Abstract
Radiologists interprets X-ray samples by visually inspecting them to diagnose the presence of fractures in various bones. Interpretation of radiographs is a time-consuming and intense process involving manual examination of fractures. In addition, clinician’s shortage in medically under-resourced areas, unavailability of expert radiologists in busy clinical settings or fatigue caused due to demanding workloads could lead to false detection rate and poor recovery of the fractures. A comprehensive study is imparted here covering fracture diagnosis with the aim to assist investigators in developing models that automatically detects fracture in human bones. The paper is presented in five folds. Firstly, we discuss data preparation stage. Second, we present various image-processing techniques used for fracture detection. Third, we analyze conventional and deep learning based techniques for diagnosing bone fractures. Fourth, we make comparative analysis of existing techniques. Fifth, we discuss different issues and challenges faced by researches while dealing with fracture detection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A fracture is a partial or complete break in the bone (Mayne 2013). High impact or force against a bone that it can structurally withstand is the substantial cause of fracture. Traumatic and stress are commonly found bone fractures in human body. Traumatic fracture is a result of automobile accident, serious fall, or intentional causes such as physical abuse whereas stress fractures are associated with repetitive load-carrying pressure to a healthy bone, common among athletes (e.g., gymnasts, dancers, long-distance runners) and military personnel. However, fracture can also occur due to several other reasons like osteoporosis, (a medical condition that weakens the bone), cancers, or a brittle bone disease known as ontogenesis imperfect. According to the World Health Organization (WHO) report, 1.66 million people suffer from hip fracture every year throughout the world and the rate is expected to rise by three to four times by year 2050 because of the worldwide increase in the number of older people (World Health Organization 2019). The rate of fracture occurrences are higher by three to four fold among women in countries with a high fracture incidence rate, and is equal among men and women in counties with low fracture prevalence (World Health Organization 2019). All bone fractures are divided into six major categories (Mayne 2013) that is depicted using Fig. 1.
Major fracture types found in bones (Mayne 2013). a Transverse, b Oblique, c Spiral, d Comminuted, e Greenstick, and f Impacted fracture
-
A.
Transverse Fracture: It is the simplest type of fracture where bone is break as a horizontal line.
-
B.
Oblique Fracture: It is a fracture type where the break is extending in a slanting direction, caused by indirect or rotational force.
-
C.
Spiral Fracture: It is a fracture type where the break spirals around the bone, common in a twisting injury.
-
D.
Comminuted Fracture: It is a fracture type where bone breaks into several pieces.
-
E.
Greenstick Fracture: It is incomplete fracture type where the broken bone is not completely separated.
-
F.
Impacted Fracture: It is a fracture type where bone breaks but the two ends of fractured bone are forced together. This produces a rather stable fracture that can heal readily but at the cost of some length lost.
X-rays, computed tomography (CT), magnetic resonance imaging (MRI) are various medical imaging modalities used for capturing images of the affected body area. A radiologist expert perform interpretation of these captured images to diagnose the disease and further recommends needed treatment. X-ray is the oldest, fastest, and most frequently used imaging modality, which examines suspected fractures by imaging internal organs of the body (https://orthoinfo.aaos.org/en/treatment/x-rays-ct-scans-and-mris). It has become a prime analytic instrument to check patients for fractures, majorly due to its wide-availability across areas where many expensive imaging modalities might not be available. Radiologists or clinicians interpret X-ray samples by visually inspecting them to identify the presence and type of fractures in various bones. The need for advanced level of imaging tools such as MRI and CT scan emerges to obtain more detailed, cross-sectional view of the bone, which might be missed during X-ray examination (https://orthoinfo.aaos.org/en/treatment/x-rays-ct-scans-and-mris). Interpretation of radiographic images is a time-consuming and intense process that involves manual examination and classification of fractures. Shortage of clinicians in medically under resourced areas, unavailability of expert radiologists in busy clinical settings and fatigue caused due to demanding workloads could lead to false detection rate and poor recovery of fractures. In addition, X-ray image interpretation is mostly performed under the supervision of only one examiner, which increases the risk of inaccurate fracture identification due to unavailability of a second opinion. 41–80% of cases reported error in diagnosing fractures due to inaccurate fracture identification (Guly 2001; Whang et al. 2013). Various studies (Krupinski et al. 2010; Waite et al. 2017; Stec et al. 2018) show how fatigue caused to the examiner because of interpreting several musculoskeletal images could lead to significantly reduced performance in detecting abnormalities. Computer Vision systems could be a potential solution to such problems if it can quickly provide a trustworthy second opinion in identifying suspicious fracture cases.
Traditionally, various low-level pixel-processing techniques such as noise reduction, segmentation and feature extraction were effectively utilized for predicting human bone fractures. Obtaining region of interests by segmenting bone regions from fleshy areas was quite popular before extracting features from the image. The first work on automatic fracture detection used Neck-Shaft Angle (NSA) as the only feature for fracture detection (Tian et al. 2003). However, local disruptions remain undetected using this technique. A new approach is proposed based on texture analysis of trabecular pattern to detect such minor disturbances (Yap et al. 2004; Lim et al. 2004). Texture features such as Gabor Orientation (GO), Intensity Gradient Direction (IGD) and Markov Random Field (MRF) are extracted to detect femur fractures in bone X-ray images (Lim et al. 2004; Lai et al. 2005; He et al. 2007). Hand bone fractures from input X-ray image is detected by locating object boundaries using sobel algorithm followed by extracting texture features using Gray Level Co-occurrence Matrix (GLCM) (Al-Ayyoub et al. 2013). GLCM (entropy, contrast, correlation and homogeneity) is used as the only feature for identifying femur fractures in bone X-rays (Chai et al. 2011). The primary tool to improve classification accuracy involves amalgamation of texture features including GLCM, GO, MRF, IGD and shape features in femur X-rays (Umadevi and Geethalakshmi 2012). Automatic crack detection in X-ray images is complicated and arduous task that demands accuracy and swiftness (Linda and Jiji 2011, 2017). Various fuzzy based approaches are investigated to identify infected sites of a crack by segmenting bone areas using fuzzy index measure (Linda and Jiji 2011). Likewise, hairline fractures are estimated by means of intensity variation concept in multiple bones by segmenting bone areas using Expectation Maximization (EM) algorithm (Zhang et al. 2001).
It is observed that the performance of the classifier is significantly improved with the introduction of multiple-classifier based systems where individual results from base classifiers are fused together (Umadevi and Geethalakshmi 2012; Yap et al. 2004; Lai et al. 2005; Mahendran and Baboo 2011b). The most prominent ensemble based models have used Neural Network (NN), Support Vector Machine (SVM), Naïve Bayes (NB) algorithms for diagnosing fractures in human bones from the year 2003 to 2015. Researchers have developed a new Stacked Random Forests-Feature Fusion (SRF-FF) technique to identify fractures in various regions like hand, foot, ankle, knee, lower leg and arm (Cao et al. 2015). Divide and conquer approach utilizes techniques that combine hierarchical SVM and texture features including GO, MRF and IGD to predict fractures (He et al. 2007). Localizing fractures in bone X-rays is significant in diagnosis, decisions and treatment planning of the patient. GO, Schmid texture feature, and proposed Contextual-Intensity (CI) features are effectively utilized to localize fractures in multiple bones (Cao et al. 2015). Distinct bones such as humerus, radius, ulna, femur, tibia, and fibula are considered as long bone, where the fracture is classified based on its type and the location where it occurred (Bandyopadhyay et al. 2016a). Based on the intensity of injury, a long bone fracture is categorized into simple and complex types while the location of fracture is recognized through fracture points identified from X-ray images (Bandyopadhyay et al. 2016c). Decision Tree (DT) and K-Nearest Neighbor (KNN) classifiers are used for fracture detection and classification respectively once break points of the leg bone is identified in the processed image (Myint et al. 2018). Various literatures (Bandyopadhyay et al. 2013, 2016a; b; c) have suggested approaches based on digital geometry, which provide a powerful tool for analyzing bone fractures from X-ray images. The digital geometry based techniques such as relaxed straightness and concavity index are effectively utilized for long bone segmentation, rectification of contour imperfections, fracture detection and fracture localization (Bandyopadhyay et al. 2016b). Various approaches like image segmentation, machine learning or deep neural networks have focused majorly on fracture detection techniques but complex fracture, which consists of multiple patterns like transverse, oblique, spiral, and comminuted etc. are either under investigated or difficult to predict. Fractures can vary from minor to complex types. The complex types are severe, which needs to be treated within a time-period to avoid complications. Complex fractures in long bones are identified, localized and visualized using linear structuring elements and Ray Cascade method in CT DICOM images (Linda and Jiji 2018). Hidden Markov Random Field– Expectation Maximization (HMRF-EM) (Zhang et al. 2001) and adaptive thresholding techniques (Singh et al. 2012) for effectively utilized for bone segmentation (Linda and Jiji 2018).
