Abstract
Tree-based intercropping (TBI) systems induce microclimate modifications that can enhance crop performance through water use efficiency. However, the extent of this efficiency is dependent on water acquisition strategies of trees in such agroforestry systems. Charting water uptake zones at the tree-crop root interface in TBI systems is essential for managing water resources, particularly with increasing water stress from a changing climate. In this study, we identify the water uptake zones of Juglans nigra (walnut) and Populus sp. (hybrid of Populus deltoids X Populus nigra clone DN-177) (poplar) intercropped with winter barley (Hordeum vulgare) in a temperate TBI system. Water isotopic analysis using δ18O was conducted on paired soil water and tree xylem water samples in early season (pre-crop) and late season (with crop) collected from the Agroforestry Research Station in Guelph, Ontario, Canada. Two approaches for identifying the water uptake zones were used: direct inference and multiple source mass-balance analysis. Both approaches showed that poplar exhibits a dominant soil water uptake zone at 20 cm in the soil profile in the early season, while the dominant walnut uptake zone was higher in the soil profile at 10 cm. Depth of water uptake was lower in the soil profile (40–70 cm) in the late season for both species. This study indicates: (i) tree species dependent water acquisition zones, and (ii) a shift in tree water acquisition to deeper in the soil profile over the growing season. Using isotopic techniques, we suggest that poplar and walnut root activity stratify below the crop root zone later in the growing season, potentially reducing competition for water resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tree-based intercropping (TBI) systems in temperate environments have received increased attention in order to achieve environmental advantages under a changing climate (Jose 2009; Schoeneberger et al. 2012). Trees in agricultural landscapes can mitigate water stress through microclimate modification by decreasing fluctuations in soil moisture, soil temperature, humidity and solar radiation (Lin 2007; Jose 2009; Stavi and Lal 2013) as well as credited with reducing evapotranspiration and improving crop water use efficiency (Kuemmel 2003; Thaler et al. 2012). Given predicted high temperatures and drier climate in future scenarios in Canada (Schindler and Donahue 2006), these modifications may be ever more important in our increasingly vulnerable agricultural sector.
Water cycling in TBI systems is comprised of complex belowground interactions where both crop and tree roots acquire water resources. Tree roots are known to partition soil water in two ways; temporally through seasonal displacement of leaves which controls transpirational water loss (Liang et al. 2011) and spatially through partitioning of roots to different active layers and horizontal spacing (Burgess et al. 2000; Tang et al. 2001; Isaac et al. 2014; Schwendenmann et al. 2015). Many studies draw conclusions on water acquisition based on inferences related to soil moisture content and performance metrics, although the mechanisms are unclear (Jose et al. 2000; Miller and Pallardy 2001; Wanvestraut et al. 2004; Senaviratne et al. 2012).
Quantification of this important yet understudied aspect of TBI systems requires new and innovative approaches to characterize water use efficiency. To date, there is limited investigation of tree water uptake zones in a temperate agroforestry scenario, presumably due to methodological limitations. Using stable isotope analysis, in this case the natural abundance of δ18O in plant and soil water, provides an effective technique for evaluating water interactions in the environment (Dawson et al. 2002; West et al. 2006; Isaac and Anglaaere 2013; Meissner et al. 2014; Schwendenmann et al. 2015). Oxygen isotopic profiles have been used since the 1960’s to demonstrate how trees use water. However, limited work has been conducted in agroforestry systems (Schwendenmann et al. 2010; Isaac et al. 2014) and even less in temperate TBI systems.
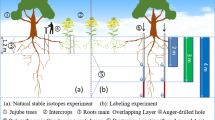
Water isotopic analysis is a useful tool for the identification of the source of plant water if two conditions are met: (i) source areas must have distinct values of isotope ratios that are consistent and significantly different from each other and greater than what would normally occur in a plant and (ii) no significant fractionation of isotopes as the water molecules travel up the plant (Ehleringer and Dawson 1992; Dawson et al. 2002). Oxygen isotopic signatures in a soil profile can exhibit differences attributed to infiltration rates, plant water uptake rates, the degree of evaporative isotopic fractionation under different vegetative covers, and processes of hydraulic redistribution (Ehleringer and Dawson 1992; Asbjornsen et al. 2008). Signatures of extracted 18O in xylem water from non-photosynthetic tissue samples of a tree are matched to the signature of δ18O in water extracted from soil horizons below an individual tree, providing a natural marker of water acquisition zones (Brunel et al. 1995).
