Abstract
The impact of climate change on fungal growth and spore production is less well documented than for allergenic pollen grains, although similar implications for respiratory tract diseases in humans occur. Fungal spores are commonly described as either “dry” or “wet” according to the type of weather associated with their occurrence in the air. This study examined the distribution of selected fungal spores (Alternaria spp., Cladosporium spp., Didymella spp., Epicoccum spp., Leptosphaeria spp. and rusts) occurring in the West Midlands of UK during 2 years of contrasting weather. Spore specimens were collected using a 7-day volumetric air sampler and then analysed with the aid of light microscopy. Distributions of spores were then studied using normality tests and Mann–Whitney U test, while relationships with meteorological parameters were investigated using Spearman’s rank test and angular-linear correlation for wind direction analysis. Our results showed that so-called wet spores were more sensitive to the weather changes showing statistically significant differences between the 2 years of study, in contrast to “dry” spores. We predict that in following years we will observe accelerated levels in allergenic fungal spore production as well as changes in species diversity. This study could be a starting point to revise the grouping system of fungal spores as either “dry” or “wet” types and their response to climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, the impact of the climate on the changes in bioaerosol concentration and composition (mainly pollen grains and fungal spores) is undergoing an open discussion (Cecchi et al. 2010; D’Amato and Cecchi 2008; D’Amato et al. 2011; Damialis et al. 2014; Kennedy and Smith 2012; Reid and Gamble 2009). As indicated by Kennedy and Smith (2012), there are several uncertainties related to the overall prediction of climate change impact on aeroallergens, i.e. (1) ambiguity related to the climate change scenarios, (2) its overall impact on pollen grain and fungal spore production, and (3) methodology of assessment of aeroallergen impact on asthma sufferers.
In Great Britain, Gange et al. (2007) examined the dynamics of 315 autumnal fruiting fungal species, recorded for over 20 years. On average, all examined species exhibited prolongation of the fruiting seasons. Comparing with records dated in the 1950s, now fungi fruit twice as long (from 33.2 ± 1.6 to 74.8 ± 7.6 days). This may have been caused by climate change observed in the UK, where mean air temperature in August has increased, while more precipitation falls in October (Met Office 2011; UKCP 2009). Some species were found to be affected solely by temperature, while others by a joint impact of both temperature and rainfall (Gange et al. 2007). Likewise, Kauserud et al. (2008) examined changes in phenology of 83 species in Norway. They also found a similar trend in extension of fruiting seasons, although the difference was found to be greater, i.e. 13.3 ± 1.2 days within a 60-year period. The growth of earlier fruiting fungi was found to be more influenced by mean air temperature compared to late fruiting species. In addition, the most significant shift in overall fungal phenology was observed between the 1980s and 2000s (Kauserud et al. 2008). That study was then extended, and similar findings were reported in Austria and Switzerland (Kauserud et al. 2012).
Climate change was also found to influence morphological changes in fungal spores (Kauserud et al. 2011). Kauserud et al. (2011) determined that spores produced at the beginning of autumn were driven towards greater water accumulation, resulting in size enlargement, while spores produced towards the end of autumn were characterised by a reduction in size. From the allergological point of view, changes in spore size are incredibly important, as the smaller the spore size, the easier spores can be inhaled and more likely that they will be deposited deeper inside the human respiratory system. Moreover, the spore enlargement in early autumn occurred during increased mean air temperature and lower precipitation (Kauserud et al. 2011). However, Kauserud et al. (2011) predicted that in a situation of simultaneous increase in mean air temperature and precipitation, the overall size of fungal spores would decrease. Therefore, three scenarios could be possible to occur in Europe, i.e. (1) asthma exacerbations may be shifted from autumn towards the winter season, (2) a reduction in hospital admissions may occur in hotter Mediterranean countries, especially in continental areas, while (3) an increase in hospital admissions may take place in central and northern Europe. However, a recent study of Damialis et al. (2014) demonstrated that the actual scenario may not necessarily be so negative, as based on a set of performed laboratory experiments, they found that although mycelia growth of allergenic fungal species, such as Alternaria alternata, Cladosporium cladosporoides or Aspergillus niger will increase, the overall spore production may in fact decrease. On the other hand, a study conducted by Wolf et al. (2010) showed that sporulation at allergenic fungi was enhanced by an increased concentration of CO2 in the air. However, the effect of increased concentrations of CO2 on the allergenic potential of fungal spores maybe more easily differentiated at the subcellular level (Kennedy and Wakeham 2015).
