Abstract
The present scoping review aimed to identify studies that investigated alexithymia, defined as a difficulty in identifying and describing one’s own emotions, in people living with HIV (PLWH).
A literature search, in line with the guidelines of PRISMA-ScR, was conducted in the following bibliographic databases: PubMed, PsycINFO, and Web of Science. The databases were queried using the following strings (using Boolean operators): (“alexithymia” OR “alexithymic”) AND (“HIV” OR “Human Immunodeficiency Virus”). In line with the eligibility criteria, fourteen articles were found.
Ten studies showed the involvement of alexithymia in disease severity (e.g., viral load levels), and adherence to antiretroviral therapy. Three studies revealed an association between alexithymia and cardiovascular disease, and three studies highlighted the implication of alexithymia in cognitive impairment.
This review revealed the complex role of alexithymia in HIV disease. A careful clinical assessment of the emotional regulation process of PLWH can provide useful prognostic information.
Resumen
La presente revisión panorámica está orientada a identificar estudios que han investigado la alexitimia, definida como la dificultad de identificar y describir las propias emociones, en personas que conviven con el VIH.
Siguiendo las directrices de PRISMA-ScR, se realizó una búsqueda bibliográfica en las siguientes bases de datos: PubMed, PsycINFO y Web of Science. Las bases de datos se consultaron utilizando las siguientes cadenas (utilizando el operador Boolean): (“alexithymia” OR “alexithymic”) AND (“HIV” OR “Human Immunodeficiency Virus”).
De acuerdo con los criterios de elegibilidad, se encontraron catorce artículos. Específicamente, diez estudios mostraron la implicación de la alexitimia en la gravedad de la enfermedad (por ejemplo, niveles de carga viral) y la adherencia a la terapia antirretroviral, tres estudios revelaron la asociación entre la alexitimia y la enfermedad cardiovascular, y tres estudios resaltaron la implicación de la alexitimia en el deterioro cognitivo.
Esta revisión reveló el complejo rol de la alexitimia en la enfermedad del VIH. Una evaluación clínica detallada del proceso de regulación emocional de las personas que viven con el VIH puede proporcionar información útil para el pronóstico.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the construct of alexithymia has become relevant in several medical settings for its implication in patients’ health [1].
Alexithymia is considered a personality trait rather than a disorder, and it is definable as a psychological construct with implications for affective processes. Patients suffering from psychosomatic diseases presented difficulties in the subjective awareness and processing of affects, their connection with specific situations, and their memories [2,3,4,5,6]. “Alexithymia construct reflects a deficit in the cognitive processing of emotions” [7], these limited capacities should not be thought of as an all-or-nothing phenomenon, but as a dimensional construct [1, 5, 8].
The characteristic aspects of alexithymia are: (1) difficulty identifying one’s own emotions and distinguishing them from physical sensations, (2) difficulty describing one’s own emotions with a restricted emotional vocabulary, (3) limited fantasy and reduced tendency to daydream, and (4) externally oriented cognitive style [5, 8]. Furthermore, people with alexithymia have limited empathic capability and relational difficulties [8]. The difficulty in mentalizing one’s own internal states could also result in impulsive or compulsive behaviors that regulate emotional activation [5]. The aetiology of alexithymia involves both genetic and environmental factors, as early life traumatic events [7, 9].
Several evidences suggest that alexithymia plays a role in the development and maintenance of psychological and physical illness conditions, resulting in poorer health outcomes and quality of life (QoL) [1,2,3, 5, 10,11,12,13]. Possibly, this occurs through various mechanisms, such as emotional dysregulation, increased vulnerability to inflammatory processes, somatosensory amplification, and the adoption of maladaptive illness behavior [10]. In fact, high levels of alexithymia have been found in patients with different medical and psychiatric conditions [11,12,13,14,15]. Concurrently, a higher prevalence of people with alexithymia was found in these clinical populations than in the general population [12,13,14,15,16].
To understand potential mechanisms and aetiology of alexithymia, several self-report instruments and interviews were developed and validated [4, 6]. In addition, clinicians can use these instruments at a screening level, allowing for an evaluation of the results into a more comprehensive clinical assessment of the patient [1].
Although an exhaustive overview of all these instruments goes beyond the scope of this review, it is useful to provide a brief description of some of them. [12, 17].
The application in research of some of these instruments, might be more difficult to achieve in research (in terms of time and interviewer training), such as the modified Beth Israel Hospital Psychosomatic Questionnaire (BIQ), an interviewer-rated inventory with two subscales (Affect Awareness and Operational Thinking), or the Toronto Structured Interview for Alexithymia, a semi-structured interview derived from Toronto Alexithymia Scale (TAS) [12, 17].
Some self-report instruments have attempted to investigate the construct of alexithymia by integrating other related aspects not present in the original definition, such as the Bermond-Vorst Alexithymia Questionnaire, that investigate the four original elements and emotionalizing, or Perth Alexithymia Questionnaire, which is based on the attention-appraisal model of alexithymia [17,18,19].
In the last few decades, the TAS has been the most widely used self-report instrument in psychological and medical research [1, 5]. TAS was developed to overcome the limitations of pre-existing unreliable tools (i.e., Schalling-Sifneos Personality Scales and Minnesota Multiphasic Personality Inventory – Alexithymia Scale) [17], and gather empirical evidence to validate the construct, supported psychometrically by a good level of reliability and acceptable construct validity [5, 20].
While The 26-item version includes four subscales corresponding to the four aspects that characterize the construct, the TAS-20 only consists of three of those: difficulty identifying feelings (DIF), difficulty describing feelings to others (DDF), and an externally oriented thinking style (EOT). Higher scores indicate higher levels of alexithymia [1, 21, 22].
From a psychosomatic medicine perspective, psychological aspects related to emotional regulation play a significant role also in adaptation to illness and adherence to treatments in chronic diseases [5, 23].
Advances in medical and pharmacological research have radically changed the nature of certain medical conditions, turning even infections (that are typically progressive and fatal in a short time) into chronic diseases that persist for decades [24, 25], at the cost of strict adherence to treatment regimens [23]. An example of the effectiveness of this scientific progress can be observed in the field of human immunodeficiency virus (HIV) infection, with significant improvements in physical health outcomes, quality of life, and life expectancy of those affected [25,26,27].
HIV infection damages the immune system by destroying CD4 cells, a subgroup of white blood cells [28].
In 2021, there were an estimated 38.4 million people living with HIV (PLWH) worldwide, including 36.7 million aged 15 years or older, 53% female and 1.5 million newly diagnosed [29, 30].
