Abstract
The lantibiotic nisin is produced by Lactococcus lactis as a precursor peptide comprising a 23 amino acid leader peptide and a 34 amino acid post-translationally modifiable core peptide. We previously demonstrated that the conserved FNLD part of the leader is essential for intracellular enzyme-catalyzed introduction of lanthionines in the core peptide and also for transporter-mediated export, whereas other positions are subject to large mutational freedom. We here demonstrate that, in the absence of the extracellular leader peptidase, NisP, export of precursor nisin via the modification and transporter enzymes, NisBTC, is strongly affected by multiple substitutions of the leader residue at position -2, but not by substitution of positions in the vicinity of this site. Export levels of precursor nisin increased by more than 70% for position -2 mutants Asp, Thr, Ser, Trp, Lys, Val and decreased more than 70% for Cys, His, Met. In a strain with leader peptidase, the Pro-2Lys and Pro-2Asp precursor nisins were less efficiently cleaved by NisP than wild type precursor nisin. Taken together, the wild type precursor nisin with a proline at position -2 allows balanced export and cleavage efficiencies by precursor nisin’s transporter and leader peptidase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
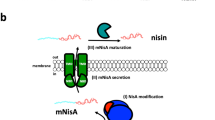
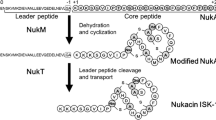
Nisin A is a peptide antibiotic produced by some Lactococcus lactis strains. It is ribosomally produced as a precursor peptide of 57 amino acids, consisting of an N-terminal leader peptide and a C-terminal core peptide (Lubelski et al. 2008). The core peptide is post-translationally modified by the nisin modification enzymes, NisB (Grag et al. 2013; Koponen et al. 2002) and NisC (Koponen et al. 2002; Li et al. 2006), before it is exported as precursor nisin by the ABC-transporter NisT. The leader peptide guides the interactions of the precursor peptide with the modification enzymes and transporter (Oman and van der Donk 2010). Outside the cell, the leader peptidase, NisP, cleaves off the leader peptide thus liberating active nisin.
Nisin is a pentacyclic peptide consisting of 21 unmodified amino acids, 2 dehydroalanines, 1 dehydrobutyrine, 1 lanthionine and 4 methyllanthionines (Gross and Morell 1971). Serines and threonines in the core peptide are dehydrated by NisB, resulting in dehydroalanines and dehydrobutyrines respectively. Subsequently, NisC catalyzes the coupling of the double bond in the dehydroamino acids to the thiol group of cysteines, forming a lanthionine (dAla-S-Ala) or methyllanthionine (dAbu-S-Ala). Nisin is a lanthionine-containing antibiotic, so-called called lantibiotic (Schnell et al. 1988). Lantibiotics are a subgroup of so-called lanthipeptides, which also comprise peptides without antibiotic activity (Arnison et al. 2013; Knerr and van der Donk 2012; Kodani et al. 2004; Willey and van der Donk 2007).
The nisin modification enzymes and transporter have a relaxed substrate specificity. This allows the use of L. lactis containing the nisin enzymes for the discovery of lanthionine-stabilized therapeutic peptides (Kluskens et al. 2005; Kuipers et al. 2004; Rink et al. 2010). Lanthionine-stabilized angiotensin-(1–7) has enhanced receptor specificity, enhanced intrinsic activity, strongly enhanced bioavailability, and potential for oral and pulmonary delivery (Kluskens et al. 2009; de Vries et al. 2010). It has multiple effective therapeutic activities for instance against acute respiratory distress syndrome (Wösten-van Asperen et al. 2011) and in the case of heart failure (Durik et al. 2012). Furthermore lanthionine-stabilized angiotensin-(1–7) and a lanthionine-containing agonist of the angiotensin type 2 receptor significantly reduce cardiopulmonary disease in hyperoxia treated neonatal rats (Wagenaar et al. 2013).
The nisin leader peptide contains a conserved FNLD sequence which is important for NisB activity (Plat et al. 2011; Khusainov et al. 2011; Mavaro et al. 2011), for NisC activity (Abts et al. 2013) and for NisT-mediated export (Plat et al. 2011). The exact mechanism(s) of lanthipeptide leader peptide-induced export out of the cell are unknown. Different mechanisms of LanT-mediated export have been discussed (Plat et al. 2013). Introduction of a negatively charged cleavage site for enterokinase, DDDDK, in the C-terminal part of the nisin leader appeared to hamper export of precursor nisin (Plat et al. 2011). We here investigated whether inserting positively charged residues in the C-terminal part of the leader peptide might modulate export via NisBTC. Position -2 in the wild type nisin leader is a helix-breaking proline. We found that substitution of this proline can have an important, amino acid-dependent impact on the efficiency of export of precursor nisin.