A joint collaboration of several governmental institutions in the UK, such as Department for Environment, Food & Rural Affairs, Met Office, Environment Agency and Natural England, resulted in estimation of future changes related to the climate in the UK and production of guideline on how to adapt to these changes (Met Office 2011; UKCP 2009). It has been estimated that air temperature will increase in summer (June–August) of +1.6 °C by the 2020s, +2.3 °C by the 2040s, and +3.9 °C by the 2080s. The increase in temperature will be also observed during winter months, but of a lesser difference comparing with summer. Summer shall also experience reduction in precipitation of −7 % by the 2020s, −13 % by the 2040s, and −27 % by the 2080s. However, an opposite trend is going to occur between December and February, where IPCC projections estimated an increase in rainfall of +6 % by the 2020s, +10 % by the 2040s, and +16 % by the 2080s.
As the discrepancy between studies and suggested scenarios regarding fungal responses towards climate change increases, we decided to analyse 2 years of fungal spore distribution measured at an urban site in England. Selected years, i.e. 2006 and 2007, were very contrasting to each other, as summer 2006 was one of the hottest, driest, and sunniest on record since 1910 until 2013 (Met Office 2012a, 2013), while summer 2007 was remembered as one of the wettest, taking into account the amount of observed precipitation and the size of flooding covering many regions of UK at that time (Marsh and Hannaford 2007; Met Office 2012b). For this purpose, we also chose six species, three of which representing so-called “dry” and three “wet” types of spores. This common classification is based on the predominant diverse weather conditions that are required for spore production, spore release and dispersal processes.
2 Materials and methods
Spore sampling was taken continuously between 1 January 2006 and 31 December 2007 at an aerobiological station in Worcester, UK (52°11′N, 2°14′W). A 7-day volumetric spore trap (Burkard Manufacturing Co., Ltd., Rickmansworth, UK) sampled air at 10 l min−1 and was processed into separate samples representing 24 h periods of time (Hirst 1952). Protocols describing sampling in detail can be found in Lacey and West (2006). Six taxa were selected for a detailed analysis, i.e. Alternaria spp. (hereafter Alternaria), Cladosporium spp. (hereafter Cladosporium), Epicoccum nigrum (hereafter Epicoccum), Didymella spp. (hereafter Didymella), Leptosphaeria spp. (Leptosphaeria), and rusts (mainly urediniospores and teliospores of Puccinia graminis, P. triticina, P. striiformis). Alternaria, Cladosporium and Epicoccum were considered to be “dry” spore types, while Didymella, Leptosphaeria and rusts were “wet” spores. The wet spores chosen were also plant pathogens where their occurrence required completed life cycles. All spores were enumerated along one central line of the 48 mm-long daily tape. Concentration of spores was calculated based on the number of observed spores, amount of air sampled within a 24 h period, and diameter of the microscope field of view under 400× magnification. This calculation converted spore counts into number of spores per cubic of air (s m−3).
A weather Link (9.0) Vantage Pro2 station (Davis, Davis Instruments, Hayward, California, USA), which was co-located with the spore trap, recorded a number of meteorological parameters. However, for the purpose of this study, only several factors were examined, i.e. air temperature (mean, minimum, and maximum), dew point temperature, leaf temperature, relative humidity, rainfall, leaf wetness, air pressure and wind direction. The meteorological data were recorded with 5 min interval in both examined years. Subsequently, all records were averaged to hourly data to match the fungal spore concentrations in a similar way as reported in previous studies (O’Connor et al. 2014; Sadyś et al. 2015).
