Abstract
This study examined feasibility, acceptability, and preliminary efficacy of an mHealth facilitated health coaching antiretroviral therapy (ART) adherence intervention. Persons living with HIV (n = 53) were randomized to an in-person adherence session and 12 months of app access and health coaching via the app (Fitbit Plus) versus single adherence session (SOC). At baseline and 1, 3, 6, and 12 months, we measured ART adherence, substance use, and depressive symptoms. We also conducted individual qualitative interviews. The intervention was found to be largely feasible and highly acceptable, with the health coach spending an average of 2.4 min per month with a participant and 76.5% of Fitbit Plus participants using the app regularly at 12 months. While most comparisons were not significant, the pattern of results was consistent with better adherence in the Fitbit Plus compared to SOC condition. Substance use was significantly associated with poorer ART adherence while depressive symptoms were not.
ClinicalTrials.gov Identifier: NCT02676128; Registered: 2/8/2016.
Resumen
En este estudio se examinó la viabilidad, aceptabilidad y la eficacia preliminar de una intervención de cumplimiento de la terapia antirretroviral (ART, por sus siglas en inglés) proporcionada por mHealth. Los pacientes con VIH (n = 53) fueron seleccionados al azar para participar en una sesión de cumplimiento presencial y para tener acceso a la aplicación y recibir asesoría médica a través de la aplicación (Fitbit Plus) durante 12 meses contra una sola sesión de cumplimiento (SOC, por sus siglas en inglés). Al comenzar y al mes 1, 3, 6 y 12, evaluamos el cumplimiento con la ART, el uso de sustancias y los síntomas de depresión; también realizamos entrevistas cualitativas individuales. Se encontró que la intervención es bastante viable y muy aceptable, con un promedio de 2.4 minutos de interacción entre el asesor médico y el participante y un 76.5% de uso de la aplicación por parte de los participantes de Fitbit Plus a los 12 meses. Si bien la mayoría de las comparaciones no fueron significativas, el patrón en los resultados fue consistente con un mayor cumplimiento en Fitbit Plus comparado con la condición SOC. El uso de sustancias se asoció significativamente con un cumplimiento de la ART más deficiente mientras que los síntomas depresivos no.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The CDC estimates that there are 1.1 million persons living with HIV (PLWH) in the United States [1], and thousands of new HIV transmissions occur each year [2]. Modern antiretroviral therapy (ART) is highly effective and allows PLWH to have longer, healthier lives [3, 4]. Despite the effectiveness of ART, only 60% of all PLWH successfully achieve viral suppression [5], and viral suppression rates among those engaged in treatment range from 81.5% to 86% [5, 6]. When examined over time, suppression can fluctuate for an individual [7,8,9]. For example, a study by Crepaz and colleagues [9] found that over 82.9% of their study sample of 265,264 PLWH were virally suppressed according to their latest viral load test, but only 62% sustained durable viral suppression over a two-year period. Even among those who are able to achieve viral suppression on two or more consecutive tests, roughly one-third have been found to experience virologic failure over the course of a 7-year study [10]. Viral suppression is significantly impacted by medication adherence; a meta-analysis found that only an estimated 63.4% of adults living with HIV achieve optimal adherence (defined as ≥ 90%) [11]. In a recently published study of ART adherence trends between 2001 and 2012, the proportion of individuals with adherence thresholds necessary for viral suppression never exceeded 60% despite improvements in adherence rates over that time period [12]. While newer ART medications can produce viral suppression at lower levels of adherence, relatively high adherence, in the range of 80–90%, is still necessary to avoid disease progression and shortened lifespan [11,12,13,14,15]. In addition, low levels of adherence can increase the risk of HIV transmission and can contribute to the development of treatment resistant strains of HIV [11, 14, 15].
Poor adherence to ART poses a significant public health problem. Therefore, considerable effort has been devoted to developing interventions to improve adherence. Several have found some degree of success at improving adherence [16, 17], with multi-component interventions demonstrating the greatest likelihood of improving ART adherence [18, 19]. However, traditional ART adherence interventions are limited in their ability to sustain behavior modification, with treatment effects diminishing over time [16, 17]. Furthermore, more intensive interventions that could potentially maintain improvements to adherence would require resources that most real-world clinical settings do not possess [17].
The need for more readily disseminable and efficient interventions that promote sustained improvements in ART adherence has sparked interest in developing efficacious mobile health (mHealth) ART adherence interventions. In some instances, mHealth interventions are an affordable and accessible means of delivering continued intervention [20]. Thus far, text message-based ART interventions have received considerable attention given that such interventions are among the least resource intensive mHealth interventions. A meta-analysis [21] observed a pattern of fairly modest but significant support for text message-based ART interventions in improving ART adherence. However, it is important to consider that many studies of this type of mHealth intervention reviewed in the meta-analysis by Finitisis [21] had relatively short follow-up periods, which is noteworthy given the well-established challenges of habituation and response fatigue among text message-based interventions [22].
There are currently hundreds of HIV-related mHealth apps marketed for PLWH available on Android and iOS platforms. mHealth interventions can be efficacious at improving ART adherence, treatment retention, and clinical outcomes, such as viral suppression [23, 24]. However, it is currently unclear which components of an mHealth intervention contribute to efficacy, and a majority of current mHealth interventions offer limited capabilities, such as functioning only as a medication reminder [23]. In addition, other content areas identified as important, such as resources for psychological and emotional support and enhanced linkage to treatment providers, are largely absent in existing apps [25]. Further, usage of mHealth apps typically declines across follow-up periods [24, 26]. In a recent prospective study of an mHealth app for PLWH, only 40% of participants were using the study mHealth app at 12 months [24]. Therefore, there is a need for more comprehensive mHealth interventions that are capable of addressing multiple self-management needs of PLWH and that simultaneously foster sustained usage.
Furthermore, a review of the ART adherence literature found that the utilization of treatment supporters resulted in better adherence [27]. Treatment supporters can be incorporated into clinical settings and can take the form of peer support sessions, home visits by nurses or counselors, case management, and provision of training in treatment support to a friend or family member of the patient. The importance of this type of adherence support has been found in other reviews of this literature as well [28, 29].
Health coaching represents a type of treatment support that has a patient-centered focus, fostering patient collaboration in the process of goal-setting [30]. Health coaches assist the patient in achieving a greater understanding of the patient’s medical condition and encourage patient accountability [30]. Recent reviews of the general literature regarding health coaching conclude that the approach shows great promise for improving health outcomes [31,32,33]. However, a majority of studies involving health coaching interventions examined outcomes for only a 6-month period; longer follow-up is needed [31].
