Abstract
Angiogenesis is a multistep process that requires highly regulated endothelial cell (EC) behavior. The transcription factor Krüppel-like factor 4 (KLF4) is a critical regulator of several basic EC functions; we have recently shown that KLF4 disturbs pathological (tumor) angiogenesis by mediating the expression of members of VEGF and Notch signaling pathways. Notch signaling is central to orchestration of sprouting angiogenesis but little is known about the upstream regulation of Notch itself. To determine the role of KLF4 in normal (developmental) angiogenesis, we used a mouse retinal angiogenesis model. We found that endothelial-specific overexpression of KLF4 in transgenic mice (EC-K4 Tg) leads to increased vessel density, branching and number of tip cell filopodia as assessed on postnatal day 6 (P6). The hypertrophic vasculature seen with sustained KLF4 overexpression is not stable and undergoes prominent remodeling during P7–P12 resulting in a normal appearing retinal vasculature in adult EC-K4 Tg mice. We find that KLF4 inhibits Delta-like 4 (DLL4) expression in the angiogenic front during retinal vascular development. Furthermore, in an oxygen-induced retinopathy model, overexpression of KLF4 results in decreased vaso-obliteration and neovascular tuft formation that is similar to genetic or pharmacologic DLL4 inhibition. Mechanistically, we show that KLF4 disables the activity of the essential Notch transcriptional activator RBP-J by interfering with binding of co-activators NICD and MAML at intron 3 of the Notch ligand DLL4. In summary, our experimental results demonstrate a regulatory role of KLF4 in developmental angiogenesis through regulation of DLL4 transcription.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiogenesis encompasses the formation of new vessels from preexisting vascular beds and involves sequential sprout formation, lumen formation, mural cell coverage, and finally, stabilization of the new network. This process is tightly regulated by hypoxia-dependent proangiogenic factors, such as vascular endothelial growth factor (VEGF), which binds to its receptors on ECs [1]. In addition to external stimuli, the dynamic regulation of endothelial cell (EC) proliferation and angiogenic sprouting also involves intrinsic signaling interactions between ECs, which are regulated by the NOTCH pathway [2,3,4]. Notch receptors are transmembrane proteins that transform extracellular signals initiated by ligands into intracellular signaling events. Ligand binding to the extracellular domain of NOTCH receptors initiates proteolytic cleavage resulting in release into the cytoplasm of a peptide termed the Notch intracellular domain (NICD). NICD translocates to the nucleus and engages transcription factor RBP-J. This interaction assists in bringing together other co-activator proteins of the mastermind-like (MAML) family. The resulting RBP-J–NICD–MAML complex then interacts with the transcription machinery, effecting activation of NOTCH-dependent target genes [5]. Delta-like ligand 4 (DLL4) is one of five mammalian Notch ligands and is a crucial regulator of sprouting angiogenesis. The interaction of DLL4 and NOTCH1 in an EC results in inhibition of DLL4 expression in the adjacent ECs, a process called lateral inhibition [6]. During sprouting angiogenesis, DLL4-mediated NOTCH activity regulates endothelial sprouting and proliferation. In the context of decreased NOTCH–DLL4 interaction, an overabundance of endothelial sprouting occurs. While the effect of DLL4 deficiency on tip versus stalk cell differentiation is a matter of continued investigation [7], it is agreed that it leads to a disturbance of the process necessary to form a functional, mature new vascular network [8,9,10]. The role of NOTCH signaling is widely appreciated in angiogenesis and vascular biology; however, the molecular mechanisms governing upstream regulation of NOTCH genes have remained elusive.
Krüppel-like factors (KLFs) are a family of evolutionarily conserved zinc finger DNA-binding transcription factors that play diverse roles during differentiation and development [11,12,13]. Previous studies identified KLF4 as critical regulator of EC homeostasis, vascular inflammation, and thrombosis [14, 15]. Recently, our group showed that sustained KLF4 expression promotes ineffective tumor angiogenesis leading to diminished tumor growth [16]. Within the background of demonstrated roles of KLF4 in endothelial homeostasis and pathologic angiogenesis, we focus herein on the role of KLF4 in developmental angiogenesis, oxygen-induced retinal vascular pathology, and the molecular mechanism of the transcriptional regulation of key factors of Notch signaling.
Methods
Mice
All mice were bred on a C57Bl/6 background. EC-K4 Tg mice were generated in our laboratory as previously described [15, 16], with constitutive expression of human KLF4 in EC-K4 Tg mice driven by the VE-cadherin promoter. For creation of the inducible endothelial KLF4 knockout mice (KLF4i∆EC), we mated KLF4 flox/flox and CDH5(PAC)CreERT2 mouse lines (kindly provided by K. Kaestner, University of Pennsylvania, USA, and R.H. Adams, University of Münster, Germany, respectively). To trigger gene inactivation in newborn mice, intraperitoneal injections of 50 µl tamoxifen (1 mg/ml in ethanol/peanut oil) were given daily on P1–P3 [17]. Robustness and endothelial specificity of KLF4 knockdown were demonstrated by QPCR and western blot analysis of mouse blood, lung, and isolated ECs (data not shown). Retinal phenotypes of mutant mice were analyzed at postnatal day P4-adulthood, as indicated in each experiment. All animals and protocols were used in accordance with the Institutional Animal Care and Use Committee of Case Western Reserve University and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Processing and analysis of retinas
Dissection and whole-mount staining of retinas were performed as previously described [17]. Enucleated eyes were fixed for 1 h in 4% paraformaldehyde, rinsed three times in PBS, dissected, and stored in methanol at − 20 °C. Immunohistochemistry of whole-mount samples was performed by using isolectin IB4 (IsoB4; Thermo Fisher Scientific, catalog #I21411, 1:200), rabbit anti-DLL4 antibody (Abcam, catalog #ab7280, 1:50), rabbit anti-desmin antibody (Abcam, catalog #ab8592, 1:100), mouse anti- α-SMA (Sigma, catalog #F3777, 1:100), and rabbit anti-collagen IV (Abcam, catalog #ab6586, 1:100). For detection, suitable specific Alexa Fluor-coupled secondary antibodies were used (Thermo Fisher Scientific, 1:1000). For the in vivo BrdU incorporation assay, 100 µg of BrdU (BD Pharmingen) per gram of body weight was injected intraperitoneally 4 h before the mice were euthanized with CO2 followed by cervical dislocation.