There is a need to broaden our current understanding of water uptake, water partitioning and interspecific water competition in agroforestry systems. Key questions include: (1) What is the dominant soil zone of source water for tree species in TBI systems, (2) Do different species have alternate strategies at the same TBI site and (3) Do trees show a seasonal difference in water uptake zones in TBI systems? The objective of this study is to chart soil water uptake zones of two tree species [Juglans nigra (walnut) and Populus sp. (hybrid of Populus deltoids X Populus nigra clone DN-177) (poplar)] at two times (early season pre-crop and late season with crop) during the growing season in a temperate TBI system. We hypothesize interspecific and seasonal based differentiation in water uptake zones and that this differentiation can be detected using the natural abundance of δ18O.
Materials and methods
Study site description
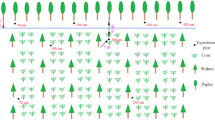
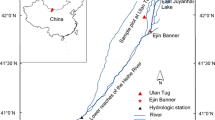
Field studies were conducted at the Agroforestry Research Station, University of Guelph, Wellington County, Ontario (43°32′49″N, 80°12′ 44″W). The research site was originally established in 1987 on 30 ha of land dedicated to studying the implementation of agroforestry systems. Twelve species of trees were planted on the site in the spring of 1988 and between 1989–1994 seven more tree species were added (Simpson 1999). Among the tree species planted were Juglans nigra (black walnut), Picea abies (Norway spruce), Quercus rubra (red oak), Thuja occidentalis and Populus nigra x deltoids cclone DN-177 (poplar hybrid) (Peichl et al. 2006). All tree species were planted in rows 12.5 or 15 m apart and a within-row spacing of 6 m for a density of 111 trees ha−1. Beginning in 1991, four different crops have been planted in the alleys between the trees. These crops were: soybean (Glycine max L.), corn (Zea mays L.), barley (Hordeum vulgare L.) and wheat (Triticum aestivum L.) (Peichl et al. 2006) with winter barley as the crop species on site during this study. The land consists of class 3 land in the Canada Land Inventory (Agriculture) designation (Gray 2000). The location is on a western slope of a drumlin resulting in the presence of glacial till with an undetermined depth of bedrock. The site has an average slope of 6 % with a maximum elevation of 346.2 m above sea level and a minimum elevation of 330.7 m above sea level (Oelbermann and Voroney 2007). Drainage varies from imperfect to moderately well-drained. The soil is classified as a gray brown luvisol in the Canadian System of Soil Classification, with a sandy loam texture consisting of 65 and, 25 % silt and 10 % clay (Oelbermann and Voroney 2007). The site has temperate climate with a hot humid summers and cold winters. Annually, the average daily temperature is 7.2°C, the average frost-free period is approximately 136 days, and annual precipitation is 830 mm, with 432 mm falling during the growing season (May–September) (Oelbermann and Voroney 2007).
Experimental design
Two sampling times were operationalized in this study: early season in the spring before planting of the crop, barley, and late season, 14 weeks later nearing crop harvest. Two criteria were used to select exact sampling times: substantial rainfall in the preceding 2 weeks (32.8 mm for early season and 75.0 mm for late season) but 2 days with no rainfall events immediately before sample collection. Daily daytime average temperatures were slightly higher in the late season sampling (16.6 °C compared to 19.6 °C). Weather data was collected from a weather station at the Guelph Turfgrass Institute adjacent to the plot.