Building on previous work in this area, the primary aim of this preliminary trial was to determine the feasibility, acceptability, and preliminary efficacy of an intervention that combines a single in-person ART adherence session with an mHealth facilitated health coaching intervention to improve ART adherence among PLWH, relative to a single in-person ART adherence session alone. Our in-person ART adherence session was based on LifeSteps [34, 35], which is grounded in the Information-Motivation-Behavioral Skills model of ART adherence [36] and combines motivational interviewing, cognitive-behavioral skills, and problem solving. The efficacy of LifeSteps for improving ART adherence is well-established [34, 35, 37, 38]. A secondary aim was to explore potential moderators of treatment effects.
Method
Overview
Details regarding the development of the intervention and study design have been published elsewhere [39]. In brief, the feasibility, acceptability, and preliminary efficacy of our intervention were tested in a parallel group, randomized controlled trial. Participants completed a baseline interview, followed by two weeks of baseline electronic pill box/bottle ART adherence monitoring. Following baseline data collection, participants were randomized, with a 1:1 ratio, to the Fitbit Plus condition (single face-to-face LifeSteps ART adherence session delivered by a health coach, followed by 12 months of access to an app and health coaching delivered via the app) or to the Standard of Care (SOC) condition (single face-to-face LifeSteps ART adherence session delivered by a health coach), which approximates the level of adherence assistance available in many HIV care settings. Participants completed follow-up interviews at 1, 3, 6, and 12 months.
Participants
The sample was recruited at a hospital-based HIV care clinic and through on-line advertisements in the northeastern United States.
Inclusion criteria were: (a) diagnosed with HIV; (b) prescribed ART for at least one month; (c) detectable viral load (> 20 copies/mL) in the past six months; (d) self-reported ART adherence of less than 100% in the past month; (e) at least 18 years of age; (f) have a smartphone that is compatible with the app used in the study. Exclusion criteria were: (a) physical or cognitive impairment that would prevent intervention completion or compromise informed consent or intervention comprehension; (b) active psychosis; (c) not fluent in English.
Measures
All measures were administered at baseline and at 1-, 3-, 6-, and 12-month follow-ups, with the exception of HIV viral load data which were collected only at baseline, 6 months, and 12 months.
Participant Characteristics
During the screening interview, information was collected regarding demographic characteristics (sex assigned at birth, gender identity, age, sexual orientation, race, ethnicity, relationship status, education level, and employment status). Characteristics used in the randomization process were assessed during the screening and baseline interviews. Blocking variables included years on ART, self-reported ART adherence in the past 30 days assessed using the Visual Analog Scale (VAS) [40], depressive symptoms assessed using the Center for Epidemiological Studies Depression Scale (CESD) [41], and substance use in the past three months, measured by the Timeline Followback (TLFB) [42].
Primary Outcomes
Given the preliminary nature of the study, the focus was on the feasibility and acceptability of the intervention and study protocol and the impact of the intervention on ART adherence measured by electronic pill boxes/bottles (EPB).
Feasibility
In order to evaluate the feasibility of our protocol, we examined the participant eligibility and study enrollment rates, number of participants recruited per month, follow-up completion rates, and health coach time spent per participant. In addition, participants in the Fitbit Plus condition completed a brief, semi-structured interview at the 12-month follow-up to evaluate the feasibility and acceptability of the intervention. Specifically, topics queried included general thoughts about the app, thoughts about the features of the app (medication reminders, secure messaging, adherence tracking, and appointment reminders), impact of having the same health coach interact with them via the app who conducted the LifeSteps session, and suggestions for improving the app.
Acceptability
Participants randomized to the treatment condition were asked to complete satisfaction questionnaires regarding the app at the 6- and 12-month follow-ups. Participants were asked about the extent to which the app helped them remember to take their ART (not at all, a little, somewhat, quite a bit, or a lot), how satisfied they were with the app (quite dissatisfied, indifferent or mildly dissatisfied, mostly satisfied, or very satisfied), and whether they would recommend the app to a friend living with HIV (no-definitely not, no-I don’t think so, yes-I think so, or yes-definitely). In addition, utilization data (months of regular app use, percentage of push notifications that resulted in a participant response, and percentage of health coach messages that received a participant response) were extracted from the app. In addition, follow-up completion rates were considered as metrics of acceptability, as well as feasibility.
EPB ART Adherence
Initially, study participants were provided with a MedSignals electronic pill box. However, we transitioned participants to the MEMS® electronic pill bottle when the MedSignals company ceased operations. No data were lost in this transition. Both devices measure daily ART adherence by recording the date and time that the device is opened. Participants were asked about any false positives and false negatives, i.e., times they opened the EPB but did not take the medication or times they took the medication but did not open the EPB. The self-report corrected EPB data constituted the main outcome variable for analysis.
Secondary Outcomes
Secondary outcomes were self-reported ART adherence and HIV viral load.
Self-reported ART Adherence
Self-reported ART adherence was assessed using the Three-Item Adherence Questionnaire developed by Wilson and colleagues [43], which was administered at baseline and at every follow-up. This measure has strong psychometric properties and is widely used in ART adherence research [43, 44]. As has recently been done by Wilson and colleagues [44], we created a composite adherence score by transforming the three items linearly to 0–100 scales (zero represents the worst adherence and 100 represents the best) and then averaging them together.
HIV Viral Load
HIV viral load (copies/mL) was examined as a biomarker of ART adherence. Blood samples were collected and tested by laboratories using standard procedures at the baseline interview and at 6- and 12-month follow-ups. The assays used by these laboratories had a lower level threshold of > 20 copies/mL. We considered < 200 copies/mL to represent viral suppression, which is a commonly used threshold for viral suppression [45]. While assays were able to detect viral load as low as 20 copies/mL, we chose < 200 copies/mL as our outcome cutoff since viral suppression at the level of < 200 copies/mL is more readily attained relative to a lower cutoff. Given our small sample size in this test of preliminary efficacy, we strove to maximize the chances of detecting an impact of the intervention on this measure. In addition, this viral load level may be more clinically meaningful in that it has well established associations with risk of transmitting HIV to others [46, 47] and with risk of virological failure [48, 49].
Potential Treatment Moderators
We examined depressive symptoms and substance use as potential moderators of treatment effects.
Depressive Symptoms
Depressive symptoms over the past week were measured using the 20-item Center for Epidemiologic Studies Depression Scale (CESD; [41]). This measure has high levels of validity, test–retest reliability, and internal consistency [50].
Substance Use
The Timeline Followback Interview for Alcohol and Drug Use (TLFB; [42]) was administered to collect daily information about number of standard drinks consumed and classes of drugs used each day for the three months prior to baseline and during the time period since the last interview at each follow-up. The TLFB measure has a high degree of reliability and validity, with coefficients usually above 0.85 [51]. Percentage of heavy drinking days (a day in which a female drank 4 or more drinks in one day or a male drank 5 or more drinks in one day) and percentage of drug use days (a day in which a participant reports using any drug on a given day) were calculated from this measure.
