Abstract
Fungal spores are among the most commonly encountered airborne biological particles, and it is widely proved that they represent a potential source of allergens involved in rhinitis and asthma. A change in temperature may influence the colonisation and growth of fungi directly through the physiology of individual organisms, or indirectly through physiological effects on their host plants or substrates and any competitors or enemies. In order to detect and monitor the evolution of the spore counts, air sampling was carried out using standard equipment (Hirst-type volumetric traps) and an identical method in several stations across France. Cladosporium has been here emphasised because of its very large contribution to the total fungal spectrum. Moreover, this taxon is of particular clinical importance because it possesses a high allergenic potential. The data from the oldest traps (Aix-en-Provence, Bordeaux, Lyon, Paris and Toulouse) were analysed on an annual base. Located at different latitudes and in different climatic areas, these five cities showed fundamentally different trends for the concentrations of Cladosporium spores: downward trend at the southernmost locations and upward trend at the other locations, whereas temperature was everywhere continuously rising over the study period. However, longer data sets are needed to be able to draw more definitive conclusions about quantitative trends in airborne fungal spore concentrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In many places all over the world, Cladosporium is the most common fungal component isolated from air (Mullins 2001), usually accounting for 30–85 % of all spores recorded. Although it can grow in a wide range of habitats and environments in nature as well as on man-made constructions and objects, this mould from the class of the anamorphic fungi (Hyde et al. 2011) occurs most abundantly on faded or rotting leaves, stems and twigs of herbaceous and woody plants. It reproduces by forming tiny fungal spores (conidia) that are normally one or two celled and occur in long, branching chains that readily disarticulate. These spores vary widely in size (3–70 × 2–56 µm), shape (rounded, barrel-like, ovoid, ellipsoid or slightly elongated), colour (black, olive-brown, dark grey, dark green or sometimes hyaline) and surface texture (smooth, a bit rough or warty). They are released actively or more often passively in the atmosphere and wind-dispersed in large numbers over long distances (Bensch et al. 2012; Money 2015). They can also remain suspended in air for periods ranging from a few hours to several days. Whereas the highly heterogeneous genus Cladosporium has been reported under over 700 names (Dugan et al. 2004) and includes more than 500 different species (Bensch et al. 2012), the most common one identified outdoors in temperate and cold climates is C. herbarum, characterised by thick-walled and verrucate conidia. Conversely, C. cladosporioides (with smooth-walled conidia) frequently dominates the indoor mycoflora. It is a common opinion that Cladosporium spores contain little or none of the major mycotoxins, but they may trigger the development, exacerbation or worsening of symptoms such as rhinoconjunctivitis, rhinosinusitis (Hamilos 2010), dry cough and/or bronchial asthma (Pringle 2013) in atopic allergy sufferers. Despite that the contribution of this genus to the pathogenesis of asthma appears to be a bit weaker than that of Alternaria (Fukutomi and Taniguchi 2015), about 60 inhalant antigens from C. herbarum have been identified by crossed immunoelectrophoresis, of which at least 36 react with IgE antibodies from patients’ sera (Aukrust 1979). Data from the European Community Respiratory Health Survey demonstrated that among adults aged 20–44 years in the general population, the prevalence of positive skin tests using Cladosporium extracts ranged from 0 to 11.9 % with an average of 1.7 % (Bousquet et al. 2007). Moreover, in many countries such as Denmark the weekly clinical symptom and medication scores were positively correlated with airborne spore counts of Cladosporium (Malling et al. 1987). The daily minimum concentration likely to trigger early allergic symptoms in sensitive subjects is estimated to be most frequently equal to or greater than 2800 or 3000 spores m−3 for Cladosporium (Rodríguez-Rajo et al. 2005; Rapiejko et al. 2007), which is much higher than that for Alternaria (about 80–100 spores m−3) (Bagni et al. 1977; Gravesen 1979; Kasprzyk et al. 2015). One of the reasons for different threshold values of Cladosporium and Alternaria spores is that volumes of these particles are different: 10–20 Cladosporium spores may correspond to one Alternaria spore (Stępalska et al. 1999). Unfortunately, as Lavaud and Dutau (2013) pointed out, for lack of adequate surveys it is still very difficult to clarify in France the relationship between Cladosporium counts, Cladosporium sensitisation, allergic rhinitis and/or asthma.
The concentration of Cladosporium spores in the air is known to fluctuate according to weather conditions. Although these spores can thrive in any temperature, the meteorological parameter displaying the strongest correlation with daily spore counts is almost always temperature. For example, Grinn-Gofroń and Rapiejko (2009) found mean temperature to be the main, if not the only explanatory factor for the day-to-day fluctuations of Cladosporium spores in the air at several Polish locations. In addition, the spores are seemingly released into the air in great numbers after it rains and in damp weather (Katial et al. 1997). The optimal conditions for high concentrations are usually recorded at temperatures ranging from 23 to 29 °C and relative humidity values of around 80 % (Rodríguez-Rajo et al. 2005). However, this is still unclear how medium- or long-term changes in climate, and particularly in temperature, affect fungal abundance in the air. Consequently, the present study was initiated to ascertain (1) whether some modifications in the atmospheric fungal spore content occurred in recent years, (2) whether these modifications can be linked to changes in climate we saw during these years and (3) whether the observed trend is uniform across the French territory or different from a climatic region to another.
2 Materials and methods
2.1 Study area
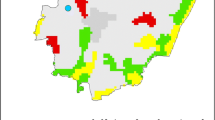
The study was conducted in the five localities of France providing the longest time series of fungal data, namely in alphabetical order Aix-en-Provence, Bordeaux, Lyon, Paris and Toulouse, with their distribution broadly corresponding to the diversity of France’s climate and biogeography as shown in Fig. 1. The height above sea level did not exceed anywhere 200 m.