Pharmacological therapy management should involve a multidisciplinary team to optimize the therapeutic benefit for the patient and prevent further spread of the infection. Highly active antiretroviral therapy (HAART) is a treatment regimen that includes a combination of antiretroviral drugs [26]. When correctly administered, an antiretroviral therapy results in rapid control of HIV, and partial restoration of immune function, preventing the onset of the complications that define the Acquired Immuno Deficiency Syndrome (AIDS) [25]. Therefore, it is particularly important to monitor the patient’s clinical, personal, and educational history to promote greater adherence to treatments [26].
Moreover, a range of non-AIDS-related conditions such as cardiovascular disease, cancer, kidney disease, liver disease, osteopenia/osteoporosis, neurocognitive disease, and mood disorders may also occur [25, 31]. For this reason, it is essential to monitor the neurocognitive status, and also the mental health of patients undergoing HAART for possible comorbidities, which may require a change in medication [26, 31, 32].
Different psychological aspects have been investigated in terms of predisposing characteristics of individuals to risk behaviors, adherence or non-adherence to treatment, and impact on QoL [31, 33]. For instance, influencing affective regulation processes and illness-related behaviors, alexithymia might have an impact on the motivation to adhere to treatments. Motivation is a common aspect of several behavioral adherence models studied in HIV [34,35,36].
Finally, particular attention has also been paid to neuropsychological issues, highlighting the prevalence of HIV-associated neurocognitive disorders (HAND) and neurophysiological aspects that could explain the causes of certain cognitive symptoms, such as fatigue and memory difficulty [37].
The brain regions, whose malfunctioning could be associated with HAND [38], might be the same areas implicated in deficits in emotion recognition and regulation.
These neuropsychological aspects appear to be associated with worse physical and mental health outcomes in PLWH [39, 40]. Indeed, damage to the frontostriatal network, including the prefrontal and anterior cingulate cortices, have been observed in HIV infection [38]. Similarly, some studies have shown that alexithymia might be related to damage in the same brain areas [8, 38].
Given these premises, this literature review aims to systematically identify studies that investigated alexithymia among PLWH and its association with other clinically significant aspects of HIV, synthesizing the results on this issue. In this way, the relevance of alexithymia in this medical condition could also be clarified, providing pointers for future research.
Methods
Protocol
To provide an overview of the findings on alexithymia in PLWH, a scoping review was conducted in line with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols extension for Scoping Review (PRISMA-ScR). A scoping review summarizes the findings related to constructs examined using heterogeneous methods and identifies aspects that future research should focus on [41, 42].
Eligibility Criteria
The PICOS scheme (participants (P), interventions (I), comparisons (C), outcomes (O), and study designs (S)) [43] was used to further define our study inclusion criteria. Studies were included if they reported data on adult PLWH, asymptomatic and symptomatic (P); those who received any type of treatment provided for this disease (I); and those with or without comparison groups (C) (i.e., healthy controls tor subgroups of patients with other diseases in comorbidities with HIV). The presence of alexithymia and its implications for mental and physical well-being has been considered as the outcome (O). Cross-sectional and longitudinal research was considered for this review (S).
This review identified academic articles that aimed to provide a measure of alexithymia [2,3,4]. All types of peer-reviewed research papers (original articles, brief reports, commentaries, letters to editors, and reviews) published in English were eligible for inclusion in this review. The exclusion criteria were as follows:
-
Papers did not focus on the implication of alexithymia.
-
Papers that did not include validated measures to investigate alexithymia.
-
Studies that used ad hoc surveys or qualitative methods.
-
Studies that have reported data for a general sample of patients with various infectious diseases, including HIV, but did not provide specific data for PLWH.
-
Papers that did not contain research data (i.e., case reports and study protocols) or did not contain complete information, such as meeting abstracts.
-
Papers published, but not peer-reviewed (i.e., gray literature) or under review at the time the search was carried out.
Information Source and Search Strategy
A literature search was conducted on November 26, 2021, in the following bibliographic databases: PubMed, PsycINFO, and Web of Science. The databases were queried using the following strings (using Boolean operators): (“alexithymia” OR “alexithymic”) AND (“HIV” OR “Human Immunodeficiency Virus”). Using this search string, 82 records were identified between 1990 and 2021 (see Fig. 1 for a flow diagram of article selection). Reports were also extracted using cross-references; however, no additional articles were found.
Studies Selection
The studies were selected by two authors (AB and AR). First, the two authors skimmed the articles by their titles and abstracts. Second, they read the full texts of the selected articles.
After the initial search, a literature search was performed again, following the steps described in the previous section, to ensure that no records were missed and/or excluded during the selection process.
Disagreements on the inclusion or exclusion of publications were discussed by all authors until an agreement was reached.
Data Extraction
All authors contributed to determine the information extracted from the studies. Two reviewers independently tracked the data and discussed the results interactively. Data items that were extracted from each included study, taking into consideration: were authors, year of publication, participants (sex and mean age), mean score and/or prevalence of alexithymia, other variables (i.e., sociodemographic, clinical, and psychological variables), neurocognitive and psychological measures used, and the main results of the studies.
The studies included in this review were grouped according to the different variables involved and associated with alexithymia. In particular, medical, psychological and neurocognitive aspects were considered.
Results
A summary of the main characteristics and findings of the 14 included studies is provided in (Table 1). The selected articles were published between 1997 and 2019. Three of these studies were conducted in the USA, five in Italy, one in Japan, one in Australia, and four did not specify the nationality of the sample.
Most of the articles had a cross-sectional study design (n = 10); all, except one [44], used biological markers; and only one [45] used functional magnetic resonance imaging (fMRI). Nine studies used TAS-20 [44,45,46,47,48,49,50,51,52], three studies used TAS-26 [53,54,55], one study used only two subscale of TAS-20 [56] and one study used a modified version of BIQ [57].
In regards to participant characteristics, the mean age of PLWH was between 34.9 [57] and 47.5 years [46], and in all studies, the sample was predominantly male. With reference to alexithymia, the prevalence among PLWH ranged from 10% [47] to 24.6% [55].
The three article study categories are presented below, separately. Note that two studies [47, 53] will appear in more than one category as they explore different variables.
Alexithymia, Disease Severity, and Neurobiological Markers Among PLWH
Among the studies that investigated the presence of alexithymia in PLWH, ten articles included both biological and psychological variables.
In the study by Lumley et al. [48], conducted on a sample of 87 PLWH, the authors related alexithymia to both subjective reports and biomedical measures of HIV. The sample had a mean age of 38.9 years and 94% were men. They found that the TAS-20 total score (r = .21; p = .05) and DIF subscale score (r = .31; p = .004) correlated positively with HIV symptoms (e.g., persistent fatigue, fever, night sweats, vision problems, rash, cough, cold/flu symptoms, muscle pain, and appetite loss) and negatively with age and income (statistical results are not available). However, no association was observed between TAS-20 total score and CD4 levels.
