Abstract
The cosmopolitan distribution of soil microarthropods and their various degrees of adaptation make them suitable tools for assessing soil ecosystem health. In this study, the microarthropod abundance of 25 home gardens in Kerala state located in South West coast of India was studied during summer and north east monsoon season of 2014. The soil microarthropods were categorized into six groups: collembola, coleoptera, hymenoptera, araeneae, acari and diplopoda. Their numbers varied from 0 to 28 per 1000 cm3 and were more abundant in the rainy season than in summer. The occurrence of these microarthropods was positively correlated to soil moisture and organic carbon and had negative correlation to soil temperature and soil pH. The presence of eu-edaphic and epi-edaphic microarthropod fauna was used to derive the soil quality index of each home garden and soil quality classes were defined. Out of the 25 home gardens, two were of good quality, 21 were of medium quality and two were of poor quality. The study supports the scope of applying the indicator value of soil microarthropods in future studies related to soil quality, management and conservation of tropical home garden ecosystems, which are facing threats of removal of canopy and unscientific land management practices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Out of the 23 % of the described soil organisms in the world, soil arthropods comprise of 85 % in which soil microarthropods coming under mesofauna are the major groups helping in the maintenance of soil quality contributing to ecosystem functioning, by acting as litter transformers and ecosystem engineers (Culliney 2013). The soil quality indication using microarthropods has been much discussed in connection with the presence of eu-edaphic fauna like collembola (Rusek 1998; Bandyopadhyaya et al. 2002; A’Bear et al. 2013). The cosmopolitan distribution of microarthropods from equator to poles and their various degrees of soil adaptations make them suitable tools for linking the human interaction with the environment, assessing the ecosystem health and studying ecosystem changes as indicated by many studies (Hodkinson et al. 1996; Andres 1999; Kautz et al. 2006). These organisms are commonly used in studying the perturbations of polluted or disturbed ecosystems (Rusek and Marshall 2000; Cortet et al. 2006; Coulson et al. 2015), assessing the progress of soil quality enhancement process (Hartley et al. 2008), monitoring soil restoration (Tizado and Nunez-Perez 2014) and evaluation of cropping systems (Olfert et al. 2002). In agro-ecosystem management studies, they are used for the comparison of similar habitats with different management practices or different habitats within the same geographical area as the degree of variation in nature-human interactions will reflect in the soil microarthropod fauna in that area, in terms of their presence, absence or change in number helping in ecosystem risk assessment and management.
Tropical home gardens are considered to be valuable traditional agro-biodiversity systems (Kumar et al. 1994; Sankar and Chandrashekara 2002; Soumya et al. 2007; Galluzzi et al. 2010). They are man-made terrestrial ecosystems, resembling a natural ecosystem but with human influence and offer valuable ecosystem functions (Fernandes and Nair 1986) like efficient nutrient cycling, low use of external inputs and has soil conservation potential (Torquebiau 1992; Jensen 1993a, b; Jose and Shanmugaratnam 1993).They are highlighted in the conservation point of view, by classifying them among in situ conservation sites in accordance with the Convention of Biological Diversity article 7, 8 and 10(c).
In India, a number of home gardens are present from the traditional times which provided benefits like food and medicine in addition to sociocultural and economic values (Mendez et al. 2001; Kumar et al. 2004; Das and Das 2005). An important property of tropical home gardens is the rich biodiversity—both natural and agro-biodiversity. The fauna of home gardens are also equally varied. The home gardens of India are replete with vegetables, ornamentals and medicinal plants (Soumya et al. 2007) in addition to a number of invertebrate and vertebrate fauna (Rahman et al. 2012). A few studies have been carried out on the soil fauna of Indian home gardens (Banerjee et al. 2009; Rahman et al. 2012). Among the soil fauna the microarthropods form a significant component playing a major role in soil organic matter recycling and soil quality enhancement; hence labelled as one of the most important factors related to soil fertility (Vreeken-Buijs et al. 1998). The soil microarthropods also contribute to complex soil food webs thus creating a ‘soil ecological balance’ needed for a sustainable soil ecosystem. These organisms can be used as indicators for assessing soil health and soil quality because they are directly involved in the soil functions and are sensitive to changes in land management thereby offering a good scope for biomonitoring (Van Straleen and Verhoef 1997; Bongers 1999).