Materials and methods
Bacterial strains and plasmids
Lactococcus lactis NZ9000 was used for expression of the modification enzymes and precursor nisin constructs. L. lactis was grown in M17 broth (Terzaghi and Sandine 1975) supplemented with 0.5% glucose (GM17) or in minimal medium (Rink et al. 2005) with or without chloramphenicol (5 μg/ml) and/or erythromycin (5 μg/ml). Prior to analysis of peptides produced in the media, cells were cultured as follows. Overnight cultures of L. lactis NZ9000, grown in GM17 broth containing antibiotics, were diluted 1/100 in minimal medium. Production of mutated precursor nisin was induced by adding supernatant of L. lactis NZ9700 1:1000, containing approximately 1 mg/l wild type nisin. Strains and plasmids are listed in Table 1.
Molecular cloning
Standard genetic manipulations were performed using established procedures (Sambrook et al. 1989). Constructs coding for mutated precursor nisin were made by amplifying plasmid pNZnisA-E3 (Kuipers et al. 2004) via round-PCR using a downstream sense primer and an upstream antisense primer. Each primer pair contained one 5′ phosphorylation and a (non-annealing) peptide-encoding tail. The pORI280 system was used for integration of the P-2K mutation on the chromosome of an industrial stain (Leenhouts et al. 1996). This mutation was confirmed by sequencing of the mutation-containing PCR fragment obtained using chromosomal DNA as template. DNA amplification was performed with Phusion DNA polymerase (Finnzymes, Finland). Digestions were performed using restriction enzymes from New England BioLabs (Ipswich, MA). Ligation of the plasmids was carried out with T4 DNA ligase (Roche, Mannheim, Germany). Electrotransformation of L. lactis was carried out as previously described using a Bio-Rad gene pulser (Richmond, CA) (Holo and Nes 1995). Nucleotide sequence analysis was performed by BaseClear (Leiden, Netherlands).
Purification
Precursor nisin was purified from culture supernatants. Minimal medium culture supernatant was diluted with an equal volume of 100 mM lactic acid (pH 2.5). The precursor peptides were subsequently purified by a single passage of the supernatant over a 5 ml HiTrap SP Sepharose cation-exchange column (GE Healthcare). Elution was performed at pH 4.0 with 1 M NaCl in 50 mM lactic acid. The fraction containing the precursor peptide was desalted on a PD10 column (GE Healthcare) and subsequently lyophilised or dried in a speed-vac. Nisin (mutants) were further purified and/or analysed via reversed-phase high-performance liquid chromatography (HPLC) on a C12 or C18 column with a gradient of 10–50% acetonitrile in 0.1% trifluoroacetic acid.
Cleavage of the leader peptide from precursor nisin
The leader peptide was cleaved off by incubating precursor nisin with 0.02 mg/ml trypsin for 30 min to 1 h at 37 °C. Alternatively, precursor nisin was incubated with NisP-expressing cells L. lactis NZ9000 pNGnisT pNGnisP cells at 30 °C.
Gel electrophoresis and quantification
Production levels of precursor nisin (mutants) were analysed via gel electrophoresis. Peptides were isolated from the supernatant of minimal medium cell cultures by TCA precipitation. Peptides were separated on tricine SDS gel (Schägger and von Jagow 1987). Analysis was performed by Coomassie staining (PageBlue™). Peptide bands were quantified by measuring the gel band density using ImageJ (Schneider et al. 2012). Cell free extract was obtained using a previously described washing procedure (Kleerebezem et al. 1997). As a control for the applied quantification, HPLC peak areas were compared for wild type precursor nisin and mutants in the medium. The chromosomal P-2K and wild type strains were compared in repeated small scale (10L) fermentations in parallel under conditions that essentially replicate commercial-scale production, including pH control during growth, inoculum ratios, and general media conditions. Samples were removed aseptically, hourly, as the culture approached stationary phase and thereafter until the end of fermentation. The samples were processed and analysed quantitatively for the presence of nisin using a validated reversed-phase analytical method using a HP HPLC System. Cleavage of wild type precursor nisin was compared with cleavage of the P-2K and P-2D mutants by incubating the precursor peptides with NisP-expressing cells. Samples were collected at different time points, and analyzed with HPLC to generate quantitative data of NisP cleavage.
