and 4 represent the number of isolates per species per type of equipment/surfaces sampled (smaller to larger circle)
Mycobacteria isolates were retrieved from the three different hospital wards. M. gordonae were isolated from the bench work table (staff area) at Hematology, and from the bedside table and bench work table at Renal Transplants. M. obuense and M. mucogenicum isolates were both recovered in the Urology ward from a sink’s drain and from a light switch, respectively.
Redundancy analysis of the bacterial diversity and the NCE-S
A redundancy analysis (RDA) was carried to obtain a graphic correlation of the contamination load of the non-critical equipment and surfaces and bacterial diversity, grouping non-critical equipment and surfaces according to the handler. The cumulative percentage variance of the species–environment relation was 90.6%. The RDA grouped the samples in two major clusters: (1) in close relation to patient that grouped in quadrants 1 and 4 except the bedside table support and bed remote; (2) mostly handled by health care staff or NCE-S of mixed use grouped together in the quadrants 2 and 3 (Fig. 3). The contribution (at more than 5%) of the microorganisms’ species to the segregation of the different clusters could not be related with one specific species or genus (Fig. 3b). The samples collected in the tap in the patients room grouped independently in quadrant 1 (patient related). The species that contributed most to this clustering belong to the genus Pseudomonas including the P. aeruginosa strain isolated in this study.
Redundancy analyses (RDA) based on the equipment microbial load. The bacterial diversity present during the sampling period. For calculating the distribution of samples, only bacterial species contributing with, at least, 5% variance were considered. Cumulative variance of species–environment relation is explained for 90.6%. Centroids, gray squares, were determined for samples according to their source, renal transplants (T), hematology (H), urology (U), and medicine wards (M), as well as their usage, mostly handled by patients (Patient), mostly handled by medical personal (Infirmary) and handled by all (Mixed). The two first principal components are plotted with the proportion of variance explained by each axe. The distribution of samples, equipment, and surfaces, (Fig. 3a) is represented along with the centroids that define such samples. Independently, are shown the bacterial species that contributed with their distribution with, at least, 5% variance, to the separation of aforementioned samples (Fig. 3b). (Asterisk) marks the position of the vectors of the strains mentioned (mostly Pseudomonas); (Hash) marks the position of the vectors of strains mentioned (most Staphylococcus and Corynebacterium)
Discussion
The monitoring of environmental occurrences of P. aeruginosa, Klebsiella spp., and NTM from a Portuguese hospital is presented as a case study, contributing with information to decision making efforts in relation to possible HAI control. The selection of the target species was related to the existence, at that hospital, of clinical cases involving these species. In this work, the monitoring of these microorganisms on surfaces and non-critical material revealed that these microbial species were not common on those environments. Nevertheless, many equipments in the wards sampled had high microbial numbers detected in non-selective conditions.
The microbial load of the non-critical equipment and surfaces in the hospital sampled did not vary during the survey. The selective medium PIA recovered bacterial strains different from P. aeruginosa in more than 50% of the samples, including other Pseudomonas species as well as Proteobacteria and Firmicutes. Moreover, the use of selective media allowed the detection of populations with low number of individuals, not visible when using non-selective media (due to the use of serial dilutions) that can be important populations related with HAI. This was the case of the strains of the genus Staphylococcus, recovered in MacConkey agar in high numbers in the Medicine A ward, related with the patient area. The patient population includes those with a wide variety of medical conditions, including infectious conditions.
Two of the target organisms of this study were isolated only once and both in the Renal Transplants ward. P. aeruginosa is highly resistant to disinfectants, able to form biofilms and may inhabit the water distribution system (Falkinham et al. 2015); therefore, it was not surprising to isolate the strain in the tap water biofilm. On the other hand, K. pneumoniae subsp. rhinoscleromatis was isolated in a used nursing tray relating its presence with the manipulation of the non-critical equipment and surfaces.
Methylobacterium spp. was in this study the bacterial genus able to colonize the largest diversity of non-critical equipment and surfaces in the wards sampled. This species, easily missed during microbiological surveillances because of its slow growth, is found in natural environments, but is also reported as the cause of health care-associated infections in individuals with reduced immunity (Lai et al. 2011). In the present study, strains from this species were isolated in dry and humid areas, clean and after use and related with all the different type of users. This persistence may be linked to its ability to form biofilms and to develop tolerance to disinfecting agents, high temperatures, and drying (Angenent et al. 2005) and may be also related to its described persistence in tap water (Kovaleva et al. 2014).
S. maltophilia was recovered several times in this study from the biofilm in the taps showing that they can subsist on the non-critical equipment and surfaces. Species that are normal inhabitants of distribution water systems are a challenge to remove since they have wide metabolic versatility, resistance, and have been shown to grow inside cells (Cateau et al. 2014). It is also possible that surfaces are continuously inoculated and organisms are spread in the environment by touch.