Retinas were isolated and collected for analysis as above. BrdU-positive cells were stained by mouse antiBrdU antibody (Cell signaling, catalog #5292, 1:100).
Microscopy and image analysis
Fluorescent images were taken using a Leica DM2000 microscope for low-magnification images and a Leica Sp8 confocal laser scanning microscope for high-magnification images. The images were processed using ImageJ (NIH, Bethesda, MD) and vascular characteristics of the retina were analyzed using Angiotool software (National Cancer Institute) [18]. Quantification is based on a minimum of six mutant and six control animals for each time point and experimental condition, and mice were litter-matched. For phenotypic analysis, low-magnification images were taken of isolectin B4-labeled control and mutant retinas. A composite picture of the whole retinal vasculature was obtained from partially overlapping images (× 10 lens) by using Photoshop CS5 (Adobe Systems) as shown previously [17]. Quantitation of percent of surface area covered by vessels (vascular density) was performed in each quadrant of the whole mount on the vascular plexus located between an artery and a vein (i.e., area coverage). Quantitation of branching points and vessel length was performed using the entire composite picture of the retina. For quantitation of sprouts and filopodia, confocal images of the angiogenic front were used. The angiogenic front was defined as the line between line connecting the bases of the sprouting ECs and line connecting the tips of filopodia. The vessels density of angiogenic front was calculated as the ratio of surface area covered by vessels per total area limited by angiogenic front on one side and a line 200 µm form angiogenic front across a whole retina. The total number of filopodia was counted per angiogenic front in each field and calculated as the ratio of the total number of filopodia at the angiogenic front line per field (250 µm). Arteries and veins were identified based on standard morphological criteria. The total length of α-SMA-positive arteries was measured in each retina from the optical nerve head in the central retina to the end of the α-SMA-positive portion of the arteries. Pericyte coverage was quantified as the desmin-positive area among the total isolectin B4-positive area. To analyze vessel remodeling, the whole-mount retinas were labeled with isolectin B4 and collagen IV. A set of high-resolution images of the central capillary plexus between an artery and a vein was taken and quantified for the ratio of collagen IV positive to isolectin B4 negative sprouts.
Oxygen-induced retinopathy and intravitreal injections
Oxygen-induced retinopathy was induced as previously described [19]. Briefly, P7 litters were placed in hyperoxia (75% oxygen) to induce pervasive capillary obliteration in the central retina. After 5 days hyperoxia (P7–P12), the litters were placed back in room air. At P17, the mice were euthanized and the retinas were processed for whole-mount staining. For short-term studies designed to evaluate the mechanisms leading to vaso-occlusion, pups were exposed to hyperoxia from P9 to P10. Five minutes prior to euthanasia, mice are perfused via jugular vein with fluorescein-labeled Griffonia Simplicifolia Lectin I (GSL; Vector Laboratories, catalog #FL-1201, 25 mg/kg) [20]. Recombinant mouse DLL4 and DLL4-Fc (R&D Systems, 200 µg/ml) was injected intravitreally (300 nl) at P6 as described previously [20] and the pups were sacrificed after 24 h to evaluate the rescue effect of Dll4 on mutant retinal vessels phenotype. Quantification of avascular area and neovascular tufts was performed as described previously [19, 21]. Briefly, vessels stained with isolectin B4 were outlined in Photoshop CS6 and the number of pixels in vascularized areas were compared to the total number of pixels of the entire retina. The morphology of neovascular tufts were identified under microscope and pixel area of pathologic neovascular tufts were quantified and compared to the total pixel area in whole retina.
Luciferase reporter assays
HUVEC were plated in 24-well plates and transfected at 60–70% confluency. pCDNA3 expression vectors (0.1 µg/well) containing either full-length KLF4 or mutant KLF4 lacking the DNA-binding zinc finger domains KLF4(−) ZnF [15] were transfected where indicated. pGL4.10 reporter plasmids expressing luciferase under the control of a promoter region consisted of the 2.0 kb region upstream of the Dll4 transcription start site (− 2008 bp), the 820 bp intron 3 of Dll4 (Intron 3), or both the 2.0 kb region and intron 3 (− 2008bp + Intron3). Renilla-expressing plasmids (0.1 μg per well) were included as an internal control to correct for transfection efficiency. Luciferase assays were analyzed using the Dual-Luciferase Reporter Assay System (Promega) at 48 h after transfection.
Quantitative PCR
All qPCR reagents used were from Light Cycler 480 kits (Roche, 04887-301001 or -352001) using probes from the Roche Universal Probe Library. Gene expression was normalized to platelet EC adhesion molecule (PECAM-1) using the ΔΔCp method and shown as relative fold-change to the respective control. Control samples are normalized to 1 and fold-change values are expressed relative to the respective control. Primer sequences are shown in Table 1.