Two tree species, poplar [Populus sp. (hybrid of Populus deltoids X Populus nigra clone DN-177)] and walnut (Juglans nigra), were subselected from the ten tree species at this study site. These two species were selected based on two criteria: (i) differentiated aboveground morphology and (ii) variable root stratification. Previous geo-imaging data using Ground Penetrating Radar at the study site demonstrated that the dominant rooting zones of poplar and walnut trees to be 1 m from the tree base and differentially distributed fine and coarse roots (Borden 2013; Borden et al. 2014). Sampled trees (n = 3 per species for early season and n = 1 per species for late season) were in close proximity (within 25 m2) to each other in order to minimize the effects of topographic features including slope and elevation.
Height, diameter at breast height (DBH) and canopy radius of each tree were collected. Mean (±S.E.) poplar and walnut tree height was 18.9 m (±2.6) and 14.8 m (±0.8), respectively. Tree DBH was larger for poplar trees at 43.6 cm (±1.9) than walnut trees at 31.3 cm (±5.0). However, the width of the tree canopy was similar for both species. The canopy width for poplar was 4.4 m (±1.5) and walnut canopy width was 4.4 m (±0.5).
Plant and soil sampling protocol
Soil samples were collected with a hand auger at a 1 m distance in the southwest direction from the base of each tree. Soil samples were taken at depths of 5, 10, 20, 30, 40, 50, 60 and 70 cm. A soil sub-sample at each depth was immediately collected in 10 ml BD vacutainer serum tubes (BD Franklin Lakes, New Jersey USA). The closed tubes were immediately sealed with parafilm to prevent evaporative fractionation and placed in a cooler. A soil sample of approximately 100–150 g was also collected at each depth interval and sealed in a bag for subsequent gravimetric moisture content analysis. A soil sub-sample was dried in a drying oven at 110 °C for 48 h and then reweighed and soil moisture content expressed as a percentage.
Non-photosynthetic tissue (tree core) was collected from each tree using a tree corer (1 cm diameter) at a tree height of 1.5 m. The trees were cored to 20 cm to ensure that the coring would reach the center of the tree. After the core was drawn from the tree, the bark was removed and the remaining core was divided into sections and 0–5 cm stem radii was subselected for analysis as the sapwood provides the most accurate representation of the water recently absorbed by roots (Busch et al. 1992; Beauchamp et al. 2013). These samples were immediately placed in vacutainers and sealed with parafilm and placed in a cooler. A total of 29 isotopic samples (24 soil water, 5 xylem water) were collected during the early season per species and 9 samples in the late season (8 soil water, 1 xylem water) per species.
Isotopic and statistical analysis
Soil samples and plant tissue samples in the tubes were refrigerated at −20 °C until they were extracted using a vacuum distillation line (West et al. 2006). The vacuum distillation line extracts all of the water of any given sample by a continuous process of heating and cooling that separates and conservatively traps all of the water out of the sample. After around 30 min of heating, vacuum distillation and trapping, all of the water has left the sample and is now trapped within the vacuum. The non-condensable gases and carbon dioxide are then removed. The isotopic composition of the soil and core water were analyzed with a Picarro H2O Cavity Ring-Down Spectrometer model L1102i (Picarro, Santa Clara, CA) at the Laboratory for Stable Isotope Sciences, Western University, Ontario, Canada and calculated as:
using Vienna Standard Mean Ocean Water (VSMOW) as the standard (Dawson 1993) with a precision of 0.2 ‰.
The use of direct matching of plant and soil isotopic signatures to determine the location of source water in a plant has been shown to be an effective method (Brunel et al. 1995). Soil depth versus δ18O was plotted for each individual tree. A vertical line was drawn on these plots at the δ18O value of the plant tissue of the tree associated with that subset of soil samples. The soil depth, which matched the tree signature was determined by the point(s) closest to this line. In the event of more than one matching value, trees of the same species were considered. The source of the soil water was the depth which was conjunct for all three trees (for a particular season).
