Abstract
Waterbirds and fish sometimes compete for macro-invertebrate prey. In Scotland, the invertivorous waterbird, the common scoter Melanitta nigra, breeds at oligotrophic lakes with few brown trout Salmo trutta. This study tested whether reducing trout biomass favours this and other invertivorous waterbirds. The study took place in Scotland’s Flow Country, where brown trout occur widely, attracting recreational anglers, though angling has declined. At four small lakes, over 7 years, trout were reduced using 25 m2 exclosures, and re-introducing traditional angling (including fish removal). Trout, macro-invertebrates and waterbirds were monitored. After angling re-introduction, trout biomass density declined by 56% (95% CLs 13–78%), but there was little lake-level change in combined macro-invertebrate biomass. However, within exclosures, macro-invertebrate biomass increased 4.7-fold (CLs 1.6–14). Analysing invertebrates in eight different groups showed lake-level increases, following angling re-introduction, for two groups (freshwater shrimps Gammarus; water-surface invertebrates). Gammarus showed the strongest response, increasing sixfold (CLs 2.2–11.6). A combined analysis was performed for the commonest invertivorous waterbirds: common scoter, mallard Anas platyrhynchos, teal A. crecca, greenshank Tringa nebularia and dunlin Calidris alpina. After angling effort increased, occurrence of these species changed little initially, but rose later: 4 years after angling began, odds of occurrence had increased 4.9-fold (CLs 2.2–11). This study supports reducing trout biomass in peatland lakes by encouraging traditional angling, to increase some macro-invertebrate groups and usage by invertivorous waterbirds. Further work should test this across more lakes alongside work investigating the origins (native or stocked) of brown trout populations in the Flow Country.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Evidence that fish and birds can compete for invertebrate prey has been found in a wide range of aquatic ecosystems, including rivers (LeBourdais et al. 2009), marshes (Hornung and Foote 2006), intertidal zones (Furness et al. 1986), the open ocean (Toge et al. 2011), and lentic systems as diverse as saline montane lakes (Hurlbert et al. 1986), aquaculture ponds (Haas et al. 2007; Kloskowski et al. 2010), oligotrophic boreal lakes (Eriksson 1979; Nummi et al. 2012) and large eutrophic lakes (Winfield et al. 1992; Winfield and Winfield 1994). Bird-fish competition is often asymmetric, with fish tending to impact birds more heavily than vice versa (Marklund et al. 2002; Nummi et al. 2016). Competitive interactions can be markedly altered by the introduction of a higher trophic level which disproportionately affects one competitor (Gurevitch et al. 2000). Management could produce a similar effect: for example, Giles (1994) and Hanson and Butler (1994) showed that reducing fish abundance by management increased both macro-invertebrate abundance and habitat use by invertivorous waterbirds. Such an approach could have important applications in nature conservation .
This study investigated how the management of invertivorous fish might be used to benefit waterbirds of conservation importance, at oligotrophic lakes in Scotland’s Flow Country, a globally important blanket bog (Joosten et al. 2016). This ~ 4000 km2 peatland landscape includes 1000s of pools and lakes, holding macro-invertebrates which are prey to breeding waterbirds like ducks Anatidae and waders (shorebirds) Charadrii (Lindsay et al. 1988). The area holds a 1453 km2 European Birds Directive Special Protection Area, in which five of the 12 designated bird species are invertivorous waterbirds. A key species is an invertivorous duck, the common scoter Melanitta nigra, for which the area holds around half the British breeding population (unpublished data, coordinated by RSPB). Small peatland lakes in the area often support populations of brown trout Salmo trutta. These typically comprise abundant small individuals, as reported by anglers, who, at many lakes, commonly catch trout weighing ~ 100–200 g. Brown trout is native to the area, and many lakes, including those in the current study, are accessible to fish dispersing through rivers and streams. However, some lakes may hold fish descended from stocking, which, while poorly documented at the lake level, is known to have taken place commonly in the region, decades ago (Frost and Brown 1967; Maitland and Campbell 1992; and local reports) and perhaps earlier.
Breeding scoters in Scotland typically utilize shallow upland lakes with abundant macro-invertebrates, foraging in shallow water near to lake shores (Hancock et al. 2016, 2019); lakes with the largest invertebrate per sample averaging over 4 mg had relatively more scoter usage than other lakes, by factors of 9 (females) or 27 (broods). In our earlier work, we also found that lakes in the scoter range with abundant macro-invertebrates tend to hold relatively few brown trout, the relationship with trout abundance being stronger for example, than that with water chemistry variables like pH or phosphate; given the potential prey overlap, the pattern of scoter lake use could therefore reflect competition with trout for the same prey resource (Hancock et al. 2016). Other duck species, and waders, often forage for similar prey in similar lake shore habitats (Cramp and Simmons 1977, 1983), including in this region (Nethersole-Thompson and Nethersole-Thompson 1986; authors’ unpublished observations). This suggested that trout could influence prey availability and hence habitat suitability for several waterbird species sharing a common macro-invertebrate prey resource.