Subsequently, seasons of fungal spore occurrence in the air were determined using the timing of the modal 90 % of spore production of a species (Nilsson and Persson 1981). Selected data were examined towards normality or its lack (Kolmogorov–Smirnov test, Kolmogorov–Smirnov test with Lilliefors correction, and Shapiro–Wilk test) after del Mar Trigo et al. (2000). As data exhibited non-normal distribution and non-linear dependence in relation to investigated weather parameters, therefore, non-parametric statistics were applied (Spearman’s rank test, angular-linear correlation, and Mann–Whitney U test). The strength of statistical association was assessed following Mukaka (2012). Microsoft Excel (2010) and GenStat (17) software were used to perform indicated above analyses.
3 Results
3.1 Changes in weather
A comparison between maximum air temperature and precipitation was prepared for the Worcester city and the Midlands region in order to widen climatic information (Table 1; Figs. 1, 2). Other meteorological parameters were presented graphically for the Worcester site only in Figs S1–S2, for the period of April–November each studied year, as fungal spore seasons of all six taxa were mainly found within this range (Table 1).
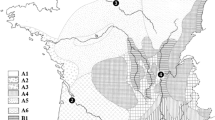
Thirty-year mean values for maximum temperature recorded in the Midlands region (grey columns) were plotted against values of maximum temperature observed in 2006 (red line) and 2007 (blue line) in Worcester, UK. The standard deviation bars represent increase in maximum temperature of +1.6 °C by the 2020s, projected by the ICPP for the UK
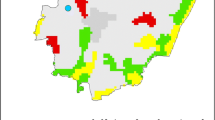
Thirty-year mean values for rainfall recorded in the Midlands region (grey columns) were plotted against values of maximum temperature observed in 2006 (red line) and 2007 (blue line) in Worcester, UK. The standard deviation bars represent increase in rainfall of +6 % by the 2020s, projected by the ICPP for winter period in the UK
Overall, the maximum temperature in Worcester was on average 0.7–1.4 °C higher than the entire region, while recorded precipitation was often a factor of 1.3–2.5 lower only 17 to 40 % of that of the region (Table 1; Fig. 1). Moreover, in 2006, the monthly mean maximum temperature of ≥20 °C was recorded between June and September both in Worcester and Midlands. The hottest month turned out to be July with an average maximum temperature of 26.7 °C (Table 1). In the following year, there were only 2 months that scored ≥20 °C in Worcester (June, August), while for most of the sites in the Midlands, this was limited to August only.
During the first year of study, precipitation events were very rare (Table 1; Fig. 2). The lowest amount of rainfall was observed in June with 6.8 mm for Worcester, and the highest in May with 78.9 mm. These values were higher for the Midlands generally by a factor of 1.3 and 2.4, respectively (Table 1). In 2006, June was the driest month in Worcester. In 2007, the driest month was April (6.2 and 8.7 mm, respectively, for Worcester and the Midlands). Rainfall increased and peaked in June–July (Table 1). In Worcester, the total amount of rain was 114.5 mm, while in the Midlands, on average, this was 144.7 mm (Table 1). Therefore, precipitation recorded in July 2007 was almost 3 times higher than in July 2006.
3.2 Spore seasonality
The Mann–Whitney U test that examined patterns of spores for similarity or its lack between 2 years of the study showed that there was no significant difference between 2006 and 2007 with regard to the overall distribution of “dry” spore types Alternaria, Cladosporium and Epicoccum spores (Table 2). The group representing so-called “wet” spores showed a statistically significant dissimilarity between years of observation (Table 2). A summary of the spore frequency distribution in the air of Worcester is given in Table 3 and Fig. 3. A spore season that corresponds to a more frequent presence of spores in the air was a subject of change between years and between species. Regardless of classification as either “dry” or “wet” type, 4 out of 6 taxa showed an earlier occurrence in 2007 compared to the previous year, one the same in both studied years and one later in 2007 (Table 3). However, an earlier beginning of the spore seasons did not correlate with an earlier end of the spore season, but actually just the opposite except for rust spores in 2007 (Table 3). These fluctuations greatly influenced the length of the spore seasons. Alternaria, Cladosporium, Epicoccum, and Leptosphaeria exhibited much shorter seasons in 2006 compared to the following year, while the season was longer for Didymella and rust spores in 2006. Overall difference between “short” and “long” spore seasons varied from 15 % (rust) to ~70 % (Leptosphaeria, Cladosporium). Interestingly, the peak concentration of all species was recorded during “shorter” rather than “longer” seasons (Table 3). Clinical threshold values that have been established for Alternaria and Cladosporium spores are 50 and 3000 s m−3, respectively. In both cases, the number of days with exceeded threshold value was much higher in 2006 (Table 4). The difference between years varied from 26 % (Alternaria) to 43 % (Cladosporium). This also had an impact on reduced number of overlapping high spore count days in 2007 (Table 4).