Chromatin immunoprecipitation assays (ChIP)
ChIP assays were performed according to the manufacturer’s instructions of a commercial ChIP assay kit (Millipore, 17-295). Briefly, HUVEC were infected with empty virus or an adenovirus overexpressing human KLF4 for 48 h, then fixed in 1% paraformaldehyde at 37 °C for 10 min. The fixed HUVEC were then washed in cold PBS containing protease inhibitors, scraped from the culture dish, and resuspended in lysis buffer. DNA was sheared to fragments between ~ 200 and 1000 bp in size using a Bioruptor sonicator (6 cycles, 30 s each cycle, “high” power). The cell lysate was collected and diluted tenfold, followed by treatment with a protein A-agarose/salmon sperm DNA slurry. The supernatant was then incubated overnight at 4 °C with 5 µg of antibody against KLF4 (Sigma, catalog #HPA002926), NICD (Abcam, catalog #ab27526), MAML1 (Cell Signaling, catalog #12166), RBP-J (Abcam, catalog #b25949), or IgG control. The immunoprecipitant was collected by pulldown with the protein A-agarose/salmon sperm DNA slurry and washed. DNA-protein cross-links were reversed with 5 M NaCl, and DNA was recovered using the Qiagen PCR purification kit. Input and immunoprecipitated products were subjected to qPCR using the primers listed in Table 2. Data are normalized to input DNA and expressed as a percentage of input.
Immunoprecipitation
HUVEC were transfected with a pCDNA3 vector containing the full-length KLF4 sequence or not (as control). After 2 days, the cells were lysed and cell extracts were precleared with protein A-agarose (50% v/v). The supernatants were then immunoprecipitated with 2 µg anti-RPB-J antibody (Abcam, ab25949) overnight at 4 °C. Protein complexes were collected by centrifugation at 12,000 rpm for 60 s at 4 °C and washed five times with RIPA buffer. Bound proteins were resolved by SDS–PAGE and transferred to a nitrocellulose membrane, followed by blotting with the anti-KLF4 (R&D, AF3640), NICD (Cell Signaling, 4147), anti-MAML1 (Cell signaling, 12166), and RPB-J (Abcam, ab25949) antibodies. The immunoblots were developed using chemiluminescence (SuperSignal West Dura Extended Duration Substrate, Thermo Scientific, USA) and visualized using the Chemidoc MP Imaging System (Bio-Rad, Hercules, CA, USA). The experiments were replicated three times.
Statistics
All data are reported as the mean ± SD. Student’s two-tailed non-paired t test were used to determine the statistical significance. The significance level was set at p ≤ 0.05 and notated by an asterisk (*).
Results
EC-specific overexpression of KLF4 results in increased sprouting angiogenesis in the retina
In mice, development of the retinal vasculature occurs during postnatal days P1–P21. We used a mouse model of retinal angiogenesis, as it is highly amenable to the investigation of the molecular mechanism of normal (developmental) angiogenesis. Perturbations of this system correlate well to human disease; for example, the mouse model of OIR is commonly used to study the retinopathy of prematurity suffered by premature infants.
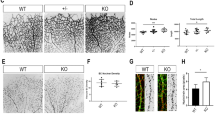
Endogenous KLF4 is expressed in arteries, veins, and microvessels of the developing retinal vasculature, as demonstrated at P6 in wild-type mice, with arterial expression appearing strongest (Fig. 1a). In order to study the effect of sustained overexpression of KLF4 during developmental angiogenesis, we used transgenic mice with endothelial-specific KLF4 overexpression driven by the VE-cadherin promoter (EC-K4 Tg) [15]. The endothelial expression of KLF4 in retinas of transgenic mice is 4–5 times that of wild-type mice (Supplemental Fig. 1), consistent with over expression we have seen in other vascular beds [15, 16]. At P6, the retinas of EC-K4 Tg mice had higher vessel density, increased vessel branching, and increased total vessel length (Fig. 1b–d). Tip cell filopodia are a key measure of angiogenesis as they indicate that ECs at the angiogenic front are responding to growth signals [22]. We found that the angiogenic front of the retinal vasculature in the EC-K4 Tg mice has more filopodia than is found in the respective control mice, suggesting excessive numbers of tip cells, leading to increased vascular density. This disturbance of normal angiogenesis appears to be related to the sustained overexpression of KLF4; mice with endothelial-specific deletion of KLF4 (KLF4i∆EC) have no vascular defects in the retinal vasculature at P6 (Supplemental Fig. 2). Combined, these data show that EC-specific overexpression of KLF4 results in hypertrophic vascular patterning in the early phase of retinal development.
EC-specific KLF4 overexpression alters retinal angiogenesis. a Endogenous KLF4 expression is seen throughout the developing retinal vasculature including arteries, veins, and capillaries. Representative confocal images of a P6 wild-type retina quadrant (left), the central vascular plexus (right upper), and angiogenic front (right lower) stained for KLF4 (white). “A” indicates artery; “V” indicates vein. b Confocal images show increased vessel density in P6 whole-mount retinas of EC-K4 Tg mice (IsoB4, white). The angiogenic front inset from the upper panel is shown enlarged in the lower panel. c High magnification of the angiogenic front shows increased number of filopodia (scored with yellow dots) in ECK4 Tg mice. whole-mount retinas at P6 were stained with IsoB4 (white). d Quantification of vasculature parameters in wild-type and EC-K4 Tg retinas as indicated in experimental procedures. Vessel length is illustrated after normalized to WT. Data are means ± SD of at least six mice per group. *p ≤ 0.05. Scale bar, a 100 µm, b 200 µm, c 25 µm
The role of KLF4 in mural cell coverage, EC proliferation, and stability of blood vessels
To describe the normal development of blood vessels in more detail, we analyzed additional factors involved in angiogenesis such as mural cell coverage and EC proliferation. Since we previously had found that endothelial KLF4 deficiency can alter vascular muscularization in hypoxia-induced pulmonary hypertension [23], we analyzed the coverage of blood vessels by mural cells during normal angiogenesis. We stained for desmin and α-smooth muscle actin (αSMA) to detect pericytes and VSMCs, respectively [2, 24]. While ECK4 Tg mice have a higher density of desmin-positive vessels compared to control (Fig. 2a, b), after normalizing to total vessel density there is no difference (data not shown), suggesting that altered EC interaction with pericytes is not associated with the vascular hypertrophy seen with sustained EC KLF4 expression. High-magnification images (Supplemental Fig. 3) show that KLF4 expression is limited to the elongated nuclei of the endothelium and is seen in both SMA-positive and -negative vessels, with possible enhanced expression at branch points. Staining of pericytes with NG2 demonstrates that the bulbous, extravascularly projecting pericyte nuclei do not express KLF4. Endothelial overexpression of KLF4 led to a decrease in the axial length of the αSMA-stained portion of the arteries in P6 retinas (Fig. 2a, c, e). This may be an abnormality that is secondary to the enhanced vascular density in the EC-K4 Tg mice, as morphologically identifiable arteries branch into and meld with the peripheral vascular pattern when less distant from the optic nerve. Alternatively, this may indicate that enhanced EC KLF4 leads to a reduction in arterial differentiation during development. In adult mice, EC KLF4 deficiency increases the severity of pulmonary vascular muscularization in hypoxia-induced pulmonary hypertension [23]. Hypoxia-induced KLF4 expression in SMC progenitors increases dedifferentiation and clonal expansion of these cells at the pulmonary arteriole muscular–unmuscular border [25]. Our (and others) current hypothesis is that KLF4 results in different vascular effects when expressed in EC versus VSMC. KLF4 is expressed by EC under basal conditions, with the most potent identified physiologic stimulus being shear stress [14, 26]. KLF4 is not significantly expressed in VSMC under basal conditions but is transiently induced under noxious stimuli such as vascular injury [27] or hypoxia as above. Interestingly, the only KLF4 signal we saw in this study that could represent VSMC expression is at a muscular–unmuscular border (Supplemental Fig. 3, left column). Complete investigation of the meaning of the differential aSMC with EC KLF4 overexpression is the focus of ongoing EC-VSMC crosstalk experiments in our laboratory.