Conversely, in the study of Fukunishi et al. [57], conducted on a sample of 81 PLWH (mean age 34.9; 81.5% men) compared to a control healthy group (HC), a significant and negative correlation was found between CD4 cell counts and BIQ (higher scores of BIQ mean greater level of alexithymia). After dividing the sample into three subgroups based on CD4 count (Group A = > 500/ul; Group B = > 200 and < 500/ul; and Group C = < 200/ul), a post hoc t test, after the analysis of variance, showed that scores on two subscales of BIQ were significantly higher for the Group A than for the Group C (Affect Awareness, t = 2.97, p < .05; and Operational Thinking, t = 2.81, p < .05). Moreover, the authors found that PLWH showed higher levels scores both in Affect Awareness subscale (t = 2.31; p = .02) and Operational Thinking subscale (t = 2.27; p = .02) than HC. Moreover, Affect Awareness subscale was significantly and negatively correlated with the Utilization (r = − .47; p < .05) and Perception subscales (r = − .49; p < .05) of Social Support. Likewise, Operational Thinking subscale was significantly and negatively correlated with the Utilization (r = − .45; p < .05) and Perception subscales (r = − .48; p < .05) of Social Support.
The correlation between alexithymia and HIV clinical markers was investigated approximately two decades later in a longitudinal study by McIntosh et al. [55]. The authors examined the possible effect of alexithymia on HIV viral load and on changes in psychological distress in a sample of 123 PLWH (65.2% men) with a mean age of 37.9 (9.2) years. They found an indirect effect of baseline TAS-26 total score on 2-year viral load, mediated by an increase in distress levels over time. Specifically, the model showed that greater levels of TAS-26 predicted higher levels of psychological distress both at the baseline (β = 0.58, p < .001) and over time (β = −0.34, p = .04). Evidence of statistical mediation was further supported by a significant indirect effect of TAS-26 on 2-year viral load as a function of greater initial levels of psychological distress (β = 0.16, p = .029). The final model, controlling for the significant covariate (i.e., baseline viral load, baseline CD4 T-helper count, medication adherence leading up to 4 days before the final time point, age, female gender, and African-American ethnicity), showed acceptable fit (CFI = 0.92; RMSEA = 0.08) and explained 33.3% of the variance in two-year viral load.
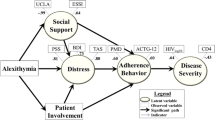
Alexithymia as a predictor of disease severity was also investigated in a study by McIntosh et al. [54] conducted on 439 PLWH (73.8% men) with a mean age of 39.2 (8.5) years. The authors explored, through a complex model, the relationship between alexithymia, psychological distress, social support, and non-active patient involvement in non-adherence to antiretroviral therapy (ART) and the relationship between non-adherence behavior and HIV disease severity. The main results showed that multiple paths within the proposed model were statistically significant: greater scores of TAS-26 were linked to lower social support (b = − 0.50, z = − 0.13.02, p < .001), greater psychological distress (b = 0.27, z = 5.36, p = < 0.001), and non-active patient involvement (b = 0.13, z = 2.85, p = < 0.01). Less active involvement in the doctor-patient relationship (b = 0.14, z = 2.72, p < .05), and greater psychological distress (b = 0.28, z = 4.51, p < .05) were associated with non-adherence behavior. In turn, poorer adherence behaviors were linked to greater disease severity (b = 0.50, z = 4.65, p < .01). The model exhibited adequately fits accounting for significant paths between the covariates of interest with observed variables and latent factors (CFI = 0.97, RMSEA = 0.04, SRMR = 0.05). Finally, the sum of all indirect paths from alexithymia to HIV disease severity was significant (b = 0.01, z = 2.60, p < .01).
Another study of McIntosh et al. [53] compared neurocognitive, neuroendocrine (assay of urinary cortisol and urinary norepinephrine), psychological variables (anxiety, depression, perceived stress), and disease progression markers (e.g., viral load) in 172 PLWH (73.2% men). The sample was divided into two groups based on alexithymia scores: 93 participants (mean age 38.7 years) with high and 79 participants (mean age 39.7 years) with low TAS-26 scores. As regards to the biological and psychological variables examined, results showed that PLWH with greater score of TAS-26 reported higher levels of stress (t = -9.5, p < .001), anxiety (t = -10.2, p < .001), and depression (t = -6.8, p < .001), a profile of higher norepinephrine to cortisol ratio (t = -2.7, p < .01) and higher HIV viral load (t = -2.1, p < .05), than participants with low TAS-26 scores. The results regarding the cognitive function will be described in more detail in the section in charge.
Landstra et al. [56] conducted a prospective longitudinal study of 291 men who have sex with men (MSM) with HIV, with a mean age of 51(9) years, undergoing anal cytological screening. They explored the link between the Identifying and describing feelings (DIDF; measured by DIF and DDF of TAS-20), psychological flexibility (PF), and mental health (depression, anxiety, and stress). When levels of baseline mental health were controlled, greater DIDF predicted increases depression (b = 0.34, p < .001), anxiety (b = 0.36, p < .001) and stress (b = 0.38, p < .001) and decreased physical QoL (b = − 0.22, p < .05). The link between PF and mental health was entirely mediated by DIDF (for more statistical data, please see the figure and table of the original paper).
Cappabianca et al. [44] explored the level of alexithymia in a total sample of 153 patients. In particular, the sample was composed of 70 PLWH (73% men) with a mean age of 46.5 (12.3) years, 26 coinfected people (HIV and Hepatitis C Virus - HCV) and 57 people with HCV. The 14.3% of PLWH had alexithymia. They found the coinfected group showed a higher score of TAS-20 than the PLWH group (p = .04). There were no significant correlations between alexithymia and socio-demographic variables both in PLWH and coinfected group. The 48.6% of PLWH had communicated with their relatives about the concerns related to the disease. There was no significant difference in the degree of alexithymia between the subjects who declared to communicate with relatives and those who did not.
The association between adherence and alexithymia was explored in a study conducted by Sofia et al. [49] on a sample of 100 PLWH (81% men) with a mean age of 45.11 (10.19) years. Particularly, this study investigated the prediction that patients who adhered or did not adhere to ART, had different levels of alexithymia, emotional recognition, emotional inhibition and emotional regulation. The authors classified participants as adherent to ART using two different methods (self-reported adherence and viral load) and then classified them into four groups: adherent by both measures, adherent only by viral load, adherent only by self-report, and non-adherent by both measures. The non-adherent by both measures group had a lower score of TAS-20 (p < .05; F/χ2 = 3.67) as well as lower emotional recognition scores (p < .05; F/χ2 = 3.94) than the adherent by both measures group.