Antimicrobial activity
Antimicrobial activity of the nisin mutants was measured against nisin-sensitive L. lactis strains. Indicator strain L. lactis LL108(pORI 280) (Kuipers et al. 1997; Leenhouts et al. 1998) or L. lactis NZ9000 pNGnisT pNGnisP (Kuipers et al. 2004) was grown in GM17 containing chloramphenicol (5 µg/ml) and erythromycin (5 µg/ml) to an optical density of 0.1 at 600 nm. After 3–6 h of incubation in microwell plates with a series of two-fold dilutions of the nisin mutant, growth inhibition was measured at 600 nm. The 50% inhibitory concentration (IC50) was obtained from the midpoint of the sigmoidal growth curve. Halo-forming capacities of (mutant) nisin-producing L. lactis NZ9000 colonies were measured using an overlay with the NisP-expressing L. lactis NZ9000 pNGnisT pNGnisP strain.
Mass spectrometry
Mass spectrometry was performed to confirm that the correct (precursor) nisin mutants were produced and, in case of leader cleavage, that the intended mutant leader peptide was produced. In case of (precursor) nisin mutants samples were pre-incubated with 1 mg/ml tris[2-carboxyethyl]phosphine (TCEP) to prevent cysteinylation of free cysteines. For all samples, and also when just the leader peptide was measured, either 1 μl of culture supernatant or purified peptide was applied to a target and allowed to dry. Spots from culture supernatants were washed once with 5 μl of Millipore water to remove the salts. Spots were subsequently overlaid with 1 μl of matrix (5 mg/ml α-cyano-4-hydroxycinnamic acid in 50% acetonitrile containing 0.1% [vol/vol] trifluoroacetic acid). Mass spectra were recorded with a Voyager-DE PRO matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectrometer (Applied Biosystems). In order to maintain high sensitivity, an external calibration was applied.
Results
Introduction of C-terminal lysines in the nisin leader peptide
To investigate the influence of positively charged residues in the C-terminal part of the leader peptide, we introduced lysines at position P-2, D-7 and S-3/P-2 (Fig. 1). Analysis of the production on a tricine SDS gel showed a nearly two-fold increased production for the P-2K mutant (Fig. 2a). Nisin mutant I+4K/L+6I (“KSI”, a positive control, (Rink et al. 2007b)) was also produced better than wild type nisin. The D-7K mutant and the S-3K/P-2K (“KKR”) double mutant showed decreased production. These data demonstrate that lysines in positions -2 or +4, which are both close to the leader peptide to core peptide border, enhance production.
Production and antimicrobial activity of nisin mutants. a A comparison of the production in the supernatant of wild type precursor nisin (wt) with precursor nisin mutants I+4K/L+6I (KSI), S-3K/P-2K (KKR, two transformants), P-2K, and D-7K. Production was measured by TCA-precipitating 1 ml of supernatant, running it over a tricine SDS gel, and subsequent staining with PageBlue. Band intensities were quantified using ImageJ and relative values are given below the peptide names. b Overlay of a duplicate gel with nisin-sensitive strain LL108pORI280. Precursor nisin peptides were cleaved by adding trypsin
Shifted trypsin-mediated cleavage of precursor nisin mutant P-2K
The antimicrobial activity of the mutants was tested by overlaying a duplicate gel with a nisin-sensitive strain. In the case of I+4K/L+6I and S-3K/P-2K precursor nisin the size of the halo correlated roughly to the amount of precursor produced (Fig. 2b).
In contrast, the relatively small halo surrounding the P-2K mutant clearly showed low activity of the P-2K mutant. In the activity assay, the leader peptide was removed from the (mutant) precursor nisin by adding trypsin in the overlay. Trypsin usually cleaves most efficiently after an Arg but it will also cleave after a Lys. We therefore investigated whether this comparatively low activity of P-2K nisin might result from incomplete removal of the leader peptide.
MALDI-TOF MS of intact P-2K precursor nisin (Fig. 3a), trypsin-cleaved P-2K precursor nisin (Fig. 3b) and NisP-cleaved P-2K precursor nisin (Fig. 3c) showed that trypsin cleaves primarily between K-2 and R-1, resulting in R-nisin (Fig. 3b). This shifted cleavage likely results from sterical hindrance from ring A. Incubating the P-2K precursor nisin with NisP-producing L. lactis cells (Kuipers et al. 2004) resulted in complete removal of the leader peptide (Fig. 3c). After incubation with the NisP-producing cells, the antimicrobial activity of the peptides was measured in a dilution assay. The P-2K-precursor-nisin-derived nisin showed a two-fold higher activity than wild type nisin (data not shown) which is consistent with its two-fold higher production level. These results show that, in this particular case of P-2K precursor nisin, trypsin has a higher affinity for the -2K position than for -1R, and that the presence of an additional N-terminal Arg decreases the antimicrobial activity of nisin. Moreover, the results demonstrate that the P-2K substitution does not hamper interaction with the modification enzymes and allows cleavage by NisP-producing L. lactis (Kuipers et al. 2004).