KLF4 alter smooth muscle cell coverage of developing vessels but does not affect endothelial cell proliferation. a Images stained to identify vascular coverage by desmin and αSMA. Destine signal is highlighted in the peripheral box, αSMA staining in the more central box. P6 whole-mount wild-type and EC-K4 Tg retinas were stained for IsoB4 (blue), α-smooth muscle actin (αSMA, red), and desmin (green). b Expanded view of the peripheral box from (a). After normalization for vascular density, there is no significant difference in the EC coverage by desmin-positive pericytes (data not shown). c Expanded view of the central box from (a). Developing arteries in EC-K4 Tg retinas have decreased smooth muscle cell coverage compared to wild type. Quantification of the smooth muscle cell coverage was performed as in Experiment Procedures and normalized to WT, shown in (e). d Proliferation of ECs was assessed by BrdU labeling (green) of wild-type and EC-K4 Tg P6 whole-mount retinas. Normalization of BrdU signal to total EC area (isolectin B4, red) demonstrates that there is no difference in EC proliferation between WT and EC-K4 Tg retinas, shown in (f). Data are means ± SD of at least four mice per group. *p ≤ 0.05. Scale bar a–c 100 µm
Importantly, the distance from the optic nerve to the angiogenic front is the same, throughout development, in wild-type and EC-K4 Tg mice. In vivo labeling with 5-bromodeoxyuridine (BrdU) shows that EC-K4 Tg mice have more BrdU-positive cells compared to control (Fig. 2d); however, after normalization for total vascular density, there is no difference between control and EC-K4 Tg (Fig. 2f), suggesting that increased EC proliferation is not the primary cause of the enhanced retinal vascular density in the EC-K4 Tg mice. In addition, the normalization of BrdU-positive cells to vascular density of angiogenic front have not showed differences between groups. These data are consistent with our previous in vitro data [16].
To determine temporal changes in the organization of the vascular network, we analyzed whole-mount retinas between P4 and P21. As described earlier, EC-K4 Tg mice have higher blood vessel density at the early phase of retinal vascular development. This is seen at days P4 and P6 in Fig. 3a. At P10 and thereafter however, the density and architecture of the retinal blood vessels in control and EC-K4 Tg mice are indistinguishable (Fig. 3a). Analysis of the retinal vasculature of adult mice showed no difference in vessel density in the adult EC-K4 Tg mice compared to control (Supplemental Fig. 4). Remodeling and maturation are later stages of sprouting angiogenesis, and vessels of the retina undergo significant remodeling during P10–P21 [28]. Regressing blood vessels leave empty sleeves of extracellular matrix deposits rich in collagen IV [29]. We found more matrix sleeves (collagen IV positive, isolectin B4 negative) in EC-K4 Tg mice at P10 and P12 (Fig. 3b, c), indicating a more active remodeling process at these time points. In summary, these data suggest that differences in mural cell association with ECs in the context of EC-KLF4 overexpression are related to the changes in vascular density rather than to primary alterations of mural cell numbers or EC proliferation.
Sustained KLF4 overexpression leads to increased vascular density in early retinal vascular development, followed by enhanced remodeling. a EC KLF4 overexpression results in increased retinal vessel density at the early stage of retinal angiogenesis as shown by images of IsoB4 stained whole-mount retinas harvested at P4 and P6. However, images collected from P10 onward show similar vascular density in WT and EC-K4 Tg mice (WT upper rows, EC-K4 Tg, lower rows). b The number of empty sleeves (collagen IV positive, isolectin negative) in the central capillary plexus is higher in EC-K4 Tg retina compared to WT, indicating enhanced remodeling. Confocal images of whole-mount isolectin B4 (green) and collagen IV (red) stained P10 retinas. c Quantification of vascular density (upper panel) and vascular remodeling (lower panel) in wild-type and EC-K4 Tg retinas. Data are means ± SD of at least six mice per group. *p ≤ 0.05. Scale bar a 200 µm, b 50 µm
The administration of DLL4 results in rescue of altered vascular development in EC-K4 Tg mice
The vascular alteration in EC-K4 Tg mice is reminiscent of that seen with DLL4 deficiency, which is characterized by enhanced angiogenic sprouting and branching at P6 [6, 20]. Thus, we assessed the amount of DLL4 by immunostaining whole-mount mouse retinas. DLL4 is largely expressed at the angiogenic front; DLL4 levels were significantly decreased in EC-K4 Tg mice compared to control (Fig. 4a). This observation is consistent with our previous data which demonstrated that sustained overexpression of KLF4 downregulates EC Dll4 in culture and in a tumor model of sprouting angiogenesis [16]. We confirmed decreased endothelial expression of Dll4 and the Notch target gene Hes1 in the EC-K4 Tg retinas (Supplemental Fig. 1). To determine whether adjustment of DLL4 protein level in the retina can ameliorate the angiogenic phenotype seen in EC-K4 Tg mice, we performed intravitreal injections of recombinant mouse DLL4 or DLL4-Fc protein at P6. Analysis of whole-mount retinas 24 h after DLL4 administration (P7) shows a reversal of the hypertrophic sprouting angiogenesis phenotype in the EC-K4 Tg mice (Fig. 4b); however it does not affect the vessels density (Supplemental Fig. 5). These data suggest that hypertrophic developmental angiogenesis in the context of sustained EC KLF4 expression is a consequence of decreased DLL4–Notch signaling.