The home gardens in Kerala, the South West coast of India where this study is undertaken are rich in vegetation which includes cultivated seasonal vegetables and natural flora of herbs and grasses interspersed with trees. There is little or scanty land management practices or input of agrochemicals. Therefore in this study, it is hypothesized that the home gardens are healthy ecosystems harboring natural and domesticated vegetation. Hence the soil microarthropod community in these home gardens can relate to the natural ecological processes, and any deviation of the community characteristics will indicate alteration of soil condition which could be a land use pattern or external input. The first part of this hypothesis, the natural variation of the microarthropod community in the home gardens of a rural locality and its probable indicator value is reported in this study.
Materials and methods
The study area
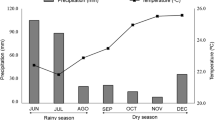
The area selected for study was Chengamanad village in the state of Kerala, India (10.1651°N–76.3623°E) which is a tropical region. This region in South India experiences typical monsoon climate with two monsoon seasons—south west monsoon (June–September), north east monsoon (October–December) and summer (January–May) with an average annual rainfall of about 3341 mm. The region has a humidity ranging from 86 to 93 %. The soil is of tropical type, enriched with humus, supporting lush vegetation. The village is one in Ernakulam district with a considerable portion under agricultural land use (Soil and Land Use Survey of India 2013). The total land area of the village is about 4335 acres (Department of Economics and Statistics 2013).
Twenty-five home gardens were selected from the village based on a simple random sampling design. They have an area ranging from 0.02 to 0.20 ha. Each home garden has a dwelling located within it surrounded by cultivated fruit trees, vegetables, shade trees and flowering plants. The high rainfall and tropical temperature favours the natural flora in between the planted vegetation. The home gardens in the study area showed clear vertical stratification of vegetation and did not contain any associated features like ponds or sacred groves. Three strata were identified based on canopy structure in which the lowest stratum was within 70 cm height represented by grasses and small herbs. The second strata which reached up to 3.5 m was represented by shrubs which included ornamental plants (flowering and foliage plants). The third layer reaching up to a height of 6 m was represented by fruit bearing plants like Papaya, Banana and Cocoa. The fourth and the topmost canopy was represented by tree species like Coconut, Mango, Jackfruit and Teak reaching up to a height of 28 m. Horizontally the land is even except for the houses. The adjacent home gardens are separated by bio-fence comprising of China rose, Jungle geranium, Bamboo and Mexican lilac. The general features of the selected home gardens are given in Table 1.
Soil sampling
The home gardens were given an identification number (home garden ID) from 1 to 25. Soil monoliths of volume 10 × 10 × 10 cm was made from each home garden in triplicate according to the TSBF protocol (Swift and Bignell 2001). Samples were transferred into poly bags and immediately shifted to lab. The sampling was done in the months of March and April (summer) and in October and November (north east monsoon) of the year 2014.
Physical and chemical analysis of soil
The different drivers like agriculture, urbanization and seasonal fluctuation affect the soil moisture, temperature, and organic carbon which can cause changes in the soil microarthropod communities (Begum et al. 2014) and hence these physicochemical factors were considered in the study. The soil analysis was done using standard procedures (Radojevic and Bashkin 2006). The soil temperature was determined in situ using digital soil thermometer (model TTX 110, Ebro make). Soil pH and moisture were determined as soon as the samples were collected. Soil pH was measured using digital pH meter (HI 98107, Hanna make). Moisture was determined by gravimetric analysis. For the estimation of soil organic carbon, the analysis was carried out using air dried and sieved soil samples by Walkley–Black method.
Microarthropod extraction
For the study of soil microarthropods, large objects like cobbles and pebbles were removed and each soil sample was transferred to Berlese tullgren funnel apparatus set up in the lab (Macfadyen 1961). The samples were kept under 25 W bulbs and a mixture of 70 % ethanol and glycerol (in the ratio 1:2) was used as the preservative into which the organisms were trapped. The extraction time was standardized for 72 h after which the extracted organisms were carefully picked up using forceps and observed under a stereomicroscope. The samples were identified and counted. The population size of microarthropods in each home garden was determined and the relative composition of the different taxa computed.