Climate zones of France (from Estienne 1979) and the five monitoring stations studied. A1 Maritime oceanic climate, A2 Atlantic littoral climate with dry summer, A3 Aquitanian climate, A4 Very wet Basque climate, A5 Oceanic climate with attenuated continental degradation (a Flemish type, b Ligerian type), A6 Oceanic climate with sharp continental degradations, B1 climate with continental affinities and sheltered climate, C1 Mediterranean climate, C2 Mediterranean mountain climate, D1 Non-Mediterranean wet or very wet climate, D2 Non-Mediterranean relatively dry climate. 1 Aix-en-Provence, 2 Bordeaux, 3 Paris, 4 Lyon, 5 Toulouse
Aix-en-Provence lies within the typical Mediterranean climatic belt, which is characterised by mild to cool, relatively wet winters, warm to hot, dry summers, high sunshine duration and occasionally strong winds. These factors favour the presence of fungal spores in the air for much of the year. Native vegetation is dominated by scrubby grassland and arboreal or shrubby evergreen sclerophyllous plants, adapted to stresses of heat and aridity.
Bordeaux’s climate is usually labelled as a temperate oceanic climate. However, the summers tend to be somewhat warmer and the winters somewhat milder than most areas of similar classification. Substantial rainfall in late spring and in summer prevents the climate from being classified as Mediterranean, so it is called “Aquitanian climate”. The surrounding vegetation is composed mainly of deciduous trees (oak or cork oak, chestnut, alder, ash and willow) not forgetting some grasslands and the 1000-year-old vineyards. To the south and west is also a low, large sedimentary basin with great forests of maritime pine.
In the Paris area, the influence of Atlantic weather systems is predominant, giving a mild and changeable climate, slightly open to continental dynamics and strongly influenced by urbanisation. Moderate rainfall events are possible at all times of the year. Synanthropic vegetation dominates in the town, where there is an abundance of green spaces in the form of parks, squares and urban or suburban woods. At some greater distance, man has substantially disturbed the climax vegetation, promoting oak instead of beech and destroying largely the forests.
Lyon has a more continental climate, exacerbated by a clear shelter effect, with lesser precipitations, harsher as well as longer winters and hotter summers than western France. The surrounding area is densely built with a large number of hedges and lawns. The flora comprises both natural plant species and introduced species, consisting in both ornamentals and crops. Typical natural plant species include grass, boxwood, oak, shrub, beech and fir.
Lastly, Toulouse occupies a middle position between the Mediterranean climate and the oceanic one, with slightly continental features, resulting in very cool winter, rainy spring and warm, rather stormy summer. The city boundaries are made up of a mosaic of fragmented forests, with downy oak and poplar as predominant species, and cultivated fields which provide suitable media for spore production.
2.2 Fungal content in the air
The aerobiological samples, where the identification and counting of Cladosporium spores were carried out, come from five 7-day recording volumetric traps of the Hirst design (Hirst 1952), manufactured by Lanzoni™, Bologna, Italy. These collectors were positioned on flat rooftops, at 20–40 m above the ground level with no nearby buildings to obstruct air circulation. They are made of a weathercock that orientates the slit for air intake according to the prevailing wind direction and a vacuum pump that sucks in a constant flow of air through the entry slit. The sucking rate was adjusted to 10 L min−1, similar to volume of air inhaled by human beings, allowing for the spore concentration to be determined at 2-h intervals. Airborne particles were trapped uninterruptedly onto a transparent tape, coated with an adhesive substance. The tape recording the impacted particles on a whole week was cut into seven equal segments, each representing a collection of 24 h, and each mounted onto glass slide. After application of a colourful reactive solution, the mean spore concentration, expressed as total number of spores per cubic metre of air (spores m−3), was carried out with the aid of optical microscopy under 400× magnification, along one longitudinal sweep per slide (Käpylä and Penttinen 1981). Spores were identified at the genus level only, since in most cases it was not possible to make species determinations. The repeatability of the counting technique was substantial, as demonstrated by Mitakakis and McGee (2000) using the example of Alternaria. Daily spore counts were derived by adding all the 2-h counts. Outside winter periods missing values in the data sets never exceeded eight consecutive days and were estimated by linearly interpolating on either side of the gap (Damialis et al. 2007). Finally, the Cladosporium Spore Index (CSI, number without unit) was calculated by summing up all daily values counted during a given year or (see below) during the main fungal season of a given year. Data of Cladosporium records were inserted in an automated computer worksheet and processed with Microsoft Excel® for Windows®. The longest time series used in this study goes back to 1999 (Bordeaux), some start in the early 2000 s (2000 in Toulouse, 2002 in Paris, 2004 at Aix-en-Provence), and the shortest time series begins in 2007 (Lyon). The last year is 2014 in all cases.
Data were sometimes analysed separately for the five selected sampling stations and sometimes pooled to give an indication of the averaged annual dynamics of Cladosporium fungal spore concentrations.
Various methods for defining the duration of the Main Spore Season (MSS) have been tried in the literature. Their common objective is to eliminate the long tails of low values at the beginning and the end of the seasons that may bias any statistical analysis. In our investigations, and in accordance with the rules proposed by Nilsson and Persson (1981) to describe the Main Pollen Season (MPS), the limits of the MSS were calculated by using the 90 % method whereby the season starts when 5 % of the total catch for the respective year has been achieved and ends when 95 % has been reached. The MSS is therefore considered as the period during which 90 % of the annual total accumulated sum of Cladosporium spores is registered. The duration of the MSS is the difference in days between the start date and the end date of the season. The value of the highest daily mean Cladosporium concentration (peak value, spores m−3) and the corresponding date (peak date) was also considered. To determine the peak date, a 3-day running average of the daily mean spore concentrations was calculated. In the only case of multiple peaks of equal height, the first occurrence was chosen as the peak day. Data related to start, end and peak day were converted to the day of the year from 1 January.
2.3 Weather-related parameters
Records of daily meteorological data were drawn from the websites http://www.infoclimat.fr/and http://meteociel.fr/. The weather stations were chosen in closest proximity to each aeroallergen monitoring station (mean 10.05 km; range 2.7–21.2 km). The record length of the meteorological characteristics of each site was the same as that of aerobiological data. The following parameters were included in the analysis, either at a daily time step or in terms of annual averages: minimum temperature T min (°C), maximum temperature T max (°C), mean temperature T mean (°C), relative humidity RH (%), cumulative rainfall R (mm), sunshine duration SD (h), mean wind speed WS (m s−1) and modal wind direction WD (16-point compass rose).