Several features such as textual, shape, edges, horizontal, vertical lines etc. were extracted from the image and supplied to the classifier for predicting the occurrence of fractures. However, this approach is now prevailed by various deep learning methods. A fastest-growing field in artificial intelligence is deep learning, which has gained enormous success in medical imaging by providing better accuracy as compared to traditional approaches (Gale et al. 2017; Lindsey et al. 2018; Chung et al. 2018; Rajpurkar et al. 2017; Kim and MacKinnon 2018). Generating large datasets by collecting and labeling radiographs from expert Radiologists is a challenging and laborious responsibility. The number of radiographs used for diagnosing fractures is increased from 500 to hundreds of thousands from year 2003 to 2018 by collecting more data from hospitals and through data augmentation. Data augmentation is extensively used to amplify datasets during training the deep learning model (Gale et al. 2017; Lindsey et al. 2018; Chung et al. 2018; Rajpurkar et al. 2017; Kim and MacKinnon 2018). A variant of deep learning architecture is Convolutional Neural Networks (CNN) that consists of convolution layer, sub-sampling layer, and fully connected layers. Feature extraction techniques took a tremendous turn when CNN model came into existence which can “learn the features” instead of handcrafting them into the system. Convolution and sub-sampling layers of CNN are part of feature learning process while fully connected layer is used for classification. The capability of the model to learn features on its own rather than handcrafting them into the system has made these architectures a great success (Lakhani and Sundaram 2017; Russakovsky et al. 2015). A 172 layers Deep Convolutional Neural Networks (DCNN) is trained to classify fracture into healthy and fractured classes in pelvis radiographs (Gale et al. 2017). Neer classification (Neer 1970) is used to classify proximal humerus fracture into four categories; greater tuberosity, surgical neck, 3-part, and 4-part by using a pre-trained ResNet-152 classifier (Chung et al. 2018). The performance of the classifiers is compared against evaluations of expert radiologist on similar test images for analyzing the practical adaptability of the model. Transfer learning is effectively utilized by fine-tuning a pre-trained model, where scarcity of resources is an obstacle for creating a successful model from scratch (Lindsey et al. 2018; Kim and MacKinnon 2018). The model is pre-trained on non-medical images (such as ImageNet) and is fine-tuned using Google’s Inception v3 network for diagnosing wrist fractures (Kim and MacKinnon 2018). However, a model is pre-trained on 100,855 bone images of several other body parts and is fine-tuned on a DCNN for detecting wrist fractures (Lindsey et al. 2018).
Computer-aided design (CAD) systems can assist medical experts by suggesting the type of treatment required for disease diagnosis thereby upgrading patient care. Fracture diagnosis system could facilitate the examination process by providing optimal results upon integration with X-ray machine software. Various reviews (Khatik 2017; Jacob and Wyawahare 2013; Kinnari and Dangar 2017; Mahendran and Baboo 2011a) focusing on fracture diagnosis in plain radiographs exists but a detailed study of traditional as well as deep learning approaches to fracture detection and classification is missing. A comprehensive study is imparted here covering fracture diagnosis with the aim to assist researchers in developing models that automatically detects fracture in several bones of human body.
2 Data preparation
The process of fracture detection and classification is divided into multiple stages. It begins with data collection, which involves collection of datasets from various hospitals or public domain followed by dataset labeling. Dataset must be prepared before feeding it into a classifier for the prediction of fracture occurrence and its corresponding class. Researchers and radiologists are putting many efforts in collecting and labeling radiographic images for research purpose. However, unavailability of the freely available standard dataset is one of the major drawbacks in comparative analysis of the existing systems due to which researchers have shown performance of the proposed model in their private datasets. After dataset collection, next major issue is the labeling process, which is an imperative stage of data pre-processing. Labeling requires annotation of radiographs by experienced radiologists, clinician or orthopedic surgeon that should be done with extreme care else, it will end up reflecting poor dataset quality and might result in reducing overall performance of the model (https://www.altexsoft.com/blog/datascience/how-to-organize-data-labeling-for-machine-learning-approaches-and-tools). The person responsible to do the labeling and time taken by him/her is the major challenge in creating a fully-fledged dataset for any classification task. A classification-based algorithm can accurately predict the outcome if the dataset is correctly mapped with extreme care and precision by a team of expertise. Although every industry has, its own regulation and governance challenges in the process of data collection, data management, and labeling that could take several months to finish but struggles of the healthcare industry are unique due to the complexity of data and extreme stringent regulations. A health institute may ask for a waiver of consent from an Institutional Review Board (IRB) study to mitigate some of these concerns, or researchers may process and anonymize DICOM data to strip away any patient health information. The source of data set, dataset split ratio, types of data collected and annotation process involved in classifying the datasets are presented in Table 1. Some freely available datasets for the task of fracture detection is presented in Table 2.
3 Image processing methods for fracture detection
The images collected in previous stage are processed using noise reduction, segmentation and feature extraction techniques for creating a convenient classification system. X-ray is the most frequently used imaging modality for fracture detection due to its painless, economic and non-invasive nature, which has gained enormous popularity in medical imaging. Poisson, Gaussian, salt and pepper noise are various type of noise artifacts commonly found in radiographs, particularly when collected in a large quantity from public domain such as internet. The need for handling such images rises largely as reducing one type of noise sometimes affects the other. Gradient, Laplacian and Sobel are frequently used methods of edge detection, which is an effective measure to determine boundaries of objects in the image. The shapes and sizes of bone are non-identical in X-ray images due to the difference in age and gender of the patients. Normalization could be used to deal with such size variations but its results are unsatisfactory as it removes important texture information in shrunken images and adds noise and artifacts in case of larger images. Hence, adaptive sampling approach is applied in various literatures to sample X-ray samples instead of scaling them (Yap et al. 2004; Lim et al. 2004; Lai et al. 2005; He et al. 2007). Adaptive sampling does not require accurate segmentation of bone contours as done by Tian et al. (2003), a slight variation of shape is accepted here. Image transforms such as wavelets and curvelets are powerful algorithms to obtain decent quality compressed images with higher PSNR (Peak Signal-to-Noise Ratio) resulting in lesser memory requirements to store medical images. Both wavelet and curvelet transforms (a multi-scale method originated from wavelets) are commonly used for medical image compression, contrast enhancement, edge detection and image registration (Tian and Ha 2004). They are used for extracting enormous set of coefficients from input image, where insignificant features are eliminated via feature selection algorithm for better or faster classification. The primary step in various image-processing applications after smoothing and edge detection is the extraction of essential features (informative representations) from the image. Feature extraction focuses on extracting image characteristics that acquires visual image attributes and the performance of the classifier is highly dependent on the perfect set of features retrieved from the image. Texture can be a useful cue for detecting diseases or tissue types in medical imaging. Visual texture is used for segmenting and discriminating objects from background that has a repeated pattern of elements with some amount of variability in element appearance and relative position. The spatial features of an image is described by its gray level, spatial distribution and amplitude, where amplitude is the simplest feature that discriminates bones tissues from X-ray images (Haralick and Shanmugam 1973). Intensity inhomogeneity and absence of sharp edges are the causes for missed out cracks while detecting fractures from X-ray images (Linda and Jiji 2017). Crack identification is significant for analyzing suspicious cases so that medical experts can suggest possible course of action within time limits (Linda and Jiji 2011). Fuzzy based image segmentation approaches are proposed for identifying cracks by employing fuzzy membership function (Linda and Jiji 2011, 2017). Image histogram is divided into three subsets to produce subset parameters and these parameters act as initial estimates to classify each pixel into one of the subsets by minimizing the fuzzy index (Linda and Jiji 2011). Their result gives promising results in detecting minute cracks from X-ray images. Hairline breakage from X-ray image is recognized by calculating intensity variation over the segmented bone regions (Linda and Jiji 2017). This approach performs better than standard approaches based on fuzzy thresholding (Linda and Jiji 2011; Mansoory et al. 2012) with an overall accuracy of 98%.
With the rise of deep learning neural networks, deep layers of Convolutional Neural Networks (CNN) replaced the task of feature extraction in digital images. CNN is a multilayered neural network, which consists of convolution layer, sub-sampling layer, and fully connected layers. Convolution and sub-sampling layers of CNN are part of feature learning process while fully connected layers are used for classification. ConvNets or CNN have the ability to learn various low level (minor details of the image e.g. lines, dots or edges etc.) and high level features (built upon low-level features to detect objects and larger shapes) through abstraction in the layers. Features are extracted using CNN in recent approaches of fracture detection and classification (Gale et al. 2017; Lindsey et al. 2018; Chung et al. 2018; Kim and MacKinnon 2018). Table 3 demonstrates relevant review findings of the image processing methods used for fracture detection in the literatures reviewed.
4 Conventional machine learning based algorithms for fracture detection
Various features including textual, shape, edges, horizontal and vertical lines extracted in previous steps are provided to classification algorithms for predicting the occurrence of bone fractures and thereby classifying it into relevant categories. Once the perfect set of features are fed into the classifier, the accuracy of fracture detection depends on the classifier selected. Hence, proper features must be extracted in order to formulate a powerful classification model. Table 4 demonstrates relevant review findings of the conventional machine learning based algorithms used for fracture detection.