Data analysis
The data analysis was divided into five parts namely analysis of soil properties, microarthropod community structure, calculation of EMI and QBS-ar scores, correlation analysis and determination of soil class values. The mean value of the soil properties like soil temperature, soil moisture, soil pH and soil organic carbon were determined for both summer and north east monsoon period and compared. The abundance of soil microarthropods in each home garden and the relative composition of the different taxa in the study area were represented graphically. The soil quality was related to the microarthropod community by two indices i.e., EMI and QBS-ar. The eco-morphological index (EMI) is an index of soil quality given to the soil microarthropods based on the morphotype of each organism. EMI values ranging from 1 to 20 are assigned to the soil microarthropods considering the morphological features like body length and edaphic adaptations like the absence of wings and visual apparatus. The EMI score uses the presence of soil microarthropod as the criterion for estimating soil quality (Yan et al. 2012), rather than their abundance. A higher EMI value refers to a higher level of adaptation of soil microarthropods. Since eu-edaphic soil microarthropods are highly adapted to soil habitat, they usually have an EMI score closer to 20 compared to the epi-edaphic and hemi-edaphic organisms having a score closer to 1 (Parisi et al. 2005). After obtaining the EMI index of all the groups, the biological soil quality index of microarthropods (QBS-ar) was calculated from the EMI scores of each location by summing up the EMI scores. According to this, the EMI and QBS-ar was calculated for both sampling seasons and graphs were plotted using the software Origin Pro version 9.0.
Pearson correlation analysis was done between various soil variables like soil temperature, soil moisture, soil organic carbon, soil pH and QBS-ar values of the home gardens during summer and north east monsoon to verify whether there was any significant relationship between soil properties and biological soil quality. The analysis was done using the software Ky Plot version 2.0. For each home garden, the mean QBS-ar index obtained was converted into the related soil quality classes, in which each site was assigned a soil class value from 0 to 6. According to this, the home gardens were classified as poor, medium and good based on the soil quality classes proposed by Parisi et al. (2003). This classification considers (1) the presence of eu-edaphic soil microarthropods like Proturans and Onychiurids and (2) the QBS-ar values as the determinants of soil quality classes.
Results
The soil of the study area was lateritic. Of about 70 % of the soils collected from home gardens, the top soil was black due to the accumulation of organic matter while the bottom soil was reddish in color. The soil type in all the home gardens were fine, mixed isohyperthermic typic udifluvent belonging to suborder udepts under inceptisols according to USDA classification.
The soil temperature in the home gardens of the village ranged from 27.3 to 32.3 °C in summer and from 26.1 to 30.9 °C during monsoon. The corresponding pH range was from 5.1 to 8.1 and 5.1 to 7.9. The soil moisture ranged from 5.92 to 19.71 % during summer and from 9.0 to 29.36 % in monsoon. The content of organic carbon was 0.78–7.02 % during summer and 0.78–5.46 % in monsoon sampling. The range, mean and standard deviation of the soil properties are given in Table 2.
The population size of the microarthropods was observed to vary from 0 to 14 per 1000 cm3 in the dry season. The organisms were not detected in the samples drawn from home gardens 5 and 6. The highest abundance occurred in home garden 18. The abundance of microarthropods was high in the wet season reaching from 2 to 28 per 1000 cm3. The site based occurrence of the total number of soil microarthropods is given in Fig. 1. According to this, the home garden ID 18 had the highest number of microarthropods in summer while home garden ID 1 scored higher during monsoon.
By examining the community structure of soil microarthropods, it was observed that the microarthropod community was composed of the groups collembola, coleoptera, hymenoptera, araeneae, acari and diplopoda. The percentage composition of soil microarthropods during the two sampling seasons is given in Fig. 2.
Accordingly, the microarthropod community was represented by six groups ranging from 33.67 % of collembola to 4.08 % of diplopoda in summer. During monsoon months, the soil fauna was represented by five groups ranging from 34.91 % of hymenoptera to 2.83 % of araeneae. Collembola was reduced to 26.89 % in monsoon.