This study aimed to test whether the pattern of higher scoter use and macro-invertebrate biomass on lakes with fewer trout reflects a causal link. If so, reducing trout populations and biomass by management could increase macro-invertebrate biomass, supporting the conservation of scoters and other invertivorous waterbird species (Hancock et al. 2020). Meanwhile, evidence on this topic is limited (ConservationEvidence.com). Therefore, during the current study, trout abundance was manipulated, to reduce biomass, and subsequent changes in macro-invertebrate biomass and waterbird lake use were measured. The study took place at four small lakes (4.1–13 ha) known to have substantial trout populations. For several years prior to the investigation, angling effort was minimal within the study lakes, with little or no fish being removed.
For the study, two trout reduction treatments were introduced, one small- and one large-scale, each at two lakes. Before and after these manipulations, measurements were made of trout and macro-invertebrate biomass, and lake use by invertivorous waterbirds. The study aimed to determine whether treatments led to (i) a reduction in trout biomass; (ii) an increase in macro-invertebrate biomass, either for all groups combined, or for more vulnerable groups; and (iii) greater lake use by invertivorous waterbirds.
Methods
Study area
The study took place on Forsinard Flows National Nature Reserve, in Scotland’s Flow Country (Fig. 1), an extensive, relatively undamaged peatland, protected under national and European law (e.g. Wildlife and Countryside Act, Birds and Habitats Directives), and a candidate World Heritage Site. The Flow Country is characterised by open upland and blanket bog landscapes with many small, oligotrophic lakes (Lindsay et al. 1988). The study lakes (Figure S1) were chosen because they were (i) rarely used by breeding scoters; (ii) within the scoter breeding range; and (iii) held abundant brown trout. Hence, they represented lakes where trout reduction might improve scoter habitat quality.
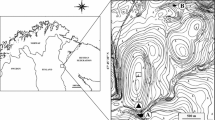
Map of the study area. The four study lakes are circled in red, and labelled with the lake codes (Table 1) and treatments (A: angling treatment; E: exclosures treatment). Forestry plantations, dating from the 1980s, are indicated in green; the remaining area (light brown) is blanket bog, or former forestry plantation being restored as blanket bog. For clarity, tracks and the railway (which passes through the small settlement of Altnabreac, shown on the map) are not shown; there are no public roads in this area. The mapped study area covers the area from approximately 3° 48′ W, 58° 22′ N, to 3°40′ W, 58°26′ N; the inset map shows northern Scotland with the study area marked as a yellow rectangle. (Color figure online)
Several waterbird species commonly forage for macro-invertebrates along shorelines of the study lakes, primarily ducks (Anatidae) and waders (shorebirds: Charadrii). The lakes are peat-stained and have low water clarity (Table 1), reducing their suitability for visual hunting piscivorous birds like divers (loons: Gaviidae; Supporting Information), and they hold no fish capable of predating adult trout. Thus, angling likely represents the main means of adult trout removal. Although angling has declined in the last 20–30 years (Headley 2005), these lakes were previously popular among anglers (Sandison 1992), sometimes with large catches removed (Adams 1889). Moreover, human exploitation of trout in the region dates back to Neolithic times (Barrett et al. 1999).
The macro-invertebrate communities of Scottish scoter lakes are typically dominated by insects like caddisflies Trichoptera, mayflies Ephemeroptera, and aquatic beetles Coleoptera; the commonest non-insect invertebrates are freshwater shrimps Gammarus spp. (Hancock et al. 2019). Some freshwater macro-invertebrates found in the Flow Country are of nature conservation importance, including species of caddisflies, water beetles and shrimps (Lindsay et al. 1988).
Three-spined sticklebacks (Gasterosteus aculeatus) and European eels (Anguilla anguilla) occur in some lakes locally, including at least some of the study lakes, but their distributions are not fully known in the area. The study lakes all have outflow streams that are large enough to allow trout dispersal, implying accessibility to trout which are native to the region; however, their trout populations may also have been influenced by undocumented trout stocking in the past.
Study design
At each lake, 10 sampling points were established around the shoreline. Point 1 was located at random; remaining points were equally spaced around the lake. At each point, most sampling took place within two adjacent 5 m × 5 m quadrats, adjoining the shoreline. Gently shelving shorelines (Table 1) meant that quadrats typically had maximum water depths around 20–25 cm. This shallow littoral zone is heavily used by foraging scoters (Hancock et al. 2019) and is a focus of other waterbird use. Quadrat substrates comprised mainly sand or gravel, with some finer and coarser substrates, and ~ 20% macrophyte cover (Figure S2). Commonly occurring macrophytes in the area are Lobelia dortmanna, Littorella uniflora, Isoetes lacustris, Myriophyllum alterniflorum and Juncus bulbosus (Robson et al. 2019). Maximum lake depths were ~ 1.5–3 m; deep-water substrates were usually peat or mud.
The study took place over 7 years: 2013–2019. The first 6 years were the main sampling years, when all forms of survey and sampling took place. In 2019, a further year’s data were gathered on bird use and angling. During each main sampling year, invertebrate sampling took place during three survey rounds, encompassing the main bird breeding period, and maintaining consistency with earlier work (Hancock et al. 2016): mid-April to mid-May (Round 1); June (Round 2) and early July to early August (Round 3). Bird surveys took place during the same period, and camera trapping extended to mid-September, to record any late season activity; however, breeding bird activity tended to peak in early June, consistent with the central date of invertebrate sampling. Trout seine netting was carried out in late summer, between mid-August and mid-September. This activity required several people for most of the day; late season timing helped avoid disturbing breeding birds during the main breeding season. This timing also preceded the trout spawning period, during which trout may commonly swim out of lakes, into streams.