Multiple source mass-balance analysis was also used where results are reported based on the frequency (as a percent of the total number of possible solutions) that a particular source (soil depth) contributes a proportion of the solution (plant water δ18O signature). Using small increments (1 % solution increments), source combinations that sum to the measured plant water isotopic signature within a tolerance level (±0.2 ‰) are counted as feasible solutions. The distribution of feasible solutions are represented as histographs. We also show the frequency distribution that a particular soil depth contributes. We report the percent that a particular soil depth contributes to a proportion of the mixture in decimal increments from 0–1 across four aggregated categories (0 signifies no contribution; 0.01–0.25 signifies 1–25 % proportion of mixture; 0.26–0.5 signifies 26–50 % proportion of mixture; 0.51–1 signifies 51–100 % proportion of mixture). The last category, 0.51–1, is the most promising to examine as the highest percentage in this category would indicate a dominant soil depth for contributing water. Similarly, the 0 category indicates the frequency that a soil depth is not contributing water to the plant water δ18O signature. Analysis was run at three aggregated soil layers of 5–10, 20–30, and 40–70 cm using the multiple source mass-balance analysis program Isosource (Phillips and Gregg 2003).
To determine difference in soil characteristics with depth under tree species and sampling time, soil moisture content and δ18O values (in early season) were subjected to analysis of variance using a general linear model (PROC GLM) in SAS (SAS Institute Inc. Cary, NC, USA). A type I error rate was set at p < 0.05 for statistical tests. Descriptive statistics (mean ± S.E.) for tree characteristics, soil moisture content and δ18O signatures were determined in SAS.
Results
Soil moisture content
Soil moisture content (SMC) varied with depth across species and sampling time (Fig. 1). A significant difference in SMC with soil depth was found in the early season under poplar (p < 0.0001) and under walnut (p = 0.0313) and in the late season under poplar (p = 0.0004) and walnut (p = 0.0004). In early season, SMC ranged from 28.6 % (±2.0) to 12.9 % (±0.5) at 5 and 70 cm depth, respectively, under poplars. Under walnut trees, SMC ranged from 21.6 % (±0.7) to 12.9 % (±0.8) at 5 and 70 cm, respectively, showing a similar decreasing SMC trend with depth as under poplar. In late season, the maximum SMC under poplars was 17.8 % soil at a 5 cm depth and the minimum was 6.1 % at a 40 cm depth, while in late season, SMC under walnuts mirrored that of early season (Fig. 1).
Soil and plant isotopic signatures
Soil δ18O values significantly decreased with increasing depth under both poplar (p = 0.0027) and walnut (p < 0.0001) in the early season (Table 1; Fig. 2) thus exhibiting the predicted pattern of a decreasing proportion of 18O through in the soil profile. However, at the 30 cm soil horizon, δ18O values tended to stabilize. Under walnut, another drop in δ18O values was detected below a 50 cm depth to below −12 ‰. Samples from the late season exhibited a smaller range in δ18O values under both tree species highlighted by a less clear trend in soil δ18O values with depth. Overall, pooled early season data from both species exhibited a slightly lower mean δ18O signature of −10.63 ‰ (±1.90) where as pooled late season soil water had a mean δ18O signature of −8.92 ‰ (±1.33), indicating some evaporative effects during the growing season.
Early and late season δ18O values in soil water in the soil profile at 1 m from a poplar and b walnut trees in a TBI system. Tree xylem δ18O values plotted with straight vertical lines to show intersection with soil isotopic values. Early season data, bars represent ±S.E. of the mean (n = 3); late season data (n = 1)
Water extracted from poplar tree plant samples exhibited a δ18O value range from −9.34 to –10.78 ‰ with a mean value of −9.90 ‰ (±0.77) in the early season and changed to −9.63 ‰ in the late season (Table 2). Water extracted from walnut tree plant samples exhibited a δ18O value range from −8.48 to −9.55 ‰ with a mean δ18O value of −8.91 ‰ (±0.57) in the early season and changed to −9.07 ‰ in the late season (Table 2).