The EMI values obtained for each home garden revealed that the average EMI ranged from 0.2 (for diplopoda) to 3.32 (for collembola) in summer. The monsoon range was from 1.24 to 4.56 for those groups excepting diplopoda which were totally absent in the collection (Fig. 3). A higher EMI value of 3.32 was reported by collembola followed by hymenoptera (2.8) and acari (2.4) during summer. The least EMI values were for coleoptera and araeneae (1.2) followed by diplopoda (0.2). During monsoon, acari had highest average EMI score of 4.9 followed by collembola, coleoptera and hymenoptera which had almost similar average EMI scores of 4.6, 4.6 and 4.5 respectively. Araeneae had least EMI score of 1.1. Diplopoda was absent in monsoon samples. Corresponding to the EMI scores, the QBS-ar was high in the monsoon in all home gardens. In two of the home gardens (ID 5 and ID 6) microarthropods were totally absent in the summer samples (Fig. 4).
The Pearson correlation analysis between soil temperature, soil moisture, soil organic carbon and soil pH against QBS-ar revealed that there are significant correlations existing between soil properties and QBS-ar during each sampling season. The results of the analysis are shown (Table 3). It is observed that the occurrence of microarthropods is negatively correlated to temperature in summer, and negatively correlated to pH in monsoon period. Moisture and organic carbon exhibited strong positive correlation with QBS-ar in both periods.
The grading of each home garden based on soil quality depending on the calculated soil class value is given in Table 4.
According to the soil quality classes defined by QBS-ar, two home gardens (HG 1 and HG 16) with QBS-ar score of 51 and 55 with soil class value 4 were classified as good quality home gardens, and 21 home gardens with soil class value 2 were classified as medium quality home gardens. Two home gardens which were found to contain only epi-edaphic or hemi-edaphic groups with soil class value 1 were classified as ‘poor quality’ home gardens.
Discussion
The natural variation of microarthropods in the home gardens was studied setting the hypothesis that they are healthy ecosystems. From the studies, it was understood that the soil type observed in the home gardens of Chengamanad village, the inceptisols are agricultural soils favouring the growth of crops and natural vegetation. Based on the range of soil pH values, these soils vary from strongly acidic to moderately alkaline (Department of Agriculture and Cooperation 2011) having silty loam to clayey loam texture. Strongly acidic soils (pH ranging from 4.6 to 5.5) were found in six home gardens during summer and five home gardens during north east monsoon. Moderately acidic soils (pH ranging from 5.6 to 6.5) were seen in 11 home gardens during summer and 12 home gardens during north east monsoon while slightly acidic soils (pH range between 6.6 and 6.9) were reported in three home gardens during summer and two home gardens during north east monsoon respectively. Moderately alkaline soils (pH ranging from 7.1 to 8.5) were reported from five home gardens during summer and six home gardens during north east monsoon. From the soil pH values of the 25 home gardens, it is observed that the dominant soils in the home gardens was moderately acidic representing 44 % of the total home gardens sampled during summer and 48 % of the total home gardens sampled during monsoon. No home gardens were reported to contain extremely acidic or extremely alkaline soils which are unfavourable to the plants and edaphic fauna. The level of soil organic carbon is such that these gardens fit into the high fertility level soils (Department of Agriculture and Cooperation 2011). The soil temperature in the home gardens was higher in summer while the moisture content was lower compared to the north east monsoon period. This could due to the reduced precipitation, increased soil evaporation and increased atmospheric temperature in summer.
The abundance of microarthropods varied across the home gardens as well as the sampling season. Collembola dominated the population in summer while hymenoptera was more important in the monsoon. The reduction in collembolan population during monsoon as observed in this study may be probably due to reduction of soil organic carbon, because other factors determining the collembolan number like soil moisture and soil temperature showed a favourable change during monsoon in the home gardens. Studies have proved that medium temperature and stable moisture conditions favour increased collembolan population by enhancing soil quality in addition to available food in the form of organic matter (Verma et al. 2014). This substantiates the importance of organic matter for collembolan survival and therefore, the leaching of organic matter from soil due to heavy rainfall may be a reason for the reduction of collembolan population during monsoon, as observed in this study. Since there is a positive correlation of collembolan population to increased availability of organic carbon as indicated by some studies (Verma and Paliwal 2010), organic matter availability may be the limiting factor in the reduction of collembolan population thereby reducing the soil quality during monsoon season. There are also studies stating that increased soil moisture do not significantly affect collembolan population (Mackay et al. 1986) which proves that besides moisture, there are certain other factors which determine soil habitat quality and thereby collembolan survival. It is also seen that hymenopteran population increased by 5.32 % and coleopteran population increased by 11.42 % in monsoon. Araenae and acari population showed a decrease of 3.29 and 2.57 % respectively during monsoon season compared to summer. Competition found among different microarthropod groups like collembola and mites during laboratory studies (Hagvar 1990) supports that the population of one microarthropod group in an ecosystem may be affected by competition with other group. Competition as reported in collembolans, areaeneae and certain hymenopterans may be a reason for reduction of collembolan population during monsoon. Another striking observation in this study is the disappearance of the faunal group diplopoda during October–November sampling. For deriving the exact reason for the disappearance of diplopods, continuous sampling and close study of the diplopodan ecology is mandatory.