Procedures
All procedures were approved by the hospital’s Institutional Review Board. Study interviews and health coach sessions were conducted in a private room at a hospital-based HIV care clinic or in a private interview room at the hospital. All study data were collected with the REDCap secure electronic data capture system.
Recruitment
Participants were recruited through an HIV care clinic and from online advertising. A HIPAA waiver was obtained for research assistants to examine the clinic schedule to identify potentially eligible participants. When research assistants identified someone who appeared to meet eligibility criteria, they emailed that patient’s provider to get permission to contact them. With this permission, potential participants were called or approached in the clinic and given a brief description of the study, and those who expressed interest in the study were screened. Additionally, advertisements for the study were posted on Craigslist and Facebook with a link to a prescreening survey. Research assistants contacted people who filled out the online prescreening survey to administer the full screening instrument.
Screening Interview
Screenings were conducted either in person at the clinic or over the phone. The screening interview included demographics questions, an item regarding cell phone ownership and type of cell phone, questions about the timing of HIV diagnosis and ART initiation, the ART adherence VAS [40] (“From a scale of 0% to 100%, how adherent would you say you were to taking your medication every day at the time you were supposed to in the last 30 days?”), and the MINI International Neuropsychiatric Interview [52]. To verify capacity to consent, we also administered the University of California, San Diego Brief Assessment of Capacity to Consent [53]. Medical charts were reviewed for an HIV diagnosis and HIV viral load levels in the past six months, and a release of information was obtained for these data for potential participants who were not part of the involved health care system.
Baseline Interview
Potential participants deemed eligible after screening were provided with information regarding the purpose of the study, study procedures, and risks and benefits associated with participation. Interested individuals were asked to provide written informed consent. Following consent, a baseline interview was conducted, during which participants: 1) completed assessments, 2) provided a blood sample, 3) and were given an EBP in which to store their ART. At the completion of the baseline interview, participants were compensated for their time in the form of a gift card worth US $25. All participants returned two weeks later for a single face-to-face ART adherence session with the study health coach. At that time, EBP data for the initial baseline monitoring period were retrieved.
Randomization
Prior to meeting with the health coach for the single face-to-face ART adherence session, participants were randomly assigned using urn randomization on a 1:1 ratio to the Fitbit Plus or SOC condition. The blocking variables controlled for during randomization were baseline level of depressive symptoms (reported on the CESD, [41]), number of years on ART, self-reported percent adherence to ART (based on the VAS, [40]), and self-reported heavy drinking or drug use during the three months prior to baseline (based on TLFB, [42]) (See Table 1 for distribution of blocking variables between groups).The study coordinator performed all randomizations. Participants were notified whether or not they would be receiving access to the app at the start of the single in-person session.
Blinding
Research assistants, who administered all screening, baseline, and follow-up interviews, were blinded to treatment allocation.
Intervention
The same health coach delivered the intervention to participants in both conditions. The health coach has a master’s degree in psychology and has five years of experience in delivering brief, manualized interventions. Both intervention components (LifeSteps and health coaching delivered via the app for those in the Fitbit Plus condition) were manualized. LifeSteps sessions were audiotaped, and messages delivered via the app were recorded in the app. Dr. Ramsey, who is a licensed clinical psychologist, provided training and supervision in the intervention components to the health coach. Training included didactics and roleplaying. Weekly supervision sessions, during which audiotapes of the LifeSteps sessions and printouts of the health coaching messages delivered via the app were reviewed, ensured that the health coach adhered to the manuals.
The face-to-face ART adherence session with the health coach was adapted from the evidence-based LifeSteps [34, 35] and occurred two weeks after the baseline interview (following two weeks of EPB ART adherence monitoring). During the LifeSteps session, the health coach used techniques based on cognitive-behavioral therapy and motivational interviewing to help participants problem solve issues regarding adherence to ART and to the HIV treatment regimen more broadly. Participants in the SOC condition only received the single in-person session with the health coach. Participants assigned to the Fitbit Plus condition received the single in-person session with the health coach and also received access to the Fitbit Plus mobile adherence app and to health coaching delivered via the app for 12 months.
At the end of the in-person session, the health coach assisted Fitbit Plus participants with downloading Fitbit Plus to their phones, programed medication reminders, and provided instructions on how to use the app. Fitbit Plus provided a daily push notification medication reminder to participants. The medication reminder asked participants to indicate ‘Yes’ or ‘No’ to having taken their medication. The health coach was able to view these data in real-time on the health coach dashboard and used the dashboard to identify individuals in need of adherence support. Fitbit Plus also included a secure two-way messaging feature, which the health coach and participants used to message each other. The messaging feature was used by the health coach to provide support, resources, and information to participants identified as needing assistance, as well as to perform weekly check-ins. Health coach adherence lapse messages were prompted by two consecutive days of missed doses, a recurring pattern of missed doses, or a mean recent adherence level below 90%. The app also features an adherence tracking component which allows users to see their recent adherence history, and users can enter upcoming appointments and schedule appointment reminders. In addition to the above features which drove the selection of this app for the study, participants could also use the app to track other behaviors (e.g., exercise). However, these additional behaviors were not monitored by the study. Fitbit Plus participants were given access to the app and received health coaching via the app for 12 months. The app is fully compliant with the Health Insurance Portability and Accountability Act and is compatible with iOS and Android operating systems.
Thirty percent of the audiotaped LifeSteps sessions, and 30% of the messages between the health coach and participants were reviewed by two independent coders (SH and JU) for competency. For the LifeSteps sessions, coders evaluated the health coach’s frequency and extensiveness (1 = Not at all, 2 = A little, 3 = Infrequently, 4 = Somewhat, 5 = Quite a bit, 6 = Considerably, 7 = Extensively) of covering each LifeSteps component and skill level (1 = Very Poor, 2 = Poor, 3 = Acceptable, 4 = Adequate, 5 = Good, 6 = Very Good, 7 = Excellent). Messages from the health coach were rated based on their responsiveness (1 = Not at all, 2 = A little, 3 = Infrequently, 4 = Somewhat, 5 = Quite a bit, 6 = Considerably, 7 = Extensively) and appropriateness (1 = Not at all, 2 = A little, 3 = Infrequently, 4 = Somewhat, 5 = Quite a bit, 6 = Considerably, 7 = Extensively). Regarding the reviewed LifeSteps sessions, the coders scored the frequency and extensiveness as ‘Considerably’ or higher and the skill level as ‘Very Good’ or higher for all LifeSteps components. Regarding the reviewed messages, timeliness of all responses from the health coach were rated as ‘Considerably’ or higher, and appropriateness of responses all received the rating of ‘Extensively.’ Rating agreement of both the LifeSteps sessions and messages was excellent (κ > 0.9).
Follow-up Interviews
Participants completed follow-up interviews 1 month, 3 months, 6 months, and 12 months after baseline. Participants were compensated in gift cards worth US $30, $35, $40, and $50 for the 1-, 3-, 6-, and 12-month interviews, respectively. At follow-up interviews, participants completed measures and provided blood samples (at 6 and 12 months) for HIV viral load testing. In addition, EPB data were retrieved at each visit.