4.1 Primary machine learning based algorithm
Some earlier works of automatic fracture detection reflects the usage of single feature as a classification parameter. For instance, the first work on automatic fracture detection used Neck-Shaft Angle (NSA) as the only feature for fracture detection (Tian et al. 2003). Radiologists considered the image as fractured if the NSA is less than 116◦. Using such type of model helped in correctly identifying 94.4% of training and 92.5% of test samples. The major reason behind 7.5% of error rate in test cases is the model’s incompetency in detecting minute changes in the femur neck-shaft angle. The upper extremity region of femur is called trabeculae and orientation of trabeculae on femur neck and head significantly changes on the event of fracture. These changes can be detected using neck-shaft angle but local disruptions remain undetected using this approach. Hence, a new approach is proposed which performs texture analysis of trabecular pattern by extracting features in femur X-rays followed by classification to detect such minor disturbances (Yap et al. 2004; Lim et al. 2004). Researchers in Yap et al. (2004) extracted Gabor features while (Lim et al. 2004; Lai et al. 2005) used Gabor orientation extracted by Yap et al. (2004) and additionally acquired Intensity Gradient Direction (IG) and Markov Random Field (MRF) texture features in femur X-rays. These features are then fed to the chosen classifier for diagnosing fractures in X-ray samples. GLCM is used as the only feature in classifying femur fractures in 30 X-ray samples and attain sensitivity and accuracy of 80% and 86.67% respectively (Chai et al. 2011).
4.2 Ensemble based classification system
Ensemble is a machine learning technique, which combines diverse models or classifiers to generate an optimal model that will best predict our wanted outcome. The basic idea behind ensemble model is to use multiple learning algorithms to obtain better predictions as oppose to traditional models that rely on a single classifier’s performance. The accuracy of the classifier depends on the perfect set of features extracted or learned from the image. However, the accuracy could further be improved by combining multiple classifiers and by integrating results of all independent classifiers. Ensemble based classification system has acquired broad range of attention in various fields such as face recognition (Antipov et al. 2016), geospatial land classification (Minetto et al. 2018), video-based face recognition system (Ding and Tao 2017), medical image segmentation (Kumar et al. 2017), wind power forecasting (Wang et al. 2017) etc. These models have manifested better accuracy (low error) by avoiding overfitting issues and by reducing bias and variance error as compared to its individual constituent classifiers. The importance of such models can be understood by the fact that various prestigious machine-learning competitions like popular Netflix challenge (Andreas et al. 2009), Knowledge Discovery in Databases (KDD) cup 2009 and Kaggle had used ensemble based model to achieve the best accuracy. The most prominent ensemble based models have used Neural Network (NN), Support Vector Machine (SVM), Naïve Bayes (NB) algorithms for diagnosing fractures in human bones from year 2003 to 2015. It is observed that the performance of the classifier is significantly improved with the introduction of multiple-classifier based systems where individual results from base classifiers are fused together (Umadevi and Geethalakshmi 2012; Yap et al. 2004; Lai et al. 2005; Mahendran and Baboo 2011b). Decision of choosing the best classifier among all competing classifiers depend on diversity among models. Choosing classifier merely based on accuracy on training data is fallacious. Some level of diversity must exists among classifiers that are part of ensemble system to make it an effective process, which can be achieved using the approaches given below (Polikar 2009):
-
1.
Using different classification algorithms for ensemble system.
-
2.
Using same classification algorithm with different instantiation or different hyper-parameter settings.
-
3.
Using different feature sets:
-
(a)
Random selection
-
(b)
Feature selection
-
(a)
-
4.
Using different training sets:
-
(a)
Bagging
-
(b)
Cross-validation
-
(a)
(a) Bagging or Bootstrap aggregating
A widely accepted technique of ensemble is non-hybrid classifier where same classification algorithm with different instantiation or different hyper-parameter settings is combined to make ensemble model. Bootstrap aggregating is one of the most intuitive and earliest ensemble based algorithms where multiple models consisting of same learning algorithms are trained with subsets of random datasets picked from the original training set with replacement. The output of the multiple-classifier or ensemble is predicted on the basis of majority voting of the constituent classifiers. Several variations of this algorithm exists which tend to enhance the performance of the model. Most popular among them works by increasing diversity among training data for individual classifiers and other is by making use of different classification algorithms (Fig. 2).
(b) Boosting
It is a simple variation of bagging technique, which strives to improve the classification model by converting weak learners into strong learners sequentially, each trying to correct its predecessor. The major difference between bagging and boosting is that bagging follows parallel training stage where each model is built independently and boosting follows sequential approach where current model architecture depends on previous classifier’s success. It is a sequential process where similar weights are assigned to the data at the beginning and are redistributed after each training stage, allowing subsequent learners to emphasize more on misclassified cases that are now attached with higher weights (Fig. 3).
Boosting is a sequential process where similar weights are assigned to the input points/data at the beginning and are selected randomly from the training set. After each training and testing, misclassified samples are identified and are attached with higher weights. In this manner, it allows subsequent learners to emphasize more on misclassified cases having higher chances to be picked for next classification
(c) Stacked ensembles
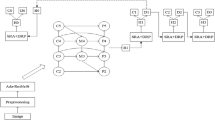
Stacking involves multi-layer of learning stages where first layer consists of base learners followed by lower level meta-learner stages, which takes base learners as input to obtain the best combination of first-level base learners. “Super learner” concept was originally developed in the year 1992 (Wolpert 1992) but its implementation with improved performance is shown for the first time in 2007 (Laan et al. 2007), which proves that stacked ensembles helps in creating optimal model for learning. A popular machine-learning algorithm is random forest, which takes a collection of weak learners (e.g. decision tree) and form a single, strong learner by following bagging technique. A fracture detection technique using 145 X-ray images with tenfold cross validation covering various regions like hand, foot, ankle, knew, lower leg and arm is developed by stacked random forests feature fusion (SRF-FF) technique (Cao et al. 2015). A four layer random forests with five decision trees is implemented in the first layer and remaining layers uses fifteen trees as shown in Fig. 4. The classifier is trained to produce confidence score maps which indicates the probability of fractures in X-ray images and then uses Efficient Subwindow Search (ESS) algorithm (Lampert et al. 2008) for localizing regions that has maximum probability of fracture occurrences. The proposed model outperforms SVM and single layer of stacked random forest in detecting and localizing fractures in X-ray images.
Flow chart of stacked random forests feature fusion (Cao et al. 2015)
Divide and conquer is another type of ensemble technique based on the concept that each sub problem is easier to solve than the whole problem. It demands larger training set and complex problem for it to produce larger clusters and thereby producing effective results. The complex problem of fracture detection is partitioned into Gini SVM’s kernel space instead of feature space as it lacks bigger training set (He et al. 2007). The process starts by training Gini SVM on training set T and calculates error on validation set V. The error obtained is used to select a new validation set V’ (subset of V) which is further classified on the basis of new SVM and training set T’ of T at the next level. This type of architecture enhances the accuracy of the SVM by ensuring that the lower-level SVM (child) always complements the performance of higher level SVM (Parent).
5 Deep learning-based algorithms for fracture detection
Deep learning is a branch of machine learning and artificial Intelligence (AI), which consists of statistical analysis algorithms, that repeatedly trains data to make predictions (Fig. 5). It is the ability of these “trained models” to automatically learn and improves from experiences to make predictions on unknown data (Kim and MacKinnon 2018). Discovering significant features, which depicts abnormalities or pattern in the data, is of great importance in machine learning approach. Traditionally, these features are crafted predominantly with the help of human expertise but, with the advancement of machine learning techniques, the models can automatically learn these features. In Radiology, skilled radiologist extract meaningful information/features from images and then interpret those features based on their expertise, experience and knowledge. Thus, it provides tremendous opportunities to apply machine-learning algorithms to make autonomous predictions on the data with similar accuracy as the radiologist expert (Kohli et al. 2017). A widely accepted computational model in the field of machine learning for finding complex patterns in the data is Artificial Neural Networks (ANN). These are the brain-inspired systems, which contemplate to imitate the human’s learning process. Neural Networks are also recognized as perceptron’s and have existed since the 1940s but have become significant part of artificial intelligence from past few decades. One reason of them become dominant in machine learning area is the advent of a technique called “backpropagation”. Backpropagation allows neural network to adjust their weights in hidden layer of neurons according to the desired output (Dormehl 2019). Intelligent Bone Fracture Detection System (IBFDS) combines image processing and neural network techniques for bone fracture detection (Dimililer 2017). Firstly, haar wavelet transform (Khashman and Dimililer 2008) is applied to enhance the image quality by reducing noise in the X-ray image followed by a feature extraction method based on Scale-Invariant Fourier Transform (SIFT) (Lowe 2004). Finally, extracted features are applied to a 3-layer back-propagation ANN, which classifies the image into fracture and non-fracture category.