Removal of leader peptide from precursor nisin P-2K. a Mass spectrometry analysis of precursor nisin containing the P-2K mutation. The [MH+] peak of 5719.01 Da corresponds to precursor nisin with the P-2K substitution; theoretical value without Met1: 5719.86 Da. b Mass spectrometry analysis after incubation with trypsin. The [MH+] peak of 3511.84 Da corresponds to R-nisin, theoretical value: 3511.38 Da. c Mass spectrometry analysis after incubation with NisP-expressing cells (Kuipers et al. 2004). The [MH+] peak of 3355.15 corresponds to nisin; theoretical value 3355.20 Da
Randomization of position -2
Is the increased production of the P-2K precursor nisin mutant positive charge-dependent or could other factors be involved? We investigated this by substituting the Pro on position -2 with other amino acids and subsequently measured production (Table 2). Interestingly, large differences were observed between production levels of the different mutant types ranging from a two-fold increase to a ten-fold decrease. Besides precursor nisin mutant P-2K, also the precursor nisin mutants P-2D, P-2T, P-2S, P-2W, and P-2V show strongly enhanced production. Very low production was measured for the P-2C, P-2H, and P-2M. Especially surprising is the high production of the P-2D mutant and the low production of the P-2H mutant. These results clearly demonstrate that the enhanced production of P-2K precursor nisin is not determined by positive charge alone.
Production stability
As an additional control, the transformation of each mutant was repeated and the precursor nisin production of the resulting mutants was measured. For ten mutation types production was satisfactorily reproducible. Only three type of mutations displayed variation in production: P-2A, P-2Q and P-2R. Strikingly P-2R mutants led to precursor nisin production which varied from very high for some transformants to very low for others. Apparently by unknown cause(s) these three specific mutations led to variable results, whereas the majority of the mutations caused stable production.
Chromosomal P-2K leader substitution
The P-2K mutation was inserted on the chromosome of a commercial nisin production strain. This strain, in contrast to the studies above, also naturally expresses NisP. Incorporation of the P-2K mutation on the chromosome of this strain was confirmed by mass spectrometry of the produced leader peptide and DNA sequence analysis. Production was compared at various time points using HPLC. At no time point did mature nisin production of P-2K mutant strain exceed that of the wild type strain. Mature nisin production by the P-2K mutant strain reached at 24 h maximally 93.4% of that of the wild type strain. Hence, we observed enhanced P-2K and P-2D precursor nisin production in cells without NisP, whereas production of mature nisin by a production strain carrying the chromosomal P-2K mutation was not increased. To investigate whether this could be caused by less efficient cleavage of P-2K precursor nisin by NisP we investigated whether or not NisP cleaves the P-2 mutant as readily as wild type precursor nisin. Preliminary data indicated that both P-2 K and P-2D precursor nisin are less efficiently cleaved by NisP than the wild type leader. Taken together, the data indicate that the wild type nisin precursor with a proline at position -2 allows balanced export- and cleavage efficiencies by precursor nisin’s transporter and leader peptidase.