Data Analyses
Overview
Preparatory analyses examined equivalence across the treatment conditions on demographic characteristics and blocking variables. Distributional properties of continuous variables were examined to determine if any transformations were necessary. Univariate regressions were utilized to determine if any of the variables collected at baseline were strongly associated (p < 0.05) with the primary and secondary outcomes. No significant associations emerged; therefore, no baseline variables were entered as covariates in models testing treatment effects.
Primary Outcomes
In order to examine the feasibility and acceptability of the intervention and protocol, we examined the eligibility rate, enrollment rate, recruitment rate, follow-up completion rates, health coach time spent per participant, intervention satisfaction ratings, and app utilization data. In addition, we analyzed qualitative data collected during the 12-month interview with Fitbit Plus participants pertaining to the feasibility and acceptability of the intervention (described below).
The preliminary test of the effects of treatment on self-report corrected EPB data at the 1-, 3-, 6-, and 12-month follow-ups was conducted using a fractional logit model estimated by general estimating equations (GEE). Treatment assignment was the primary between-group independent variable in this model. An unstructured correlation matrix was selected as it is most appropriate for small sample sizes. Time (data collection points) was entered as a within-subjects predictor, as we assumed adherence rates would have a tendency to decrease over time. Lastly, the treatment assignment by time interaction term was entered in the model to examine the extent to which treatment differences changed over the follow-up period. Analysis of covariance (ANCOVA) was then employed to estimate treatment effect sizes at each follow-up time point. For each ANCOVA, treatment group was entered as the predictor variable, and adherence rate captured during the two-week monitoring period was entered as a covariate.
Secondary Outcomes and Exploratory Analyses
The GEE analysis strategy outlined above was employed to evaluate the impact of Fitbit Plus, relative to SOC, on the secondary outcomes (self-reported adherence and HIV viral load). As with the EPB data, ANCOVA was used to estimate treatment effect sizes for self-reported adherence at each follow-up time point. In these cases, treatment assignment was entered as the predictor variable, and baseline composite adherence score was entered as a covariate. Viral load was dichotomized as suppressed vs. unsuppressed. Chi-squares were used to estimate treatment effect sizes for viral load at the 6- and 12-month follow-up.
This was an exploratory study, with a sample size that was not sufficient to formally test moderation. In lieu of formal tests of moderation, generalized linear mixed models were used to explore associations between both depressive symptoms and substance use and EPB ART adherence over time. Percentage of heavy drinking days, percentage of drug use days, and depressive symptoms were entered as predictors in three separate models, along with time, and a predictor by time interaction term.
Qualitative Analyses
Recordings of the semi-structured interviews conducted with Fitbit Plus participants at the 12-month follow-up were transcribed by the research team. Transcriptions were uploaded into NVivo 12 [54], and thematic analysis was performed. A coding structure was developed which contained a list of overarching thematic codes and subcodes, focused on the feasibility and acceptability of the intervention components, and was applied to the transcripts. Two coders (SH and JU) independently coded each transcript using this coding structure, with excellent agreement (κ > 0.9).
Results
Baseline Characteristics
Participants were 53 (38 men, 15 women) persons living with HIV. Participants were 46.7 years of age on average and ranged in age from 20 to 70 years old. Sixty-six percent of participants identified as White, 24.5% of participants identified as Black, 3.8% identified as American Indian/Alaskan Native, and 5.7% identified as more than one race. In terms of ethnicity, 13.2% of participants described themselves as Hispanic/Latinx. Participant demographic characteristics and baseline values of blocking variables for the entire sample and between conditions are reported in Table 1.
Feasibility
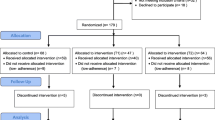
The intervention and protocol were both found to be highly feasible. First, the eligibility criteria did not appear to be overly restrictive. Of the 132 potential participants screened for the study, 94 (71%) were eligible (see Consort Diagram in Fig. 1). Of those 94 who were eligible, 65 (69%) were consented and completed the baseline interview. However, 12 participants did not attend the LifeSteps session and therefore were not randomized, resulting in a final sample of 53 (56% of those who were eligible). There were no demographic differences between eligible individuals who enrolled and those who did not, with the exception of mean age (47 years of age for enrolled vs. 40 years of age for not enrolled). Toward the end of the study, we determined that we needed to supplement clinic-based recruitment with online recruitment due to simultaneous recruitment for multiple studies (including other adherence studies) in the clinic. Once this additional recruitment mechanism was in place, we were able to recruit 12 participants per month. In addition, follow-up rates were 100% at 1 month, 94% at 3 months, 89% at 6 months, and 89% at 12 months. Importantly, follow-up rates did not differ by treatment condition. Finally, the intervention was delivered in an efficient manner. The health coach spent a mean of 2.4 min per month communicating with each Fitbit Plus participant.
Based on the qualitative data gathered through the semi-structured interviews with Fitbit Plus participants at 12 months, use of the app was feasible for most participants. One participant stated, "I thought just taking the pill, watching the graph for progress was good and straightforward." Other participants noted the convenience of having the app on their phones (e.g., "Well I liked that it was on my phone, so it was accessible.”). Most participants did not have any suggested changes (e.g., "I don't think it's missing anything. I mean I like it the way it is."). However, some participants did encounter minor technical issues. Participants noted that sometimes the notifications from the app were not loud enough or were easy to miss, the app would sometimes log participants out without them realizing that this had happened, or it would crash and have to be reset. No participant voiced concerns about confidentiality. The most frequently cited suggestion to improve app feasibility, which may also improve acceptability, was adding a snooze feature for days that participants sleep in or are unable to take their medication at their usual time (e.g., “But it would have been really great, like if I could have designed the app, I would’ve given a second reminder an hour later.”). Lastly, some participants suggested incorporating graphs into the medication tracking feature to make the data easier to comprehend.
Acceptability
Regarding the acceptability of the intervention, a majority of the Fitbit Plus participants (87.5%) reported that they were mostly or very satisfied with the app. Further, all Fitbit Plus participants said that they would recommend the app to friends living with HIV. Only one participant (6.3%) reported that the app did not at all help them remember to take their ART. Further, 76.5% of Fitbit Plus participants were still using the app on a regular basis (≥ 50% of days) at 12 months. Over the course of the 12-month follow-up period, participants used the app regularly for a mean of 9.5 months and responded to 86.5% of push notifications.
Examination of the qualitative data collected from Fitbit Plus participants revealed that, overall, participants found the app to be acceptable, reporting “I thought it was very helpful-it was like my mini secretary.” In particular, participants found the medication reminders to be beneficial. One participant noted “The thing that I liked most about the app was that it reminded me to take my HIV medication on time and by pressing the reminder every day it really scheduled me to be responsible with my health.” Participants also mentioned that they appreciated the additional features of the app, such as medication adherence tracking and the ability to add appointment reminders (e.g., “It can let you chart, I can look back and sometimes I was kind of surprised that I had missed more than I realized. So it was good for me to be mindful of that.”).