Soil water uptake zones: direct inference
Using a direct inference approach, plant water extracted from poplar in the early season exhibited the above mentioned mean δ18O signature of −9.90 ‰ (±0.77) which corresponded to a water uptake zone at a soil depth of 20 cm (Fig. 2a; Table 3). In the late season, plant water extracted from poplar had a δ18O signature of −9.63 ‰ corresponded to a water uptake zone at a soil depth of between 40 and 70 cm (Fig. 2a). Similarly, using the direct method approach, early season walnut exhibited a δ18O signature of −8.91 ‰ (±0.57) which corresponded to a soil depth of 10 cm (Fig. 2b; Table 3). In the late season, similar to poplar, walnut plant water δ18O signature of −9.07 ‰ corresponded to a soil depth lower in the soil profile at 40 and 70 cm (Fig. 2b; Table 3).
Soil water uptake zones: multiple source mass-balance analysis
With the multiple source mass-balance approach, poplar trees in the early season (Fig. 3a) and late season (Fig. 3c) exhibited broad water uptake from multiple soil depths. In contrast, walnut trees exhibited a very narrow range of feasible source contributions in both early (Fig. 3b) and late (Fig. 3d) season. When examining the relative contribution of each soil depth, the 20–30 cm aggregated depth contributed the highest frequency to the solutions (28.02 %) in over 50 % of the mixture for the poplar water δ18O signature (Table 4). However, the 5–10 cm aggregated depth also contributed a high frequency to the solutions (89.46 %) but in only 26–50 % of the mixture (Table 4). This multiple source mass-balance analysis revealed that poplar trees in the late season had the highest frequency (45.84 %) from the 20–30 cm aggregated depth and the next highest (34.54 %) from the 40–70 cm aggregated depth in over 50 % of the mixture (Table 4). In contrast, for walnut tress, 5–10 cm aggregated depth contributed 100 % frequency to the solutions in the early season and 40–70 cm aggregated depth contributed 100 % frequency to the solutions in the late season in the over 50 % of the mixture (Table 4).
Histographs of the frequency of proportional contributions from each aggregated soil depth (5–10 cm, 20–30 cm and 40–70 cm) to the mixture (plant water signature) derived from multiple source mass-balance analysis for a early season poplar; b early season walnut; c late season poplar; d late season walnut in a TBI system
Discussion
Numerous studies have demonstrated that tree roots have the ability to modify the location of water acquisition based on belowground resources, aboveground demand and competition avoidance with neighbouring plants (Hutching and John 2004; Kulmatiski et al. 2010; Mulia et al. 2010; Schwendenmann et al. 2015). Yet, charting the patterns of water acquisition in-situ is limited, particularly in diverse agroecosystems. In this study, the direct inference approach revealed a dominant water acquisition zone at a soil depth of 20 cm for poplar in the early season. However, the dominant water acquisition zone for walnut trees in the early season was at a soil depth of 10 cm. This interspecific difference in early season water acquisition zones tracks with known root distribution patterns of these species. Walnut trees generally have a deep taproot that can reach a 2 m depth but with large lateral roots near the surface (Buck et al. 1999; Gauthier and Jacobs 2011). In contrast, Gray (2000), at the same study site, showed poplar rooting zones concentrated (56 %) within 2 m from the tree but throughout the soil profile. Fine root presence was minimal at the soil surface and reached a maximum at a depth of 15–20 cm (Gray 2000; Thevathasan and Gordon 2004), thus matching our isotopically derived water uptake zone. Using the same approach but with annual crops, Wang et al. (2010) also showed strong differentiation in water uptake zones between species at the same site, relating this difference to species-based root physiology. Similarly, Schwendenmann et al. (2014) clearly show species-specific water uptake strategies for trees in monocultures with the δ18O natural abundance method.
In this study, there is an unambiguous difference in the soil water isotopic profile under both poplar and walnut trees in late season as compared to early season. The seasonal difference in soil water isotopic profiles may be due in part to differences in seasonal precipitation inputs (Tang and Feng 2001). The soil water from the soil surface to 30 cm reflected the combined effects of spring precipitation and the selective evaporation of lighter isotopes. However, in late season there may have been a significant summer precipitation event, what Tang and Feng (2001) label a “soil water reset” which exchanged the soil water from the winter equilibrium state, replacing it with summer precipitation.