NTM are widely dispersed in the environment, vary greatly in their ability to cause disease, and are not spread from person to person. In the present study, M. gordonae was isolated from a work bench and also in a bedside table. Although considered a rarely disease-associated organism, this species was predominant in patients from a hospital in Greece (Panagiotou et al. 2014). In the Urology ward of the present hospital, diverse Mycobacteria were recovered. One of the strains was M. mucogenicum which has been also previously isolated in a study analysing samples from bottled drinking water, and from ice (Covert et al. 1999). Here we demonstrate that these microorganisms can persist, at least for some time, in the environment, on surfaces touched by hospital staff, patients, and others. The isolation of NTM from dry surfaces (light switch) can open a new perspective since it was not described before.
In this hospital environment, complex contamination relationships occur, and some patterns seem to emerge when data were treated by redundancy analysis. Based on microbial composition of the environment, it was possible to separate samples in close relation with the patient area from the remaining samples. The microbial community around the patients did not seem to be related with the patient’s disease, although patients from Urology infirmary grouped slightly apart. Methylobacterium species were shared by the different groups of non-critical equipment and surfaces.
This study shows that the preferential users of the non-critical equipment and surfaces seem to be important contributors to the microbial community of the space. The most recovered bacterial species are colonizers of the water distribution system, therefore it is possible that the water points and biofilms in taps also contribute as dispersion hotspots in this hospital.
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W et al (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Angenent LT, Kelley ST, St Amand A, Pace NR, Hernandez MT (2005) Molecular identification of potential pathogens in water and air of a hospital therapy pool. Proc Natl Acad Sci USA 102:4860–4865. doi:10.1073/pnas.0501235102
Anzai Y, Kim H, Park JY, Wakabayashi H, Oyaizu H (2000) Phylogenetic affiliation of the pseudomonads based on 16S rRNA sequence. Int J Syst Evol Microbiol 50:1563–1589. doi:10.1099/00207713-50-4-1563
Bessonneau V, Mosqueron L, Berrubé A, Mukensturm G, Buffet-Bataillon S, Gangneux JP et al (2013) VOC contamination in hospital, from stationary sampling of a large panel of compounds, in view of healthcare workers and patients exposure assessment. PLoS ONE 8:e55535. doi:10.1371/journal.pone.0055535
Cateau E, Maisonneuve E, Peguilhan S, Quellard N, Hechard Y, Rodier MH (2014) Stenotrophomonas maltophilia and Vermamoeba vermiformis relationships: bacterial multiplication and protection in amoebal-derived structures. Res Microbiol 165:847–851. doi:10.1016/j.resmic.2014.10.004
Covert TC, Rodgers MR, Reyes AL, Stelma GN. (1999) Occurrence of nontuberculous mycobacteria in environmental samples. Appl Environ Microbiol 65:2492–2496. http://www.ncbi.nlm.nih.gov/pubmed/10347032
de Abreu PM, Farias PG, Paiva GS, Almeida AM, Morais PV (2014) Persistence of microbial communities including Pseudomonas aeruginosa in a hospital environment: a potential health hazard. BMC Microbiol 14:118. doi:10.1186/1471-2180-14-118
Falkinham J, Hilborn E, Arduino M, Pruden A, Edwards MA (2015) Epidemiology and ecology of opportunistic premise plumbing pathogens: Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa. Environ Health Perspect 110:A174. doi:10.1289/ehp.1409385
Fernandez-Rendon E, Cerna-Cortes JF, Ramirez-Medina M, Helguera-Repetto C, Rivera-Gutierrez S, Estrada-Garcia T et al (2012) Mycobacterium mucogenicum and other non-tuberculous mycobacteria in potable water of a trauma hospital: a potential source for human infection. J Hosp Infect 80:74–76. doi:10.1016/j.jhin.2011.10.003
Høiby N (2011) Recent advances in the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. BMC Med 9:32. doi:10.1186/1741-7015-9-32
Jukes TH, Cantor CR (1990) Evolution of protein molecules, vol 33. Academic Press, New York, pp 21–132
Kovaleva J, Degener JE, van der Mei HC (2014) Methylobacterium and its role in health care-associated infection. J Clin Microbiol 52:1317–1321. doi:10.1128/JCM.03561-13
Lai CC, Cheng A, Liu WL, Tan CK, Huang YT, Chung KP et al (2011) Infections caused by unusual Methylobacterium species. J Clin Microbiol 49:3329–3331. doi:10.1128/JCM.01241-11
Leverstein-Van Hall M, Blok HEM, Paauw A, Fluit AC, Troelstra A, Mascini EM et al (2006) Extensive hospital-wide spread of a multidrug-resistant Enterobacter cloacae clone, with late detection due to a variable antibiogram and frequent patient transfer. J Clin Microbiol 44:518–524. doi:10.1128/JCM.44.2.518-524.2006