Trout reduction treatments were planned to start in 2014, the second study year, and this timing was achieved for exclosures. However, a change in angling tenancy delayed the start of the angling treatment until 2015. Exclosures were constructed in February 2014, therefore, all years from 2014 were post-treatment years. Angling took place largely in mid-summer (July and early August), after most invertebrate and bird surveys, but before trout seine netting. Therefore, for the angling treatment, the post-treatment period was considered to start in 2016 for invertebrate and bird responses, and in 2015 for trout responses as measured by seine netting.
Each of the two trout reduction treatments was applied at two of the four lakes, such that all four treatment combinations were present among the study lakes (Table 1; Fig. 1). Treatments took place at two spatial scales, with angling applied at the whole lake scale, and exclosures constructed at the quadrat scale.
Prior to the study, the lakes had been unfished or only rarely fished for several years (~ 0–3 angling visits per lake per year) and commonly managed on a ‘catch and release’ basis (captured trout being returned, alive). Contrasting with this, the angling treatment introduced for this study comprised ~ 10 angling excursions per lake per year, each of a few rod-hours, with all captured trout being killed and removed. Consistent with some guidance (Youngson et al. 2003; Lewin et al. 2006), there were no size restrictions on fish removal, although the choice of tackle (fly, hook) influenced sizes of fish taken. This treatment is termed ‘traditional trout angling’, being similar to typical twentieth century practices locally, described by older anglers and relevant literature (e.g. Bridgett 1924). The angling treatment was carried out by experienced fly fishers from the local angling club.
Trout exclosures, 5 m × 5 m (Figure S3), constructed at two lakes (Table 1; Fig. 1), were planned at alternate sampling points among the 10 at each lake. However, at both lakes, one point was unsuitable for exclosure construction (due to the presence of large boulders, or deep soft peat). Therefore, four exclosures were built per lake. Each sampling point had two adjacent 5 m × 5 m quadrats adjoining the shore (above), and at points chosen for exclosures, an exclosure was built around the left-hand side quadrat, viewing from the shore. Exclosures comprised a frame of untreated wood, fitted with ~ 10 mm mesh to a height of ~ 1 m above the lake-bed. Given typical shoreline slopes (Table 1), the outer edges of exclosures typically had depths around 20–25 cm.
Although there was only a single lake in each treatment combination, the work included at least one lake-year in each category, before and after treatment, with a control lake, allowing a practical approach to measuring effects that was realistically achievable alongside large-scale nature conservation management (Ockendon et al. 2021). Although highly replicated and long-term paired-series designs would be preferable, these are challenging to deliver in practice; indeed even simple Before-After-Control-Impact designs like this one are not often achieved in similar projects, despite their advantages (Christie et al. 2019).
Field methods
Invertebrate sampling
During each sampling round at each lake, invertebrates were surveyed at all 10 sample points, using six different sampling methods (Figures S4b–g), consistent with recommendations to use multiple methods to characterize lake macro-invertebrate communities (Schilling et al. 2009). Four methods were those used in previous work (Hancock et al. 2016): stone sweeps (pond-net sweeps under shoreline stones); surface sweeps (standardized pond-net sweeps of the water’s surface); sediment grabs (grab samples of soft sediment); and colonization traps (placed on the lake-bed for colonization by invertebrates between survey rounds). For this study, two further methods were added: visual counts (1 min lake-bed observations using an aquascope, counting invertebrates seen in size and taxonomic categories); and funnel traps (collecting invertebrates caught in traps set to sample three-spined sticklebacks).
All sampling methods were conducted twice per point, once in each quadrat, except grab samples, which took place in deeper water to obtain soft sediments; these were done once per point. Mesh sizes of pond nets, bag-sieves (used to process grab samples), and the lower size threshold for visual counts, were 1 mm. Samples were preserved in 70% ethanol in the field and later sorted in the laboratory, identified using Croft (1986), usually to family level for common groups and late instars. Body lengths were measured, allowing biomass estimation from published length-mass regressions, as in previous work (Hancock et al. 2016).
Fish surveys and angling records
Seine netting and mark-recapture methods were used to estimate trout populations (Figure S4h). Each lake was surveyed twice, a few days apart, using a 37.5 m long, ~ 3 m deep seine net made of knotless nylon mesh, with a mesh size of 6.5 mm in the central 12.5 m and 14 mm in the wings. Seine netting always took place along the same stretches of shoreline. These seine netting zones (around one third of the lake perimeter) had gently shelving substrates mainly of gravel and pebble and were reasonably clear of large boulders. The seine net was loaded into a small inflatable boat and deployed by wading, setting the net in an approximate semi-circle, starting about 10 m from the shore. A series of adjacent sets of the net were made until a suitable catch (aiming for at least 50 fish per day) had been obtained. Trout captured were transferred to mechanically aerated holding bins, then lightly anaesthetized using a solution of 30 ppm Benzocaine, weighed, measured (fork length), photographed and marked by fin-clipping. A sample of at least five scales was collected from each fish for ageing. After a period of recovery in holding bins to ensure that equilibrium was re-established, all trout were returned to the lake. The proportion of fish caught on the second survey each lake-year, bearing the fin-clip mark from the first survey, was used to estimate trout population size using mark-recapture methods (Southwood and Henderson 2000). Population was converted to biomass per ha using mean trout mass and lake area.