Discussion
Lanthipeptide leader peptides are very interesting since each single leader peptide functionally interacts with proteins as different as a serine/threonine dehydratase, a cyclase, a transporter and –if present- a leader peptidase (Plat et al. 2013). Here we studied the role of C-terminal mutations in the nisin leader peptide on export. Previous observations (Plat et al. 2011) showed decreased production when substituting C-terminal residues in the leader peptide of nisin with negatively charged residues. Therefore we aimed in this study at enhancing production levels by mutating C-terminal amino acids of the leader peptide into lysines. A strong increase in production was measured for the nisin P-2K mutant. By contrast the D-7K did not cause increased production nor did the simultaneous introduction of -2K and -3K, indicating the relevance of the selective mutagenesis of the -2 position. Substituting the Pro at position -2 with other amino acids showed a more complicated picture: P-2D causes enhanced production; P-2H causes reduced production. Interestingly, also P-2T caused enhanced production and precursor nisin Q also contains a Thr at position -2. In an old pioneer study P-2V and P-2G did not have any detectable effect on mature nisin production (van der Meer et al. 1994), whereas in the present study both these mutations caused increased production of precursor nisin, respectively 1.70 ± 0.24 and 1.36 ± 0.12 (Table 2). In addition to the inherent differences between studying production of mature nisin and precursor nisin, the observed production differences may result from the here used improved two-plasmid expression system. The modulation of export depends on the -2 leader site and is amino acid dependent. This precludes that modulated production is caused by more optimal or less optimal codons. Furthermore the reproducibility of the data for plasmid-containing cells that result from different transformations also precludes that modulated production is caused by heterogeneity of lactococcal cells. Our data indicate that production of wild type precursor nisin, containing a Pro at position -2, is average compared to production of P-2X precursor nisin mutants. Taken together the data indicate that the helix breaking proline-2 might be relevant for optimal NisP-mediated cleavage but possibly not optimal for export of precursor nisin.
Lanthipeptide leader peptides are much more hydrophilic than the lanthipeptides themselves. Most class I leader peptides end up in the extracellular medium after cleavage from the core peptide by LanP or other proteases (Kuipers et al. 2004; Stein and Entian 2002). Outside the FNLD box a large mutational freedom has been demonstrated without affecting the capacity to modify substrate. Even a 6His tag could be introduced (Plat et al. 2011). Residues between the FNLD box and the core peptide might function as a spacer (Plat et al. 2011). The same hypothesis has been suggested for a class III lanthipeptide labyrinthopeptin (Müller et al. 2011). A large part of the diverse leaders of class II ProcM substrates is dispensable (Zhang et al. 2014). Replacement of the conserved FNLD sequence in the nisin leader peptide by four alanines eliminates the capacity to induce NisB activity (Plat et al. 2011) by eliminating binding to NisB (Khusainov et al. 2011; Mavaro et al. 2011). Furthermore, this leader peptide with the FNLD box replaced by four alanines, hardly induced any export. Deleting a sequence in NisB itself, which resembles the FNLD sequence, also eliminated NisB activity (Khusainov et al. 2011). The co-crystal structure of NisB and precursor nisin together with exciting mechanistic information provided an explanation of the importance of the leader peptide FNLD box for interaction with NisB (Ortega et al. 2014).
Experiments using Isothermal Titration Calorimetry (ITC) and a combination of Size Exclusion Chromatography (SEC) with Multi-Angle Light Scattering (MALS) analysis have demonstrated that NisC binds the FxLx motif of the nisin leader peptide (Abts et al. 2013). Replacement with all alanines of six regions of 2–4 leader peptide amino acids followed by co-purification with NisB and NisC yielded a more complex and detailed picture on binding and modification and indicated that leader regions LVSV(-14-11), STKD(-22-19) and especially FNLD(-18-15) contributed to interaction with NisB and NisC (Khusainov et al. 2013). In addition replacement of PR(-2-1) with alanines strongly reduced co-purification of NisB and NisC (Khusainov et al. 2013). The latter implies that it can not be excluded that replacement of P-2 might indirectly affect export by modulated interaction of the leader with the modification enzymes.
A propensity to form an alpha helical structure has been demonstrated for the leader peptides of nisin, lacticin 481 (Patton et al. 2008) and nukacin (Nagao et al. 2009) and predicted for other leader peptides (Oman and van der Donk 2010). The nisin leader peptide showed a random coil in aqueous solution (van den Hooven et al. 1997), but adopts a helical form in a mixture of trifluoroethanol and water (Beck-Sickinger and Jung 1993; Lian et al. 1992). However, the nisin leader peptide binds to NisB as an antiparallel-strand (Ortega et al. 2014). Introduction of a proline in position -8 or -6 or -4 in lacticin 481 abolished LctM synthetase activity, while introduction of a proline in position -5 reduced LctM synthetase activity (Patton et al. 2008). Introduction of a proline in position -8 or -9 in the nukacin leader, abolished, respectively reduced antimicrobial activity in the supernatant (Nagao et al. 2009). Mutational freedom, except for the introduction of proline, appeared to be high for any position. Proline has a turn propensity and thereby disturbs alpha helical structure (Piela et al. 1987). On the contrary, here we eliminated a helix-breaking proline residue at position -2 of the nisin leader peptide by substituting it for other amino acids. Replacement of proline for Asp, Thr, Ser, Trp, Lys and Val enhanced export without compromising the extent of NisB- and NisC-mediated modification.