Intravitreal administration of DLL4 rescues the hypertrophic EC-K4 Tg retinal vascular phenotype. a EC KLF4 overexpression leads to decreased DLL4 levels in the angiogenic front of the developing retinal vasculature. Confocal images of the angiogenic front of representative whole-mount retinas from wild-type and EC-K4 Tg mice at P6 (left). Quantification of the relative DLL4 levels is shown to the right. The retinal vasculature was stained with isolectin B4 (red) and DLL4 (green). b Intravitreal injection of DLL4 at P5 leads to normalization of filopodia number within 24 h. Images are of whole-mount retinas of wild-type and EC-K4 Tg mice at P6 (harvested 24 h after administration of vehicle or DLL4 (50 ng) was injected intravitreally). Quantification of filopodia is shown to the right. Data are means ± SD of at least four mice per group. *p ≤ 0.05. Scale bar a 50 µm; b 25 µm
Sustained overexpression of KLF4 results in reduction of avascular area and neovascular tufts induced by relative hyperoxia
Although the cause and pathogenesis of various human ischemic retinopathies varies, the last stage is very similar and is characterized by avascular areas and the formation of neovascular tufts which project towards the vitreous and are a hallmark of proliferative retinopathies [30].The OIR model has been widely used to study the response of retinal blood vessels to hyperoxia and to determine the molecular factors involved in subsequent neovascular tuft formation [30,31,32,33]. The animal OIR model includes two phases: (1) initial capillary vaso-obliteration induced by ambient hyperoxia at P7–P12, which leads to avascular areas in the retina, and (2) retinal hypoxia upon subsequent return to room air at P12–P17, with excessive release of angiogenic factors that results in the formation of neovascular tufts. In EC-K4 Tg mice, 24 h of hyperoxia (P7, 75% O2) results in decreased vaso-constriction than in wild-type mice as demonstrated by greater retinal perfusion at P8 (Fig. 5a). After 5 days of hyperoxia (P7–P12) however, control and EC-K4 Tg mice demonstrate a similar degree of vessel loss (Fig. 5b, left panels). Finally, after return to room air for 5 days (P12–P17), EC-K4 Tg mice demonstrated less vessel loss and fewer neovascular tufts (Fig. 5b, right panels). In similar experiments performed in an inducible endothelial KLF4 knockout mouse strain (KLF4i∆EC), no significant differences in vaso-obliteration or neovascular tuft formation are seen compared to control mice (Supplemental Fig. 6). Thus, endothelial-specific overexpression of KLF4 results in decreased vaso-obliteration and neovascular tuft formation. This is similar, though less profound, to the phenotype seen with genetic or pharmacological inhibition of DLL4 [34], likely due to the reduction, rather than ablation, of DLL4 expression in our model.
EC KLF4 overexpression ameliorates oxygen-induced retinopathy. a EC KLF4 overexpression leads to preservation of retinal perfusion after a short exposure of perinatal mice to hyperoxia. Mice were exposed to 75% O2 for 24 h from P9 to P10 and then euthanized. Perfused vessels are demonstrated by intravascular fluorescein-labeled Griffonia Simplicifolia lectin signal (GSL perfusion, green) and the entire vasculature demonstrated by staining of the whole-mount specimens after fixation (IsoB4 staining, red). Areas with red IsoB4 signal but lacking the green IsoB4 signal are non-perfused. Quantification of the non-perfused area is shown below images. b After exposure to 5 days of hyperoxia (P7–P12), loss of retinal vascularity is similar in wild-type and EC-K4 Tg pups; however, 5 days after return to normoxia (P17), EC-K4 Tg mice have significantly less severe pathological changes demonstrated by decreased avascular area and fewer neovascular tufts. Retinas were stained with isolectin B4. Quantification of the avascular area and neovascular areas was performed as in experimental procedures is shown below images. Data are means ± SD of at least four mice per group. *p ≤ 0.05. Scale bar a, b: 500 µm
KLF4 interferes with RBP-J activator activity and thereby downregulates DLL4 transcription
The human DLL4 gene is located on chromosome 15 and composed of 11 exons (Fig. 6a), coding for a protein of ~ 70 kDa. While there are little data elucidating the Dll4 gene transcription in angiogenesis, recent reports show that intron 3 of Dll4 is a well-conserved non-coding region and regulates DLL4 transcription through the VEGF/MAPK pathway [35,36,37,38]. We previously demonstrated, in vivo and in vitro, that KLF4 regulates the expression of key members of the NOTCH signaling pathway, and we reported on several putative KLF4 binding sites in the upstream of the mRNA start site of DLL4 transcription [16]. To establish experimentally the effect of KLF4 on the 2 kb upstream and intron 3 regions, we independently cloned and inserted these regions into luciferase reporter plasmids. Transient transcription experiments were performed with these plasmids in HUVEC co-transfected with control or KLF4 expression plasmids. As illustrated in Fig. 6b, c KLF4 has a different effect on the classical upstream “promoter” region versus the intronic enhancer region of DLL4. KLF4 activates the luciferase reporter driven by the 2 kb region upstream of the DLL4 transcriptional start site but inhibits reporter activity of the plasmid containing the sequence from intron 3 of the DLL4 gene (Fig. 6b, c). As our in vivo and cell culture data [16] demonstrated that KLF4 overexpression leads to inhibition of the endogenous DLL4 gene, we suggest that the intronic site comprises the important location for KL4-mediated regulation of DLL4. RBP-J is a transcriptional regulator which can act as a repressor or activator of transcription of Notch signaling genes when not bound or when in the complex with NICD, respectively [16, 39]. To assess the effect of the binding sites for KLF4 and RBP-J on expression, we mutated the putative binding sites in intron 3 and performed luciferase assays with these mutated reporter plasmids and KLF4 expression plasmids coding for full-length KLF4 or for KLF4 lacking the zinc finger domain.