Three-spined sticklebacks were sampled during each invertebrate sampling round using one funnel trap (Figure S4g) per quadrat for 20 min (giving 20 trap-hours per lake-year). Sticklebacks captured were measured (fork length) and released.
To measure angling effort and catch at angling treatment lakes, anglers completed a ‘catch return’ form after each excursion, recording lake, date, number of anglers, hours fishing, and numbers of trout caught and removed by 1 cm size classes. No angling took place at the two lakes assigned to the non-angling treatment.
Waterbird recording
To record waterbirds, camera traps were deployed at each lake (Figure S4i). Cameras were sited 2–3 m from the shore, facing north, viewing the shoreline of a sheltered bay. Bird records were collated for the period 15 April to 15 September inclusive, in all study years (2013–2019). Cameras were visited approximately fortnightly (mean 15.9 days, s.e. 0.6) to change memory cards and check batteries. During these short visits, the lake was checked for birds, by scanning with binoculars and walking part of the shore. Additional short bird survey visits were carried out (five per lake per year, in all study years, 2013–2019), using similar search methods, as part of a long-running standard waterbird monitoring programme. These ‘short survey visits’ usually involved one observer (mean 1.2 observers, s.e. 0.3) for less than an hour (mean 0.49 h, s.e. 0.02). During invertebrate and fish survey days (eight per lake per year, during 2013–2018), birds were also recorded. These ‘long survey visits’ lasted several hours (mean 6.0 h, s.e. 0.09), involved a few observers (mean 4.0 observers, s.e. 0.2), and covered much or all of the lake perimeter.
Data analysis
Trout biomass
Trout numbers and biomass within each lake were estimated for each year using seine netting data. Firstly, the trout population for each lake-year was estimated from mark-recapture data using the Lincoln index, adjusted for small samples (Southwood and Henderson 2000: Eqs. 3.25, 3.26). Secondly, mean individual trout body mass was calculated for that lake-year, using the first capture event for any fish caught more than once. Biomass density was calculated as the product of trout population and mean body size, divided by lake area. Lake-year biomass density was right-skewed, therefore, it was loge-transformed for analysis. Because netting surveys in different lake-years varied markedly in the numbers of trout caught, estimates of population and hence biomass varied markedly in accuracy (see Results). Hence, a weighted analysis was used, in which the biomass density estimate for each lake-year was weighted by the reciprocal of its estimated variance (Quinn and Keough 2002; Supporting Information). Also, the study period included some exceptionally cold and warm spells (e.g. the seventh coldest spring in north Scotland since 1910 (2013) and two of the three warmest Mays (2017 and 2018): Met Office 2021). Such temperature variation might affect trout behaviour and populations (Jonsson and Jonsson 2011) and hence blur treatment effects. Therefore, mean water surface temperature was included as a covariate in analyses of trout biomass density, to help control for this source of variation.
To test whether trout biomass density changed in association with the angling treatment, a generalized linear mixed model (GLMM: Stroup 2013) was used, with lake-year as the unit of analysis, fitted using the GLIMMIX procedure in SAS (SAS 2014). The response (y) variable was loge (biomass density), with a normal error distribution. The explanatory (x) variables were water surface temperature, treatment (assigned to angling, or not), period (before or after angling) and their interaction. Lake and year were fitted as random effects. The reciprocal of estimated variance in y, at the lake-year level, was used as a weight variable. The x-variable of interest was treatment × period, which tested whether trout biomass density declined in lakes where angling took place, relative to corresponding changes at lakes without angling.
Combined invertebrate biomass
To investigate treatment effects on combined macro-invertebrate biomass, data were analysed at two spatial scales: quadrat and lake, testing the effect of trout reduction by exclosures and angling, respectively. Each analysis combined data within 1 year, hence the units of replication were quadrat-year and lake-year, respectively. The quadrat-year analysis included a factor to represent lake-years with angling, but interpretation focussed on the exclosures effect. Similarly, the lake-year analysis included a factor to represent lake-years with exclosures present, but interpretation focussed on the angling effect. Each analysis tested whether experimental fish reduction reduced macro-invertebrate biomass, for all taxa combined.
The timing of the treatments differed: exclosures were in place in 2014, but the angling not until 2015. Angling largely took place after invertebrate sampling each year, therefore, it was not expected to affect invertebrate data until the following year. Thus, for invertebrate analyses, baseline versus post-treatment periods were 2013 versus 2014–2018 for the exclosures treatment, but 2013–2015 versus 2016–2018 for the angling treatment.
In these analyses, the following treatment variables were included as fixed effects: treatment (exclosure quadrat, angling lake), period (before or after treatment) and treatment × period. This last (interaction) term measured how changes between periods in macro-invertebrate biomass differed between treatments; it was therefore the key estimate of responses by combined macro-invertebrate biomass to fish reduction treatments. The following additional fixed x-variables were also included to compensate for sources of variation other than treatment: both analyses: water temperature (included for similar reasons to trout analyses); quadrat-year analysis: angling lake-years, quadrat position (left or right); lake-year analysis: exclosure lake-years.