Deep learning is a branch of machine learning and machine learning is a branch of artificial learning and perform tasks which requires human intelligence (Meyer et al. 2018)
Deep learning is the advancement of artificial neural networks, which consists of multiple hidden layers and provides greater levels of abstraction. With the rise of deep neural network, the accuracy of predicting a task has improved in a tremendous manner by incorporating deep layers into the model that allows system to learn complex data (Kim 2016). The rise of deep learning in healthcare sector is driven by various factors such as; (1) availability of large datasets, which became possible due to rapid accumulation of electronic data in the form of Electronic Medical Records (EMRs), (2) GPU advancement providing better performance with graphics and videos, (3) progress in deep learning algorithm due to incorporating multiple layers in deep learning architecture (Shen et al. 2017).
A variant of deep learning architecture is Convolutional Neural Networks (CNN), which consists of convolution layer, sub-sampling layer, and fully connected layers as depicted in Fig. 6. Feature learning techniques took a tremendous turn when CNN model came into existence which can “learn the features” instead of handcrafting them into the system. Convolution and sub-sampling layers of CNN are part of feature learning process while fully connected layer is used for classification. It has emerged as a recent breakthrough in machine learning and accomplished humongous success across various fields of medical image analysis, such as image segmentation, image registration, image fusion, image annotation, genomics etc. Radiologists uses a blend of perception, memory, pattern recognition, and cognitive reasoning for interpreting radiographic studies. Numerous distractors affect their performance, which ultimately leads to increasing workloads and fatigues. Hence, developing systems or tools that automatically detects abnormalities or patterns in the musculoskeletal radiographs without human intervention would improve patient’s security (Waite et al. 2017). A model known as AlexNet developed by Krizhevsky et al. (2017) brought out a revolution in the field of computer vision, which gave a new insight to deep CNN. It was originally developed to compete in the ImageNet competition whose general architecture is similar to LeNet-5 (LeCun et al. 1998) but is substantially larger. It became successful in convincing Computer Vision community for its usefulness after securing first position in the ImageNet competition. Use of effective regularization parameters, data augmentation approaches, rectified linear unit and, the use of graphics processing units for meeting computing requirements helped in bringing out this revolution. It was recognized as one of the top 10 milestones of 2013 in deep learning (Birks et al. 2016). Deep architecture of a CNN is its biggest strength, which allows extraction of discerning features at different layers of abstractions (Krizhevsky et al. 2017; Szegedy et al. 2016).
Schematic representation of the CNN architecture, consisting of convolution layer, sub-sampling layer and fully connected layers where convolution and sub-sampling layers are part of feature learning process and fully connected layer is used for classification (Prabhu 2018)
A large amount of training data is required to train a Deep Convolutional Neural Network (DCNN) from scratch and it becomes difficult to ensure proper convergence of the model due to absence of huge amount of training data especially in medical imaging, where data is kept heavily protected due to privacy concerns. The process of creating new data with minor alterations such as flips, rotation, mirroring, translations etc. from our existing dataset is known as data augmentation that helps in reducing data insufficiency and overfitting problems. The ability of CNN architectures to detect and classify fractures even when they are placed in different orientations is mitigating data scarcity issues, which is the major cause of hindrance to the deep neural network architectures. It is due to data augmentation techniques that the number of radiographs used for diagnosing fractures is amplified from 500 to hundreds of thousands from year 2003 to 2018. Only few published works (shown in Table 5) related to fracture diagnosis have applied augmentation techniques to their private datasets. This approach could successfully be applied in upcoming work to enhance the performance of the classifier. Another promising alternative to data scarcity problem is to fine-tune a CNN, which is pre-trained on a different network architecture (Birks et al. 2016). Pre-trained CNN is the state-of-the-art image classification network trained on millions of images of a particular domain running for several weeks on multiple servers and then used in a different domain of interest. This approach has become extremely useful for researchers where scarcity of resources is an obstacle for creating a successful model from scratch. These large pre-trained models can be utilized to obtain highly sophisticated and powerful set of features needed for required domain of interest (Greenspan et al. 2016). A study is performed where two models of CNN are compared. One model is trained from scratch and the other is a pre-trained model, which is further fine-tuned on the required domain (Birks et al. 2016). The performance of both the model is compared on distinct medical imaging applications such as classification, detection, and segmentation. A fine-tuned CNN model efficiently outperformed the CNN model trained from scratch in the best case and performed similar to CNN model in the worst case. Therefore, fine-tuning of existing model could offer a practical way to reach the best performance for the application at hand based on the amount of available data. Table 6 demonstrates relevant review findings of the deep learning based algorithms used for fracture detection.
Six board-certified expert radiologists from Stanford hospital develop a very large dataset of 40,561 X-ray images consisting of elbow, finger, hand, humerus, forearm, shoulder, and wrist. They manually examined all X-ray samples and labeled them as abnormal and normal (Rajpurkar et al. 2017). The model is pre-trained using ImageNet (Deng et al. 2009) and fine-tuned on 169 layer dense CNN based on (Huang et al. 2017) for end-to-end classification. Hyper-parameters such as Adam parameters (Kingma and Ba 2015) and mini-batches of size 8 is used in achieving similar performance as the best radiologist in detecting abnormalities on finger and wrist bones but the model reflected lower performance than the worst radiologist in forearm, shoulder and humerus samples. Their model is performing similar to the worst radiologist in finger and elbow studies and performing better in remaining samples. An Inception V3 network (Szegedy et al. 2016), which is pre-trained on ImageNet Large Visual Recognition Challenge (ILSVRC) (Russakovsky et al. 2015), is used to automatically detect fractures in 11,112 wrist X-ray images (Kim and MacKinnon 2018). They have additionally used 100 unused wrist images exhibiting 50% fracture prevalence for testing their model and results obtained are comparable with that of the experts with an AUC of .954. However, both approaches are limited to only wrist images, which can further be extended to other bones for its practical adaptability. Proximal humerus fractures are detected and classified into greater tuberosity, surgical neck, 3-part, and 4-part classes based on Neer (Neer 1970) classification (Chung et al. 2018). Microsoft’s ResNet-152 is used for pre-training and fine-tuning the model for detecting and classifying fractures in 1891 radiographs collected from various hospitals of Korea. The model outperformed the performance of general physician and general orthopedist in detecting humerus fractures and reflected equivalent performance to shoulder orthopedist (Chung et al. 2018). 100,855 radiographs of 12 different body parts are used to acquire random model parameters by pre-training the model on medical images instead of non-medical images (Lindsey et al. 2018). DCNN is used to fine-tune the model on 31,490 wrist images by initializing the model with the parameters obtained from a pre-trained model. The first output of the model is the probability of the presence of fracture in the image and the other output identifies location and extent of the fracture. Finally, the trained model is tested on two different test sets where first set consists of 3500 images, randomly collected from training and validation set and second set consists of 1400 unseen images collected over a period of 3 months from the same hospital. A controlled experiment is additionally executed to test the accuracy of emergency clinician with and without model’s assistance and revealed that the emergency clinician involved in providing X-ray interpretation experienced a reduction rate of 47% by taking assistance of the trained model.
6 Multi-fracture identification techniques for bone X-ray images
The study of multi-fracture classification techniques is extremely significant for speedy recovery of the patient. However, the field of orthopedic surgery and traumatology have investigated scarcity of techniques in classifying fractures despite its huge importance to public health (Chung et al. 2018). Fractures can vary from simple to complex types based on its location and complexity (Mayne 2013). Complex types can become severe for which imperative treatment is required to avoid further complications. Majorly found fractures are addressed in the introduction section and its types are outlined in Fig. 1. Long bone consists of upper and lower extremity region and fracture existing in these bones are identified in several literatures (Chung et al. 2018; Al-Ayyyoub and Al-Zghool 2013; Myint et al. 2018; Mansoory et al. 2012; Linda and Jiji 2018; Mahmoodi 2011). Often researchers accomplish the task of fracture classification in two phases where the fracture is identified in first phase, and is classified into multiple categories in the second phase. Authors have categorized long bone fractures into five classes: normal, greenstick, spiral, transverse and comminute using computer vision techniques such as DT, SVM, NB, and NN, out of which SVM outperforms all other classifiers with an accuracy of more than 85% under tenfold cross validation (Al-Ayyyoub and Al-Zghool 2013). Lower leg bones known as tibia is considered for carrying out fracture classification task by utilizing K-Nearest Neighbor (KNN) approach (Myint et al. 2018). The fracture is identified as one of the four possible labels; normal, oblique, transverse and comminute whereas harris corner points are used to identify fracture locations in tibia images. Cohen’s Kappa assessment is effectively used for measuring the correctness of KNN classifier with an accuracy of 82% (Myint et al. 2018). Proximal humerus fractures are detected and classified into greater tuberosity, surgical neck, 3-part, and 4-part classes based on Neer (Neer 1970) classification (Chung et al. 2018). 1891 radiographs collected from various hospitals of Korea are divided into 10 partitions, where one of the partitions is kept for test set and Microsoft’s ResNet-152 is used for pre-training and fine-tuning the model for detecting and classifying fractures. The model outperformed the performance of general physician and general orthopedist in detecting humerus fractures and reflected equivalent performance to shoulder orthopedist (Chung et al. 2018). CT DICOM images are taken as input to identify complex fractures by utilizing linear structuring elements (Linda and Jiji 2018). Various 2D slices of 3D input image are enhanced by removing noise and sharpening the edges using 2D anisotropic diffusion filter (Mahmoodi 2011; Mendrik et al. 2009). Hidden Markov Random, Field–Expectation Maximization (HMRF-EM) (Zhang et al. 2001) and adaptive thresholding techniques (Singh et al. 2012) are used for segmenting bone regions from fleshy areas followed by segregation of fractured area using template-matching technique (Jurie and Dhome 2001). Complex fractures are identified and located by means of linear structuring elements in the image. Finally, the fracture is visualized in 3D using Ray Cascade method (Sathik et al. 2015). The performance of the proposed approach is validated against expert radiologist data, providing an overall sensitivity and specificity of 95% and 97% respectively where 57 patients suffered from small to severe bone fractures out of 70 test images (Linda and Jiji 2018).