In order to facilitate a better understanding of spore behaviour in air, we calculated the Continentality Index CI, which expresses the range between the maximum temperature and the minimum temperature for the period considered, here the MSS, and is given by the formula CI = T max−T min (Valencia-Barrera et al. 2002).
2.4 Statistical treatment
SigmaStat® program version 3.5.1 (Systat Software, Inc.) was used for statistical processing of the data. A significance level (p) of 0.05 was adopted to be critical.
Before computing any statistical analysis, all the data sets were subjected to a Shapiro–Wilk test (Shapiro and Wilk 1965) to verify whether their frequency distribution could be fitted to a normal distribution. It was found that, if the daily aeromycological series approximated a Gaussian curve, the yearly data (which were the only ones to be included in the correlation analyses) were normally distributed (Shapiro–Wilk’s test with p > 0.05) in three of our stations and were approaching the normality (Shapiro–Wilk’s test with 0.047 < p < 0.05) in the other two (Lyon and Toulouse). We therefore considered there was no downside to applying parametric statistical analyses (Limpert et al. 2008). In particular, although the number of observations was small (<30), we did not follow the example of Pidek et al. (2015) who used the nonparametric Spearman’s correlation coefficient instead of the Pearson’s coefficient in order to establish the relationship between the annual Cladosporium count values and the annual atmospheric conditions.
In addition to the intensity and timing of the peak concentration, the following characteristics of the Cladosporium MSS were examined: intensity (CSI), seasonality, onset, end and duration.
In the same way as Ziello et al. (2012), Smith et al. (2014) and Ugolotti et al. (2015) and many other authors did with regard to pollen, all the above-mentioned seasonal characteristics and the corresponding meteorological parameters were linearly regressed against the increase in time to detect possible trends in certain fungal and climatic data over recent years. Trend analysis was performed on annual base only. The following parameters were calculated: Pearson product–moment correlation coefficient (r), goodness of fit (determination coefficient R 2) and probability level (p). A graphical comparison was also performed between the annual mean temperature and average concentration of Cladosporium spores.
Lastly, as set out above, Pearson’s correlation coefficients (r) were used to examine whether there were any clear associations between the climate variable trends and the Cladosporium spore concentration trends during the MSS—what does not assume that one variable caused the other.
3 Results
The diurnal temperature range made it possible to distinguish between one station under strong oceanic influence (Bordeaux, CI = 9.1) and four stations with reduced maritime influences and progressively continental characteristics (in increasing order Toulouse, Aix-en-Provence, Paris, and Lyon with CI’s ranging from 9.4 to 12.4).
3.1 Characterisation of MSS
The main characteristics of Cladosporium spore records are reported in Table 1. For the whole period under investigation, the lowest concentration of Cladosporium (CSI) during the MSS was on average noted in Bordeaux (230,276—giving an average of 1175 spores m−3 day−1), whereas the highest value occurred in Paris (719,562—implying an average of 4112 spores m−3 day−1). The scores varied from place to place by a factor of 1 to more than 3, with an average of 493,770. Lyon appeared to be very close to Paris, while Toulouse and Aix-en-Provence occupied middle positions. In spite of large year-to-year and site-to-site fluctuations, these figures were 20–38 times those of Alternaria and represented up to 62 % of the entire load of identified fungal propagules in the air.
Not only the spores from the genus Cladosporium were the most common components of the airborne mycoflora at all the monitoring stations, but they were present practically year round (Fig. 2). However, their concentration was low or very low in winter and early spring, without ever exceeding 741 spores m−3 day−1, for instance, in Paris during the month of February. Inversely, the concentration hit very high levels in the summer and early fall months. This clearly monomodal distribution pattern was rather different to the bimodal pattern observed for “all fungi”, which usually exhibited a gradual take-off of concentrations in May and June, a first peak at the end of July, a pronounced drop in August and a second peak between September and November (not shown). The Cladosporium MSS lasted between 160 days in Lyon and 220 days in Aix-en-Provence, with an average of 187 days (from calendar day 115 to calendar day 301, in other words from 25 April to 28 October in a non-leap year) for all the stations together. Aix-en-Provence had both the earliest start and the latest end of MSS, while Lyon had the most delayed onset and the earliest end. The period exceeding the threshold of 2800 spores m−3 day−1 had an elapsed time of more than 2.5 months.
The highest levels were registered mainly in mid-July (Fig. 2), but at certain years and places they might happen on a date falling between mid-June and early September. The absolute peak for the entire study period occurred everywhere in the middle of summer (July or August), but the corresponding values varied considerably from site to site, with daily concentrations approximately three times higher in Paris and Bordeaux than in Aix-en-Provence and Toulouse. It should also be stressed that no correlation existed between CSI and peak values since the two localities with the highest peaks had the minimum and the maximum CSI, respectively.
3.2 Observed MSS and climate trends over the study period
Only a few trends were clearly identified compared to the total number of tests performed (Table 2).
In general terms, there was in the five referred locations an upward tendency in the mean annual temperature with an increase ranging from +0006 °C per year in Bordeaux, what is very inconsequential, to +0050 °C per year in Lyon, what is 8 times higher. As evidenced by Figures 3, 4, 5, 6 and 7, the proportion of variance explained in the dependent variable (temperature) by variations in the independent variable (year numbers) ranged from 0.8 % in Bordeaux till 6.6 % in Lyon. However, none of the analysed characteristics of temperature had a statistically significant temporal trend. Moreover, while the regression of T min and T mean against time was always positive, its sign was variable in the case of T max. It is also difficult to take a definitive view on the rainfall, which had a consistently rising trend in Paris (p < 0.05) and in Aix-en-Provence (not significant) and a slight falling, not significant trend in the three other sampling stations.
The only significant finding as regards the main characteristics of the fungal season was a trend towards increasing Cladosporium counts (CSI) in Bordeaux (p < 0.05), without any significant links to the start date, duration, nor end date of the MSS. At most, it might be conceded that a slight trend towards an earlier start to the MSS was observed in Bordeaux, Lyon, Paris and Toulouse, as a tendency towards a later end date was recorded in Aix-en-Provence, though variations were not significant in any case.