Invertebrates were sampled using several different methods; at each sampling unit, the value from each method was included as a separate row of data, modelling ‘method’ as a random effect. Analyses were carried out using GLMMs with the following random effects: sampling method, lake, year, lake × year (both analyses); sampling point, quadrat, sampling point × year and quadrat × year (quadrat-year analysis). The y-variable for each analysis was the biomass (mg) of macro-invertebrates recorded by that sampling method at that spatial unit, per sampling visit during that year, with a normal error distribution. Data were right-skewed, so were loge-transformed for analysis. Since some zero values were present, a constant was added (equal to the lowest recorded nonzero value) prior to log-transformation.
Biomass of different macro-invertebrate groups
Because different taxa might differ markedly in their responses to trout reduction, further analyses were carried out in which macro-invertebrates were grouped into eight taxon groups (Table 2). It was considered that each group would comprise animals that shared commonalities of behaviour, location within the lake and/or taxonomy, which might affect vulnerability to fish predation: for example, taxa typically living within the sediment or in protective cases were considered less vulnerable than those commonly active in open water. The assignment of taxa to these groupings was based on our own observations, local angler knowledge, and literature on brown trout diet in lakes (e.g. Frost and Brown 1967; Headley 2005; Martínez-Sanz et al. 2010; Jonsson and Jonsson 2011; Sanchez-Hernandez and Amundsen 2015; Milardi et al. 2016a, b).
To analyse responses by these invertebrate taxon groups, biomass was summed within each group across all sampling methods at the lake-year or quadrat-year level and divided by the number of samples, giving mean biomass per sample. These data were then analysed separately for each taxon group, using GLMMs, similarly to combined invertebrate analyses. For these data, square-root transformation gave a good fit to normal distribution of residuals. Random effects were as for combined biomass models (above), except that method was not needed here since data were too sparse (too many zeros) at the taxon group level for modelling using separate data row for each method. As in the combined biomass analyses, the treatment × period interaction term was the key test of the focal taxon group’s response to trout reduction.
Waterbird lake use
To analyse responses by waterbirds, data were first collated from the three types of survey: short survey visits, long survey visits, and camera traps. For short and long survey visits, an occurrence of a particular bird species was defined as its first occurrence on a survey visit. Survey effort was the number of visits of that type during that lake-year. For camera trap data, an occurrence was the first photograph showing the focal species at that lake on a particular date (Rich et al. 2016). Survey effort was the number of days that lake-year, when the camera trap was operational.
Firstly, waterbird responses were analysed across all years. This was done using a logistic GLMM, modelling the number of bird occurrences, adjusted for survey effort, in relation to treatment, across all lakes, years, species and survey types. The unit of replication was a species, recorded by a survey type, during a lake-year. Data were analysed for all invertivorous waterbird species that occurred at least 50 times during the study. There were five such species (see Results). The recorded, breeding season macro-invertebrate diet for these species (Cramp and Simmons 1977; 1983) and for scoters Melanitta species (summarized in Hancock et al. 2019) comprises 14 prey taxa, of which half are recorded for at least four of these bird taxa, implying a high degree of prey overlap. These five species were included in a single analysis, modelling ‘species’ as a random effect; exclusion of rare species allowed a reasonable fit to normality for the ‘species’ random effect estimates. In each row of data, the number of occurrences of a particular species was the y-variable, and survey effort (see above) was the binomial denominator; in effect, this modelled frequency of occurrence. The fixed effect x-variables of interest were treatment (angling or not), period (before or after angling) and treatment × period. As in other analyses, this last term estimated the angling treatment effect on frequency of waterbird occurrence, controlling for changes at non-angling lakes. Analyses also included a categorical variable representing lake-years with exclosures present, in case this affected bird occurrence (considered unlikely). The following random effects were included to account for correlation among different observations: lake, year, species, survey type, and their two- and three-level interactions.
Secondly, the above analyses were performed separately for each post-treatment year, because these bird species could show lagged responses to changes in food availability following trout reduction, due to their high breeding site fidelity as adults (e.g. Johnson and Grier 1988; Jackson 1994), potentially slowing changes in breeding distribution.
In general, across data analyses, P values are presented, with effect sizes and confidence intervals to help interpretation. In some cases, one-tailed tests might have been appropriate (e.g. when estimating the effect of trout reduction in their typical prey groups), but for simplicity, two-tailed tests were used throughout.
Further information on study methods is given in Supporting Information.