6.1 Digital geometry based approaches for fracture identification
Senior citizens often suffer from long bone fractures due to conditions such as osteoporosis, stress, and sudden fall. Various literatures (Bandyopadhyay et al. 2013, 2016a; b; c) have suggested approaches based on digital geometry, which provide a powerful tool for analyzing bone fractures from X-ray images. A precise fracture detection approach relies on accurate segmentation of bone areas from the fleshy regions in X-ray image and the quality of fracture detection depends on the sharpness and clarity of the bone contour. However, the intensity based segmentation method becomes inaccurate if the pixel belonging to the bone region and fleshy areas has overlapping intensity regions. Therefore, an entropy based thresholding technique is proposed to accurately segment the bone areas from its surrounding fleshy regions in bone X-ray images (Bandyopadhyay et al. 2016a). The authors successfully implemented spatial filtering techniques based on morphology to remove the noise and spurious edge problem in the image followed by bone contour enhancement using multilevel LOCO (open–close and close–open) (Schulze and Pearce 1993) in scanned analog images (Bandyopadhyay et al. 2016a).
An entropy image is generated by separating the brighter bone regions from relatively darker bone areas in the original image, which is used as a preprocessed image for the task of fracture detection and classification in long bones (Bandyopadhyay et al. 2013, 2016b; c). Relaxed straightness and concavity index techniques of digital geometry are effectively utilized for long bone segmentation, rectification of contour imperfections, fracture detection and fracture localization (Bandyopadhyay et al. 2016b). A rapid change is observed in clock and anticlockwise directions in the chain code while traversing a contour nearby fractured region of long bones. Further, the fracture is categorized into simple and complex types based on diaphyseal, proximal and distal regions in the bone X-ray samples. They have additionally developed a software tool to make the entire procedure interactive and automated for which the demonstration is available in the given link [http://oldwww.iiests.ac.in/it-abiswa.-research]. Fracture points identified from X-ray images are successfully employed in categorizing fracture into upper (proximal), middle (diaphyseal) and lower (distal) regions of long bones (Bandyopadhyay et al. 2013). The classification of six long bones named as humerus, radius, ulna, femur, tibia, and fibula are based upon the type of fracture and its location in the X-ray image. Müller AO classification guidelines (Müller et al. 1990) are used for the first time in the classification of long bone fractures by utilizing digital geometry based techniques that appeared in their prior works (Bandyopadhyay et al. 2016a, b, c). However, the line of fracture and its location identified in (Bandyopadhyay et al. 2016a, b, c) are prone to certain inaccuracies when a closer view of fractured regions are observed in X-ray images. The authors have introduced a new concept based on relative concavity analysis to overcome this problem (Bandyopadhyay et al. 2013). Fracture is localized by integrating fracture points and chain-code representing digital-geometric concept such as concativity points and relative concativity. A software tool based on MATLAB is developed for classifying fracture into three major classes namely simple, complex and greenstick. 100 test images are taken as input out of which the system correctly classifies 97 and 92 images in the first and second level respectively (Table 7).
7 Performance evaluation
After successfully applying pre-processing, feature engineering, and feature selection approaches, followed by implementing a model and getting some output in forms of a probability or a class, the next step is to find out how effective is the model based on some metric using test datasets. Different performance metrics have been used to evaluate machine-learning algorithms for fracture detection and classification. It includes log-loss, accuracy, sensitivity, specificity, precision, recall, F-measure, AUC, and ROC etc. It is extremely important to estimate the performance of the classifier by finding out the wrong predictions on the test dataset. The major reasons for evaluating the predicting capability of any classification model is 1) to estimate the generalized performance, 2) to increase the predictive capability of a classifier by refining the model parameters and selecting the best performing model from the algorithm’s hypothesis space. Several standard performance measures applied in literatures from year 2003 to year 2018 are discussed in this section.
7.1 Confusion matrix
It is one of the simplest and intuitive metrics for determining the correctness and accuracy of a classifier, used in binary or multi-class classification problem. It is a table, which is used to describe the performance of a classifier on a test set for which the true values are known. Various machine-learning algorithms are analyzed to predict the presence or absence of fracture in human bones. Figure 7 represents a confusion matrix where the row corresponds to a value predicted by the machine-learning algorithm and column indicates the known truth. True Positive (TP), True Negative (TN), False Positive (FP) and False Positive (FN) values of confusion matrix are defined below:
-
1.
True positive: Patients have fracture and correctly identified as “fractured” by the algorithm (TP).
-
2.
True Negative: Patients does not have fracture and correctly identified as “healthy” by the algorithm (TN).
-
3.
False positive: Patients does not have fracture but incorrectly identified as “fracture” by the algorithm (FP).
-
4.
False Negative: Patients do have fracture but incorrectly identified as “healthy” by the algorithm (FN).
-
5.
Accuracy: It is the total number of correct predictions (both fracture and healthy) divided by total number of samples in the dataset.
7.2 Accuracy paradox in classification problem
Choosing the right metrics for classification tasks is extremely important in accessing a model. Accuracy alone is not reliable to select a classifier due to classification imbalance problem where one category represents the overwhelming majority of the data samples (Brownlee 2014). Another imbalanced classification problem occurs when the rate of disease in the public is very low. Consider a classification task where out of 150 patients, 25 patients are diagnosed with fracture and 125 patients are healthy, known as actual truth-value. Following two problems may exists when accuracy alone is used for prediction task—(a) all patients detected healthy: a model that only predicted healthy cases in patients achieves an accuracy of (125/150)*100 or 83.3%. This represents high accuracy and if it is used alone for decision support system to inform doctors, it would wrongly inform 25 patients of no occurrence of fracture (high False Negatives) making it a terrible model. (b) All patients detected with fracture: a model that only predicted the presence of fracture in patients achieves an accuracy of (25/150)*100 or 16.6%. If it is used alone for decision support system to inform doctors, it would wrongly inform 125 patients of the occurrence of fracture (high False Positives) making it a terrible model. Recall and precision are other performance matrix used for eliminating this accuracy paradox in classification problem (Brownlee 2014).
Recall/Sensitivity
It is the ability of a classifier to find all the relevant cases within a dataset.
Precision
It measures the quality of our predictions only based on what our predictor claims to be positive.
Specificity
It is total number of true negative assessments divided by number of all negative assessments.
Sensitivity measures the ability of the model to predict fractured cases and specificity measures the ability of the model to predict healthy cases.
F1 score
While recall measures the ability to find all relevant instances in a dataset, precision expresses the proportion of the data points the model says relevant actually were relevant. We can maximize either precision or recall at the expense of the other metric. For example, during preliminary fracture detection phase, we would want to correctly detect fractures in all the patients (high recall) and can accept a low precision rate if the cost of the follow-up examination is not significant. However, we may also find an optimal blend of precision and recall using F1 score that is the combination of recall and precision (Koehrsen 2018).
Receiver operating characteristics curve (ROC)
It predicts the probability of a binary classifier. It is the plot of false positive rate (x-axis), which is the same as precision and true positive rate (Y-axis), which is the same as recall. Designing confusion matrices for all possible threshold values is a challenging task, ROC graphs provides a simpler way to summarize all the information. The shape of the ROC curve contains a lot of information (Fig. 8):
Receiver Operating Characteristics Curve (ROC) (Stephanie 2016)
-
1.
A point at (1, 1) means that our model correctly classifies all fractured samples but incorrectly classifies all samples that were not fractured.
-
2.
Diagonal line where (True Positive Rate = False Positive Rate), shows that the proportion of correctly classified fractured samples is similar to proportion of incorrectly classified healthy samples.
-
3.
A point at (0, 0) results in zero True Positives and zero False Negatives.
AUROC (Area under the receiver operating characteristics)
Area under curve (AUC–ROC) is a performance measure for classification problems at various thresholds settings. ROC is a probability curve and AUC is the capability of the model in making a distinction between various classes. AUC makes it easier to compare one ROC curve to another, the more the area the better is the performance. Although ROC graphs are drawn using true positive rates and false positives rates, other matrices perform the same task. For example, false positive rates can be replaced with precision. Almost all literature surveyed here used confusion matrix based performance matrix for analyzing the classifier’s performance. Table 8 depicts comparative analysis of the model proposed by authors against radiologist’s interpretation on the dataset where the performance of the radiologist is assumed to be 100% on almost all the comparisons. Researchers have shown the performance of the proposed model in their private datasets due to the unavailability of the standard dataset.