While examining the seasonal variation in soil microarthropod numbers, the number of organisms in the home gardens was found to be higher in October–November than in April–May. The soil properties are reported to control the survival of the microarthropods. During summer, the increase in soil temperature will lead to increased soil evaporation, resulting in soil moisture deficit thereby reducing the number of less tolerant soil organisms (Kardol et al. 2011). Increased soil moisture during monsoon is found to have a favourable effect on the soil microarthropod population. An increasing soil pH in monsoon is unfavourable to the organisms, but the positive effects of increased moisture and lower temperature supports higher population. According to Kadrol et al. (2011) soil moisture is a limiting factor for the survival of soil microarthropods and many species of microarthropods try to escape from extreme dryness by burrowing deep into the soil during drought conditions. This substantiates the reduction of number of organisms in summer when compared to that of monsoon.
During summer the average EMI value was higher for the group collembolans followed by hymenoptera, acari coleoptera, araeneae and diplopoda. Since collembolans are euedaphic soil microarthropod fauna found in about 95 % of the home gardens, a higher EMI value was obtained. The hymenopteran group which ranked next to collembola in EMI score was epiedaphic fauna which did not show much specialization for a true soil life as compared to collembola. Acari and coleoptera were not found in all home gardens and their presence was smaller when compared to collembolans. Since EMI score is a reflection of the presence of euedaphic soil microarthropod fauna in soil ecosystem, the home gardens with a higher EMI score represented better quality soil ecosystems containing soil microarthropods of the groups collembolan and acari. There is a general increase in EMI values during monsoon compared to summer due to reduction in soil temperature and increase in soil moisture favouring the survival of soil microarthropods. The difference is highly prominent in coleopteran population with a threefold increase in average EMI values in monsoon than in summer. Acari population also showed a twofold increase in the average EMI value in monsoon when compared to summer. Diplopodans, which are less abundant in summer showed the least EMI score of 0.2. In general by examining the EMI scores, it may be concluded that the soils in the home gardens of Chengamanad village favoured the presence of euedaphic fauna than epiedaphic fauna during monsoon.
The highest QBS value as reported by home garden 1 during both sampling seasons followed by home garden 16 indicates that home garden 1 was with the best soil quality compared to other 24 home gardens. Compared to summer, the QBS-ar values showed an increase during monsoon in all the 25 home gardens which indicates that the conditions required for the survival soil microarthropods showed a favourable change during monsoon. These observations point to the direct influence of seasonal changes on the survival of soil microarthropods and consequently on the QBS-ar value.
It was observed that the home gardens grouped as good quality were the oldest home gardens in the study area without much human intervention and rigorous management practices and favours the sustenance of eu-edaphic fauna; while the home gardens grouped as medium quality were subjected to management practices like constant cleaning and removal of soil litter. Those home gardens under soil class 1 and 2 also had canopy cover of less than 50 % when compared with the home gardens coming under soil class 4 in which the canopy cover was about 90 %. The canopy cover as well as soil litter may be having a shielding effect on the microhabitat of the soil microarthropods, enhancing the soil habitat quality by encouraging the survival of the eu-edaphic fauna.
Conclusion
The use of soil microarthropods for the assessment of soil quality is an easier approach when compared to frequent chemical analysis. Our results prove that soil microarthropods can be effectively used for the soil quality assessment of the tropical home gardens of smaller areas like villages.