Furthermore, to examine the relationship among TAS-20, emotional recognition, emotional inhibition, emotional regulation scores, and years of infection with the different measures of adherence separately, discriminant function analyses were performed to predict group members. Predicting categorization on the basis of viral load, the model was significant (Wilks’ λ = 0.932, χ2 = 6.848, df = 1, p < .01), and only the TAS-20 made a significant contribution to this prediction. Of the cases, 72% were correctly classified into their original categories.
The presence of alexithymia in HIV infection has been studied differently by Clark et al. [45] in an fMRI study. This study was conducted on 28 PLWH and 25 healthy adults. Participants were divided into two groups based on the high or low presence of early life stress (ELS). The PLWH High-ELS group had a mean age of 49.0 (10.2) years and 53.8% were men. The PLWH Low-ELS group had a mean age of 46.3 (8.9) years, and 60% were men.
The study aimed to investigate the independent and combined effects of HIV status and exposure on the amygdala activity. Secondly, it aimed to explore whether abnormalities in the amygdala reactivity are associated with elevated neuropsychiatric symptoms (including alexithymia).
Analyses revealed that the PLWH High-ELS group reported significantly higher neuropsychiatric symptom levels than HC High-ELS (t = 2.16, p = .04, Cohen’s d = 0.94). Furthermore, PLWH High-ELS participants reported significantly higher levels of depression (t[16.8] = 3.74, p < .01, Cohen’s d = 1.48), anxiety (t[13.1] = 2.27, p = .04, Cohen’s d = 0.92), apathy (t= 2.08, p = .05, Cohen’s d = 0.80), and alexithymia (t = 3.03, p < .01, Cohen’s d = 1.15) than PLWH Low-ELS participants. Finally, ELS-related abnormalities in the amygdala activity were significantly correlated with higher levels of alexithymia (t = -2.99, p = .01) in PLWH.
Immune parameters are other interesting markers related to alexithymia. Temoshok et al. [50] explored the association between alexithymia, type C coping (intended as a maladaptive coping pattern characterized by a lack of emotional expression and communication of emotions and needs), cardiovascular reactivity, recovery from stress, and relevant immune parameters (the b-chemokines macrophage inflammatory protein (MIP-1α/b) and interleukin 6 (IL-6), involved in HIV progression. The sample of 200 PLWH (48.7% men) had a mean age of 44.5 (25 − 6) years.
In short, after adjustment for age, CD4 + count, methadone use, and cardiovascular medications, TAS-20 score was associated significantly with MIP-1α (b = 0.304, R2 = 0.09, p < .001) but not with MIP-1b or IL-6 production. Moreover, Type C coping and alexithymia were not significantly correlated. Finally, there were no significant correlations between heart rate reactivity and recovery, and alexithymia.
Alexithymia and Cardiovascular Disease Among PLWH
Three articles that explored the association between cardiovascular diseases (CVD) and alexithymia in PLWH were found. Parruti et al. [51] evaluated the predictors of increased intima media thickness of carotid arteries (c-IMT) and carotid plaque(s) (CPs) in a sample composed of 201 PLWH (76% men) with a mean age of 45.1 (10.1) years. Among the psychological variables, depression and type-D personality traits were assessed. A linear regression analysis showed that increasing age (b = − 0.086, p < .001), total cholesterol (b = − 0.022, p = .001), and alexithymia (b = − 0.164, p = .032) remained significantly associated with c-IMT. Moreover, a logistic regression showed that increasing age (OR 1.72, 95% CI: 1.36–2.19, p = .001), total cholesterol (OR 1.10, 95% CI:1.05–1.16, p < .001), current smoking (OR 2.74; 95% CI: 1.16–6.44, p = .021), and TAS-20 (OR 2.63; 95% CI:1.17–5.89, p = .019) remained significantly associated with CPs (threshold ≥ 1.5 mm). Finally, in the Cox proportional hazards model, age (HR, 1.55, 95% CI: 1.19–2.02, p = .001), current smoking (3.87, 95% CI: 1.25–12.0, p = .019) and TAS-20 (5.58, 95% CI: 1.44–21.6, p = .013) were the only factors significantly associated with vascular events.
A few years later, Vadini et al. [52] conducted a cross-sectional analysis to evaluate the predictors of CPs and a prospective cohort analysis of 712 PLWH (mean age of 46.1(10.1) years, 75.3% men) to explore the determinants of vascular events (VEs) and all-cause mortality (ACM) during the 10-year observation period.
Multivariable logistic regression analyses were used to evaluate potential independent predictors of the presence of CPs. They defined the regression model including a priori several potential confounders (age, gender, body mass index, smoking, hypertension, diabetes, one among total cholesterol, physical activity, alcohol/drug abuse, educational level, and infection duration), because of their known association with CPs, and including other eventually significant variables, which were selected using a stepwise forward process.
Among the predictors, only male sex, increasing age, total cholesterol, hypertension, years lived with HIV, and alexithymia remained significantly associated with CPs. In particular, subjects with a TAS-20 score > 50 (presence of alexithymia) consistently showed an OR of 4.9 (95% CI: 2.9–8.5; p < .001) of having CPs.
The incidence rate ratio of VEs was 5.8 (95% CI: 2.9–11.7) per 100 person-years among patients with alexithymia versus patients without alexithymia. In the Cox proportional hazard model, age (HR, 1.07; 95% CI: 1.02–1.10; p = .002), current smoking (HR, 2.02; 95% CI: 1.01–4.04; p = .044), hypertension (HR, 2.23; 95% CI: 1.15–4.32; p = .017), and TAS-20 (HR, 3.66; 95% CI: 1.80–7.44; p < .001) were the only significantly associated factors.
The incidence rate of ACM was 6.2 (95% CI: 2.7–15.7) per 100 person-years in patients with alexithymia. In the Cox proportional hazard model, age (HR, 1.05; 95% CI: 1.0–1.08; p = .023), alcohol abuse (HR, 2.52; 95% CI: 1.09–5.81; p = .030), AIDS diagnosis (HR, 1.96; 95% CI: 0.98–3.93; p = .05), suboptimal HAART adherence (HR, 2.62; 95% CI: 1.14–6.0; p = .022), and TAS-20 (HR, 4.93; 95% CI: 1.64–9.00; p = .002) were the only factors significantly associated with ACM.
Finally, the study of Ciccarelli et al. [47] explored the prevalence of alexithymia and its association with cardiovascular risk factors and cognitive impairment in 140 PLWH (47 median age; 72.8% men) undergoing antiretroviral therapy compared to 35 healthy subjects matched for age, education, and gender.