Despite the difference in soil water δ18O values below 30 cm, there was very little difference between the plant xylem δ18O values measured in the early and late season. The δ18O value for the deeper soil water was closer to that of the plant samples for the late season thus both the direct inference and the multiple source mass-balance approaches indicate water uptake deeper in the soil profile without a significant change in the plant sample isotopic value. Using the direct matching approach with confirmation from the mass-balance approach, walnut and poplar water acquisition shifted deeper in the soil profile to the 40–70 cm depth later in the growing season. This is not uncommon as other studies, using this approach, documented shifts in water uptake to deeper in the soil profile over time (Wang et al. 2010).
It should be noted that the mass-balance approach also indicated that the 20-30 cm depth strongly contributed to the late season poplar plant signature. The direct inference approach gives a clear match in cases where the tree signature is within the isotopic range of the soil profile and in situations where there is exclusively one soil depth with the same signature (Asbjornsen et al. 2007; Wang et al. 2010; Isaac and Anglaaere 2013). The multiple source mass-balance mixing model can be used to account for the possibility that the plant tissue water is a mixture of various soil water sources. Inconsistencies in the slope of the gradient and possibility of multiple zones of uptake makes this a valuable tool in this study. A combined approach using direct matching and multiple source mass-balance analysis of data has been used in several studies to date (Asbjornsen et al. 2007; Wang et al. 2010; Zhang et al. 2011). With this approach, we show that unlike walnut, poplar acquired soil water over a larger range of the soil profile. This is indicative of root foraging strategies (Cahill et al. 2010), a pattern of soil water acquisition that might have been misinterpreted if the direct inference approach had been used exclusively.
Interestingly, our estimated soil water uptake zones do not necessarily track with soil moisture content. This suggests that shifts in water uptake zones could be highly related to the root neighborhood (Hoekstra et al. 2014; Isaac et al. 2014). Crop (barley) rooting depth is approximately between 7 and 10 cm, potentially influencing root activity in the soil profile over the growing season. Others have documented some, although not significant, complementarity in water uptake strategies in tree-based multi-species systems (Schwendenmann et al. 2015). Such species-based differentiated root activity allows for more accurate management for niche partitioning of water uptake zones.
Conclusions
Knowledge of the active rooting zones of trees selected for use in TBI systems is critical for understanding the potential of interspecific competition in these multispecies systems. Agroforestry research has been limited in techniques to clearly chart belowground processes and the use of 18O isotopic techniques with both direct inference between plants and soil signatures or with multiple source mass balance models provides a unique tool to assess these dynamics. As shown here, poplar and walnut have alternate strategies at the same TBI site. However, both tree species show a strong seasonal difference in water uptake zones evidenced by the root activity drop deeper in the soil profile later in the season, showing alterations in water uptake zones. This is important as it demonstrates a clear ability to adapt to environmental stimuli, essential to maintain agroecosystem resiliency in the face of a changing climate.