Many participants found the messaging feature helpful because it allowed them to effortlessly ask questions, stating “I liked it. I mean I could talk back and forth if I had a problem or whatever. It was hands on so it was really good for me.” The participants uniformly enjoyed interacting with the health coach via the app. Notable quotes included: “Oh I appreciated him. Checking in on me once in a while. I felt that he was staying on top of things and my wellbeing. He was concerned about my wellbeing, I appreciated that.” Further, Fitbit Plus participants appreciated having met the person on the other end of the app. When asked to imagine an intervention in which they would not have met the health coach in person prior to interacting with him via the app, participants made comments such as: 1) “I would feel like I was talking to a robot…seeing him face to face it helped me.” 2) “I have a sense of his self…I got to see him and I got to experience what he had to tell me.” 3) “I was more likely to interact with him because I’d met him. If I didn’t know who he was, I wouldn’t have interacted with the person…” 4) “The fact that I worked with him in the study and such at the beginning it did make me feel more comfortable. And meeting him in person, he’s a very friendly person, so it was really easy to chat with him over the app.”
Preliminary Efficacy
Self-Report Corrected EPB Data
Results from data analysis of self-report corrected EPB adherence data are presented in Table 2. Briefly, no main effect of time or interaction effect were detected. The grand estimated mean of adherence among Fitbit Plus participants was 83.4% (SE = 3.6%) compared to 72.9% (SE = 5.1%) among SOC participants (p = 0.09). Post-hoc pairwise comparisons of treatment group differences in adherence at each follow-up point revealed a trend of higher ART adherence among Fitbit Plus participants compared to SOC participants (see Fig. 2). Importantly, analysis of the raw, uncorrected EPB data reflects the same pattern in results, increasing our confidence in the findings (See Fig. 3 in Appendix for results from raw, uncorrected EPB data). Small effect sizes were detected at the 1-, 6-, and 12-month follow-up (η2 = 0.05, η2 = 0.05, and η2 = 0.06, respectively). In addition, a medium effect size was detected at the 3-month follow-up (η2 = 0.11).
Self-Reported ART Adherence
Analysis of self-reported ART adherence revealed no interaction effect of treatment group and time. A main effect of time and a non-significant trend of treatment group were observed (See Table 2). Participants’ composite adherence score increased over time (p = 0.03). The mean composite adherence score at each time point was 78.8 (SE = 2.5) at baseline, 85.1 (SE = 2.6) at the 1-month follow-up, 80.3 (SE = 3.4) at the 3-month follow-up, 81.2 (SE = 3.3) at the 6-month follow-up, and 81.2 (SE = 3.6) at the 12-month follow-up. Although non-significant, the grand mean composite adherence score was higher among Fitbit Plus participants (85.1, SE = 3.1) compared to SOC (77.5, SE = 3.5) (p = 0.10). Post-hoc pairwise comparisons of treatment group differences at each follow-up revealed that Fitbit Plus participants reported non-significantly higher composite adherence scores compared to SOC participants at the 1-, 3-, and 6-month follow-ups (see Fig. 4). At the 12-month follow-up, there was virtually no difference between the mean composite adherence score among Fitbit Plus participants and SOC participants. Small effect sizes were detected at each of the follow-ups. Specifically, the treatment effect sizes at the 1-, 3-, 6-, and 12-month follow-ups were η2 = 0.01, η2 = 0.06, η2 = 0.01, and η2 = 0.01, respectively.
Viral Load. Results from data analysis of viral load are presented in Table 2. No main effect of group or time or interaction effect emerged. The grand mean percentage of Fitbit Plus participants with unsuppressed viral loads throughout the study was 9.4% (SE = 4.6%) compared to 17.2% (SE = 6.8%) of SOC participants (p = 0.34). Specifically, at baseline, 8.0% (SE = 5.2%) of Fitbit Plus participants were unsuppressed compared to 15.4% (SE = 7.1% of SOC participants (p = 0.4). At the 6-month follow-up, 10.2% (SE = 6.1%) of Fitbit Plus participants were unsuppressed compared to 16.6% (SE = 7.7%) of SOC participants (p = 0.52). At the 12-month follow-up, 10.6% (SE = 6.0%) of Fitbit Plus participants were unsuppressed compared to 19.9% (SE = 8.5%) of SOC participants (p = 0.37). Small effect sizes were detected at the 6- and 12-month follow-ups (ϕ = 0.14 and ϕ = 0.22, respectively).
Exploratory Analyses of Potential Moderators of Treatment
Generalized linear mixed models were used to explore associations between both depressive symptoms and substance use and EPB ART adherence over time. Percentage of heavy drinking days, percentage of drug use days, and depressive symptoms were entered as predictors in three separate models, along with time, and a predictor by time interaction term. In the percentage of heavy drinking days model, time and the percentage of heavy drinking days by time interaction term were not significantly associated with adherence. However, a negative association between heavy drinking days and adherence emerged, β = − 1.2, SE = 0.3, 95% CI − 1.9, − 0.6. In the percentage of drug use days model, time and the percentage of drug use days interaction term were not significantly associated with adherence. Percentage of drug use days was negatively associated with adherence, β = − 1.3, SE = 0.4, 95% CI − 2.1, − 0.5. Finally, in the depressive symptoms model, no associations of depressive symptoms, time, or the depressive symptoms and time interaction term emerged.
Discussion
This study examined the feasibility, acceptability, and preliminary efficacy of an mHealth facilitated health coaching intervention aimed at improving ART adherence. The intervention and protocol were largely found to be feasible. The eligibility criteria were not overly restrictive, and our dual recruitment methods yielded a reasonable number of participants per month. In addition, our retention rates ranged from 89% to 100% at the various follow-up points. We were also able to deliver the intervention efficiently, with the health coach devoting 2.4 min per month, on average, to each Fitbit Plus participant. However, the loss of approximately 18.5% of participants prior to the LifeSteps session does raise some feasibility concerns. In addition, there were some minor technical issues identified during the qualitative interviews that slightly diminished app ease of use. The most commonly cited feasibility concern regarding the app was the lack of a snooze function for the medication push notifications.
The app also demonstrated a high level of acceptability, with the vast majority of Fitbit Plus participants reporting that they were satisfied with the app, would recommend it to a friend living with HIV, and found it helpful in remembering to take their ART. In addition, over three-quarters of the participants were still using the app regularly at 12 months, and participants used the app regularly for 9.5 of the 12 months and responded to 84.5% of push notifications, on average. The qualitative data were also consistent with a high degree of acceptability. In particular, participants appreciated the medication reminders and other app features, including appointment reminders and adherence tracking. Regarding the app secure messaging feature, participants liked the ease with which they were able to communicate with the health coach and felt more comfortable doing so given that they had met the health coach during the LifeSteps session.