Results
Fish: angling, trout biomass, and other fish species
At the two lakes where angling was used to remove trout during the treatment period, there were six to 15 angling excursions per year (mean 8.8, s.e. 0.95), each with one or two anglers: a mean of 3.9 rod-hours per excursion (s.e. 0.73). In the five angling years, the two lakes with angling, Loch na Cloiche and Lochan nam Breac, had, respectively, averages of 148 (s.e. 27) and 152 (s.e. 20) fish caught and removed per year: means of 4.2 (s.e. 0.52) and 5.0 (s.e. 0.80) trout per rod-hour. Mean lengths of trout caught averaged 22.4 (s.e. 0.54) and 17.3 (s.e. 0.84) cm, respectively. Such fish would have estimated individual weights of ~ 122 g and ~ 57 g, respectively, using the study’s overall length–weight regression from seine-netted trout (\(\log_{e}\) (weight (g)) = 2.92 × \(\log_{e}\) (length (mm)) − 11.0). These estimated weights would imply ~ 1.4 kg ha−1 and ~ 2.1 kg ha−1 removed per year, by angling, from Loch na Cloiche and Lochan nam Breac, respectively. Seine-netted trout at these lakes averaged somewhat smaller than fish taken by angling, at 17.5 (s.e. 0.76) and 16.3 (s.e. 0.81) cm for Loch na Cloiche and Lochan nam Breac, respectively. (Means and standard errors given here were calculated at the lake-year level). There was a weak (non-significant) tendency for trout caught per rod-hour to fall during the study, and their mean lengths to rise (Figure S5; Table S1).
Each lake-year, between 34 and 229 trout were caught by seine netting, giving lake-year estimates in the following ranges: trout populations, ~ 80 to ~ 780; mean weights: 30–210 g; biomass densities: ~ 0.6 to ~ 10 kg ha−1 (Figure S6). There were few old fish: most were aged as 1 + (45%) or 2 + (39%), with only 1.0% aged as 4 +; relative frequencies of year-classes varied strongly between lake-years (Figure S7). At lake-years with angling, trout lengths tended weakly to average higher at a given age (by ~ 24 mm); however, this difference was highly variable (s.e. 23 mm) and not significant (Table S2).
The introduction of angling was associated with a significant (P = 0.032: Table 3) reduction in trout biomass density, by an estimated 56% (95% confidence intervals 14–78%) (Table 3, Fig. 2). In lake-years with angling, trout biomass averaged around 1.7 kg ha−1, compared to around 3.6 kg ha−1 without angling.
The effect of angling on brown trout biomass per ha. The introduction of angling resulted in a significant decline in trout biomass per ha (see Results, and Table 3). The lakes with angling introduced were Loch na Cloiche (lake code CLOI) and Lochan nam Breac LNBR); the other lakes, with no angling, were Clar Loch (CLAR) and Loch Talaheel (TALA)
Sticklebacks were occasionally recorded in funnel traps, but only at Loch na Cloiche and Lochan nam Breac (36 and 2 records, respectively). Due to small samples, these data were not analysed formally, however, there was no clear contrast between pre-angling (7.3, s.e. 3.5) and post-angling (4.7, s.e. 2.0) mean counts per year at Loch na Cloiche. During seine netting at Loch na Cloiche, an average of 66 sticklebacks were caught (year-wise s.e. 54) per set of the net in the pre-angling period and 29 (s.e. 18) in the post-angling period (t test comparing periods at the lake-year level: P = 0.39; n = 6).
During seine netting, European eels were occasionally observed (11 records) and released, confirming occurrence at Loch na Cloiche and Clar Loch; however, eel abundance was not measured.
Composition of macro-invertebrate samples
The composition of samples by lake (Fig. 3a) showed some commonalities, such as the prevalence of caddisfly and mayfly larvae, making up 50–72% of sampled biomass (depending on lake), and beetles, Diptera larvae and terrestrial insects, making up a further 12–19% of biomass. Other groups varied more strongly between lakes, such as shrimps Gammaridae (8.5% of biomass at Loch na Cloiche), leeches Hirudinae (23% at Loch na Cloiche) and water boatmen Corixidae (13% at Loch Talaheel).
The composition of macro-invertebrate samples. a Comparing the four study lakes (lake codes: CLAR, Clar Loch; CLOI, Loch na Cloiche; LNBR: Lochan nam Breac; TALA, Loch Talaheel). b Comparing the six sampling methods (method codes: VISU, visual counts; TRAP, funnel traps; NETD, surface sweeps; NETS, stone sweeps; COLO, colonization traps; GRAB, sediment grab). The miscellaneous (aquatic) category comprised adult stages of aquatic insects, gastropods, common but small invertebrates like mites, Cladocera and pea mussels, and various rarer groups
As expected, different sampling methods tended to sample different components of the macro-invertebrate fauna (Fig. 3b). Visual counts and funnel traps were characterized by active invertebrates like corixids, making up 32% and 49% of biomass, respectively. Surface sweeps caught many more terrestrial insects (14% of biomass) than other methods. Stone sweep and colonization trap samples were somewhat similar, dominated by caddisfly larvae (39% and 50% of biomass, respectively), but included a wide range of other groups. Sediment grab samples held more Diptera larvae and oligochaete worms (19% of biomass each), than other methods. Mayfly larvae varied least between methods, making up a significant proportion (10–31% of biomass) under all methods.
Trout reduction and combined macro-invertebrate biomass
Examining macro-invertebrate biomass separately by sampling method suggested that most methods tended to record more biomass within exclosures than in adjacent open quadrats (Fig. 4a). This pattern was most pronounced for methods focussing on active, exposed invertebrates: visual counts and funnel traps.