3.3 Impact of weather on spore distribution
Impact of the weather on spore distribution was studied by an analysis of ten meteorological parameters and performing two tests for association analysis (Table 5; Fig. S3).
3.3.1 “Dry” spores (Alternaria, Cladosporium, and Epicoccum)
Presence and concentration levels of Alternaria spores in the air were mainly dependent on temperature parameters, and these relationships were found in both years of study, although their strength increased from low to moderate in 2007 (Table 5). Dew point temperature had no statistically significant association with Alternaria. Other parameters revealed either low or negligible relationships with the presence of Alternaria spores in the air of Worcester, regardless of their statistical significance (Table 5).
The distribution of Cladosporium spores exhibited a limited analogy to the fluctuations of all examined temperature parameters, which was more visible during the first year of study (Table 5). The strongest association achieved by Cladosporium was found with leaf temperature in 2006 at r s = 0.45 (Table 5). In 2007, the influence of these and remaining weather factors was significantly reduced (except for dew point temperature).
Out of three “dry” spore types, Epicoccum showed statistically significant (1) positive relationships with maximum temperature, minimum temperature, mean temperature, leaf temperature, and air pressure, and (2) negative relationship with relative humidity at the moderate level with correlation coefficient values varying from r s = ±0.51 to r s = ±0.60 (Table 5). Impact of these factors was mainly detected in 2006, while it disappeared completely in the following year (Table 5).
3.3.2 “Wet” spores (Didymella, Leptosphaeria, and rusts)
The second investigated type of spores also revealed heterogeneity within the group (Table 5). During the dry year, 2006, Didymella showed (1) moderate association with rainfall only, (2) low with relative humidity, leaf wetness, and air pressure, and (3) negligible with other factors (Table 5). In the wetter year, 2007, dependence on precipitation remained unchanged, while it increased from low to moderate in the case of leaf wetness and relative humidity (Table 5). Regardless of the changes in the overall weather between 2006 and 2007, the impact of most temperature parameters on distribution of Didymella spores was found to be negligible.
Similarly to Didymella, Leptosphaeria was not dependent on maximum, minimum, mean, leaf, and dew point temperature (Table 5). Influence of rainfall, air pressure, and leaf wetness was exhibited, however, at the moderate level from r s = ±0.51 to r s = ±0.54 in the drier year, but not in wetter (Table 5). Other investigated weather factors did not have any impact on fluctuations in Leptosphaeria distribution.
Finally, in both studied years, none of the analysed weather factors seemed to affect rusts, as all associations were found as negligible or low (Table 5). The strongest (and negative) relationship was with rainfall in 2007 (Table 5).
4 Discussion
In comparison with tree pollen production, which was found to be produced earlier and last longer due to climate change (Cecchi et al. 2010), our findings showed that climate change has similar impact on many fungi (Table 3). These results are therefore in agreement with previous findings for Basidiomycetes examined by Kauserud et al. (2008, 2012).