References
Asbjornsen H, Mora G, Helmers MJ (2007) Variation in water uptake dynamics among contrasting agricultural and native plant communities in the Midwestern U.S. Agric Ecosyst Environ 121:343–356
Asbjornsen H, Shepherd G, Helmers M, Mora G (2008) Seasonal patterns in depth of water uptake under contrasting annual and perennial systems in the corn belt region of the Midwestern U.S. Plant Soil 308:69–92
Beauchamp K, Mencuccini M, Perks M, Gardiner B (2013) The regulation of sapwood area, water transport and heartwood formation in sitka spruce. Plant Ecol Divers 6:45–56
Borden KA (2013) Tree roots in agroforestry: evaluating biomass and distribution with ground penetrating radar. M.Sc. Thesis. University of Toronto
Borden KA, Isaac ME, Thevathasan NV, Gordon AM, Thomas SC (2014) Estimating coarse root biomass with ground penetrating radar in a tree-based intercropping system. Agrofor Syst 88:657–669
Brunel JP, Walker GR, Kennett-Smith AK (1995) Field validation of isotopic procedures for determining sources of water used by plants in a semi-arid environment. J Hydrol 167:351–368
Buck LE, Lassoie JP, Fernandes ECM (1999) Agroforestry in sustainable agricultural system. CRC Press LLC, Boca Raton
Burgess SSO, Adams MA, Turner NC, Ward B (2000) Characterisation of hydrogen isotope profiles in an agroforestry system: implications for tracing water sources of trees. Agric Water Manage 45:229–241
Busch DE, Ingraham NL, Smith SD (1992) Water uptake in woody riparian phreatophytes of the southwestern United States: a stable isotope study. Ecol Appl 2:450–459
Cahill JF, McNickle GG, Haag JJ, Lamb EG, Nyanumba SM, St. Clair CC (2010) Plants integrate information about nutrients and neighbors. Science 328:1657
Dawson TE (1993) Hydraulic lift and water use by plants: implications for water balance, performance and plant-plant interactions. Oecologia 95:565–574
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu K (2002) Stable isotopes in plant ecology. Annu Rev Ecol Syst 33:507–559
Ehleringer R, Dawson E (1992) Water uptake by plants: perspectives from stable isotope composition. Plant Cell Environ 15:1073–1082
Gauthier MM, Jacobs DF (2011) Walnut (Juglans spp.) Ecophysiology in response to environmental stresses and potential acclimation to climate change. Ann For Sci 68:1277–1290
Gray GRA (2000) Root distribution of hybrid poplar in a temperate agroforestry intercropping system. M.Sc. Thesis. University of Guelph
Hoekstra NJ, Finn JA, Hofer D, Luscher A (2014) The effect of drought and interspecific interactions on depth of water uptake in deep- and shallow-rooting grassland species as determined by δ18O natural abundance. Biogeosciences 11:4493–4506
Hutchings MJ, John EA (2004) The effects of environmental heterogeneity on root growth and root/shoot partitioning. Ann Bot 94:1–8
Isaac ME, Anglaaere LCN (2013) An in-situ approach to detect tree root ecology: linking ground-penetrating radar imaging to isotope-derived water acquisition zones. Ecol Evol 3:1330–1339
Isaac ME, Anglaaere LCN, Borden K, Adu-Bredu S (2014) Intraspecific root plasticity in agroforestry across edaphic conditions. Agric Ecosyst Environ 185:16–23
Jose S (2009) Agroforestry for ecosystem services and environmental benefits: an overview. Agrofor Syst 76(1):1–10
Jose S, Gillespie AR, Seifert JR, Biehle DJ (2000) Defining competition vectors in a temperate alley cropping system in the midwestern USA: 2. Competition for water. Agrofor Syst 48:41–59
Kuemmel B (2003) Theoretical investigation of the effects of field margin and hedges on crop yields. Agric Ecosyst Environ 95:387–392
Kulmatiski A, Beard KH, Verweij RJ, February EC (2010) A depth-controlled tracer technique measures vertical, horizontal and temporal patterns of water use by trees and grasses in a subtropical savanna. New Phytol 188:199–209
Liang WL, Kosugi K, Mizuyama T (2011) Soil water dynamics around a tree on a hillslope with or without rainwater supplied by stemflow. Water Resour Res 47:1–16
Lin BB (2007) Agroforestry management as an adaptive strategy against potential microclimate extremes in coffee agriculture. Agric For Meteorol 144:85–94
Meissner M, Köhler M, Schwendenmann L, Hölscher D, Dyckmans J (2014) Soil water uptake by trees using water stable isotopes (δ2H and δ18O) − a method test regarding soil moisture, texture and carbonate. Plant Soil 376:327–335