Authors reported that PLWH and HC did not differ in TAS-20 total score. Regarding cardiovascular factors, participants with alexithymia had a higher prevalence of diabetes (21% vs. 3%, p = .035) and hypertension (36% vs. 13%, p = .037) than participants without alexithymia. The results regarding cognitive function will be described in more detail in the next section.
Alexithymia and Cognitive Function Among PLWH
A large body of research has highlighted the presence of impaired cognitive function in patients with HIV, but only three studies have focused on its association with alexithymia.
The study of Ciccarelli et al. [47] (the description of sample and aim is in the paragraph above) found that the proportion of individuals meeting research criteria for HAND was higher in the group with alexithymia compared to the one without alexithymia (57% vs. 26%, p = .021). Moreover, participants with alexithymia showed worse performance on psychomotor speed functioning compared to individuals without alexithymia (50% vs. 11%, p = .003), while the two groups did not show a significant difference regarding cognitive performance in other domains. At multivariate analysis, TAS-20 score (OR 1.08; 95% CI: 1.00-1.17; p = .037) showed an independent association with a higher risk of an abnormal psychomotor speed.
Bogdanova et al. [46] examined the association between alexithymia and cognitive function in 34 PLWH (mean age 47.5; 73.5% men) in early asymptomatic stage (“all PLWH were Stage A as determined by the CDC (1993) guidelines”) and compared them to 34 HC adults matched for socio-demographic variables.
All participants completed measures of alexithymia, apathy, depression, and a neuropsychological battery (attention, executive function, visuospatial ability, naming abilities, and story memory).
Alexithymia was present in 20% of PLWH, while no presence of alexithymia was found in HC. The TAS-20 total score was significantly and negatively correlated with scores on measures of attention and working memory (digit span r = − .36, p < .025 and spatial span r = − .44, p < .025), category fluency (animals r = − .43, p < .025), spatial reasoning (RCPM r = − .45, p < .025) and visuospatial organization (BVSQB Draw r = − .39, p < .025; BVSQB Copy r = − .42, p < .025).
Furthermore, TAS-20 correlated significantly with several subscales on the HIV MOS self-report measure: General Health [r(34) = − 0.46, p < .009], Cognitive Function [r(34) = − 0.62, p < .0001], and Health Distress [r(34) = − 0.57, p < .001].
Consistent with Bogdanova et al. [46], the study of McIntosh et al. [53] (the description of sample and aim is in the paragraph above) found a significant association between alexithymia and neurocognitive function.
Results showed that higher scores on the TAS-26 were associated with worse performance on the executive task-switching measure of the Trails Making Test (r( )= 0.40, p < .01). In particular, the difference in the trial time of completion (B-A) was significantly higher in the high alexithymia group than in the low alexithymia group (t(33) = 2.07, p < .05).
The regression model demonstrated that greater score in difficulty describing feelings (DDF, TAS-26) predicted greater difference in time to completion between the TMT-B and TMT-A, (b = 2.18, p < .01). The general linear model, accounting for the effects of demographic variables (age, gender, ethnicity, education, income), disease severity, and DDF on TMT B–A difference time of completion, is significant (F(12,171) = 2.04, p < .05) and explains the 13% of the variance.
Discussion
The present scoping review of 14 articles provided an overview about the possible association between alexithymia and medical, biological, psychological and neuropsychological variables in PLWH. Specifically, ten studies showed that alexithymia is associated with both disease severity and adherence to HAART. Three studies showed a relationship between alexithymia and CVD, and three studies highlighted a relation between alexithymia and cognitive impairment.
Whether or not alexithymia plays a direct role in HIV disease severity is still an open question, and studies have provided different data in this field.
For example, difficulty in identifying feelings was positively correlated with HIV symptoms [48], and a significant and negative association was found between alexithymia and CD4 levels [57] and with some immune parameters (MIP-1α) [50]. Research on HIV pathogenesis has focused on the balance between cytokines that either stimulate (inflammatory cytokines) or inhibit (b-chemokines) HIV replication in vivo [58]. An increased production of these b-chemokines (MIP-1α/b) is associated with a more favorable clinical status, disease-free HIV infection, and protection from infection [59, 60].
Furthermore, in one study no association was found between alexithymia and viral load [48], while in another one emerged an indirect association between alexithymia and HIV disease progression, mediated by psychological distress [55]. This result is not surprising, considering that alexithymia and depression are two constructs that are closely associated both in the general population and in chronic diseases [11, 61].
Moreover, a large body of literature has shown that depression leads to worse clinical outcomes in terms of decreased CD4 T-cells, increased viral load, and a greater risk of mortality [62, 63]. It is well known that in depression there is a hyperfunctioning of the hypothalamic–pituitary–adrenal axis, which leads to increased secretion of cortisol. Elevated cortisol alters the T lymphocyte production of cytokines, which in turn might accelerate HIV disease progression by triggering the destruction of CD4 lymphocytes and stimulating virus replication [59, 60].
Alexithymia, as seen so far, appears to be linked not only with biological marker changes but also with HIV-related neurological alterations.
For example, the findings of Clark et al. [45], showing that low amygdala activation levels correlated significantly with higher levels of alexithymia, are relevant in light of previous studies that have observed significant associations between volumetric abnormalities in affective brain regions and markers of historical HIV disease severity [64, 65].
The severity of HIV disease assumes a different meaning in the HAART era due to its close association with patient adherence behavior. Adherence to HAART is an important health behavior and it is related to a reduced chance of drug resistance and lower viremia in PLWH. Different psychosocial factors have been associated with non-adherence behavior [32, 35]. Among these factors, alexithymia seems to have both a direct [49] and indirect [54] association with adherence behavior. In particular, alexithymia is indirectly positively associated with doctor-patient relationship that in turn influenced the non-adherence behavior. [54].
Therefore, these findings also underlined the role of interpersonal communication in adherence behavior. Accordingly, PLWH were found reluctant to communicate with friends and family about their disease concerns [44]. Indeed, not only PLWH experienced lower levels of perceived social support than healthy people, but PLWH with high levels of alexithymia tended also to avoid the use of social relationships as a personal resource to cope with their disease condition [57].
Another interesting topic that this scoping review has addressed is the association between alexithymia and CVD in PLWH.
Although it is well known that PLWH have a higher risk of CVD than the general population [66], the link between psychological factors and CVD has been poorly examined. Previous studies shed light on the possible role of alexithymia in cardiovascular risk in PLWH [47, 51, 52].
Alexithymia is associated with cardiovascular events [51, 52], and with a higher risk of diabetes and hypertension [47]. In line with the available literature, these findings showed that the presence of alexithymia increases the likelihood of cardiovascular problems, even in healthy populations [67, 68].
Cognitive impairment is the most common central nervous system consequence of HIV infection [69]. Although previous studies have shown that the prevalence of HIV-associated dementia and minor cognitive motor disorders has significantly declined in the HAART era, these conditions still represent important cerebral HIV complications [70].