KLF4 inhibits Dll4 transcription via binding to a site in intron 3. a Schematic of the human Dll4 gene. The black arrowhead indicates the transcriptional start site and orientation, black boxes represent exons. Two regions were assessed for transcriptional activity in luciferase reporter assays; the upstream − 2008 bp region and intron 3. Relative locations of sequences identified as possible binding sites for KLF4 and RBP-J are shown in the expanded region above. Luciferase constructs with mutation of either the RBP-J or KLF4 binding sequence are illustrated to the upper right, with mutated site indicated by gray box. Results from transient transcription assays show that KLF4 increases transcriptional activity of the 2008 bp region of the Dll4 gene but inhibits transcriptional activity of the intron 3 region (b). c KLF4 is required for inhibition of RBP-J mediated transcriptional activity of the intron 3 region. Decreased activity of the construct with the mutated RBP-J site is assumed to be due to lack of association of endogenous RBP-J. The KLF4(−) ZnF construct lacks the DNA-binding domain of KLF4. d Transactivation of intron 3 by NICD requires an intact RBP-J site. KLF4 inhibits NICD-mediated, RBP-J-dependent transactivation. Assays were performed in HUVEC. Expression vectors for full-length KLF4, KLF4 lacking the DNA-binding domain (KLF4(−) ZnF), and NICD were used in (b–d). e In ChIP assays, we demonstrate that KLF4 is the only transcription factor of those assessed in this experiment that associates measurably with the KLF4 binding site. On the other hand, NICD, RBP-J, and MAML associate with the RBP-J site, as shown previously. KLF4 inhibits the association of NICD and MAML to the RBP-J site but does not alter RBP-J association. HUVEC lysate was subjected to immunoprecipitation using an antibody against the transcription proteins listed on the X-axis or non-immunized serum as a negative control, followed by qPCR with primers flanking either the intron 3 KLF4 or RBP-J site. f In the immunoprecipitation assay, we demonstrated that KLF4 overexpression in HUVEC cells decreased RBP-J binding with NICD and MAML proteins. Data represent means ± SD of 3 independent experiments.*p ≤ 0.05, comparing Con to KLF4 or Con to NICD condition; ζp ≤ 0.05, comparing NICD to NICD + KLF4 condition
(KLF4(−) ZnF) required for binding to DNA. These assays confirm that an intact RBP-J site is required for RBP-J-mediated transactivation, and that KLF4 is required for KLF4-mediated inhibition of transactivation by RBP-J (Fig. 6d). In addition, we also show that KLF4 inhibits transactivation by the co-activator NICD (Fig. 6e). To assess the effect of these sites on DLL4 expression, we performed chromatin immunoprecipitation (ChIP) assays in HUVEC to confirm the effect of KLF4 on association of Notch transcription factor with the intron 4 KLF4 and RBP-J sites. We found that only KLF4 binds to the KLF4 binding site, whereas RBP-J, NICD, MAML, and KLF4 associate with RBP-J site (Fig. 6f). Moreover, overexpression of KLF4 does not alter binding of RBP-J, but it does decrease the association of NICD and MAML with the intron 3 RBP-J binding site. However, we performed immunoprecipitation of RBP-J protein in HUVEC cells (Fig. 6f). Immunoprecipitation data showed that RBP-J binds MAML and NICD proteins in control group. After KLF4 overexpression, RBP-J does not bind MAML and NICD proteins but bind KLF4 protein. Combined, these results show that endothelial KLF4 regulates endothelial DLL4 expression through a highly conserved site in intron 3 of the DLL4 gene.
Discussion
Sprouting angiogenesis is a dynamic multi-phase process involving EC tip and stalk cell determination, proliferative vascular patterning, and finally a remodeling phase that culminates in a pruned, mature structure that provides a new path for blood circulation. We have previously shown that sustained endothelial KLF4 overexpression causes a profound dysregulation of tumor sprouting angiogenesis, markedly limiting tumor growth due to deficient perfusion [16]. We provided mechanistic data indicating that this vascular alteration occurred via inhibition of NOTCH–DLL4 signaling by KLF4, leading to an imbalance of tip versus stalk cell development. Importance of this finding was supported by the KLF–Notch interaction being evolutionarily preserved; in Drosophila, Krüppel (Kr) determines the fate of neural tip cells in developing malpighian tubules, and Kr expression is controlled by Notch-dependent (Delta) signaling [16, 37]. As there are significant signaling and morphological differences between tumor and normal developmental angiogenesis [16, 40], we performed the current study to explore the role of KLF4 in developmental angiogenesis. Here we show, using a mouse retinal model, that endothelial-specific KLF4 overexpression results in excessive sprouting and branching during the early stage of vascular development. Interestingly, this vascular phenotype is not stable, and through enhanced processes of remodeling, the mature retinal vasculature of EC-KLF4 Tg mice is morphologically indistinguishable from WT.