For both sites of Toulouse (Fig. 3) and, to a lesser degree, Aix-en-Provence (Fig. 4), a slow decline in Cladosporium spore counts was recorded over the study period, at a rate of, respectively, 2.5 and 1.3 % per year during the MSS, although T mean was on rise, as mentioned earlier (respectively, +0.027 °C and +0.036 °C per year). Thus, in Aix-en-Provence, 2010 was both the coolest year and the one with the highest load in Cladosporium. In the three other considered cities, the amounts of Cladosporium spores tended to increase in line with the gradual increase in temperatures, the trend being barely noticeable in Lyon (Fig. 5), but much clearer in Paris (Fig. 6) and even more in Bordeaux (Fig. 7). In the capital for instance, the warmest year (2011) was also the year with the highest load in Cladosporium. The proportion of variance in the Cladosporium scores explained by the year numbers varied from 2.7 % in Bordeaux to 15.6 % in Toulouse, including 7.3 % in Aix-en-Provence.
3.3 Correlation between Cladosporium and climate trends
Some of the most relevant coefficients of correlation calculated on an annual base between particular climatic parameters and concurrent concentrations of Cladosporium spores are included in Table 3. Of the various weather-related variables analysed, air temperature (be they T min, T mean, or T max) seemed to be the most commonly associated with the inter-annual course of Cladosporium spores in the atmosphere, although only the fungal series of Bordeaux correlated rather strongly (positively and significantly) with T mean. It must also be emphasised that the T mean/CSI correlation was very weakly negative for Aix-en-Provence, but positive for all other stations, including Toulouse.
Most other variables indicated weak levels of correlation, suggesting that climate had very little or no effect on the year-to-year Cladosporium counts. The analysis revealed that, except in Paris, the concentration of Cladosporium spores was indirectly proportional to the annual rainfall amount, a wet year corresponding with a lower concentration of fungal spores in the atmosphere; the correlation was strong and significant in both Aix-en-Provence and Bordeaux. Lastly, the correlations with relative humidity, sunshine duration, wind speed and wind direction were close to zero, with the notable exception of Aix-en-Provence where the annual number of hours of sunshine displayed a strong positive and significant correlation with the annual Cladosporium spore concentrations, as evidenced by an “explained variance” of more than 37 %.
4 Discussion
The aim of this study was to examine the temporal variations in an extended spectrum of characteristics of airborne Cladosporium seasons recorded at 5 French localities with diverse climatic conditions and to investigate the relationship, if any, between the conidia levels and the climate evolution. The stations provided different number of yearly periods comprised between 1999 and 2014, depending on the existing aerobiological data. It was the first attempt to show in France, on an annual base and for almost a decade or more than a decade, the climate sensitivity of this fungal genus of greatest clinical interest—not to mention the fact that Cladosporium infection in crops prompts considerable economic losses.
Many of the obtained results are in close agreement with the previously reported findings, though almost all past studies were conducted in other parts of the world. It has been a long time since some pioneering surveys of the occurrence of conidia belonging to Cladosporium genus have demonstrated their dominance in comparison with other spores (Adams 1964; Goodman et al. 1966; Nogales et al. 1985; Hjelmroos 1993; Herrero and Zaldivar 1997; Stępalska et al. 1999). A large number of recent aeromycological papers (Mitakakis and Guest 2001; Shelton et al. 2002; Ho et al. 2005; Stępalska and Wołek 2005; Grinn-Gofroń 2009; Recio et al. 2012; Sadyś et al. 2015a, b; Vélez-Pereira et al. (2015) (1995–2013) provide similar results, even if one of the reasons for this is that any aerobiological studies routinely identify only a limited number of fungal genera (Aira et al. 2012).
Comparing the results obtained from the five stations, the rather low counts of Bordeaux may be linked to the proximity to the ocean. According to various researchers (Rodríguez-Rajo et al. 2005; Aira et al. 2008, 2012), the spores of Cladosporium have their highest presence in the most continental centres, with concentrations being up to four times lower in coastal than in inland areas. The available substrate for fungal growth is smaller in coastal cities, where halophytic vegetation is probably not as susceptible as intensive crops to fungal infection; to this must be added the fact that the prevailing winds come mainly from the ocean, which do not provide any substrate for fungal development. It follows that Cladosporium spore concentrations tend to gradually increase as the Continentality Index CI (Valencia-Barrera et al. 2002) rises.
In terms of temporal distribution, airborne Cladosporium spore concentrations are reported to peak between late spring and early fall in temperate climates (Kurkela 1997; Hjelmroos 1993; Thibaudon and Lachasse 2006; Grinn-Gofroń and Strzelczak 2008; Grinn-Gofroń 2009; O’Connor et al. 2014), including the Mediterranean area (Aira et al. 2012; Vélez-Pereira et al. (2015) 1995–2013). This finding is not unexpected, given that mean temperature clearly follows a similar pattern. It is only in the most hot and arid areas, as the south-eastern Iberian Peninsula, that spore concentrations tend to decline at the height of summer, which leads to a bimodal seasonality, with two peaks of highest incidence in spring and fall.
As regards the potential impact of climate change, it is no wonder that only a few significant trends were identified compared to the total number of tests performed, because the inter-annual variability of the characteristics studied was particularly high (Hollins et al. 2004), while the size of the data sets was genuinely small. Due to the short length of available series, we did not find any significant trends in the salient features of the Cladosporium seasons (i.e. start date, peak date, end date, duration and peak value). Notwithstanding this, the general increase in annual temperatures observed during recent years in our five stations is consistent with the reported instances of warming worldwide (Hansen et al. 2006). By contrast, the findings on the relationships with Cladosporium levels might appear more problematic. In three locations, the yearly quantities of spores tended to increase with the temperature during the period of interest, while they tended to decrease in the remaining two. Yet, Aix-en-Provence and Toulouse are below the 44th parallel, while the other sites are above it. Consequently, these cities are climatically different.