Regarding the association between alexithymia and cognitive functioning, there were significant correlations between alexithymia and performance on several measures of cognitive functioning in PLWH.
Alexithymia is significantly associated with worse performance in psychomotor speed [47], attention and working memory, category fluency, spatial reasoning, and visuospatial organization [46]. Moreover, difficulty in describing feelings is a significant predictor of executive dysfunction [53]. Accordingly, the literature has shown that alexithymia is associated with less efficient executive function also in the general population [71, 72].
In conclusion, the present scoping review showed significant association between alexithymia and disease severity, adherence behavior, CVD, and cognitive impairment in PLWH.
Moreover, it is interesting to note that this review showed the use of the TAS as the most widely used tool for measuring alexithymia even among PLWH.
These results should also be interpreted with caution owing to the limitations of the included studies. First, most studies adopted a cross-sectional design, which does not allow the establishment of any causal direction between alexithymia and HIV-related diseases. Second, only four studies recruited a group of healthy controls as a comparison for PLWH. Third, most of them recruited a sample with a high prevalence of males, which does not allow the generalization of the results to the female population. Finally, only eight studies investigated the association between alexithymia and other psychological constructs in PLWH.
Despite these limitations, the present scoping review represents, to the best of our knowledge the first contribution to the summary of available evidence on the involvement of alexithymia in HIV infection and HIV-related diseases. However, further evidence is necessary to replicate and confirm these results.
Future research should implement new directions to clarify the role of alexithymia in HIV disease through longitudinal studies, and allowing comparison with control groups. Furthermore, even though among PLWH, 53% are female [29], it seems surprising that studies have so far focused more on the male population. It would be useful to improve gender heterogeneity of the sample in the future.
Furthermore, it could be interesting to investigate the association between alexithymia and other psychological constructs such as attachment style, personality characteristics, and emotional regulation, which, as widely suggested in the literature, are correlated with alexithymia [14, 31, 33].
From a clinical perspective, careful clinical assessment of the emotional regulation process of PLWH can provide relevant and useful prognostic information. Identifying the different pathways through which alexithymia is related with the severity of the disease, makes it possible to detect subjects most likely to develop a worse clinical status. Consequently, providing psychological treatments, in addition to the “treatment as usual”, focused on the emotional regulation process, could help PLWH improve their socio-cognitive ability, maintain adherence behavior, and enhance their quality of life.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Bagby RM, Parker JDA, Taylor GJ. Twenty-five years with the 20-item Toronto Alexithymia Scale [published online ahead of print, 2020 Jan 23]. J Psychosom Res. 2020;131:109940. https://doi.org/10.1016/j.jpsychores.2020.109940.
Sifneos PE. The prevalence of ‘alexithymic’ characteristics in psychosomatic patients. Psychother Psychosom. 1973;22(2):255–62. https://doi.org/10.1159/000286529.
Nemiah JC, Freyberger H, Sifneos PE. Alexithymia: A view of the psychosomatic process. In: Hill OW, editor. Modern trends in psychosomatic medicine. 3th vol. London: Butterworths; 1976. pp. 430–9.
Sifneos PE. Alexithymia, clinical issues, politics and crime. Psychother Psychosom. 2000;69(3):113–6. https://doi.org/10.1159/000012377.
Taylor GJ, Bagby RM, Parker JD. The alexithymia construct. A potential paradigm for psychosomatic medicine. Psychosomatics. 1991;32(2):153–64. https://doi.org/10.1016/s0033-3182(91)72086-0.
López-Muñoz F, Pérez-Fernández F. A History of the Alexithymia Concept and Its Explanatory Models: An Epistemological Perspective. Front Psychiatry. 2020;10:1026. https://doi.org/10.3389/fpsyt.2019.01026. Published 2020 Jan 31.
Taylor GJ, Bagby RM. Psychoanalysis and empirical research: the example of alexithymia. J Am Psychoanal Assoc. 2013;61(1):99–133. https://doi.org/10.1177/0003065112474066.
Messina A, Beadle JN, Paradiso S. Towards a classification of alexithymia: primary, secondary and organic. J Psychopathol. 2014;20:38–49.
Jørgensen MM, Zachariae R, Skytthe A, Kyvik K. Genetic and environmental factors in alexithymia: a population-based study of 8,785 Danish twin pairs. Psychother Psychosom. 2007;76(6):369–75. https://doi.org/10.1159/000107565.
Lumley MA, Neely LC, Burger AJ. The assessment of alexithymia in medical settings: implications for understanding and treating health problems. J Pers Assess. 2007;89(3):230–46. https://doi.org/10.1080/00223890701629698.
Di Tella M, Castelli L. Alexithymia in Chronic Pain Disorders. Curr Rheumatol Rep. 2016;18(7):41. https://doi.org/10.1007/s11926-016-0592-x.
Carrozzino D, Porcelli P. Alexithymia in Gastroenterology and Hepatology: A Systematic Review. Front Psychol. 2018;9:470. https://doi.org/10.3389/fpsyg.2018.00470. Published 2018 Apr 6.
De Vries AM, Forni V, Voellinger R, Stiefel F. Alexithymia in cancer patients: review of the literature. Psychother Psychosom. 2012;81(2):79–86. https://doi.org/10.1159/000330888.
Evren C, Cagil D, Ulku M, et al. Relationship between defense styles, alexithymia, and personality in alcohol-dependent inpatients. Compr Psychiatry. 2012;53(6):860–7. https://doi.org/10.1016/j.comppsych.2012.01.002.
Lemche AV, Chaban OS, Lemche E. Alexithymia as a risk factor for type 2 diabetes mellitus in the metabolic syndrome: a cross-sectional study. Psychiatry Res. 2014;215(2):438–43. https://doi.org/10.1016/j.psychres.2013.12.004.
Mattila A. Alexithymia in Finnish general population. Tampere University Press; 2009.
Taylor GJ, Bagby RM, Luminet O. Assessment of alexithymia: Self-report and observer-rated measures. In: Bar-On R, Parker JDA editors. The handbook of emotional intelligence. San Francisco: Jossey-Bass, 2000. Pp. 301 – 19
Vorst HCM, Bermond B. Validity and reliability of the Bermond–Vorst alexithymia questionnaire. Pers Individ Differ, 2001;30(3):413–434. https://doi.org/10.1016/S0191-8869(00)00033-7.
Preece D, Becerra R, Robinson K, Dandy J, Allan A. The psychometric assessment of alexithymia: Development and validation of the Perth Alexithymia Questionnaire. Pers Individ Differ. 2018;132:32–44. https://doi.org/10.1016/j.paid.2018.05.011.