The effect of trout exclosures on invertebrate biomass across all taxa. a Sampled biomass by method, for sample points with exclosures, after exclosures had been constructed, at exclosure quadrats and adjacent open quadrats. Note that the y-axis differs by method. Quadrats with exclosures present (white bars with bold outline) tended to hold more biomass than adjacent, open quadrats (grey bars), under most sampling methods. b Fitted mean biomass per sample, across all methods, from the quadrat-year statistical model of invertebrate biomass (Table 4) (with standard errors). Note that the y-axis differs in the two periods, and uses a logarithmic scale. Across all sampling methods combined, there was a highly significant increase in biomass following exclosure construction, compared to changes in quadrats where no exclosure was built (labelled as “Other quadrats”) (see Results, and Table 4). Due to unequal sample sizes, the overlap of errors bars is poorly related to significance level. These charts are based on the two lakes where exclosures were constructed: Clar Loch (lake code: CLAR) and Lochan nam Breac (LNBR)
Statistical analysis of the exclosures treatment across all sampling methods showed that exclosures were associated with a 4.7-fold (95% CLs 1.6–14) increase in combined macro-invertebrate biomass (Fig. 4b), a highly significant difference from corresponding values in open quadrats (Table 4: P = 0.0044). Invertebrate biomass was also higher in the post-treatment period generally (Table 4: P = 0.03), perhaps reflecting cold conditions in 2013, the single pre-exclosures baseline year. Conversely, the angling treatment, tested at the lake-year level, had no effect on combined macro-invertebrate biomass (Table 4: P = 0.71).
There was no evidence that invertebrate responses became more positive in later years of the study (correlation between year and treatment × period estimates from analyses including single, post-treatment years: exclosures: r = 0.24, P = 0.70, N = 5; angling: r = − 0.21, P = 0.86, N = 3). Nor was there evidence that exclosures strongly affected physical conditions (wave height, water temperature: Table S3).
Trout reduction and different macro-invertebrate groups
Four of the eight macro-invertebrate groups investigated showed signs of increasing in biomass following trout reduction (Table 5; Fig. 5). The strongest evidence of increase (P = 0.011) was for shrimps Gammaridae at the lake-year level, associated with the introduction of angling. There was weaker evidence of positive effects of trout reduction on surface-layer insects (associated with angling, at the lake-year scale, P = 0.067), and both exposed and concealed larvae (associated with exclosures, at the quadrat-year scale, P = 0.065, P = 0.085, respectively). Conversely, pea mussels Sphaeriidae declined at the lake-year scale in association with angling (P = 0.012). Putative trout predation risk was weakly related to these results, as shown by three of the significant positive results being among the highest listed three groups in Fig. 5, and the only significant negative effect in the lowest listed group.
The effect of trout reduction on eight groups of macro-invertebrate taxa (described in Table 2). The figure shows the fitted treatment × period parameter estimates (Table 5) for each taxon group, indicating the effect of trout reduction by angling at the lake scale (filled circles) or by exclosures at the quadrat scale (open circles). Positive values indicate that the biomass of this group increased after trout reduction. Groups are listed in order of putative vulnerability to trout predation, with more vulnerable groups at the top of the chart. Significant (P < 0.05) effects are indicated by an asterisk; near-significant effects (0.05 < P < 0.1) are indicated by a bracketed asterisk
Effect sizes of these group-specific changes were estimated by setting pre-treatment biomass per sample to its mean value (Table 5). This implied that angling was associated with a sixfold increase in shrimp biomass per sample (95% CLs 2.2–11.6), and a weaker, 1.3-fold increase in biomass of surface-layer insects (1.0–1.7). Similarly, exclosure construction was associated with 2.6- and 2.9-fold increases in biomass of exposed and concealed larvae, respectively (confidence limits: 0.93–5.2, and 0.82–6.3). Conversely, pea mussel biomass decreased following angling, by a factor of 2.2 (1.2–3.3).
Body mass size distributions of sampled macro-invertebrates were plotted graphically (Figure S8). Although not analysed formally, the presence of exclosures was associated with higher abundance in larger size classes (0.125–32 mg) compared to adjacent open quadrats; no such pattern was observed in the baseline period, prior to exclosure construction (Figure S8a). At the whole lake level, however, there were no clear differences in body size distribution associated with the introduction of angling (Figure S8b).
Trout reduction and lake use by waterbirds
During each lake-year, bird survey effort comprised, on average, 140 camera trap days (s.e. 2.5), 10.4 short survey visits (s.e. 0.55) and 6.8 long survey visits (s.e. 0.56). Survey effort declined slightly during the study, largely due to increasing rates of camera trap malfunction as the cameras got older (operational camera trap days falling on average by 1.6 days per lake per year).
There were five regularly occurring (over 50 records in total) waterbird species that are wholly or largely invertivorous in the breeding season: the ducks common scoter (58 occurrences), teal (862) and mallard (254), and the waders greenshank (210) and dunlin (65). These species were included in analyses. Observations and camera trap images from these species supported the idea that they spend most of their time at study lakes foraging, or in related activities (e.g. locomotion); however, this was not quantified in detail. A further nine invertivorous waterbird species occurred too infrequently for analysis.