Whereas the relation between the other major class of clinically relevant outdoor aero-allergens, namely airborne pollen grains, and global warming possesses considerable and expanding literature (Newnham 1999; Spieksma et al. 2003; Besancenot and Thibaudon 2012), it is surprising how little work has been performed on the potential impacts of climate change on allergenic fungi species and especially on spore production (Beggs 2004; Kinney 2008; Gobakken 2010; Barnes et al. 2013). This mainly reflects the rareness of routine mould monitoring data, but probably also the complicated relationships between climate factors, mould growth, spore release, and airborne measurements. As a result, there is only limited and inconsistent evidence of trends in mould production (Cecchi et al. 2010). Certainly, a meticulous study conducted in Derby, UK, suggested a clear trend towards increasing annual Alternaria counts, especially after 1992 (Corden and Millington 2001), but the link with changes in temperature remained speculative. Moreover, Corden et al. (2003) have established that Alternaria spore abundance varies largely from one location to another depending on regional vegetation and other local factors among which temperature is not always playing the key role. With regard to Cladosporium, the paucity of information regarding the impact of climate change is even more striking. However, in cold or relatively cool climates, Hjelmroos (1993) and Katial et al. (1997) suggested an upward linear trend in mean spore concentrations over time in parallel with a positive trend in temperature, respectively, in Stockholm, Sweden, and in Denver, USA.
However, the situation would be quite different in warm climates. In Thessaloniki, Greece, where a significant rise in air temperature has been observed over the period 1987–2005, mean annual Cladosporium concentrations have fallen considerably and significantly (−62 %, namely −3.26 % per year, p < 0.05) between the years 1987–1992 and the years 1999–2005, the negative trend having been reinforced since 1999 (Damialis et al. 2015a). By simulating different environmental regimes, Damialis et al. (2015b) have demonstrated experimentally that most fungal species, including Cladosporium, displayed dramatic growth responses, with faster mycelium growth and lower spore production at higher temperatures. This might explain why trends in different directions from city to city were recorded in our study. The only exception noted by Damialis et al. (2015b) was the indoor mould C. cladosporioides, which increased exponentially its spore production as air temperature rises, with an optimum for the temperature projection in 2100. As described earlier for Alternaria (Thibaudon and Lachasse 2006), the latitude has an uncontroversial impact on the concentration of spores of Cladosporium, as well as on the mould growth and dispersal. Several Spanish studies (Sabariego et al. 2000; Rodríguez-Rajo et al. 2005) demonstrated that average temperatures of 23–29 °C and a relative humidity of around 80 % correspond to optimal conditions for high Cladosporium spore counts. Such meteorological conditions are relatively less present in the Mediterranean area, where the humidity is low during the main season of Cladosporium. This is not the case in the oceanic and continental areas, where the humidity is higher. As a result, the optimal meteorological conditions for the development and release of the mould spores are usually achieved during the summer in these climates. This being so, certain Mediterranean areas, as Catalonia, would be characterised according to Vélez-Pereira et al. 2015 (1995–2013) by a positive significant tendency of Cladosporium counts. The authors are asking about the important differences in the results from Damialis et al. (2015a) and theirs. The assumptions made involve the amount of precipitation, the vegetation type and degree of arable lands in the surroundings. It needs to be explored, but at first sight it is hard to understand why Aix-en-Provence has much more in common with Thessaloniki than with Catalonia.
Climatic conditions may affect spore production in diverse ways, directly or indirectly through their effect on the substrates colonised by the fungus. The analysis of the statistical correlation of various annual climatic variables with the annual occurrence of Cladosporium spores in the atmosphere of our five monitoring station showed that the most important variable studied was T mean, which markedly influenced the production of fungal spores. These results concur with the findings of previous studies that have viewed mean temperature as the most suitable predictor of the Cladosporium spore concentrations (Katial et al. 1997; Hollins et al. 2004; Grinn-Gofroń and Rapiejko 2009; Oliveira et al. 2009). Aira et al. (2012) reported that relatively warm years favour the rapid growth of many Cladosporium species and induce sporulation in the absence of limiting factors such as inadequate nutrient availability. Moreover, more broadly, increasing temperatures usually provide the environmental dryness needed for conidia release into the atmosphere (Hollins et al. 2004).
Changes in rainfall patterns are less marked than temperature variations. Yet we found that, apart from Paris, lower CSI was expected in years with greater rainfall, as has already been shown by Katial et al. (1997) in Denver, Hollins et al. (2004) in Britain, Aira et al. (2008) in the north-western Iberian Peninsula, Oliveira et al. (2009) in Portugal or Aira et al. (2012) in the whole Iberian Peninsula. The washout effect of heavy and intense rain may prompt a sharp drop in airborne spore concentrations. The only exceptions are the driest areas, where rainfall increases soil moisture levels, favouring saprophytic growth and Cladosporium sporulation (Kurkela 1997); however, such conditions are unknown in France.
Still, the major weakness of our study lies in the fact that it has been conducted over a too short time scale, varying in length from 8 to 16 years according to locations. Particularly given the strong year-to-year fluctuations, longer periods of observations are needed to arrive to more definitive conclusions about trends in airborne Cladosporium concentrations. Thus, it is surely no coincidental that the only significant trend in CSI was noted in the city (Bordeaux) offering the longest data series (16 years).
5 Conclusion
Fungal spores are an important, although often underestimated, cause of allergic sensitisation and seasonal allergy symptoms, including acute respiratory failures. For this reason, the knowledge of fungal concentrations over the time may be of value in the clinical management of allergies (Hollins et al. 2004; Grinn-Gofroń and Bosiacka 2015). But at present, there is much that remains unknown about changes in production, dispersion and allergen content of fungal spores, which may be region- and species-specific (Cecchi et al. 2010). As regards Cladosporium in particular, a species with high spore allergenic potential and great atmospheric abundance, increasing trends were found in the oceanic areas with or without continental degradation, and decreasing trends in the Mediterranean or submediterranean areas. Depending on the context, the same warming can trigger more or less airborne spore production and alter the distribution timing and pattern in one or other direction. Longer aerobiological monitoring periods and more sites recording the fungal data are imperatively required to corroborate these preliminary results and survey the increasing or decreasing levels of certain pollen types that could exert an impact in terms of public health.