Taylor GJ, Ryan D, Bagby RM. Toward the development of a new self-report alexithymia scale. Psychother Psychosom. 1985;44(4):191–9. https://doi.org/10.1159/000287912.
Bagby RM, Taylor GJ, Parker JDA. Construct validity of Toronto Alexithymia Scale. Psychother Psychosom. 1988;50(1):29–34. https://doi.org/10.1159/000288097.
Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale–I. Item selection and cross-validation of the factor structure. J Psychosom Res. 1994;38(1):23–32. https://doi.org/10.1016/0022-3999(94)90005-1.
de Ridder D, Geenen R, Kuijer R, van Middendorp H. Psychological adjustment to chronic disease. Lancet. 2008;372(9634):246–55. https://doi.org/10.1016/S0140-6736(08)61078-8.
Fauci AS, Folkers GK. Toward an AIDS-free generation. JAMA. 2012;308(4):343–4. https://doi.org/10.1001/jama.2012.8142.
Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–33. https://doi.org/10.1016/S0140-6736(13)61809-7.
Eggleton JS, Nagalli S. Highly Active Antiretroviral Therapy (HAART). In: StatPearls. Treasure Island (FL): StatPearls Publishing; November 25, 2021.
Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS ONE. 2013;8(12):e81355. https://doi.org/10.1371/journal.pone.0081355. Published 2013 Dec 18.
WHO-World Health Organization. Health topic: HIV/AIDS. https://www.who.int/health-topics/hiv-aids#tab=tab_1. N.d. [Retrieved 2022 Feb 28].
HIV.gov. The Global HIV/AIDS Epidemic. https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics 2021 Nov 30. [Retrieved 2022 Aug 2].
WHO-World Health Organization. The global health observatory: HIV/AIDS. https://www.who.int/health-topics/hiv-aids#tab=tab_1 2022 July. [Retrieved 2022 Aug 2].
Remien RH, Stirratt MJ, Nguyen N, Robbins RN, Pala AN, Mellins CA. Mental health and HIV/AIDS: the need for an integrated response. AIDS. 2019;33(9):1411–20. https://doi.org/10.1097/QAD.0000000000002227.
Ortego C, Huedo-Medina TB, Llorca J, et al. Adherence to highly active antiretroviral therapy (HAART): a meta-analysis. AIDS Behav. 2011;15(7):1381–96. https://doi.org/10.1007/s10461-011-9942-x.
Farias OO, Alexandre HO, Lima ICV, Galvão MTG, Hanley-Dafoe R, Santos VDF. Attachment styles of People Living with HIV/AIDS. Cien Saude Colet. 2020;25(2):495–504. https://doi.org/10.1590/1413-81232020252.11852018.
Amico KR, Mugavero M, Krousel-Wood MA, Bosworth HB, Merlin JS. Advantages to Using Social-Behavioral Models of Medication Adherence in Research and Practice. J Gen Intern Med. 2018;33(2):207–15. https://doi.org/10.1007/s11606-017-4197-5.
Andrews L, Friedland G. Progress in HIV therapeutics and the challenges of adherence to antiretroviral therapy. Infect Dis Clin North Am. 2000;14(4):901–28. https://doi.org/10.1016/s0891-5520(05)70139-2.
Porcelli P, Taylor G. Alexithymia and Physical Illness: A Psychosomatic Approach. In: Luminet O, Bagby R, Taylor G. editors. Alexithymia: Advances in Research, Theory, and Clinical Practice. Cambridge: Cambridge University Press; 2018. pp. 105–26. https://doi.org/10.1017/9781108241595.009.
Muñoz-Moreno JA, Cysique LA, Rourke SB. Neuropsychiatric Disorders, Emotional Disturbances, and Their Associations with HIV-Associated Neurocognitive Disorder. Curr Top Behav Neurosci. 2021;50:347–66. https://doi.org/10.1007/7854_2021_233.
Baldonero E, Ciccarelli N, Fabbiani M, et al. Evaluation of emotion processing in HIV-infected patients and correlation with cognitive performance. BMC Psychol. 2013;1(1):3. Published 2013 Feb 27. https://doi.org/10.1186/2050-7283-1-3.
Brandt CP, Jardin C, Sharp C, Lemaire C, Zvolensky MJ. Main and interactive effects of emotion dysregulation and HIV symptom severity on quality of life among persons living with HIV/AIDS. AIDS Care. 2017;29(4):498–506. https://doi.org/10.1080/09540121.2016.1220484.
Heggeness LF, Brandt CP, Paulus DJ, Lemaire C, Zvolensky MJ. Stigma and disease disclosure among HIV + individuals: the moderating role of emotion dysregulation. AIDS Care. 2017;29(2):168–76. https://doi.org/10.1080/09540121.2016.1204419.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. Published 2021 Mar 29. https://doi.org/10.1136/bmj.n71.
Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–73. https://doi.org/10.7326/M18-0850.
Thomas J, Kneale D, McKenzie JE, Brennan SE, Bhaumik S. Chapter 2: Determining the scope of the review and the questions it will address. In Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated 2021 Feb). Cochrane. Available from www.training.cochrane.org/handbook. Accessed 10 November 2021.
Cappabianca A, Vitale M, Madonia S, et al. Alexithymia in HIV, HCV and coinfections. Infez Med. 2019;27(1):46–52.
Clark US, Sweet LH, Morgello S, Philip NS, Cohen RA. High early life stress and aberrant amygdala activity: risk factors for elevated neuropsychiatric symptoms in HIV + adults. Brain Imaging Behav. 2017;11(3):649–65. https://doi.org/10.1007/s11682-016-9542-5.
Bogdanova Y, Díaz-Santos M, Cronin-Golomb A. Neurocognitive correlates of alexithymia in asymptomatic individuals with HIV. Neuropsychologia. 2010;48(5):1295–304. https://doi.org/10.1016/j.neuropsychologia.2009.12.033.
Ciccarelli N, Baldonero E, Milanini B, et al. Cognitive impairment and cardiovascular disease related to alexithymia in a well-controlled HIV-infected population. Infez Med. 2019;27(3):274–82.
Lumley MA, Tomakowsky J, Torosian T. The relationship of alexithymia to subjective and biomedical measures of disease. Psychosomatics. 1997;38(5):497–502. https://doi.org/10.1016/S0033-3182(97)71427-0.
Sofia SA, Lysaker PH, Smith E, Celesia BM, Dimaggio G. Therapy Adherence and Emotional Awareness and Regulation in Persons With Human Immunodeficiency Virus. J Nerv Ment Dis. 2018;206(12):925–30. https://doi.org/10.1097/NMD.0000000000000901.