In the UK, Corden and Millington (2001) investigated the distribution of Alternaria at an urban site in Derby from 1970 to 1998. They found a gradual increase in spore concentration, which accelerated since 1992 onwards. Fungi started fruiting earlier, resulting in extension of spore seasons. In addition to that, a number of days with high spore levels of clinical importance, i.e. ≥50 s m−3 (Frankland and Davies 1965), also exhibited an upward trend (Corden and Millington 2001). These findings were in line with results obtained by Damialis et al. (2015), who also observed an ascending tendency for Alternaria spore levels in Greece between 1987 and 2005. However, other taxa revealed a contrary drift (Damialis et al. 2015). Reduction in spore concentration was the highest for Botrytis (−85.4 %), a “wet” type of spores. Other fungi exhibited losses of 62.1 % (Cladosporium), 25.9 % (Epicoccum) and 10.1 % (Leptosphaeria), (Damialis et al. 2015). It must be stressed, however, that throughout the examined period of time, the minimum air temperature has increased on average from 10.8 to 12.2 °C (Damialis et al. 2015).
With regard to the length of fungal spore seasons, Damialis et al. (2015) noticed a later onset and earlier end. This was relevant for Alternaria, Epicoccum, and Leptosphaeria but not entirely for Cladosporium (Damialis et al. 2015). Results of our study based on 2 years of contrasting weather conditions showed that seasons were shorter, when optimal conditions for fungal growth and spore production occurred (Table 3). As the right conditions were met, fungi accelerated their mycelial growth and spore production was elevated for 18–329 % compared to an adverse year (Table 3). However, this led to exhaustion of nutrients and energy over the time. This was true for all six examined taxa, regardless of their environmental stipulations. Spore production by Alternaria, Aspergillus, Botrytis, Cladosporium, and Epicoccum species was also examined by Damialis et al. (2014) under laboratory conditions. They found that although higher temperature facilitated mycelia growth, especially those of Alternaria and Epicoccum, the spore production was mostly inhibited except for Cladosporium cladosporioides (Damialis et al. 2014). Hence, they concluded that fungi extend their growth stage until depletion of food stocks, and at the very last minute start sporulation (Damialis et al. 2014). Interpretation of such results cannot be unequivocal, as others could argue that differences between years in spore levels could derive from other factors, such as changes in wind direction (O’Connor et al. 2014; Sadyś et al. 2014) and/or changes in land use in the vicinity of the sampling site (Corden and Millington 2001).
This study also demonstrated that so-called “wet” spores were more sensitive to dry weather conditions than so-called “dry” spores to wet weather conditions (Table 2). The latter group seemed to be less frequently observed in the air due to excess of precipitation that resulted in lack of release or washing spores out from the air rather than having a significant impact on their biology (Pakpour et al. 2015). Having said that, it is more likely that along with an increase in mean air temperature, an increase in plant diseases caused by fungi with biology similar to those of Alternaria, Cladosporium, and Epicoccum shall occur. The overall amount of Alternaria spores in the atmosphere may, however, remain constant due to the occurrence of similar species, which have different optimal conditions. For example, the sporulation by A. brassicae and A. brassicicola on naturally infected leaf discs of oilseed rape and cabbage required humidities equal to or higher than 91.5 % and 87 % r.h., respectively (Humperson-Jones and Phelps 1989). The optimum temperatures for sporulation were 18–24 °C for A. brassicae and 20–30 °C for A. brassicicola at which temperatures both fungi produced spores within 12–14 h (Humperson-Jones and Phelps 1989).
Other diseases, if already at their optimum temperature most of the time, for example rusts will start their sporulation earlier in the year (West et al. 2012). Urediniospores and aeciospores of rust fungi, such as P. graminis, require direct contact with water sources in order to germinate (Ziska et al. 2011). Precipitation also plays a key role in splash dispersal of spores at local and regional scales, while drought periods prevent rust epidemics (Lacey 1996; West et al. 2012; Ziska et al. 2011). Furthermore, it must be also stressed that grouping fungi based solely on the weather conditions, when their spores are seen more frequently in the air, can be misleading, as for example Sadyś et al. (2015) classified rust spores as so-called " dry" spores upon their maximum contribution to the total spore load and associated weather conditions recorded throughout a 5-year survey. In contrast to the previously reported findings, this study gives foundation for their affiliation to the group of “wet” type spores based on twofold increased spore count observed in 2007 compared with 2006 (Table 3). In addition to that, Epicoccum spores are released, thanks to hydrostatic pressure of turgid cells (Lacey 1981). This mechanism is often described as active, water-demanding. In contrast, spores of Alternaria and Cladosporium species require either light rain showers or very high relative humidity in order to be discharged (Meredith 1962, 1963). This type of spore release mechanism is considered as active dry (Lacey 1981). Increased levels of Cladosporium conidia in the air after light rain showers were often reported worldwide (Hjelmroos 1993; Oliveira et al. 2009; Şakiyan and İnceoğlu 2003). A loss of water by conidiophores results in fact twisting and formation of gas bubbles that once burst enables spore discharge (Lacey 1981; Meredith 1962, 1963).