This was a pilot study and was not powered to find statistically significant differences in outcomes between treatment conditions. Rather, the goal was to examine the pattern of outcomes and to estimate effect sizes to inform the design of a fully powered clinical trial. The pattern of results on all three outcome variables, our primary outcome of EPB adherence and our secondary outcomes of self-reported adherence and HIV viral load, were consistent with better adherence in the Fitbit Plus condition relative to the SOC condition at all follow-up time points with the exception of self-reported adherence at 12 months (where the two groups had virtually identical levels of adherence). While most comparisons did not reach statistical significance, effect sizes at the follow-up time points were in the small to medium range across the three outcomes. We believe that the intervention impacted outcomes despite its efficient use of health coach time because the app allowed for targeted and timely intervention for those participants who were experiencing an adherence lapse.
Our intervention shares similarities with other interventions that have employed mHealth as a means for patients living with HIV to access health counseling as needed. For, example, Puig and colleagues [55] developed an app for adults 60 and over living with HIV. The app provided medication and appointment reminders, educational material, and links to obtain health counseling if desired by participants. Once reminders were sent to participants to use the app, there was a high degree of engagement with the app, and participants reported a high level of app acceptability. However, the app did not appear to impact adherence and clinical outcomes. Other studies have used real-time monitoring of adherence to trigger a health counseling intervention, as was done with our intervention. One of these studies, conducted in the US, used an electronic pill box that transmitted adherence data in real-time to prompt a phone call from a counselor [56]. Participants in this feasibility and acceptability study reported a high degree of acceptability on most measures. However, consistent with our intervention protocol, the authors concluded that it might be more useful to provide intervention in response to multiple missed doses or a pattern of missed doses rather than periodic single missed doses given the relative forgiveness of modern ART regimens. Another study, conducted in China, found that real-time monitoring of adherence that triggered a text message and counseling at the next clinic visit resulted in better ART adherence, relative to the control condition, during the intervention period [57]. In a similar vein, Haberer and colleagues [58] found that scheduled text reminders that, after three months, progressed to texts prompted by late or missed doses with text message notification to social supporters for adherence lapses of more than 48 h resulted in better ART adherence relative to a control condition in a sample from Uganda. This intervention was found to be feasible and had a high degree of perceived utility among participants [59]. Results were more mixed in a study conducted in South Africa [60], where text messages in response to adherence lapses monitored in real time had no effect on ART adherence or viral load but did reduce treatment interruptions lasting 72 h or longer. Taken together, our findings and those of similar studies suggest a generally high degree of feasibility and acceptability for interventions that prompt an adherence counseling intervention in response to an adherence lapse and some support for the efficacy of these types of interventions, particularly those that go beyond a text message response and prompt an intervention from a counselor or social support individual.
Our exploratory examination of the association between substance use and EPB ART adherence yielded results that are consistent with substance use as a potential moderator of treatment effects. Specifically, heavy drinking and drug use were each associated with poorer adherence at a statistically significant level. This finding is consistent with the extant literature in this area [61,62,63,64,65], as well as our finding of a significant daily association between both heavy drinking and drug use and missed ART dose for a given day during the two-week baseline EPB monitoring for this study [66].
However, the results from our exploratory examination of the association between depressive symptoms and EPB ART adherence were not consistent with depressive symptoms as a potential moderator of treatment effects. This finding runs counter to the association between higher levels of depressive symptoms and poorer ART adherence that is typically found in the literature [67,68,69]. Our failure to find this association may be due, at least in part, to a relative lack of variability in depressive symptom levels between participants and across time points. This finding merits further examination.
Based on the results of this preliminary trial, we have made a number of modifications to our intervention. Most notably, our future trials will use an app that has a snooze feature for medication reminders and more interpretable adherence history tracking tools for participants. In addition, our future work will use an app that also has a secure video chat feature to enhance the personal connection with the health coach that participants viewed as impactful. We have also modified our health coach manual to include more information about how to assist participants in customizing push notifications received from the app in order to avoid the technical challenges that some of the participants in this study experienced. Lastly, given the potential for substance use and mental health to serve as barriers to adherence, we have added a module in the LifeSteps session that directly addresses these issues. While we did not find an association between level of depressive symptoms and poorer ART adherence in this study, this association is well established in the literature [51,52,53]. Therefore, we believe that the intervention will be improved with the addition of a module that address both substance use and mental health.
As with any study, the results of the current study should be evaluated within the context of its strengths and limitations. There are a number of features of this study that contributed to its methodological rigor: a randomized controlled design that yielded groups that were balanced on demographic and other baseline characteristics; a rigorous training, supervision, and fidelity rating process that ensured that the manualized intervention was delivered in a competent and protocol-adherent manner; the blinding of research assistants to treatment condition; and the inclusion of both an objective measure of ART adherence (EPB data) and a biomarker of ART adherence (HIV viral load). A further strength of this study was the collection and analysis of qualitative data using rigorous qualitative methodology; these data have been invaluable in the refinement of the intervention.
This study has a number of limitations. Its chief limitation pertains to the sample size. This was a preliminary study that was not powered to find statistically significant differences between groups or to perform formal moderation analyses. Further, our design did not equate for health coach contact time across treatment conditions. Rather, we chose to use a single LifeSteps session as our comparison condition because it approximates standard of care in most HIV treatment settings. As such, the results of a fully powered study using this design could inform HIV treatment settings of the benefit they are likely to achieve in the form of improved ART adherence among their patients should they elect to adopt our intervention. As a result, we viewed our comparison condition as a pragmatic choice. In addition, we included both newly diagnosed and established patients. These groups may be quite different in important ways. Lastly, the generalizability of the findings may be limited to the region in which the study was conducted and to characteristics of our sample, namely a predominantly White sample who do not identify as Hispanic/Latinx. Generalizability may also be somewhat limited due to the requirement that participants own a smartphone compatible with the study app.
Conclusions
In summary, this study establishes the feasibility and acceptability of our mHealth facilitated health coaching intervention aimed at improving medication adherence among PLWH. In addition, the pattern of results is consistent with intervention preliminary efficacy. Results also suggest that addressing heavy drinking and drug use will be key to maximizing impact on ART adherence. Given the high levels of feasibility and acceptability, promising preliminary efficacy, and potential scalability, this intervention warrants further investigation in a fully powered trial that would ideally be conducted at multiple sites and consist of a sample with a high level of diversity.
Availability of Data and Material
Data will be placed in a repository after this manuscript is accepted for publication.
Code Availability
Not applicable.
Reference Lists
Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveillance Supplemental Report. 2019;24(1).
Centers for Disease Control and Prevention. Diagnoses of HIV Infection in the United States and Dependent Areas, 2017. HIV Surveillance Report. 2018;29.