References
A’Bear AD, Boddy L, Jones TH (2013) Bottom-up determination of soil collembola diversity and population dynamics in response to interactive climatic factors. Oecologia 173(3):1083–1087
Andres P (1999) Ecological risks of the use of sewage sludge as fertilizer in soil restoration: effects on the soil microarthropod populations. Land Degrad Develop 10(1):67–77
Bandyopadhyaya I, Choudhuri DK, Ponge JF (2002) Effects of some physical factors and agricultural practices on Collembola in a multiple cropping programme in West Bengal (India). Eur J Soil Biol 38(1):111–117
Banerjee S, Sanyal AK, Bhaduri AK (2009) Studies on soil microarthropod population in three different garden plots of 24 Parganas, West Bengal-a preliminary report. Rec Zool Surv India 109(4):1–9
Begum F, Bajracharya RM, Sitaula BK, Sharma S, Ali S, Ali H (2014) Seasonal dynamics and land use effect on soil microarthropod communities in the mid-hills of Nepal. Int J Agron Agric Res 5(2):114–123
Bongers T (1999) The maturity index, the evolution of nematode life history traits, adaptive radiation and cp-scaling. Plant Soil 212:13–22
Cortet JR, Elmholt S, Coeurdassier M, Scheifler R, Krogh PH (2006) Interspecific relationships among soil invertebrates influence pollutant effects of phenanthrene. Environ Toxicol Chem 25(1):120–127
Coulson SJ, Fjellberg A, Melekhina EN, Taskaeva AA, Lebedeva NV, Belkina OA, Seniczak S, Seniczak A, Gwiazdowicz DJ (2015) Microarthropod communities of industrially disturbed or imported soils in the High Arctic; the abandoned coal mining town of Pyramiden, Svalbard. Biodivers Conserv 24(7):1671–1690
Culliney T (2013) Role of arthropods in maintaining soil fertility. Agriculture 3:629–659
Das T, Das AK (2005) Inventorying plant biodiversity in homegardens: a case study in Barak Valley. Curr Sci 89(1):155
Department of Agriculture and Cooperation (2011) Soil testing in India Ministry of Agriculture, Government of India
Department of Economics and Statistics (2013) Panchayath level statistics—2011 Ernakulam district, Government of Kerala
Fernandes ECM, Nair PKR (1986) An evaluation of the structure and function of tropical homegardens. Agric Syst 21:279–310
Galluzzi G, Eyzaguirre P, Negri V (2010) Home gardens: neglected hotspots of agro-biodiversity and cultural diversity. Biodivers Conserv 19:3635–3654
Parisi V, Menta C, Gardi, C, Jacomini C (2003) Evaluation of soil quality and biodiversity in Italy: the biological quality of soil index (QBS) approach. Agricultural impacts on soil erosion and soil biodiversity: developing indicators for policy analysis Rome. In: Proceedings from an OECD expert meeting, pp 1–12
Hagvar S (1990) Reactions to soil acidification in microarthropods: is competition a key factor. Biol Fertil Soils 9(2):178–181
Hartley W, Uffindell L, Plumb A, Rawlinson HA, Putwain P, Dickinson NM (2008) Assessing biological indicators for remediated anthropogenic urban soils. Sci Total Environ 405(1):358–369
Hodkinson ID, Coulson SJ, Webb NR, Block W (1996) Can high Arctic soil microarthropods survive elevated summer temperatures? Funct Ecol 10:314–321
Jensen M (1993a) Soil conditions, vegetation structure and biomass of a Javanese homegarden. Agrofor Syst 24:171–186
Jensen M (1993b) Productivity and nutrient cycling in a Javanese homegarden. Agrofor Syst 24:187–201
Jose D, Shanmugaratnam N (1993) Traditional homegardens of Kerala: a sustainable human ecosystem. Agrofor Syst 24(2):203–213
Kardol P, Reynolds WN, Norby RJ, Classen AT (2011) Climate change effects on soil microarthropod abundance and community structure. Appl Soil Ecol 47(1):37–44
Kautz T, Lopez-Fando C, Ellmer F (2006) Abundance and biodiversity of soil microarthropods as influenced by different types of organic manure in a long-term field experiment in Central Spain. Appl Soil Ecol 33(3):278–285
Kumar BM, Nair PR (2004) The enigma of tropical homegardens. Agrofor Syst 61(1–3):135–152
Kumar BM, George SJ, Chinnamani S (1994) Diversity, structure and standing stock of wood in the homegardens of Kerala in Peninsular India. Agrofor Syst 25:243–262