Considering model simulations and possible scenarios of climate change projected by the Intergovernmental Panel on Climate Change, except for a rise in the air temperature (1.1–6.4 °C warmer by the end of this century), more precipitation will occur in Northern Europe, including the UK (Allali et al. 2007). Currently, the plague of indoor mould in Europe has been estimated for 10–50 %, and it is constantly growing (Heseltine and Rosen 2009). Other kinds of buildings that possibly will be invaded by mould are of public use, i.e. schools, health care centres, and work offices (Heseltine and Rosen 2009). In a group of the most invasive indoor moulds, species of Alternaria, Cladosporium, Epicoccum, and Phoma (asexual stage of Leptosphaeria) were listed by the WHO (Heseltine and Rosen 2009). Although these fungi may encounter unfavourable weather conditions for their growth and reproduction in outdoor environments, we may expect their migration into indoors and their further adaptation to new habitats. On the other hand, it could be argued that throughout application of sustainable architecture and air-moisture-controlling devices, this scenario will never take place (Airaksinen et al. 2004; Codina et al. 2008).
Factors that may contribute towards high fungal spore levels in the air, such as (1) nutrient availability, (2) increased concentration of air pollutants such as CO2, (3) impact of mowing grasses and harvesting infected crops, or (4) changes in land use in the vicinity of the spore trap, were excluded from the overall discussion, herein, although they should not be neglected completely (Corden and Millington 2001; Damialis et al. 2014; Skjøth et al. 2012; Ziska et al. 2008).
5 Conclusion
In conclusion, several observations were made, i.e.:
-
1.
fungal spore seasons were shorter, when fungi encountered propitious weather conditions that facilitated their growth, spore production, release, and dispersal;
-
2.
spore concentration mostly rose by a minimum factor of 2, when favourable weather conditions occurred, although some species showed greater increase (Didymella);
-
3.
taking into account predicted weather conditions for the UK by IPCC, we may expected elevated levels of highly allergenic Alternaria and Cladosporium species, as well as other fungi (e.g. Epicoccum) within following next two decades.
References
Airaksinen, M., Pasanen, P., Kurnitski, J., & Seppänen, O. (2004). Microbial contamination of indoor air due to leakages from crawl space—A field study. Indoor Air, 14(1), 55–64.
Allali, A., Bojariu, R., Diaz, S., Elgizouli, I., Griggs, D., Hawkins, D. et al. (eds) (2007). Climate change 2007: Synthesis report. An assessment of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge.
Cecchi, L., D’Amato, G. D., Ayres, J. G., Galan, C., Forastiere, F., Forsberg, B., et al. (2010). Projections of the effects of climate change on allergenic asthma: The contribution of aerobiology. Allergy, 65(9), 1073–1081.
Codina, R., Fox, R. W., Lockey, R. F., DeMarco, P., & Bagg, A. (2008). Typical levels of airborne fungal spores in houses without obvious moisture problems during a rainy season in Florida, USA. Journal of investigational Allergology and Clinical Immunology, 18(3), 156–162.
Corden, J. M., & Millington, W. M. (2001). The long-term trends and seasonal variation of the aeroallergen Alternaria in Derby, UK. Aerobiologia, 17, 127–136.
D’Amato, G. D., & Cecchi, L. (2008). Effects of climate change on environmental factors in respiratory allergic diseases. Clinical and Experimental Allergy, 38, 1264–1274.