Chen LF, Hoy J, Lewin SR. Ten years of highly active antiretroviral therapy for HIV infection. Medical J Aust. 2007;186(146):151.
The Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: A collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–9.
Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data--United States and 6 dependent areas, 2016. HIV Surveillance Supplemental Report. 2018;23(4).
Nance RM, Delaney JAC, Simoni JM, Wilson IB, Mayer KH, Whitney BM, et al. HIV viral suppression trends over time among HIV-infected patients receiving care in the United States, 1997 to 2015: A cohort study. Ann Intern Med. 2018;169(6):376–84.
Mugavero MJ, Napravnik S, Cole SR, Eron JJ, Lau B, Crane HM, et al. Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis. 2011;53(9):927–35.
Marks G, Patel U, Stirratt MJ, Mugavero MJ, Mathews WC, Giordano TP, et al. Single viral load measurements overestimate stable viral suppression among HIV patients in care: Clinical and public health implications. J Acquir Immune Defic Syndr. 2016;73(2):205–12.
Crepaz N, Tang T, Marks G, Mugavero MJ, Espinoza L, Hall HI. Durable viral suppression and transmission risk potential among persons with diagnosed HIV infection: United States, 2012–2013. Clin Infect Dis. 2016;63(7):976–83.
Gunn JKL, Patterson W, Anderson BJ, Swain C. Understanding the risk of human immunodeficiency virus (HIV) virologic failure in the era of undetectable equals untransmittable. AIDS Behav. 2021.
Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM. Adherence to antiretroviral therapy and virologic failure: A meta-analysis. Medicine (Baltimore). 2016;95(15):e3361.
Youn B, Shireman TI, Lee Y, Galárraga O, Wilson IB. Trends in medication adherence in HIV patients in the US, 2001 to 2012: An observational cohort study. J Int AIDS Soc. 2019;22(8):e25382.
Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30.
Bangsberg DR. Preventing HIV antiretroviral resistance through better monitoring of treatment adherence. J Infect Dis. 2008;197(Suppl 3):S272–8.
Lima VD, Harrigan R, Bangsberg DR, Hogg RS, Gross R, Yip B, et al. The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. J Acquir Immune Defic Syndr. 2009;50(5):529–36.
Locher C, Messerli M, Gaab J, Gerger H. Long-term effects of psychological interventions to improve adherence to antiretroviral treatment in HIV-infected persons: A systematic review and meta-analysis. AIDS Patient Care STDS. 2019;33(3):131–44.
Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: Translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7:44–51.
Torres-Robles A, Wiecek E, Tonin FS, Benrimoj SI, Fernandez-Llimos F, Garcia-Cardenas V. Comparison of interventions to improve long-term medication adherence across different clinical conditions: A systematic review with network meta-analysis. Front Pharmacol 2018;0(1454).
Kanters S, Park JJH, Chan K, Socias ME, Ford N, Forrest JI, et al. Interventions to improve adherence to antiretroviral therapy: A systematic review and network meta-analysis. Lancet HIV. 2017;4(1):e31–40.
Iribarren SJ, Cato K, Falzon L, Stone PW. What is the economic evidence for mHealth? A systematic review of economic evaluations of mHealth solutions. PLoS ONE. 2017;12(2):e0170581.
Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): A meta-analysis of randomized controlled trials. PLoS ONE. 2014;9(2):e88166.
Muench F, Baumel A. More than a text message: Dismantling digital triggers to curate behavior change in patient-centered health interventions. J Med Internet Res. 2017;19(5):e147.
Cooper V, Clatworthy J, Whetham J, Consortium E. mHealth interventions to support self-management in HIV: A systematic review. Open AIDS J. 2017;11:119–32.
Dillingham R, Ingersoll K, Flickinger TE, Waldman AL, Grabowski M, Laurence C, et al. PositiveLinks: A mobile health intervention for retention in HIV care and clinical outcomes with 12-month follow-up. AIDS Patient Care STDS. 2018;32(6):241–50.
Muessig KE, Pike EC, LeGrand S, Hightow-Weidman LB. Mobile phone applications for the care and prevention of HIV and other sexually transmitted diseases: A review. Med Internet Res. 2013;15(1):e1.
Lee K, Kwon H, Lee B, Lee G, Lee JH, Park YR, et al. Effect of self-monitoring on long-term patient engagement with mobile health applications. PLoS ONE. 2018;13(7):e0201166.
Chaiyachati KH, Ogbuoji O, Price M, Suthar AB, Negussie EK, Barnighausen T. Interventions to improve adherence to antiretroviral therapy: A rapid systematic review. AIDS. 2014;28(Suppl 2):S187–204.
Amico KR, Orrell C. Antiretroviral therapy adherence support: Recommendations and future directions. J Int Assoc of Provid AIDS Care. 2013;12(2):128–37.
Sandelowski M, Voils CI, Chang Y, Lee EJ. A systematic review comparing antiretroviral adherence descriptive and intervention studies conducted in the USA. AIDS Care. 2009;21:953–66.
Wolever RQ, Simmons LA, Sforzo GA, Dill D, Kaye M, Bechard EM, et al. A systematic review of the literature on health and wellness coaching: Defining a key behavioral intervention in healthcare. Glob Adv Health Med. 2013;2(4):38–57.
Dejonghe LAL, Becker J, Froboese I, Schaller A. Long-term effectiveness of health coaching in rehabilitation and prevention: A systematic review. Patient Educ Couns. 2017;100(9):1643–53.
Hill B, Richardson B, Skouteris H. Do we know how to design effective health coaching interventions: A systematic review of the state of the literature. Am J Health Promot. 2015;29(5):e158–68.
Kivela K, Elo S, Kyngas H, Kaariainen M. The effects of health coaching on adult patients with chronic diseases: A systematic review. Patient Educ Couns. 2014;97:147–57.
Safren SA, Otto MW, Worth J. Life-steps: Applying cognitive-behavioral therapy to patient adherence to HIV medication treatment. Cogn Behav Pract. 1999;6:332–41.
Safren SA, Otto MW, Worth J, et al. Two strategies to increase adherence to HIV antiretroviral medication: Life-steps and medication monitoring. Behav Res Ther. 2001;39:1151–62.
Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25:462–73.
Safren SA, O’Cleirigh CO, Tan JY, Raminani SR, Reilly LC, Otto MW, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28:1–10.
Safren SA, O’Cleirigh CO, Bullis JR, Otto MS, Stein MD, Pollack MH. Cognitive Behavioral Therapy for Adherence and Depression (CBT-AD) in HIV-infected injection drug users: A randomized controlled trial. J Consult Clin Psychol. 2012;80:404–15.
Ramsey SE, Ames EG, Uber J, Habib S, Clark S. mHealth application to improve HIV medication adherence: Protocol to establish feasibility, acceptability, and preliminary efficacy. JMIR Res Protoc. 2019;8(11):e15356.
Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16:269–77.
Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401.
Sobell LC, Sobell MB. Timeline Followback user’s guide: A calendar method for assessing alcohol and drug use. Toronto, Ontario, Canada: Addiction Research Foundation; 1996.
Wilson IB, Lee Y, Michaud J, Fowler FJ Jr, Rogers WH. Validation of a new three-item self-report measure for medication adherence. AIDS Behav. 2016;20(11):2700–8.
Wilson IB, Tie Y, Padilla M, Rogers WH, Beer L. Performance of a short, self-reported adherence scale in a probability sample of persons using HIV antiretroviral therapy in the United States. AIDS. 2020.
Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data-United States and 6 dependent areas, 2018. HIV Surveillance Supplemental Report. 2020;25(2).
Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171–81.
Bavinton BR, Pinto AN, Phanuphak N, Grinsztejn B, Prestage GP, Zablotska-Manos IB, et al. Viral suppression and HIV transmission in serdiscordant male couples: An international prospective, observational, cohort study. Lancet HIV. 2018;5(8):e438–77.
(ART-CC) ATCC, Vandenhende M, Ingle S, May M, Chene G, Zangerle R, et al. Impact of low-level viremia on clinical and virological outcomes in treated HIV-1-infected patients. AIDS. 2015;29(3):373–83.
Boillat-Blanco N, Darling KEA, Schoni-Affolter F, Vuichard D, Rougemont M, Fulchini R, et al. Virological outcome and management of persistent low-level viremia in HIV-1-infected patients: 11 years of the Swiss HIV Cohort Study. Antivir Ther. 2015;20(2):165–75.
Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiological Studies-Depression(CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12(2):277–87.
Sobell LC, Sobell MB, Buchan G, Cleland PA, Fedoroff I, Leo GI. The reliability of the Timeline Followback method applied to drug, cigarette, and cannabis use. Presented at the 30th Annual Meeting of the Association for Advancement of Behavior Therapy. New York, NY; 1996.
First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition. New York: New York State Psychiatric Institute; 1997.
Jeste D, Palmer B, Appelbaum P, Golshan S, Glorioso D, Dunn L, et al. A new brief instrument for assessing decisional capacity for clinical research. Arch Gen Psychiatry. 2007;64(8):966–74.
QSR International Pty Ltd. NVivo qualitative data analysis program. 2018.
Puig J, Echeverría P, Lluch T, Herms J, Estany C, Bonjoch A, et al. A specific mobile health application or older HIV-infected patients: Usability and patient’s satisfaction. Telemed J E Health. 2021;27(4):432–40.
Pellowski JA, Kalichman SC, White D, Amaral CM, Hoyt G, Kalichman MO. Real-time medication adherence monitoring intervention: Test of concept in people living with HIV infection. J Assoc Nurses AIDS Care. 2014;25(6):646–51.
Sabin LL, DeSilva MB, Gill CJ, Zhong L, Vian T, Xie W, et al. Improving adherence to antiretroviral therapy with triggered real-time text message reminders: The China adherence through technology study. J Acquir Immune Defic Syndr. 2015;69(5):551–9.
Haberer JE, Musiimenta A, Atukunda EC, Musinguzi N, Wyatt MA, Ware NC, et al. Short message service (SMS) reminders and real-time adherence monitoring improve antiretroviral therapy adherence in rural Uganda. AIDS. 2016;30(8):1295–300.
Musiimenta A, Atukunda EC, Tumuhimbise W, Pisarski EE, Tam M, Wyatt MA, et al. Acceptability and feasibility of real-time antiretroviral therapy adherence interventions in rural Uganda: Mixed-method pilot randomized controlled trial. JMIR Mhealth Uhealth. 2018;6(5):e122.
Orrell C, Cohen K, Mauff K, Bangsberg DR, Maartens G, Wood R. A randomized controlled trial of real-time electronic adherence monitoring with text message dosing reminders in people starting first-line antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;70(5):495–502.
Kahler CW, Liu T, Vaughn B, Pinkston MM, Kojic EM, Onen N, et al. Direct and indirect effects of heavy alcohol use on clinical outcomes in a longitudinal study of HIV patients on ART. AIDS Behav. 2017;21(7):1825–35.
Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Cook RL, et al. A temporal and dose response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29(7):1190–7.
Malta M, Strathdee SA, Magnanini MMF, Bastos FI. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: A systematic review. Addiction. 2008;103(8):1242–57.
Rosen MI, Black AC, Arnsten JH, Goggin K, Remien RH, Simoni JM, et al. Association between use of specific drugs and antiretroviral adherence: Findings from MACH14. AIDS Behav. 2013;17(1):142–7.
Cohn SE, Jiang H, McCutchan JA, Koletar SL, Murphy RL, Robertson KR, et al. Association of ongoing drug and alcohol use with non-adherence to antiretroviral therapy and higher risk of AIDS and death: results from ACTG 362. AIDS Care. 2011;23(6):775–85.
Ramsey SE, Ames EG, Uber J, Habib S, Clark S, Waldrop-Valverde D. Same-day associations between substance use and medication nonadherence among persons living with HIV. Subst Abuse. 2019;13:1178221819878751.
Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: A review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2):181–7.
Tyer-Viola LA, Corless IB, Webel A, Reid P, Sullivan KM, Nichols P, et al. Predictors of medication adherence among HIV-positive women in North America. J Obstet Gynecol Neonatal Nurs. 2014;43(2):168–78.
Sauceda JA, Johnson MO, Saben P. Nonadherence as four-day antiretroviral therapy interruptions: Do depression and race/ethnicity matter as much in the modern antiretroviral therapy era? AIDS Behav. 2016;20(11):2624–8.
Acknowledgements
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R34MH108431. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study is registered on ClincalTrials.gov, NCT02676128.
Funding
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R34MH108431 to Dr. Susan Ramsey. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the manuscript as follows: Study conception/design: SER and EGA; Data collection: JU and SH; Data analysis/interpretation of results: SER and EGA; Drafting of manuscript: SER, EGA, and JU. All authors reviewed the results, participated in the critical revision of the article, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Susan Ramsey has an Investigator Sponsored Research Agreement with Gilead Science, Inc. for the provision of medication for another trial.
Ethical Approval
This protocol was approved by the Lifespan IRB.
Consent to Participate
All participants signed a written informed consent statement.
Consent for Publication
Consent was obtained to publish data in a manner in which identification could not occur.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Fig. 3.
Rights and permissions
About this article
Cite this article
Ramsey, S.E., Ames, E.G., Uber, J. et al. A Preliminary Test of an mHealth Facilitated Health Coaching Intervention to Improve Medication Adherence among Persons Living with HIV. AIDS Behav 25, 3782–3797 (2021). https://doi.org/10.1007/s10461-021-03342-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-021-03342-5