With respect to removal of the leader peptide, different cases occur within lanthipeptides. Most Class I leader peptides are cleaved off extracellularly by a dedicated peptidase LanP (Plat et al. 2013) or in the case of subtilin (Stein and Entian 2002) by non-dedicated extracellular peptidases. Also transport of the nisin leader peptide itself, if it is produced without any attached core peptide, has been demonstrated (Rink et al. 2007a). In contrast the leader peptide of Class II lanthipeptides is cleaved off by an intracellular peptidase domain within the transporter. Hence, most Class I transporters export the leader peptide, whereas class II transporters do not.
NisT has been reported to be present as a dimer (Ortega et al. 2014; Siegers et al. 1996). We previously presented three models of the NisT-mediated export (Siegers et al. 1996). A tentative model involves binding of the FNLD box to NisT, which induces ATP-driven segregation of the two NisT molecules and opening of a NisT-pore, through which the N-terminal site of the core peptide first diffuses to the outside of the cell. Alternatively, the C-terminus of the peptide moves first out of the cell, after the “knock on the door” effectuated by the binding of the FNLD box to NisT. A third possibility might be that the FNLD box binds to NisT after which an ATP-driven pumping of the whole peptide with N- to C-terminal directionality moves out of the cell.
In conclusion, the present study demonstrates that mutagenesis of position -2 of the nisin leader peptide strongly modulates export of precursor nisin. Future research will establish the mechanistic role of this leader site in export, the order of the export of the leader and the core peptide and the overall mechanism of leader peptide-induced transport via NisT.
References
Abts A, Montalban-Lopez M, Kuipers OP, Smits SH, Schmitt L (2013) NisC binds the FxLx motif of the nisin leader peptide. Biochemistry 52:5387–5395
Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, Camarero JA, Campopiano DJ, Challis GL, Clardy J, Cotter PD, Craik DJ, Dawson M, Dittmann E, Donadio S, Dorrestein PC, Entian KD, Fischbach MA, Garavelli JS, Göransson U, Gruber CW, Haft DH, Hemscheidt TK, Hertweck C, Hill C, Horswill AR, Jaspars M, Kelly WL, Klinman JP, Kuipers OP, Link AJ, Liu W, Marahiel MA, Mitchell DA, Moll GN, Moore BS, Müller R, Nair SK, Nes IF, Norris GE, Olivera BM, Onaka H, Patchett ML, Piel J, Reaney MJ, Rebuffat S, Ross RP, Sahl HG, Schmidt EW, Selsted ME, Severinov K, Shen B, Sivonen K, Smith L, Stein T, Süssmuth RD, Tagg JR, Tang GL, Truman AW, Vederas JC, Walsh CT, Walton JD, Wenzel SC, Willey JM, van der Donk WA (2013) Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat Prod Rep 30:108–160
Beck-Sickinger AG, Jung G (1993) Synthesis and conformational analysis by CD spectroscopy of lantibiotic leader, pro- and pre-peptides. Liebigs Ann Chem 1993:1125–1131
de Vries L, Reitzema-Klein CE, Meter-Arkema A, van Dam A, Rink R, Moll GN, Akanbi MH (2010) Oral and pulmonary delivery of thioether-bridged angiotensin-(1–7). Peptides 31:893–898
Durik M, Van Veghel R, Kuipers A, Rink R, Haas Jimoh Akanbi M, Moll GN, Danser AHJ, Roks AJM (2012) The effect of the thioether-bridged, stabilized angiotensin-(1–7) analogue cyclic ang-(1–7) on cardiac remodeling and endothelial function in rats with myocardial infarction. Int J Hypertens. doi:10.1155/2012/536426
Grag N, Salazar-Ocampo LM, van der Donk WA (2013) In vitro activity of the nisin dehydratase NisB. Proc Natl Acad Sci USA 110:7258–7263
Gross E, Morell JL (1971) The Structure of Nisin. J Am Chem Soc 93:4634–4635
Holo H, Nes IF (1995) Transformation of Lactococcus by Electroporation. Methods Mol Biol 47:195–199
Khusainov R, Heils R, Lubelski J, Moll GN, Kuipers OP (2011) Determining sites of interaction between precursor nisin and its modification enzymes NisB and NisC. Mol Microbiol 82:706–718
Khusainov R, Moll GN, Kuipers OP (2013) Identification of distinct nisin leader peptide regions that determine interactions with the modification enzymes NisB and NisC. FEBS Open Bio 3:237–242