D’Amato, G. D., Rottem, M., Dahl, R., Blaiss, M. S., Ridolo, E., Cecchi, L., et al. (2011). Climate change, migration, and allergic respiratory diseases: An update for the allergist. WAO Journal, 4, 121–125.
Damialis, A., Mohammad, A. B., Halley, J. M., & Gange, A. C. (2014). Fungi in a changing world: Growth rates will be elevated, but spore production may decrease in future climates. International Journal of Biometeorology,. doi:10.1007/s00484-014-0927-0.
Damialis, A., Vokou, D., Gioulekas, D., & Halley, J. M. (2015). Long-term trends in airborne fungal-spore concentrations: A comparison with pollen. Fungal Ecology, 13, 150–156.
Del Mar Trigo, M., Toro, F. J., Recio, M., & Cabezudo, B. (2000). A statistical approach to comparing the results from different aerobiological stations. Grana, 39(5), 252–258.
Frankland, A. W., & Davies, R. R. (1965). Allergy to mold spores in England. Le Poumon et le coeur, 21, 11–31.
Gange, A. C., Gange, E. G., Sparks, T. H., & Boddy, L. (2007). Rapid and recent changes in fungal fruiting patterns. Science, 316, 71.
Heseltine, E., & Rosen, J. (2009). World Health Organisation guidelines for indoor air quality: Moulds and dampness. Copenhagen: WHO.
Hirst, J. (1952). An automatic volumetric spore trap. Annals of Applied Biology, 39, 257–265.
Hjelmroos, M. (1993). Relationship between airborne fungal spore presence and weather variables. Cladosporium and Alternaria. Grana, 32, 40–47.
Humperson-Jones, F. M., & Phelps, K. (1989). Climatic factors influencing spore production in Alternaria brassicae and Alternaria brassicicola. Annals of Applied Biology, 114, 449–458.
Kauserud, H., Heegaard, E., Büntgen, U., Halvorsen, R., Egli, S., Senn-Irlet, B., et al. (2012). Warming-induced shift in European mushroom fruiting phenology. PNAS, 109(36), 14488–14493.
Kauserud, H., Heegaard, E., Halvorsen, R., Boddy, L., Høiland, K., & Stenseth, N. C. (2011). Mushroom’s spore size and time of fruiting are strongly related: Is moisture important? Biology Letters, 7, 273–276.
Kauserud, H., Stige, L. C., Vik, J. O., Økland, R. H., Høiland, K., & Stenseth, N. C. (2008). Mushroom fruiting and climate change. PNAS, 105(10), 3811–3814.
Kennedy, R., & Smith, M. (2012). Effects of aeroallergens on human health under climate change. In S. Vardoulakis & C. Heaviside (Eds.), Health effects of climate change in the UK 2012. Current evidence, recommendations and research gaps (pp. 83–96). London: Health Protection Agency.
Kennedy, R., & Wakeham, A. J. (2015). Centenary Editorial: Measuring biological particles in the air using the Hirst type spore trap: Aerobiology in the age of genomics. Annals of Applied Biology, 166, 1–3. doi:10.1111/aab.12192.
Lacey, J. (1981). The aerobiology of conidial fungi. In G. T. Cole (Ed.), Biology of conidial fungi (pp. 373–416). New York: Academic Press.
Lacey, J. (1996). Spore dispersal—Its role in ecology and disease: the British contribution to fungal aerobiology. Mycological Research, 100(6), 641–660.
Lacey, M. E., & West, J. (2006). The air spora. A manual for catching and identifying airborne biological particles., Dordrecht: Springer.
Marsh, T. J., & Hannaford, J., (2007). The summer 2007 floods in England and Wales—A hydrological appraisal. Wallingford: Centre for Ecology & Hydrology.
Meredith, D. S. (1962). Some components of the air-spora in Jamaican banana plantations. Annals of Applied Biology, 50(3), 577–594.
Meredith, D. S. (1963). Violent spore release in some fungi imperfecti. Annals of Botany, 27(1), 39–47.