Cohen’s kappa statistic (k)
Many a times collecting and interpreting research or laboratory data such as collecting and labeling X-Ray images rely on multiple experts or clinicians in healthcare industries. Therefore, it is extremely important to have an agreement among multiple observers working on the interpretations of these samples. One such measurement of calculating the agreement and disagreement is Cohen’s kappa statistic (k). It gives a quantitative measure of the agreement on a situation where two or more independent observers are evaluating the same process (Sim and Wright 2005). For example: two radiologists are interpreting 100 X-ray images for the presence and absence of fracture in a patient simultaneously and mutually agree that fracture is diagnosed in 60 cases and not diagnosed in 15 cases, offering 75% agreement in total. This total observed agreement is calculated by adding diagonal values as shown in Fig. 9.
Here, T is the total number of observations, C and R represents column and row respectively.
However, random evaluation of patients by observers would sometimes lead to agreement just by chance and this quantity is measured by using expected or chance agreement Pc or Pe. It is calculated by multiplying positive answers of observer-1 and observer-2 and adding them to the negative answers of observer-1 and observer-2, all divided by total number of observed samples.
Here, S represents summation and Pe is evaluated as .6.
Now, we finally calculate kappa volume using the given formula:
Here, K = .375.
According to the commonly used scale presented in Table 9, both the radiologists have a minimal agreement (k = .375) in diagnosing fractures for the above example.
Sensitivity of 45.45% is observed when Neck-Shaft Angle (NSA) is used as the only feature in the first ever approach of fracture detection in 2003 (Tian et al. 2003). The poor fracture detection rate is obtained because NSA alone could not detect significant changes observed during the event of fracture. This problem is overcome by using several feature-classifier combinations and ensemble based approaches and resulted in better performance (Yap et al. 2004; Lim et al. 2004; Lai et al. 2005). SVM, NN, DT and NB algorithms are used independently in earlier approaches such as (Lim et al. 2004), which is later combined by using boosting and bagging techniques and successfully achieved the accuracy of 91.8% on 98 hand X-ray images (Al-Ayyoub et al. 2013). Tibia fractures are detected, classified and localized in human bones using Decision Tree and K-Nearest Neighbor algorithms (Myint et al. 2018). This is the first work proposed which performs all three activities after successfully applying Unsharp Masking (USM) in the input image. However, they have used only 40 images to train the model including all class types, which is not sufficient for practical adaptability of the model.
8 Discussions
Fracture detection in radiographs has significant clinical importance. There was an estimated backlog of 200,000 plain radiographs and 12,000 cross-sectional studies in the year 2012 (Cliffe et al. 2016). These figures demand desperate improvements in reporting efficiency and workflow management to mitigate harm caused to patients due to delayed or missed diagnosis. Automatic abnormality detection or localization techniques could help radiologists fight fatigue largely. Radiologists can further prioritize diagnosis and treatment according to the abnormality type detected by the automated system. Most often, the first point of contact for any patient in case of fracture is non-orthopedic surgeons or inexperienced clinicians who often lack expertise in detecting fractures (Chung et al. 2018). Hence, it is quite usual that fractures are misdiagnosed during interpretation of X-Ray images. Radiologists or clinicians manually examine the X-rays for the existence of fractures and its type. The interpretation and classification of radiographic images is a time-consuming and intense process, which could be solved using automated fracture classification models.
Majority of the published work of fracture detection and classification is focused on either single anatomical region or a single type of fracture in different anatomical regions. An ideal model would be the one that is able to detect different type of fractures in various anatomical regions. Collecting hundreds of thousands of radiographs, providing correct labels to these X-ray images and feeding enough training data to the models is an arduous task in medical imaging but predicting the fracture using a trained model take less than a second in a modern computer. The major challenge in achieving multi-label classification on different anatomical regions is the availability of the labeled dataset. A promising alternative to solve this problem is to fine-tune a CNN, which is pre-trained on a different network (Birks et al. 2016). Researchers can use these large pre-trained models to obtain highly sophisticated and powerful set of features needed for the domain of interest. Instead of pre-training the model on millions of non-radiology images, the model can be trained on multiple bone X-ray images such as ankle, knee, neck, hip etc. This way we could better initialize model parameters, which can be later used for training the required X-ray images (Lindsey et al. 2018). This approach is similar to transfer learning with the slight difference that we pre-train the model on several types of bone X-ray images instead of non-medical images. This system can also be useful in emergency conditions where such models may provide assistance to the radiologists for diagnosing fracture. The more complex a fracture type is, easier it will become for CNN to classify it as compared to human, as humans face difficulty in interpreting complex bone structures. A machine such as CNN remains consistent in its performance and does not lack concentration while interpreting several complex X-ray structures and it can even surpass human level performance when provided with sufficient amount of training data. In addition, it is a human tendency to predict correct output in familiar shapes as opposed to the one whose fracture configuration is less familiar. Hence, a CNN can potentially be trained with humongous training data with all possible cases ranging from simpler fracture structure to the most complex ones which is more than any orthopedics will even encounter in his/her life.
The decision-making in clinical examination is a complex process that is based on accurate evaluation of clinical findings using diagnostic tests and reference standard data. A gold standard study may refer to an experimental model that has been thoroughly tested and has a reputation in the field as a reliable method (Cardoso et al. 2014). Whenever a classifier is compared against actual or known data provided by radiologist, the performance of radiologist is considered as 100% accurate, which means that the radiologist involved in interpreting hundreds of images correctly detects and classifies fracture in all samples with zero error rate. However, there may be a chance that the radiologist involved in the interpretation of images could not provide the best set of results or do not agree with each other in case of multiple observers. It is extremely important to have an agreement among multiple observers dedicated to interpret the sample images. One such measurement of calculating the agreement and disagreement is Cohen’s kappa statistic (k). It gives us a quantitative measure of the agreement in situation where two or more independent observers are evaluating the same thing. It is only in 2017 when a gold standard is created for abnormality detection in X-ray samples including multiple anatomical regions using Cohen’s kappa statistic (Rajpurkar et al. 2017). Gold standard is created by randomly choosing three expert radiologists out of six board certified radiologists and the label is selected based on majority voting. Cohen’s kappa statistic is used to compare both radiologists and proposed model’s performance with the gold standard (Rajpurkar et al. 2017). Likewise, a gold standard could be created for a labeled fracture dataset for the purpose of fracture detection and classification that is globally available and acceptable. This would enable researchers to compare their proposed model with the Gold standard for better performance analysis. Kappa statistics is used for classifying tibia fractures into four categories with the accuracy of 83% but no such gold standard is observed here for accuracy measurement while calculating kappa statistic (Myint et al. 2018). A similar approach is observed where performance of two models and performance of a group of general orthopedics, general physicians and specialized orthopedics are evaluated on test set for diagnosing and classifying humerus fractures (Chai et al. 2011). The adopted CNN based model outperformed human groups in detecting and classifying fractures except for one category of fracture.
Overfitting issues can be mitigated by including only relevant features necessary for the model. Cropping our region of interest from the input image and exploring several network architectures including dropout could be a solution to overfitting problem. An additional label termed as “indecisive” could be included along with “normal” or “abnormal” fracture classes in the case where radiologist experts indicate indecisiveness in detecting the fracture. This way the examiner would review it for the second time (Kim and MacKinnon 2018). If any result is ‘unknown’ or ‘uncertain’ during the examination of fracture then such cases must either be excluded or assigned a new label such as “uncertain” before including them in training data (Gale et al. 2017). Assigning them ‘uncertain’ would help radiologists and researchers to identify that the patient has to undergo further level of treatment and can go for CT or MRI, as to accurately identify the fracture or fracture type.
9 Conclusion
Various fracture detection and classification approaches have been proposed which includes data preparation, image pre-processing stage followed by feature extraction and classification. The interpretation and classification of radiographic images by expert radiologists is a time-consuming and intense process, which could be solved using automated fracture classification models. The major obstacle in the development of a high performance classification model is the lack of labeled training dataset as pointed by many researchers cited in this survey. We have tried to provide a number of ideas and perspectives to explore that could help in developing an ideal model, which can detect different type of fractures in various anatomical regions.