References
Adams, K. F. (1964). Year to year variation in the fungus spore content of the atmosphere. Acta Allergologica, 19(1), 11–50. doi:10.1111/j.1398-9995.1964.tb03220.x.
Aira, M. J., Rodríguez-Rajo, F. J., Fernández-González, M., Seijo, C., Elvira-Rendueles, B., Gutiérrez-Bustillo, M., et al. (2012). Cladosporium airborne spore incidence in the environmental quality of the Iberian Peninsula. Grana, 51(4), 293–304. doi:10.1080/00173134.2012.717636.
Aira, M. J., Rodríguez-Rajo, F. J., & Jato, V. (2008). 47 annual records of allergenic fungi spore: Predictive models from the NW Iberian Peninsula. Annals of Agricultural and Environmental Medicine, 15(1), 91–98.
Aukrust, L. (1979). Crossed radioimmunoelectrophoretic studies of distinct allergens in two extracts of Cladosporium herbarum. International Archives of Allergy and Applied Immunology, 58(4), 375–390. doi:10.1159/000232217.
Bagni, B., Davies, R. R., Mallea, M., Nolard, N., Spieksma, F. T., & Stix, E. (1977). Sporenkonzentrationen in Städten der Europäischen Gemeinschaft (EG). II. Cladosporium- und Alternaria-Sporen. Acta Allergologica, 32(2), 118–138. doi:10.1111/j.1398-9995.1977.tb02617.x.
Barnes, C. S., Alexis, N. E., Bernstein, J. A., Cohn, J. R., Demain, J. G., Horner, E., et al. (2013). Climate change and our environment: The effect on respiratory and allergic disease. Journal of Allergy and Clinical Immunology: In Practice, 1(2), 137–141. doi:10.1016/j.jaip.2012.07.002.
Beggs, P. J. (2004). Impacts of climate change on aeroallergens: Past and future. Clinical and Experimental Allergy, 34(10), 1507–1513. doi:10.1111/j.1365-2222.2004.02061.x.
Bensch, K., Braun, U., Groenewald, J. Z., & Crous, P. W. (2012). The genus Cladosporium. Studies in Mycology, 72(1), 1–401. doi:10.3114/sim0003.
Besancenot, J. P., & Thibaudon, M. (2012). Changement climatique et pollinisation. Revue des Maladies Respiratoires, 29(10), 1238–1253. doi:10.1016/j.rmr.2012.07.007.
Bousquet, P. J., Chinn, S., Janson, C., Kogevinas, M., Burney, P., & Jarvis, D. (2007). Geographical variation in the prevalence of positive skin tests to environmental aeroallergens in the European Community Respiratory Health Survey I. Allergy, 62(3), 301–309. doi:10.1111/j.1398-9995.2006.01293.x.
Cecchi, L., D’Amato, G., Ayres, J. G., Galan, C., Forastiere, F., Forsberg, B., et al. (2010). Projections of the effects of climate change on allergic asthma: The contribution of aerobiology. Allergy, 65(9), 1073–1081. doi:10.1111/j.1398-9995.2010.02423.x.
Corden, J. M., & Millington, W. M. (2001). The long-term trends and seasonal variation of the aeroallergen Alternaria in Derby, UK. Aerobiologia, 17(2), 127–136. doi:10.1023/A:1010876917512.
Corden, J. M., Millington, W. M., & Mulllins, J. (2003). Long-term trends and regional variation in the aeroallergen Alternaria in Cardiff and Derby UK—Are differences in climate and cereal production having an effect? Aerobiologia, 19(3), 191–199. doi:10.1023/B:AERO.0000006529.51252.2f.
Damialis, A., Halley, J. M., Gioulekas, D., & Vokou, D. (2007). Long-term trends in atmospheric pollen levels in the city of Thessaloniki, Greece. Atmospheric Environment, 41(33), 7011–7021. doi:10.1016/j.atmosenv.2007.05.009.
Damialis, A., Mohammad, A. B., Halley, J. M., & Gange, A. C. (2015a). Fungi in a changing world: Growth rates will be elevated, but spore production may decrease in future climates. International Journal of Biometeorology, 59(9), 1157–1167. doi:10.1007/s00484-014-0927-0.
Damialis, A., Vokou, D., Gioulekas, D., & Halley, J. M. (2015b). Long-term trends in airborne fungal-spore concentrations: A comparison with pollen. Fungal Ecology, 13, 150–156. doi:10.1016/j.funeco.2014.09.010.
Dugan, F. M., Schubert, K., & Braun, U. (2004). Check-list of Cladosporium names. Schlechtendalia, 11, 1–103.
Estienne, P. (1979). La France, I. Généralités, Région du Nord. Paris: Masson.
Fukutomi, Y., & Taniguchi, M. (2015). Sensitization to fungal allergens: Resolved and unresolved issues. Allergology International, 64(4), 321–331. doi:10.1016/j.alit.2015.05.007.
Gobakken, L. R. (2010). Effects of global climate change on mould growth—Interactions of concern. In 41 st Annual Meeting of the International Research Group on Wood Protection, Biarritz, 9–13 May 2010 (pp. 1–11). Stockholm: IRG Secretariat.
Goodman, D. H., Northey, W. T., Leathers, C. R., & Savage, T. H. (1966). A study of airborne fungi in the Phoenix, Arizona, metropolitan area. Journal of Allergy, 38(1), 56–62. doi:10.1016/0021-8707(66)90074-8.
Gravesen, S. (1979). Fungi as a cause of allergic disease. Allergy, 34(3), 135–154. doi:10.1111/j.1398-9995.1979.tb01562.x.
Grinn-Gofroń, A. (2009). The occurrence of Cladosporium spores in the air and their relationships with meteorological parameters. Acta Agrobotanica, 62(2), 111–116. doi:10.5586/aa.2009.032.
Grinn-Gofroń, A., & Bosiacka, B. (2015). Effects of meteorological factors on the composition of selected fungal spores in the air. Aerobiologia, 31(1), 63–72. doi:10.1007/s10453-014-9347-1.