Notch signaling through DLL4 expression is integral in coordination of the dynamic phenotypic and functional changes of ECs as they interconvert between tip and stalk cell roles during sprouting angiogenesis. Moreover, aspects of Notch signaling regulate remodeling and maturation of arterial retinal vessels [16, 40,41,42] as well veins and capillaries [16, 28]. We do not know if the enhanced remodeling leading to normalization of the vascular tree in our EC-KLF4 Tg mice is related to the early inhibition of DLL4 expression, or if it is an entirely independent process. A literature search did not reveal published data documenting the time course of retinal vascular remodeling in DLL4-deficient mice beyond the proliferative phase. While alteration of Notch signaling has no effect on cell proliferation, the activity of NOTCH signaling in arterial cells does show a direct correlation with arterial smooth muscle coverage [16, 28].
KLFs are evolutionarily conserved zinc finger DNA-binding transcription factors that play varied roles during cell differentiation, normal development, and pathology [8,9,10]. KLFs 4 and 2 are particularly closely related by sequence and are expressed in ECs. They display partially overlapping regulation and function: their expression is induced by hypoxia and shear stress, and in turn they modulate diverse inflammatory and vasoactive stimuli [14, 15]. The pattern of KLF expression can change in these conditions and expression of certain KLFs may overlap at some point [13, 43]. Recently, Sangwung et al. showed that a single allele of either Klf4 or Klf2 is necessary and sufficient for maintenance of vascular integrity [13, 43]. Thus, the fact that EC-specific KLF4 knockdown results in no change in sprouting developmental angiogenesis in our model suggests functional compensation by other Krüppel-like factors.
In our developmental angiogenesis experiments, as in others, it is unknown whether the phenotypic abnormalities seen during early retinal angiogenesis lead to functional consequences. As the hyperoxia-induced retinopathy model mimics the perfusion and vessel pathologies seen in the retinopathy of prematurity which lead to vision loss, we used this model to predict the functional consequences of endothelial KLF4 overexpression during a pathological insult. Our data indicate that KLF4 acts not only to maintain blood vessel perfusion but also to limit capillary regression and tuft formation induced by exposure to hyperoxia. In these settings, attenuation of vessel loss, improving flow in existing vessels, and regulation of neoangiogenesis after hypoxic insult might have beneficial effects. These effects of KLF4 on blood vessels are likely correlated with inhibition of the DLL4–NOTCH pathway.
The profound effect of DLL4–NOTCH signaling on sprouting angiogenesis enhances the interest in determining the molecular mechanism by which this occurs. Earlier studies have shown that VEGF (mediated by VEGF receptors) upregulates Dll4, and that activation of both integrin and phosphatidylinositol 3-kinase/Akt signaling transmit the effect of VEGF on Dll4 expression [35, 44, 45]. Foxc proteins are essential for vascular development and are involved in arterial specification; they have also been shown to directly bind to an − 3.7 kb upstream promoter region of Dll4 [46]. VEGF-induced Dll4 expression is enhanced by Foxc1 and Foxc2 [47], and the integrin-mediated upregulation of Dll4 in particular appears dependent upon the transcription factor Foxc2. More recently, an in silico search of the Dll4 locus identified a highly conserved enhancer region in the third intron that can direct arterial specification independent of the endogenous (upstream) promoter [37]. Combined loss of SOXF and RBP-J binding sites in the intron 3 enhancer leads to ablation of Dll4 expression and arterial specification. The function of SOXF endothelial transcription factors is incompletely defined, but pleiotropic and redundant roles of the vertebrate sox7, sox17, and sox18 proteins have been found during cardiovascular development [48]. Of interest, the Dll3 intron 3 enhancer does not contain functional binding sites for Foxc transcription factors, and works independently of the endogenous promoter, raising the possibility that Foxc and SOX have independently regulated roles and function in Dll4 regulation [5, 37, 49, 50]. In the current work, we demonstrate that KLF4 differentially regulates the transcriptional activity of a DLL4 upstream promoter region and the intron 3 enhancer. While both regions are highly evolutionarily conserved and have binding sites for KLF4, we postulate that the inhibitory effect of KLF4 on DLL4 expression is mediated primarily by the 91 bp site in intron 3. Our data support KLF4-mediated inhibition of DLL4 expression via disruption of the RBP-J–NICD–MAML complex in intron 3 of DLL4, with resultant dysregulation of developmental angiogenesis. These data represent an important refinement of the understanding of the molecular mechanism of Notch signaling in developmental angiogenesis and may contribute a new tool for differentiating the functions of other transcription factors involved in cardiovascular development.
Together, our previous data and the current work demonstrate the need for dynamic expression of EC KLF4 during both pathological and developmental angiogenesis. When EC KLF4 expression is sustained, Notch signaling through DLL4 is disrupted and a hyperdense vasculature ensues. In tumor angiogenesis, this limits tumor growth. In developmental angiogenesis, this effect appears to resolve during enhanced remodeling and no functional adverse consequences are apparent in the adult animal. In fact, sustained KLF4 expression during oxygen-induced retinopathy displays potentially protective phenotypic features. KLF4 is a critical regulator of EC homeostasis, vascular inflammation, and thrombosis. The data herein provide another fundamental reason for the discovery of pharmacological agents that modulate the expression of EC KLF4 for amelioration of human vascular disease.