Kleerebezem M, Beerthuyzen MM, Vaughan EE, de Vos WM, Kuipers OP (1997) Controlled gene expression systems for lactic acid bacteria: transferable nisin-inducible expression cassettes for Lactococcus, Leuconostoc, and Lactobacillus spp. Appl Environ Microbiol 63:4581–4584
Kluskens LD, Kuipers A, Rink R, de Boef E, Fekken S, Driessen AJ, Kuipers OP, Moll GN (2005) Post-translational modification of therapeutic peptides by NisB, the dehydratase of the lantibiotic nisin. Biochemistry 44:12827–12834
Kluskens LD, Nelemans SA, Rink R, de Vries L, Meter-Arkema A, Wang Y, Walther T, Kuipers A, Moll GN, Haas M (2009) Angiotensin-(1-7) with thioether Bridge: an angiotensin-converting enzyme-resistant, potent angiotensin-(1-7) analog. J Pharmacol Exp Ther 328:849–854
Knerr PJ, van der Donk WA (2012) Discovery, biosynthesis, and engineering of lantipeptides. Annu Rev Biochem 81:479–505
Kodani S, Hudson ME, Durrant MC, Buttner MJ, Nodwell JR, Willey JM (2004) The SapB morphogen is a lantibiotic–like peptide derived from the product of the developmental gene ramS in Streptomyces coelicolor. Proc Natl Acad Sci USA 101:11448–11453
Koponen O, Tolonen M, Qiao M, Wahlström G, Helin J, Saris PE (2002) NisB is required for the dehydration and NisC for the lanthionine formation in the post-translational modification of nisin. Microbiology 148:3561–3568
Kuipers OP, de Ruyter PG, Kleerebezem M, de Vos WM (1997) Controlled overproduction of proteins by lactic acid bacteria. Trends Biotechnol 15:135–140
Kuipers A, de Boef E, Rink R, Fekken S, Kluskens LD, Driessen AJ, Leenhouts K, Kuipers OP, Moll GN (2004) NisT, the transporter of the lantibiotic nisin, can transport fully modified, dehydrated, and unmodified precursor nisin and fusions of the leader peptide with non-lantibiotic peptides. J Biol Chem 279:22176–22182
Leenhouts K, Buist G, Bolhuis A, ten Berge A, Kiel J, Mierau I, Dabrowska M, Venema G, Kok JA (1996) General system for generating unlabelled gene replacements in bacterial chromosomes. Mol Gen Genet 253:217–224
Leenhouts K, Bolhuis A, Venema G, Kok J (1998) Construction of a food-grade multiple-copy integration system for Lactococcus lactis. Appl Microbiol Biotechnol 49:417–423
Li B, Yu JP, Brunzelle JS, Moll GN, van der Donk WA, Nair SK (2006) Structure and mechanism of the lantibiotic cyclase involved in nisin biosynthesis. Science 311:1464–1467
Lian LY, Chan WC, Morley SD, Roberts GC, Bycroft BW, Jackson D (1992) Solution structures of nisin a and its two major degradation products determined by NMR. Biochem J 283:413–420
Lubelski J, Rink R, Khusainov R, Moll GN, Kuipers OP (2008) Biosynthesis, immunity, regulation, mode of action and engineering of the model lantibiotic nisin. Cell Mol Life Sci 65:455–476
Mavaro A, Abts A, Bakkes PJ, Moll GN, Driessen AJ, Smits SH, Schmitt L (2011) Substrate recognition and specificity of the NisB protein, the lantibiotic dehydratase involved in nisin biosynthesis. J Biol Chem 286:30552–30560
Müller WM, Ensle P, Krawczyk B, Süssmuth RD (2011) Leader peptide-directed processing of labyrinthopeptin A2 precursor peptide by the modifying enzyme LabKC. Biochemistry 50:8362–8373
Nagao J, Morinaga Y, Islam MR, Asaduzzaman SM, Aso Y, Nakayama J, Sonomoto K (2009) Mapping and identification of the region and secondary structure required for the maturation of the nukacin ISK-1 prepeptide. Peptides 30:1412–1420
Oman TJ, van der Donk WA (2010) Follow the leader: the use of leader peptides to guide natural product biosynthesis. Nat Chem Biol 6:9–18
Ortega MA, Hao Y, Zhang Q, Walker MC, van der Donk WA, Nair SK (2014) Structure and mechanism of the tRNA-dependent lantibiotic dehydratase NisB. Nature 517:509–512
Patton GC, Paul M, Cooper LE, Chatterjee C, van der Donk WA (2008) The importance of the leader sequence for directing lanthionine formation in lacticin 481. Biochemistry 47:7342–7351
Piela L, Némethy G, Scheraga HA (1987) Proline-induced constraints in alpha-helices. Biopolymers 26:1587–1600