References
Al-Ayyoub M, Hmeidi I, Rababah H (2013) Detecting hand bone fractures in X-ray images. J Multimed Process Technol (JMPT) 4(3):155–168. https://doi.org/10.13140/RG.2.1.2645.8327
Al-Ayyyoub M, Al-Zghool D (2013) Determining the type of long bone fractures in X-ray images. WSEAS Trans Inf Sci Appl 10(6):261–270
Andreas T, Jahrer M, Bell RM, Park F (2009) The BigChaos solution to the Netflix Grand Prize
Antipov G, Berrani S, Dugelay J (2016) Minimalistic CNN-based ensemble model for gender prediction from face images. Pattern Recognit Lett. https://doi.org/10.1016/j.patrec.2015.11.011
Bandyopadhyay O, Biswas A, Chanda B, Bhattacharya BB (2013) Bone contour tracing in digital X-ray images based on adaptive thresholding. In: Proceedings, 5th international conference on pattern recognition and machine intelligence, volume LNCS 8251, pp 465–473. https://doi.org/10.1007/978-3-642-45062-4_64
Bandyopadhyay O, Biswas A, Bhattacharya BB (2016a) Automatic segmentation of bones in X-ray images based on entropy measure. Int J Image Graph. https://doi.org/10.1142/s0219467816500017
Bandyopadhyay O, Biswas A, Bhattacharya BB (2016b) Long-bone fracture detection in digital X-ray images based on digital-geometric techniques. Comput Methods Programs Biomed 123:2–14. https://doi.org/10.1007/978-3-319-07148-0_19
Bandyopadhyay O, Biswas A, Bhattacharya BB (2016c) Classification of long-bone fractures based on digital-geometric analysis of X-ray images. Pattern Recognit Image Anal 26(4):742–757. https://doi.org/10.1134/S1054661816040027
Birks JS, L’Allier PL, Gingras MA (2016) Convolutional neural networks for medical image analysis: full training or fine tuning? IEEE Trans Med Imaging 35(5):1299–1313. https://doi.org/10.1109/TMI.2016.2535302
Brownlee J (2014) Classification accuracy is not enough: more performance measures you can use. https://machinelearningmastery.com/classification-accuracy-is-not-enough-more-performance-measures-you-can-use. Accessed 22 Apr 2019
Cao Y, Wang H, Moradi M, Prasanna P, Syeda-Mahmood TF (2015) Fracture detection in X-ray images through stacked random forests feature fusion. In: Proceedings—international symposium on biomedical imaging, pp 801–805. https://doi.org/10.1109/isbi.2015.7163993
Cardoso JR et al (2014) What is gold standard and what is ground truth? Dental Press J Orthod 19(5):27–30. https://doi.org/10.1590/2176-9451.19.5.027-030.ebo
Chai HY, Wee LK, Swee TT, Hussain S (2011) Gray-level co-occurrence matrix bone fracture detection. WSEAS Trans Syst 10(1):7–16. https://doi.org/10.3844/ajassp.2011.26.32
Chung SW, Han SS, Lee JW (2018) Automated detection and classification of the proximal humerus fracture by using deep learning algorithm. Acta Orthop 89(4):468–473. https://doi.org/10.1080/17453674.2018.1453714
Cliffe H et al (2016) Summary of The Royal College of Radiologists’ (RCR) reporting backlog surveys and assessment of potential causes and solutions. Clin Radiol 71:1–30. https://doi.org/10.1016/j.crad.2016.06.060
Deng J, Dong W, Socher R, Li L, Li K, Fei-fei L (2009) ImageNet: a large-scale hierarchical image database. In: IEEE conference on computer vision and pattern recognition, 20–25 June 2009, Miami, FL, USA. https://doi.org/10.1109/cvpr.2009.5206848
Dimililer K (2017) IBFDS: intelligent bone fracture detection system. Procedia Comput Sci 120:260–267. https://doi.org/10.1016/j.procs.2017.11.237
Ding C, Tao D (2017) Trunk-Branch Ensemble Convolutional Neural Networks for Video-based Face Recognition. IEEE Trans Pattern Anal Mach Intell. https://doi.org/10.1109/TPAMI.2017.2700390
Dormehl L (2019) What is an artificial neural network? Here’s everything you need to know. https://www.digitaltrends.com/cool-tech/what-is-an-artificial-neural-network. Accessed 13 Feb 2019
Gale W, Oakden-Rayner L, Carneiro G, Bradley AP, Palmer LJ (2017) Detecting hip fractures with radiologist-level performance using deep neural networks. Comput Vis Pattern Recognit. arXiv:1711.06504
Greenspan H, Ginneken BV, Summers RM (2016) Guest editorial deep learning in medical imaging: overview and future promise of an exciting new technique. IEEE Trans Med Imaging 35(5):1153–1159. https://doi.org/10.1109/TMI.2016.2553401
Guly H (2001) Diagnostic errors in an accident and emergency department. Emerg Med J (EMJ) 18(4):263–269. https://doi.org/10.1136/emj.18.4.263
Haralick RM, Shanmugam K (1973) Textural features for image classification. IEEE Trans Syst Man Cybern 3(6):610–621. https://doi.org/10.1109/TSMC.1973.4309314
Hari CV, Jojish JV, Gopi S, Felix VP (2010) Mid-point Hough transform: a fast line detection method. In: Annual IEEE India conference, 18–20 Dec. 2009. https://doi.org/10.1109/indcon.2009.540945
He JC, Leou WK, Howe TS (2007) Hierarchical classifiers for detection of fractures in X-ray images. In: Kropatsch WG, Kampel M, Hanbury A (eds) Computer analysis of images and patterns. CAIP 2007. Lecture Notes in Computer Science, vol 4673. Springer, Berlin. https://doi.org/10.1007/978-3-540-74272-2_119
How to organize data labeling for machine learning: approaches and tools (2018). https://www.altexsoft.com/blog/datascience/how-to-organize-data-labeling-for-machine-learning-approaches-and-tools. Accessed 26 Jan 2019
Huang G, Liu Z, Maaten LVD, Weinberger KQ (2017) Densely connected convolutional networks. In: IEEE conference on computer vision and pattern recognition (CVPR), 21–26 July 2017, Honolulu, HI, USA, IEEE, pp 2261–2269. http://doi.org/10.1109/CVPR.2017.243
Jacob NE, Wyawahare MV (2013) Survey of bone fracture detection techniques. Int J Comput Appl 71(17):31–34. https://doi.org/10.5120/12452-9342
Jurie F, Dhome M (2001) A simple and efficient template matching algorithm. In: Eighth IEEE international conference on computer vision, 7–4 July 2001, Vancouver, BC, Canada, IEEE, pp 544–549. http://doi.org/10.1109/ICCV.2001.937673
Khashman A, Dimililer K (2008) Image compression using neural networks and Haar wavelet. WSEAS Trans Signal Process 4(5):330–339
Khatik I (2017) A study of various bone fracture detection techniques. Int J Eng Comput Sci 6(5):21418–21423. https://doi.org/10.18535/ijecs/v6i5.38
Kim KG (2016) Book review: Deep learning, healthcare informatics. Research 22(4):351–354. https://doi.org/10.4258/hir.2016.22.4.351
Kim DH, MacKinnon T (2018) Artificial intelligence in fracture detection: transfer learning from deep convolutional neural networks. Clin Radiol 73(5):439–445. https://doi.org/10.1016/j.crad.2017.11.015
Kingma D, Ba J (2015) A method for stochastic optimization. In: International conference on learning representations, San Diego
Kinnari P, Dangar Y (2017) A survey for fracture detection in hand bone X-ray images. Int J Adv Res Innov Ideas Educ 3(1):1529–1533. ISSN(O)-2395-4396
Koehrsen W (2018) Beyond accuracy: precision and recall. https://towardsdatascience.com/beyond-accuracy-precision-and-recall-3da06bea9f6c. Accessed 9 Apr 2019
Kohli M, Prevedello LM, Filice RW, Geis JR (2017) Implementing machine learning in radiology practice and research. Am J Roentgenol 208(4):754–760. https://doi.org/10.2214/ajr.16.17224
Krizhevsky A, Sutskever I, Hinton GE (2017) ImageNet classification with deep convolutional neural networks. Adv Neural Inf Process Syst 60(6):84–90. https://doi.org/10.1145/3065386
Krupinski EA, Berbaum KS, Caldwell RT, Schartz KM, Kim J (2010) Radiology workdays reduce detection and accommodation accuracy. J Am Coll Radiol 7(9):698–704. https://doi.org/10.1016/j.jacr.2010.03.004
Kumar A et al (2017) An ensemble of fine-tuned convolutional neural networks for medical image classification. IEEE J Biomed Health Inform 21(1):31–40. https://doi.org/10.1109/JBHI.2016.2635663
Kuncheva L, Whitaker E (2001) Ten measures of diversity in classifier ensembles: limits for two classifiers. In: Proceedings of IEEE workshop on intelligent sensor processing, Birmingham, vol 10, pp 1–10
Laan MJVD, Polley EC, Hubbard AE (2007) Super Learner. https://doi.org/10.2202/15446115.1309
Lai V, Lum F, Leow WK, Chen Y (2005) Combining classifiers for bone fracture detection in X-ray images. In: IEEE international conference on image processing 2005, Genova, Italy, IEEE. https://doi.org/10.1109/icip.2005.1529959
Lakhani P, Sundaram B (2017) Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology 284(2):574–582
Lampert CH, Blaschko MB, Hofmann T (2008) Beyond sliding windows: object localization by efficient subwindow search. In: IEEE conference on computer vision and pattern recognition, 23–28 June 2008, Anchorage, AK, USA. https://doi.org/10.1109/cvpr.2008.4587586