References
Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438(7070):932–936
Benedito R et al (2009) The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137(6):1124–1135
Bentley K et al (2014) The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat Cell Biol 16(4):309–321
Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7(9):678–689
Borggrefe T, Oswald F (2009) The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci 66(10):1631–1646
Hellstrom M et al (2007) Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445(7129):776–780
Pitulescu ME et al (2017) Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nat Cell Biol 19(8):915–927
Li JL et al (2007) Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res 67(23):11244–11253
Noguera-Troise I et al (2006) Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444(7122):1032–1037
Phng LK, Gerhardt H (2009) Angiogenesis: a team effort coordinated by notch. Dev Cell 16(2):196–208
McConnell BB, Yang VW (2010) Mammalian Kruppel-like factors in health and diseases. Physiol Rev 90(4):1337–1381
Bieker JJ (2001) Kruppel-like factors: three fingers in many pies. J Biol Chem 276(37):343558
Tetreault MP, Yang Y, Katz JP (2013) Kruppel-like factors in cancer. Nat Rev Cancer 13(10):701–713
Hamik A et al (2007) Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem 282(18):13769–13779
Zhou G et al (2012) Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J Clin Invest 122(12):4727–4731
Hale AT et al (2014) Endothelial Kruppel-like factor 4 regulates angiogenesis and the Notch signaling pathway. J Biol Chem 289(17):12016–12028
Pitulescu ME et al (2010) Inducible gene targeting in the neonatal vasculature and analysis of retinal angiogenesis in mice. Nat Protoc 5(9):1518–1534
Zudaire E et al (2011) A computational tool for quantitative analysis of vascular networks. PLoS ONE 6(11):e27385
Smith LE et al (1994) Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 35(1):101–111
Lobov IB et al (2007) Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA 104(9):3219–3224
Connor KM et al (2009) Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc 4(11):1565–1573
Gerhardt H et al (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161(6):1163–1177
Shatat MA et al (2014) Endothelial Kruppel-like factor 4 modulates pulmonary arterial hypertension. Am J Respir Cell Mol Biol 50(3):647–653
Ribatti D, Nico B, Crivellato E (2011) The role of pericytes in angiogenesis. Int J Dev Biol 55(3):261–268
Sheikh AQ et al (2015) Smooth muscle cell progenitors are primed to muscularize in pulmonary hypertension. Sci Transl Med 7(308):308ra159
Davies PF et al (2013) The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res 99(2):315–327
Shankman LS et al (2015) KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21(6):628–637
Ehling M et al (2013) Notch controls retinal blood vessel maturation and quiescence. Development 140(14):3051–3061
Baluk P et al (2003) Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol 163(5):1801–1815
Stahl A et al (2010) The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci 51(6):2813–2826
Scott A, Fruttiger M (2010) Oxygen-induced retinopathy: a model for vascular pathology in the retina. Eye (Lond) 24(3):416–421
Gu X et al (2002) Effects of sustained hyperoxia on revascularization in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci 43(2):496–502
Okuno Y et al (2012) Pathological neoangiogenesis depends on oxidative stress regulation by ATM. Nat Med 18(8):1208–1216
Lobov IB et al (2011) The Dll4/Notch pathway controls postangiogenic blood vessel remodeling and regression by modulating vasoconstriction and blood flow. Blood 117(24):6728–6737
Liu ZJ et al (2003) Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol 23(1):14–25
Hainaud P et al (2006) The role of the vascular endothelial growth factor-Delta-like 4 ligand/Notch4ephrin B2 cascade in tumor vessel remodeling and endothelial cell functions. Cancer Res 66(17):8501–8510
Sacilotto N et al (2013) Analysis of Dll4 regulation reveals a combinatorial role for Sox and Notch in arterial development. Proc Natl Acad Sci USA 110(29):11893–11898
Wythe JD et al (2013) ETS factors regulate Vegf-dependent arterial specification. Dev Cell 26(1):45–58
High FA, Epstein JA (2008) The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet 9(1):49–61
Ziyad S, Iruela-Arispe ML (2011) Molecular mechanisms of tumor angiogenesis. Genes Cancer 2(12):1085–1096
Swift MR, Weinstein BM (2009) Arterial-venous specification during development. Circ Res 104(5):576–588
Gridley T (2007) Notch signaling in vascular development and physiology. Development 134(15):2709–2718
Sangwung P et al (2017) KLF2 and KLF4 control endothelial identity and vascular integrity. JCI Insight 2(4):e91700
Lee TH et al (2006) Integrin regulation by vascular endothelial growth factor in human brain microvascular endothelial cells: role of alpha6beta1 integrin in angiogenesis. J Biol Chem 281(52):40450–40460
Estrach S et al (2011) Laminin-binding integrins induce Dll4 expression and Notch signaling in endothelial cells. Circ Res 109(2):172–182
Seo S, Kume T (2006) Forkhead transcription factors, Foxc1 and Foxc2, are required for the morphogenesis of the cardiac outflow tract. Dev Biol 296(2):421–436
Hayashi H, Kume T (2008) Foxc transcription factors directly regulate Dll4 and Hey2 expression by interacting with the VEGF-Notch signaling pathways in endothelial cells. PLoS ONE 3(6):e2401
Lilly AJ, Lacaud G, Kouskoff V (2017) SOXF transcription factors in cardiovascular development. Semin Cell Dev Biol 63:50–57
Caolo V et al (2010) Feed-forward signaling by membrane-bound ligand receptor circuit: the case of NOTCH DELTA-like 4 ligand in endothelial cells. J Biol Chem 285(52):40681–40689
Diez H et al (2007) Hypoxia-mediated activation of Dll4–Notch–Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Exp Cell Res 313(1):1–9
Acknowledgements
We are grateful for the support and encouragement provided by Dr. Mukesh K. Jain. We thank Dr. Christina Antonopoulos-Buzzy (Case Western Reserve University School of Medicine) for kindly providing technical support, discussion, and review of the manuscript. We thank Kevin P. Montgomery for assistance with figures. This work is supported by NIH R01HL113570.
Author information
Authors and Affiliations
Contributions
EB designed, performed, and analyzed experiments, and drafted the manuscript. HZ, JP, and MAS provided input into experimental results and reviewed the manuscript. RHA provided crucial reagents and reviewed the manuscript. AH conceived and coordinated the studies, designed, and analyzed the experiments, and revised the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Additional information
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Boriushkin, E., Zhang, H., Becker, M. et al. Kruppel-like factor 4 regulates developmental angiogenesis through disruption of the RBP-J–NICD–MAML complex in intron 3 of Dll4. Angiogenesis 22, 295–309 (2019). https://doi.org/10.1007/s10456-018-9657-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-018-9657-y