Macfadyen A (1961) Improved funnel-type extractors for soil arthropods. J Anim Ecol 30:171–184
Mackay WP, Silva S, Lightfoot DC, Pagani MI, Whitford WG (1986) Effect of increased soil moisture and reduced soil temperature on a desert soil arthropod community. Am Midl Nat 16:45–56
Mendez VE, Lok R, Somarriba E (2001) Interdisciplinary analysis of homegardens in Nicaragua: micro-zonation, plant use and socioeconomic importance. Agrofor Syst 51:85–96
Olfert O, Johnson GD, Brandt SA, Thomas AG (2002) Use of arthropod diversity and abundance to evaluate cropping systems. Agron J 94(2):210–216
Parisi V, Menta C, Gardi C, Jacomini C, Mozzanica E (2005) Microarthropod communities as a tool to assess soil quality and biodiversity: a new approach in Italy. Agric Ecosyst Environ 105:323–333
Radojevic M, Bashkin VN (2006) Practical environmental analysis. Royal Society of Chemistry, RSC, Cambridge
Rahman PM, Varma RV, Sileshi GW (2012) Abundance and diversity of soil invertebrates in annual crops, agroforestry and forest ecosystems in the Nilgiri biosphere reserve of Western Ghats, India. Agrofor Syst 85(1):165–177
Rusek J (1998) Biodiversity of Collembola and their functional role in the ecosystem. Biodivers Conserv 7(9):1207–1219
Rusek J, Marshall VG (2000) Impacts of airborne pollutants on soil fauna. Annu Rev Ecol Syst 31:395–423
Sankar S, Chandrashekara UM (2002) Development and testing of sustainable agroforestry models in different agroclimatic zone of Kerala with emphasis on socio-cultural, economic, technical and institutional factors affecting the sector. Research Report 234, Kerala Forest Research Institute, Peechi
Soil and Land Use Survey of India (2013) Inventory of soil & land resource of Ernakulam District, Kerala using remote sensing techniques. Report No. SRM:—12, Department of Agriculture & Co-operation, Ministry of Agriculture, Government of India
Soumya M, Nair PKR, Long AJ (2007) An assessment of ecological diversity in home gardens: a case study from Kerala State, India. J Sustain Agric 29(4):135–153
Swift M, Bignell D (2001) Standard methods for assessment of soil biodiversity and land use practice. International Centre for Research in Agroforestry, Bogor
Tizado EJ, Nunez‐Perez E (2014) Terrestrial arthropods in the initial restoration stages of anthracite coal mine spoil heaps in northwestern Spain: potential usefulness of higher taxa as restoration indicators. Land Degrad Develop. doi:10.1002/ldr.2280
Torquebiau E (1992) Are tropical agroforestry home gardens sustainable? International Centre for Research in Agroforestry (ICRAF). Agric Ecosyst Environ 41:189–207
Van Straleen N, Verhoef H (1997) The development of a bioindicator system for soil acidity based on arthropod pH preferences. J Appl Ecol 34:217–232
Verma D, Paliwal AK (2010) Effects of springtails community on plant growth. Biol Forum Int J 2(2):70–72
Verma D, Yadav RK, Kumar M (2014) Effect of ecological factors on population density of collembola in Agra. J Environ Appl Biores 2(1):25–28
Vreeken-Buijs MJ, Hassink J, Brussard L (1998) Relationships on soil microarthropods biomass with organic matter and pore size distribution in soil under different land use. Soil Biol Biochem 30:97–106
Yan S, Singh AN, Fud S, Liao C, Wanga S, Li Y, Cui Y, Huf L (2012) A soil fauna index for assessing soil quality. Soil Biol Biochem 47:158–165
Acknowledgments
The research is supported under the Junior Research Fellowship from Kerala State Council for Science, Technology and Environment (KSCSTE), Kerala, India. We also thank Cochin University of Science and Technology, Kerala, India for providing the research facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lakshmi, G., Joseph, A. Soil microarthropods as indicators of soil quality of tropical home gardens in a village in Kerala, India. Agroforest Syst 91, 439–450 (2017). https://doi.org/10.1007/s10457-016-9941-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-016-9941-z