Plat A, Kluskens LD, Kuipers A, Rink R, Moll GN (2011) Requirements of the engineered leader peptide of nisin for inducing modification, export, and cleavage. Appl Environ Microbiol 77:604–611
Plat A, Kuipers A, Rink R, Moll GN (2013) Mechanistic aspects of lanthipeptide leaders. Curr Protein Pept Sci 14:85–96
Rink R, Kuipers A, de Boef E, Leenhouts KJ, Driessen AJ, Moll GN, Kuipers OP (2005) Lantibiotic structures as guidelines for the design of peptides that can be modified by lantibiotic enzymes. Biochemistry 44:8873–8882
Rink R, Wierenga J, Kuipers A, Kluskens LD, Driessen AJ, Kuipers OP, Moll GN (2007a) Dissection and modulation of the four distinct activities of nisin by mutagenesis of rings A and B and by C-terminal truncation. Appl Environ Microbiol 73:5809–5816
Rink R, Wierenga J, Kuipers A, Kluskens LD, Driessen AJ, Kuipers OP, Moll GN (2007b) Production of dehydroamino acid-containing peptides by Lactococcus lactis. Appl Environ Microbiol 73:1792–1796
Rink R, Arkema-Meter A, Baudoin I, Post E, Kuipers A, Nelemans SA, Akanbi MH, Moll GN (2010) To protect peptide pharmaceuticals against peptidases. J Pharmacol Toxicol Methods 61:210–218
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, p 545
Schägger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to IMAGEJ: 25 years of image analysis. Nat Methods 9:671–675
Schnell N, Entian KD, Schneider U, Gotz F, Zahner H, Kellner R, Jung G (1988) Precursor peptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulphide-rings. Nature 333:276–278
Siegers K, Heinzmann S, Entian KD (1996) Biosynthesis of lantibiotic nisin. Posttranslational modification of its precursor peptide occurs at a multimeric membrane-associated lanthionine synthetase complex. J Biol Chem 271:12294–12301
Stein T, Entian KD (2002) Maturation of the lantibiotic subtilin: matrix-assisted laser desorption/ionization time-of-flight mass spectrometry to monitor precursors and their proteolytic processing in crude bacterial cultures. Rapid Commun Mass Spectrom 16:103–110
Terzaghi BE, Sandine WE (1975) Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol 29:807–813
van den Hooven HW, Rollema HS, Siezen RJ, Hilbers CW, Kuipers OP (1997) Structural features of the final intermediate in the biosynthesis of the lantibiotic nisin. Influence of the Leader Peptide. Biochemistry 36:14137–14145
van der Meer JR, Rollema HS, Siezen RJ, Beerthuyzen MM, Kuipers OP, de Vos WM (1994) Influence of amino acid substitutions in the nisin leader peptide on biosynthesis and secretion of nisin by Lactococcus lactis. J Biol Chem 269:3555–3562
Wagenaar GT, Laghmani EH, Fidder M, Sengers RM, de Visser YP, de Vries L, Rink R, Roks AJ, Folkerts G, Walther FJ (2013) Agonists of MAS oncogene and Angiotensin II type 2 receptors attenuate cardiopulmonary disease in rats with neonatal hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol Physiol 305:L341–L351
Willey JM, van der Donk WA (2007) Lantibiotics: peptides of diverse structure and function. Annu Rev Microbiol 61:477–501
Wösten-van Asperen RM, Lutter R, Specht PA, Moll GN, van Woensel JB, van der Loos CM, van Goor H, Kamilic J, Florquin S, Bos AP (2011) Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin ii receptor antagonist. J Pathol 225:618–627
Zhang Q, Yang X, Wang H, van der Donk WA (2014) High divergence of the precursor peptides in combinatorial lanthipeptide biosynthesis. ACS Chem Biol 9:2686–2694
Acknowledgements
Sander Smits and Lutz Schmitt are gratefully acknowledged for helpful discussions on mechanistic models for transport.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that this article content has no conflict of interest.
Rights and permissions
About this article
Cite this article
Plat, A., Kuipers, A., Crabb, J. et al. Mutagenesis of nisin’s leader peptide proline strongly modulates export of precursor nisin. Antonie van Leeuwenhoek 110, 321–330 (2017). https://doi.org/10.1007/s10482-016-0802-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-016-0802-6