Grinn-Gofroń, A., & Rapiejko, P. (2009). Occurrence of Cladosporium spp. and Alternaria spp. spores in Western, Northern and Central-Eastern Poland in 2004–2006 and relation to some meteorological factors. Atmospheric Research, 93(4), 747–758. doi:10.1016/j.atmosres.2009.02.014.
Grinn-Gofroń, A., & Strzelczak, A. (2008). Artificial neural network models of relationships between Cladosporium spores and meteorological factors in Szczecin (Poland). Grana, 47(4), 305–315. doi:10.1080/00173130802513784.
Hamilos, D. L. (2010). Allergic fungal rhinitis and rhinosinusitis. Proceedings of the American Thoracic Society, 7(3), 245–252. doi:10.1513/pats.200909-098AL.
Hansen, J., Sato, M., Ruedy, R., Lo, K., Lea, D. W., & Medina-Elizade, M. (2006). Global temperature change. Proceedings of the National Academy of Sciences, 103(39), 14288–14293. doi:10.1073/pnas.0606291103.
Herrero, B., & Zaldivar, P. (1997). Effects of meteorological factors on the levels of Alternaria and Cladosporium spores in the atmosphere of Palencia, 1990–1992. Grana, 36(3), 180–184. doi:10.1080/00173139709362606.
Hirst, J. M. (1952). An automatic volumetric spore trap. Annals of Applied Biology, 39(2), 257–265. doi:10.1111/j.1744-7348.1952.tb00904.x.
Hjelmroos, M. (1993). Relationship between airborne fungal spore presence and weather variables: Cladosporium and Alternaria. Grana, 32(1), 40–47. doi:10.1080/00173139309436418.
Ho, H. M., Rao, C. Y., Hsu, H. H., Chiu, Y. H., Liu, C. M., & Chao, H. J. (2005). Characteristics and determinants of ambient fungal spores in Hualien, Taiwan. Atmospheric Environment, 39(32), 5839–5850. doi:10.1016/j.atmosenv.2005.06.034.
Hollins, P. D., Kettlewell, P. S., Atkinson, M. D., Stephenson, D. B., Corden, J. M., Millington, W. M., et al. (2004). Relationships between airborne fungal spore concentration of Cladosporium and the summer climate at two sites in Britain. International Journal of Biometeorology, 48(3), 137–141. doi:10.1007/s00484-003-0188-9.
Hyde, K. D., McKenzie, E. H. C., & KoKo, T. W. (2011). Towards incorporating anamorphic fungi in a natural classification—Checklist and notes for 2010. Mycosphere, 2(1), 1–88.
Käpylä, M., & Penttinen, A. (1981). An evaluation of the microscopal counting methods of the tape in Hirst-Burkard pollen and spore trap. Grana, 20(2), 131–141. doi:10.1080/00173138109427653.
Kasprzyk, I., Rodinkova, V., Šaulienė, I., Ritenberga, O., Grinn-Gofroń, A., Nowak, M., et al. (2015). Air pollution by allergenic spores of the genus Alternaria in the air of central and eastern Europe. Environmental Science and Pollution Research, 22(12), 9260–9274. doi:10.1007/s11356-014-4070-6.
Katial, R. K., Zhang, Y., Jones, R. H., & Dyer, P. D. (1997). Atmospheric mold spore counts in relation to meteorological parameters. International Journal of Biometeorology, 41(1), 17–22. doi:10.1007/s004840050048.
Kinney, P. L. (2008). Climate change, air quality, and human health. American Journal of Preventive Medicine, 35(5), 459–467. doi:10.1016/j.amepre.2008.08.025.
Kurkela, T. (1997). The number of Cladosporium conidia in the air in different weather conditions. Grana, 36(1), 54–61. doi:10.1080/00173139709362591.
Lavaud, F., & Dutau, G. (2013). Spores fongiques atmosphériques et allergies respiratoires. Revue Française d’Allergologie et d’Immunologie Clinique, 53(7), 567–568. doi:10.1016/j.reval.2013.10.003.
Limpert, E., Burke, J., Galán, C., del Mar Trigo, M., West, J. S., & Stahel, W. A. (2008). Data, not only in aerobiology: How normal is the normal distribution? Aerobiologia, 24(3), 121–124. doi:10.1007/s10453-008-9092-4.
Malling, H. J., Dreborg, S., & Weeke, B. (1987). Diagnosis and immunotherapy of mould allergy, part VI. Allergy, 42(4), 305–314. doi:10.1111/j.1398-9995.1987.tb02214.x.
Mitakakis, T. Z., & Guest, D. I. (2001). A fungal spore calendar for the atmosphere of Melbourne, Australia, for the year 1993. Aerobiologia, 17(2), 171–176. doi:10.1023/A:1011028412526.
Mitakakis, T. Z., & McGee, P. A. (2000). Reliability of measures of spores of Alternaria and pollen concentrations in air over two towns in rural Australia. Multiple sites for Burkard sampling. Grana, 39(2–3), 141–145. doi:10.1080/001731300300045300.
Money, N. P. (2015). Spore production, discharge and dispersal. In S. C. Watkinson, L. Boddy, & N. P. Money (Eds.), The Fungi (3rd ed., pp. 67–97). London: Academic Press.
Mullins, J. (2001). Microorganisms in outdoor air. In B. Flannigan, R. A. Samson, & J. D. Miller (Eds.), Microorganisms in home and indoor work environments: Diversity, health impacts, investigation and control (pp. 3–16). London: Taylor & Francis.
Newnham, R. M. (1999). Monitoring biogeographical response to climate change: The potential role of aeropalynology. Aerobiologia, 15(2), 87–94. doi:10.1023/A:1007595615115.
Nilsson, S., & Persson, S. (1981). Tree pollen spectra in the Stockholm region (Sweden), 1973–1980. Grana, 20(3), 179–182. doi:10.1080/00173138109427661.
Nogales, M. T., Galán Soldevilla, C., Ruíz de Clavijo, J. E., & Dominguez Vilches, E. (1985). Variación estacional del contenido de esporas de Cladosporium en la atmósfera de Córdoba. Anales de la Asociación de Palinólogos de Lengua Española, 2, 339–345.