Neely AN (2000) A survey of gram-negative bacteria survival on hospital fabrics and plastics. J Burn Care Res 21:523–527
Nielsen P, Fritze D, Priest FG (1995) Phenetic diversity of alkaliphilic Bacillus strains: proposal for nine new species. Microbiology 141:1745–1761. doi:10.1099/13500872-141-7-1745
Panagiotou M, Papaioannou AI, Kostikas K, Paraskeua M, Velentza E, Kanellopoulou M et al (2014) The epidemiology of pulmonary nontuberculous mycobacteria: data from a general hospital in Athens, Greece, 2007–2013. Pulm Med 2014:1–9. doi:10.1155/2014/894976
Pendleton JN, Gorman SP, Gilmore BF (2013) Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther 11:297–308. doi:10.1586/eri.13.12
Pitcher DG, Saunders N, Owen RJ (1989) Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol 8:151–156. doi:10.1111/j.1472-765X.1989.tb00262.x
Radomski N, Cambau E, Moulin L, Haenn S, Moilleron R, Lucas FS (2010) Comparison of culture methods for isolation of nontuberculous mycobacteria from surface waters. Appl Environ Microbiol 76:3514–3520. doi:10.1128/AEM.02659-09
Rainey FA, Ward-Rainey N, Kroppenstedt RM, Stackebrandt E (1996) The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int J Syst Bacteriol 46:1088–1092. doi:10.1099/00207713-46-4-1088
Ramos T, Dedesko S, Siegel J, Gilbert J, Stephens B (2015) Spatial and temporal variations in indoor environmental conditions, human occupancy, and operational characteristics in a new hospital building. PLoS ONE 10:e0118207. doi:10.1371/journal.pone.0118207
Richardson ET, Morrow CD, Kalil DB, Bekker LG, Wood R (2014) Shared air: a renewed focus on ventilation for the prevention of tuberculosis transmission. PLoS ONE 9:e96334. doi:10.1371/journal.pone.0096334
Schau HP (1986) I. Gylstorff (Herausgeber), Infektionen durchMycoplasmatales. 568 S., 39 Abb., 72 Tab. Jena 1985. VEB Gustav Fischer Verlag. M 260,00. J Basic Microbiol 26:224. doi:10.1002/jobm.3620260410
Struve C, Krogfelt KA (2004) Pathogenic potential of environmental Klebsiella pneumoniae isolates. Environ Microbiol 6:584–590. doi:10.1111/j.1462-
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599. doi:10.1093/molbev/msm092
Tan TY, Tan JSM, Tay H, Chua GH, Yong Ng LS, Syahidah N (2013) Multidrug-resistant organisms in a routine ward environment: differential propensity for environmental dissemination and implications for infection control. J Med Microbiol 62:766–772. doi:10.1099/jmm.0.052860-0
Tang JW (2009) The effect of environmental parameters on the survival of airborne infectious agents. J R Soc Interface 6:S737–S746. doi:10.1098/rsif.2009.0227.focus
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Umscheid C, Mitchell MD, Doshi J, Agarwal R, Williams K, Brennan PJ (2011) Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol 32:101–114. doi:10.1086/657912
Van den Mooter M, Swings J (1990) Numerical analysis of 295 phenotypic features of 266 Xanthomonas strains and related strains and an improved taxonomy of the genus. Int J Syst Bacteriol 40:348–369. doi:10.1099/00207713-40-4-348
Wiener-Well Y, Galuty M, Rudensky B, Schlesinger Y, Attias D, Yinnon AM (2011) Nursing and physician attire as possible source of nosocomial infections. Am J Infect Control 39:555–559. doi:10.1016/j.ajic.2010.12.016
Acknowledgements
This work was financial supported by Instituto Piaget, Portugal, under the project “Estudo da prevalência e da variabilidade genética de estirpes de Pseudomonas aeruginosa e de Mycobacterium sp. em ambiente hospitalar” and by Fundação para a Ciência e Tecnologia, Portugal under the project Projecto PTDC/BIA-MIC/114958/2009- “ArsTOOL-Construção de um bioacumulador para biorremediação de arsénio” Pedro Farias was supported by an Instituto Piaget fellowship. Susana Alarico was supported by FCT fellowship SFRH/BPD/43321/2088. We thank the directing office and staff of the Hematology, Medicine A, and Urology/Renal Transplants wings as well as Dr. Filomena Coelho from the Centre for Infection Control Office.
Author’s Contributions
Conception of the project and responsible for obtaining funding support PVM, AFA, NE. Bacterial work performed by PF, FG, CM, PVM, mycobacterium work performed by DR, SA, FG, NE. First draft produced by PVM, PF, writing and discussing of the manuscript by PF, PVM, SA, NE. All authors agree to act as guarantors of the work, ensuring that questions related to any part of the work are appropriately investigated and resolved.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Geadas Farias, P., Gama, F., Reis, D. et al. Hospital microbial surface colonization revealed during monitoring of Klebsiella spp., Pseudomonas aeruginosa, and non-tuberculous mycobacteria. Antonie van Leeuwenhoek 110, 863–876 (2017). https://doi.org/10.1007/s10482-017-0857-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-017-0857-z