Temoshok LR, Waldstein SR, Wald RL, et al. Type C coping, alexithymia, and heart rate reactivity are associated independently and differentially with specific immune mechanisms linked to HIV progression. Brain Behav Immun. 2008;22(5):781–92. https://doi.org/10.1016/j.bbi.2008.02.003.
Parruti G, Vadini F, Sozio F, et al. Psychological factors, including alexithymia, in the prediction of cardiovascular risk in HIV infected patients: results of a cohort study. PLoS ONE. 2013;8(1):e54555. https://doi.org/10.1371/journal.pone.0054555.
Vadini F, Sozio F, Madeddu G, et al. Alexithymia Predicts Carotid Atherosclerosis, Vascular Events, and All-Cause Mortality in Human Immunodeficiency Virus-Infected Patients: An Italian Multisite Prospective Cohort Study. Open Forum Infect Dis. 2019;6(9):ofz331. https://doi.org/10.1093/ofid/ofz331. Published 2019 Jul 27.
McIntosh RC, Ironson G, Antoni M, Kumar M, Fletcher MA, Schneiderman N. Alexithymia is linked to neurocognitive, psychological, neuroendocrine, and immune dysfunction in persons living with HIV. Brain Behav Immun. 2014;36:165–75. https://doi.org/10.1016/j.bbi.2013.10.024.
McIntosh RC, Ironson G, Antoni M, Fletcher MA, Schneiderman N. Alexithymia. Assertiveness and Psychosocial Functioning in HIV: Implications for Medication Adherence and Disease Severity. AIDS Behav. 2016;20(2):325–38. https://doi.org/10.1007/s10461-015-1126-7.
McIntosh RC, Ironson G, Antoni M, et al. Psychological Distress Mediates the Effect of Alexithymia on 2-Year Change in HIV Viral Load. Int J Behav Med. 2017;24(2):294–304. https://doi.org/10.1007/s12529-016-9602-7.
Landstra JM, Ciarrochi J, Deane FP, Hillman RJ. Identifying and describing feelings and psychological flexibility predict mental health in men with HIV. Br J Health Psychol. 2013;18(4):844–57. https://doi.org/10.1111/bjhp.12026.
Fukunishi I, Hirabayashi N, Matsumoto T, Yamanaka K, Fukutake K. Alexithymic characteristics of HIV-positive patients. Psychol Rep. 1999;85(3 Pt 1):963–70. https://doi.org/10.2466/pr0.1999.85.3.963.
Garzino-Demo A, Moss RB, Margolick JB, et al. Spontaneous and antigen-induced production of HIV-inhibitory beta-chemokines are associated with AIDS-free status. Proc Natl Acad Sci U S A. 1999;96(21):11986–91. https://doi.org/10.1073/pnas.96.21.11986.
Cocchi F, DeVico AL, Yarchoan R, et al. Higher macrophage inflammatory protein (MIP)-1alpha and MIP-1beta levels from CD8 + T cells are associated with asymptomatic HIV-1 infection. Proc Natl Acad Sci U S A. 2000;97(25):13812–7. https://doi.org/10.1073/pnas.240469997.
Garzino-Demo A. Chemokines and defensins as HIV suppressive factors: an evolving story. Curr Pharm Des. 2007;13(2):163–72. https://doi.org/10.2174/138161207779313696.
Honkalampi K, Hintikka J, Tanskanen A, Lehtonen J, Viinamäki H. Depression is strongly associated with alexithymia in the general population. J Psychosom Res. 2000;48(1):99–104. https://doi.org/10.1016/s0022-3999(99)00083-5.
Hartzell JD, Janke IE, Weintrob AC. Impact of depression on HIV outcomes in the HAART era. J Antimicrob Chemother. 2008;62(2):246–55. https://doi.org/10.1093/jac/dkn193.
Arseniou S, Arvaniti A, Samakouri M. HIV infection and depression. Psychiatry Clin Neurosci. 2014;68(2):96–109. https://doi.org/10.1111/pcn.12097.
Clark US, Cohen RA, Sweet LH, et al. Effects of HIV and early life stress on amygdala morphometry and neurocognitive function. J Int Neuropsychol Soc. 2012;18(4):657–68. https://doi.org/10.1017/S1355617712000434.
Clark US, Walker KA, Cohen RA, et al. Facial emotion recognition impairments are associated with brain volume abnormalities in individuals with HIV. Neuropsychologia. 2015;70:263–71. https://doi.org/10.1016/j.neuropsychologia.2015.03.003.
So-Armah K, Benjamin LA, Bloomfield GS, et al. HIV and cardiovascular disease. Lancet HIV. 2020;7(4):e279–93. https://doi.org/10.1016/S2352-3018(20)30036-9.
Aluja A, Malas O, Urieta P, Worner F, Balada F. Biological correlates of the Toronto Alexithymia Scale (TAS-20) in cardiovascular disease and healthy community subjects. Physiol Behav. 2020;227:113151. https://doi.org/10.1016/j.physbeh.2020.113151.
Grabe HJ, Schwahn C, Barnow S, et al. Alexithymia, hypertension, and subclinical atherosclerosis in the general population. J Psychosom Res. 2010;68(2):139–47. https://doi.org/10.1016/j.jpsychores.2009.07.015.
Rackstraw S. HIV-related neurocognitive impairment–a review. Psychol Health Med. 2011;16(5):548–63. https://doi.org/10.1080/13548506.2011.579992.
Ferrando S, van Gorp W, McElhiney M, Goggin K, Sewell M, Rabkin J. Highly active antiretroviral treatment in HIV infection: benefits for neuropsychological function. AIDS. 1998;12(8):F65–70. https://doi.org/10.1097/00002030-199808000-00002.
Zhang L, Zhu C, Ye R, et al. Impairment of conflict processing in alexithymic individuals. Neurosci Lett. 2011;504(3):261–4. https://doi.org/10.1016/j.neulet.2011.09.043.
Santorelli GD, Ready RE. Alexithymia and Executive Function in Younger and Older Adults. Clin Neuropsychol. 2015;29(7):938–55. https://doi.org/10.1080/13854046.2015.1123296.
Acknowledgements
The authors would like to thank “Editage” for the English editing service.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: Annunziata Romeo, Agata Benfante; Supervision: Annunziata Romeo; Roles/Writing - original draft: Annunziata Romeo, Agata Benfante; Writing - review & editing: Annunziata Romeo, Agata Benfante.
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
The authors declare that they have no conflict of interest.
Ethics Approval
Not applicable.
Consent to Participate (include appropriate consent statements)
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Benfante, A., Romeo, A. Alexithymia Among People Living with HIV: A Scoping Review. AIDS Behav 27, 1926–1941 (2023). https://doi.org/10.1007/s10461-022-03926-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-022-03926-9