O’Connor, D. J., Sadyś, M., Skjøth, C. A., Healy, D. A., Kennedy, R., & Sodeau, J. R. (2014). Atmospheric concentrations of Alternaria, Cladosporium, Ganoderma and Didymella spores monitored in Cork (Ireland) and Worcester (England) during the summer of 2010. Aerobiologia, 30(4), 397–411. doi:10.1007/s10453-014-9337-3.
Oliveira, M., Ribeiro, H., Delgado, L., & Abreu, I. (2009). The effects of meteorological factors on airborne fungal spore concentration in two areas differing in urbanization level. International Journal of Biometeorology, 53(1), 61–73. doi:10.1007/s00484-008-0191-2.
Pidek, I. A., Poska, A., & Kaszewski, B. M. (2015). Taxon-specific pollen deposition dynamics in a temperate forest zone, SE Poland: The impact of physiological rhythmicity and weather controls. Aerobiologia, 31(2), 219–238. doi:10.1007/s10453-014-9359-x.
Pringle, A. (2013). Asthma and the diversity of fungal spores in air. PLoS Pathogens, 9(6), e1003371. doi:10.1371/journal.ppat.1003371.
Rapiejko, P., Stanlaewicz, W., Szczygielski, K., & Jurkiewicz, D. (2007). Progowe stężenie pyłku roślin niezbędne do wywołania objawów alergicznych/Threshold pollen count necessary to evoke allergic symptoms. Otolaryngologia Polska, 61(4), 591–594.
Recio, M., del Mar Trigo, M., Docampo, S., Melgar, M., García-Sánchez, J., Bootello, L., et al. (2012). Analysis of the predicting variables for daily and weekly fluctuations of two airborne fungal spores: Alternaria and Cladosporium. International Journal of Biometeorology, 56(6), 983–991. doi:10.1007/s00484-011-0509-3.
Rodríguez-Rajo, F. J., Iglesias, I., & Jato, V. (2005). Variation assessment of airborne Alternaria and Cladosporium spores at different bioclimatical conditions. Mycological Research, 109(4), 497–507. doi:10.1017/S0953756204001777.
Sabariego, S., Díaz de la Guarda, C., & Alba, F. (2000). The effect of meteorological factors on the daily variation of airborne fungal spores in Granada (Southern Spain). International Journal of Biometeorology, 44(1), 1–5. doi:10.1007/s004840050131.
Sadyś, M., Kennedy, R., & West, J. S. (2015a). Potential impact of climate change on fungal distributions: Analysis of 2 years of contrasting weather in the UK. Aerobiologia,. doi:10.1007/s10453-015-9402-6.
Sadyś, M., Strzelczak, A., Grinn-Gofroń, A., & Kennedy, R. (2015b). Application of redundancy analysis for aerobiological data. International Journal of Biometeorology, 59(1), 25–36. doi:10.1007/s00484-014-0818-4.
Shapiro, S. S., & Wilk, M. B. (1965). An analysis of variance test for normality (complete samples). Biometrika, 52(3–4), 591–611. doi:10.2307/2333709.
Shelton, B. G., Kirkland, K. H., Flanders, W. D., & Morris, G. K. (2002). Profiles of airborne fungi in buildings and outdoor environments in the United States. Applied and Environmental Microbiology, 68(4), 1743–1753. doi:10.1128/AEM.68.4.1743-1753.2002.
Smith, M., Jäger, S., Berger, U., Šikoparija, B., Hallsdottir, M., Sauliene, I., et al. (2014). Geographic and temporal variations in pollen exposure across Europe. Allergy, 69(7), 913–923. doi:10.1111/all.12419.
Spieksma, F. T. M., Corden, J. M., Detandt, M., Millington, W. M., Nikkels, H., Nolard, N., et al. (2003). Quantitative trends in annual totals of five common airborne pollen types (Betula, Quercus, Poaceae, Urtica, and Artemisia), at five pollen-monitoring stations in Western Europe. Aerobiologia, 19(3), 171–184. doi:10.1023/B:AERO.0000006528.37447.15.
Stępalska, D., Harmata, K., Kasprzyk, I., Myszkowska, D., & Stach, A. (1999). Occurrence of airborne Cladosporium and Alternaria spores in Southern and Central Poland in 1995–1996. Aerobiologia, 15(1), 39–47. doi:10.1023/A:1007536513836.
Stępalska, D., & Wołek, J. (2005). Variation in fungal spore concentrations of selected taxa associated to weather conditions in Cracow, Poland, in 1997. Aerobiologia, 21(1), 43–52. doi:10.1007/s10453-004-5877-2.
Thibaudon, M., & Lachasse, C. (2006). Alternaria, Cladosporium: Dispersion atmosphérique, rythmes nycthéméral et saisonnier. Revue Française d’Allergologie et d’Immunologie Clinique, 46(3), 188–196. doi:10.1016/j.allerg.2006.01.025.
Ugolotti, M., Pasquarella, C., Vitali, P., Smith, M., & Albertini, R. (2015). Characteristics and trends of selected pollen seasons recorded in Parma (Northern Italy) from 1994 to 2011. Aerobiologia, 31(3), 341–352. doi:10.1007/s10453-015-9368-4.
Valencia-Barrera, R. M., Comtois, P., & Fernández-González, D. (2002). Bioclimatic indices as a tool in pollen forecasting. International Journal of Biometeorology, 46(4), 171–175. doi:10.1007/s00484-002-0138-y.
Vélez-Pereira, A. M., De Linares, C., Delgado, R., & Belmonte, J. (2015). Temporal trends of the airborne fungal spores in Catalonia (NE Spain), 1995–2013. Aerobiologia. 1–15. doi: 10.1007/s10453-015-9410-6.
Ziello, C., Sparks, T. H., Estrella, N., Belmonte, J., Bergmann, K. C., Bucher, E., et al. (2012). Changes to airborne pollen counts across Europe. PLoS One, 7(4), e34076. doi:10.1371/journal.pone.0034076.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sindt, C., Besancenot, JP. & Thibaudon, M. Airborne Cladosporium fungal spores and climate change in France. Aerobiologia 32, 53–68 (2016). https://doi.org/10.1007/s10453-016-9422-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-016-9422-x