LeCun Y et al (1998) Gradient based learning applied to document recognition. Proc IEEE 86(11):2278–2324. https://doi.org/10.1109/5.72679
Lim SE, Xing Y, Chen Y, Leow WK, Howe TS, Png MA (2004) Detection of femur and radius fractures in X-ray images. In: Second international conference on advances in medical signal and information processing, pp 399–404
Linda H, Jiji GW (2011) Crack detection in X-ray images using fuzzy index measure. Appl Soft Comput 11:3571–3579. https://doi.org/10.1016/j.asoc.2011.01.029
Linda H, Jiji GW (2017) Hairline breakage detection in X-ray images using data fusion. Multimedia Tools and Applications. 77(6):1–16. https://doi.org/10.1007/s11042-017-5286-5
Linda CH, Jiji GW (2018) Hierarchical approach to detect fractures in CT DICOM images, section C: computational intelligence, machine learning and data analytics. Comput J 61(7):1099–1108. https://doi.org/10.1093/comjnl/bxy023
Lindsey R, Daluiski A, Chopra S et al (2018) Deep neural network improves fracture detection by clinicians. Proc Natl Acad Sci 115(45):11591–11596. https://doi.org/10.1073/pnas.1806905115
Lowe DG (2004) Distinctive image features from scale-invariant keypoints David. Int J Comput Vision 60(2):91–110. https://doi.org/10.1023/B:VISI.0000029664.99615.94
Mahendran SK, Baboo SS (2011a) Automatic fracture detection using classifiers: a review. Int J Comput Sci Issues 8(6):340–345
Mahendran SK, Baboo SS (2011b) An enhanced Tibia fracture detection tool using image processing and classification fusion techniques in X-ray images. Global J Comput Sci Technol 11(14):23–28
Mahmoodi S (2011) Anisotropic diffusion for noise removal of band pass signals. Elsevier Signal Process 91(5):1298–1307. https://doi.org/10.1016/j.sigpro.2010.12.018
Mansoory MS, Ashtiyani M, Sarabadani H (2012) Automatic crack detection in eggshell based on SUSAN edge detector using fuzzy thresholding. World Appl Sci J 18(11):1602–1608. https://doi.org/10.5539/mas.v5n6p117
Mayne R (2013) How to identify fracture types. https://allthingsaafs.com/tag/how-to-identify-fracture-types. Accessed 7 Apr 2019
McHugh ML (2012) Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 22(3):276–282. https://doi.org/10.11613/BM.2012.031
Mendrik M, Vonken EJ, Rutten A, Viergever MA, Ginneken BV (2009) Noise reduction in computed tomography scans using 3D anisotropic hybrid diffusion with continuous switch. IEEE Trans Med Imaging 28(10):1585–1594. https://doi.org/10.1109/TMI.2009.2022368
Meyer P et al (2018) Survey on deep learning for radiotherapy. Comput Biol Med 98:126–146. https://doi.org/10.1016/j.compbiomed.2018.05.018O
Minetto R, Segundo MP, Sarkar S (2018) Hydra: an ensemble of convolutional neural networks for geospatial land classification. Comput Vis Pattern Recognit. arXiv:1802.03518
Müller ME, Koch P, Nazarian S, Schatzker J (1990) The comprehensive classification of fractures of long bones. Springer, Berlin. 73(4):202. https://doi.org/10.1007/978-3-642-61261-9
Myint WW, Tun KS, Tun HM (2018) Analysis on leg bone fracture detection and classification using X-ray. Mach Learn Res 3(3):49–59. https://doi.org/10.11648/j.mlr.20180303.11
Neer CS (1970) Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg 52(6):1077–1089
Pisano ED et al (1998) Contrast limited adaptive histogram equalization image processing to improve the detection of simulated spiculations in dense mammograms. J Digit Imag 11(4):193–200. https://doi.org/10.1007/BF03178082
Polikar R (2009) Ensemble learning. Scholarpedia 4(1):2776. https://doi.org/10.4249/scholarpedia.2776
Prabhu (2018) Understanding of convolutional neural network (CNN)—deep learning. https://medium.com/@RaghavPrabhu/understanding-of-convolutional-neural-network-cnn-deep-learning-99760835f148. Accessed 7 Dec 2018
Rajan J, Kaimal MR (2006) Image denoising using wavelet embedded anisotropic diffusion (WEAD). In: IET international conference on visual information engineering, pp 26–28. https://doi.org/10.1049/cp:20060597
Rajpurkar P et al (2017) MURA: large dataset for abnormality detection. In: 1st conference on medical imaging with deep learning, MIDL 2018 (2017). arXiv:1712.06957
Russakovsky O, Deng J et al (2015) ImageNet large scale visual recognition challenge. Int J Comput Vis 115(3):211–252. https://doi.org/10.1007/s11263-015-0816-y
Sathik MM, Mehaboobathunnisa R, Thasneem AAH, Arumugam S (2015) Ray casting for 3D rendering—a review. Int J Innov Eng Technol 5:121–124
Schulze MA, Pearce JA (1993) Linear combinations of morphological operators: the midrange, pseudomedian, and loco filters. In: Proceedings of IEEE international conference on acoustics, 27–30 April, Minneapolis, USA, IEEE, pp 1520–6149. https://doi.org/10.1109/icassp.1993.319746
Shah T (2017) About train, validation and test sets in machine learning. https://tarangshah.com/blog/2017-12-03/train-validation-and-test-sets. Accessed 27 Dec 2018
Sharan V, Keshari N, Mondal T (2014) Biomedical image denoising and compression in wavelet using MATLAB. Int J Innov Sci Mod Eng (IJISME) 2(6):9–13
Shen D, Wu G, Suk HI (2017) Deep learning in medical image analysis. Annu Rev Biomed Eng 19(1):221–248. https://doi.org/10.1146/annurev-bioeng-071516-044442
Sim J, Wright CC (2005) The Kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther 85(3):257–268. https://doi.org/10.1093/ptj/85.3.257
Singh TR, Roy S, Singh OI, Sinam T, Singh KM (2012) A new local adaptive thresholding technique in binarization. Int J Comput Sci 8(6):271–277 arXiv:1201.5227
Stec N, Arje D, Moody A, Krupinski E, Tyrrell P (2018) A systematic review of fatigue in radiology: is it a problem? Am J Roentgenol 210(4):799–806. https://doi.org/10.2214/AJR.17.18613
Stephanie (2016) C-statistic: definition, examples, weighting and significance. https://www.statisticshowto.datasciencecentral.com/c-statistic. Accessed 12 Apr 2019
Szegedy C, Vanhoucke V, Ioffe S, Shlens J, Wojna Z (2016) Rethinking the inception architecture for computer vision. In: IEEE conference on computer vision and pattern recognition (CVPR), IEEE, pp 2818–2826. https://doi.org/10.1109/cvpr.2016.308
Teixeira L, Celes W, Gattass M (2008) Accelerated corner-detector algorithms. In: Proceedings of the British machine vision conference. http://doi.org/10.5244/C.22.62
Tian D, Ha M (2004) Applications of wavelet transform in medical image processing. In: Proceedings of 2004 international conference on machine learning and cybernetics (IEEE Cat. No.04EX826), pp 26–29. https://doi.org/10.1109/icmlc.2004.1382071
Tian TP et al (2003) Computing neck-shaft angle of femur for X-ray fracture detection. In: Petkov N, Westenberg MA (eds) Computer analysis of images and patterns. CAIP 2003. Lecture Notes in Computer Science, vol 2756. Springer, Berlin
Umadevi N, Geethalakshmi SN (2012) Multiple classification system for fracture detection in human bone X-ray images. In: Third international conference on computing, communication and networking technologies (ICC-CNT’12), 26–28 July 2012, Coimbatore, India, IEEE. https://doi.org/10.1109/icccnt.2012.6395889
Waite S, Scott J, Gale B, Fuchs T, Kolla S, Reede D (2017) Interpretive error in radiology. Am J Roentgenol 208(4):739–749. https://doi.org/10.2214/ajr.16.16963
Wang H et al (2017) Deep learning based ensemble approach for probabilistic wind power forecasting. Appl Energy 188:56–70. https://doi.org/10.1016/j.apenergy.2016.11.111
Whang J, Baker SR, Patel R, Luk L, Castro A (2013) The causes of medical malpractice suits against radiologists in the United States. Radiology 266(2):548–554. https://doi.org/10.1148/radiol.12111119
Wolpert DH (1992) Stacked generalization. Neural Netw 5(2):241–259. https://doi.org/10.1016/S0893-6080(05)80023-1
World Health Organization (2019). https://www.who.int/nutrition/topics/5_population_nutrient/en/index25.html. Accessed 14 Mar 2019
X-rays, CT Scans and MRIs- OrthoInfo-AAOS (2019). https://orthoinfo.aaos.org/en/treatment/x-rays-ct-scans-and-mris. Accessed 14 Jan 2019
Yap DWH et al (2004) Detecting femur fractures by texture analysis of trabeculae. In: Proceedings—international conference on pattern recognition 3, pp 730–733. http://doi.org/10.1109/ICPR.2004.1334632
Zhang Y, Brady M, Smith S (2001) Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans Med Imaging 20:45–57. https://doi.org/10.1109/42.906424
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Joshi, D., Singh, T.P. A survey of fracture detection techniques in bone X-ray images. Artif Intell Rev 53, 4475–4517 (2020). https://doi.org/10.1007/s10462-019-09799-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10462-019-09799-0