Miller AW, Pallardy SG (2001) Resource competition across the crop-tree interface in a maize-silver maple temperate alley cropping stand in Missouri. Agrofor Syst 53:247–259
Mulia R, Dupraz C, Noordwijk M (2010) Reconciling root plasticity and architectural ground rules in tree root growth models with voxel automata. Plant Soil 337:77–92
Oelbermann M, Voroney RP (2007) Carbon and nitrogen in a temperate agroforestry system: using stable isotopes as a tool to understand soil dynamics. Ecol Eng 29:342–349
Peichl M, Thevathasan NV, Gordon AM, Huss J, Abohassan RA (2006) Carbon sequestration potentials in temperate tree-based intercropping systems, southern Ontario, Canada. Agrofor Syst 66:243–257
Phillips DL, Gregg JW (2003) Source partitioning using stable isotopes: coping with too many sources. Oecologia 136:261–269
Schindler DW, Donahue WF (2006) An impending water crisis in Canada’s Western Prairie provinces. Proc Natl Acad Sci USA 103:7210–7216
Schoeneberger M, Bentrup G, de Gooijer H, Soolanayakanahally R, Sauer T, Brandle J, Zhuo X, Current D (2012) Branching out: agroforestry as a climate change mitigation and adaptation tool for agriculture. J Soil Water Conserv 67:128–136
Schwendenmann L, Veldkamp E, Moser G, Holscher D, Kohler M, Clough Y et al (2010) Effects of an experimental drought on the functioning of a cacao agroforestry system, Sulawesi, Indonesia. Glob Change Biol 16:1515–1530
Schwendenmann L, Pendall E, Sanchez-Bragado R, Kunert N, Hölscher D (2015) Tree water uptake in a tropical plantation varying in tree diversity: interspecific differences, seasonal shifts and complementarity. Ecohydrology 8:1–12. doi:10.1002/eco.1479
Senaviratne GMA, Udawatta RP, Nelson KA, Shannon K, Jose S (2012) Temporal and spatial influence of perennial upland buffers on corn and soybean yields. Agron J 104:1356–1362
Simpson JA (1999) Effects of shade on corn and soybean productivity in a tree based intercrop system. M.Sc. Thesis. University of Guelph, Guelph, Ontario
Stavi I, Lal R (2013) Agroforestry and biochar to offset climate change: a review. Agron Sustain Dev 33:81–96
Tang K, Feng X (2001) The effect of soil hydrology on the oxygen and hydrogen isotopic compositions of plants’ source water. Earth Planet Sci Lett 185:355–367
Thaler S, Eitzinger J, Tranka M, Dubrovsky M (2012) Impacts of climate change and alternative adaptation options on winter wheat yield and water productivity in a dry climate in Central Europe. J Agric Sci 150:537–555
Thevathasan NV, Gordon AM (2004) Ecology of tree intercropping systems in the north temperate region: experiences from southern Ontario, Canada. Agrofor Syst 61:257–268
Wang P, Song X, Han D, Zhang Y, Liu X (2010) A study of root water uptake of crops indicated by hydrogen and oxygen stable isotopes: a case in Shanxi province, China. Agric Water Manage 97:475–482
Wanvestraut RH, Jose S, Nair PKR, Brecke BJ (2004) Competition for water in a pecan (carya illinoensis K. Koch)–cotton (Gossypium hirsutum L.) Alley cropping system in the southern United States. Agrofor Syst 60:167–179
West AG, Patrickson SJ, Ehleringer JR (2006) Water extraction times for plant and soil materials used in stable isotope analysis. Rapid Commun Mass Spectrom 20:1317–1321
Zhang C, Zhang J, Zhao B, Zhu A, Zhang H, Huang P, Li X (2011) Coupling a two-tip linear mixing model with a δD–δ18O plot to determine water sources consumed by maize during different growth stages. Field Crop Res 123:196–205
Acknowledgments
We thank the faculty, staff, and students at the University of Guelph Agroforestry Research Station and the Laboratory for Stable Isotope Sciences, Western University, Ontario, Canada. We thank anonymous journal reviewers. Funding was provided by the Agriculture and Agri-Food Canada’s Agricultural Greenhouse Gases Program, and the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Link, C.M., Thevathasan, N.V., Gordon, A.M. et al. Determining tree water acquisition zones with stable isotopes in a temperate tree-based intercropping system. Agroforest Syst 89, 611–620 (2015). https://doi.org/10.1007/s10457-015-9795-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-015-9795-9