Met Office. (2011). Climate: Observations, projections and impacts. http://www.metoffice.gov.uk/media/pdf/t/r/UK.pdf.
Met Office. (2012a). Record breaking heat and sunshine—July 2006. http://www.metoffice.gov.uk/climate/uk/interesting/july2006.
Met Office (2012b). Heavy rainfall/flooding—June 2007. http://www.metoffice.gov.uk/climate/uk/interesting/june2007.
Met Office. (2013). Warmest, driest and sunniest summer since 2006. http://www.metoffice.gov.uk/news/releases/archive/2013/early-summer-stats.
Mukaka, M. M. (2012). Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Medical Journal, 24(3), 69–71.
Nilsson, S., & Persson, S. (1981). Tree pollen spectra in the Stockholm region (Sweden), 1973–1980. Grana, 20(3), 179–182.
O’Connor, D. J., Sadyś, M., Skjøth, C. A., Healy, D. A., Kennedy, R., & Sodeau, J. R. (2014). Atmospheric concentrations of Alternaria, Cladosporium, Ganoderma and Didymella spores monitored in Cork (Ireland) and Worcester (England) during the summer of 2010. Aerobiologia, 30, 397–411.
Oliveira, M., Ribeiro, H., Delgado, J. L., & Abreu, I. (2009). Seasonal and intradiurnal variation of allergenic fungal spores in urban and rural areas of the North of Portugal. Aerobiologia, 25, 85–98.
Pakpour, S., Li, D.-W., & Klironomos, J. (2015). Relationships of fungal spore concentrations in the air and meteorological factors. Fungal Ecol., 13, 130–134.
Reid, C. E., & Gamble, J. L. (2009). Aeroallergens, allergic disease, and climate change: Impacts and adaptation. EcoHealth, 6, 458–470.
Sadyś, M., Skjøth, C. A., & Kennedy, R. (2014). Back-trajectories show export of airborne fungal spores (Ganoderma sp.) from forests to agricultural and urban areas in England. Atmospheric Environment, 84, 88–99.
Sadyś, M., Strzelczak, A., Grinn-Gofroń, A., & Kennedy, R. (2015). Application of redundancy analysis for aerobiological data. International Journal of Biometeorology, 59, 25–26.
Şakiyan, N., & İnceoğlu, Ö. (2003). Atmospheric concentrations of Cladosporium Link and Alternaria Nées in Ankara and the effects of meteorological factors. Turkish Journal of Botany, 27, 77–81.
Skjøth, C. A., Sommer, J., Frederiksen, L., & Karlson, U. G. (2012). Crop harvest in Denmark and Central Europe contributes to the local load of airborne Alternaria spore concentrations in Copenhagen. ACP, 12(22), 11107–11123.
UKCP. (2009). UK climate projections. http://ukclimateprojections.metoffice.gov.uk/.
West, J. S., Townsend, J. A., Stevens, M., & Fitt, B. D. L. (2012). Comparative biology of different plant pathogens to estimate effects of climate change on crop diseases in Europe. European Journal of Plant Pathology, 133, 315–331.
Wolf, J., O’Neil, N. R., Rogers, C. A., Muilenberg, M. L., & Ziska, L. H. (2010). Elevated atmospheric carbon dioxide concentrations amplify Alternaria alternata sporulation and total antigen production. Environmental Health Perspectives, 118, 1223–1228.
Ziska, L. H., Blumenthal, D. M., Runion, G. B., Hunt, E. R, Jr, & Diaz-Soltero, H. (2011). Invasive species and climate change: An agronomic perspective. Climate Change, 105, 13–42.
Ziska, L. H., Epstein, P. B., & Rogers, C. A. (2008). Climate change, aerobiology, and public health in the northeast United States. Mitigation and Adaptation Strategies for Global Change, 13, 607–613.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sadyś, M., Kennedy, R. & West, J.S. Potential impact of climate change on fungal distributions: analysis of 2 years of contrasting weather in the UK. Aerobiologia 32, 127–137 (2016). https://doi.org/10.1007/s10453-015-9402-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-015-9402-6