Occurrence of the five focal waterbird species was variable (Fig. 6a), but over time, there was a slight decline in recorded occurrence for some species at some lakes, potentially influenced by the slight decline in survey effort (see above; note that, bird analyses statistically compensated for variations in effort, see Data Analysis). Teal was the most regularly recorded species (up to 121 occurrences per lake-year), followed by mallard and greenshank as middle ranked species (up to 31 and 26 occurrences per lake-year, respectively). Dunlin and common scoter were less common (up to 8 and 7 occurrences per lake-year, respectively). At lakes without angling, records declined by 1.2 occurrences per year, averaged across the five species and the 7-year period. Meanwhile, at lakes where angling was introduced, occurrences remained broadly level (rising by 0.1 occurrence per year, on average).
Waterbird occurrence at the study lakes. a The observed pattern of occurrence for the five most regularly recorded (over 50 occurrences) species, summing occurrences from all forms of survey (long and short survey visits, and camera traps). On each species chart, for each lake, there are seven adjacent bars representing the seven study years, 2013–2019. b The modelled effects of trout reduction by angling on the occurrence of invertivorous waterbirds (see also Table 6). Fitted treatment × period interaction terms, which estimate the effect of treatment as: the change in occurrence at treatment lakes, comparing before and after treatment, relative to the corresponding change at control lakes. Positive values of the fitted log odds ratio indicate an increase in occurrence associated with treatment, controlling for any parallel changes at lakes which were not treated. Statistical significance is indicated as follows: *** P < 0.001; * P < 0.05; (*) = P < 0.1
Statistical analysis of occurrences for the five waterbird species, accounting for variation in survey effort, found no significant effect of trout reduction by angling, when combining all post-treatment years (2016–2019: Table 6). However, analyses of each post-treatment year separately implied a strong increase in effects of angling over time: for the last 2 years of the study, there was a significant positive association between trout reduction by angling and occurrence of these invertivorous waterbirds (Fig. 6b). In the final year of the study, the fitted loge(odds ratio) of 1.59 (s.e. 0.41) indicated a 4.9-fold increase in the odds of these species occurring (95% CLs 2.2–10.9). Lake-year estimates of occurrence in this year suggested a 5.6-fold increase in occurrence for these species at lakes with angling, relative to corresponding changes at other lakes.
Among piscivorous bird species, only grey heron Ardea cinerea was regularly recorded (190 records overall); a further six species were much rarer (only 45 records in total). For these rarer species (in sum), but not for grey heron, recorded occurrence showed a decline in association with the introduction of angling, relative to corresponding changes at non-angling lakes (Figure S9).
Discussion
Reducing trout abundance and biomass: exclosures and angling
Trout reduction took place at two scales (quadrat, lake) using two methods (exclosures, angling). This follows the recommendations (e.g. Carpenter et al. 2010) to investigate lake processes using complementary approaches, combining mesocosms with whole lake studies. Although exclosures completely exclude fish, they are not viable forms of management and may be influenced by artefacts, such as edge effects or changes in the physical environment (Marklund et al. 2002; Holomuzki et al. 2010). Meanwhile, angling is clearly a management approach, but one which might not quickly produce a strong enough change in trout abundance, that would be required to measure subsequent effects within the study time frame. However, consistent results across both spatial scales would support interpretation.
In this study, skilled anglers making several well-timed excursions per season captured ~ 40–60% of standing trout biomass each year. General patterns in lake fisheries (Downing and Plante 1993) suggest this level of trout removal would exceed sustainable yield. Hence, over time, it should reduce overall biomass, and this was achieved, in line with the management objective. Angling was also found to be time-efficient, capturing ~ 400 g of trout biomass per person-hour, in comparison with seine netting surveys, which captured ~ 70 g per person-hour.
This study has shown that the removal of brown trout by angling can have significant impacts on overall population numbers and biomass of trout. These results are supported by those of Almodóvar and Nicola (2004) for stream-dwelling brown trout and Parker et al. (2007) for lacustrine bull trout (Salvelinus confluentus), both of whom recorded a rise in fish abundance after angling had ceased. This accords with traditional brown trout management at upland Scottish lakes, where angling has long been used to reduce numbers, with the intention of reducing intraspecific competition to increase the availability of larger fish for recreational anglers (Bridgett 1924; Frost and Brown 1967; Headley 2005).
Although, in this study, angling halved trout biomass density, there was marked variation in year-to-year population estimates, making it harder to measure treatment differences accurately. Trout age-class composition also varied strongly between lake-years, perhaps reflecting variability in recruitment, which could be linked to weather events affecting spawning habitats (for example, 2016 was the 12th driest autumn since 1910 in North Scotland, with 66% of average rainfall: Met Office 2021), or fish skipping spawning in some years (Frost and Brown 1967; Jonsson and Jonsson 2011).
Trout reduction and macro-invertebrates
Trout exclosures showed that combined macro-invertebrate biomass was several times higher when trout were excluded, and this is similar to results from other presence-absence contrasts. For example, Schilling et al. (2009) found 13 times more macro-invertebrate biomass in fishless lakes compared to those stocked with brook trout Salvelinus fontinalis. Increases within exclosures appeared most marked in the larger sizes classes of invertebrates, which may be of disproportionate value as prey to foraging waterbirds at lakes like these (discussed in detail in Hancock et al. 2016). This result has parallels with other studies showing changes in invertebrate size classes in association with changes in fish predation (e.g. Nummi et al. 2006).
At the whole lake level, however, trout biomass reduction by angling was not associated with any overall increase in macro-invertebrate biomass, across all taxonomic groups combined. A few possible reasons might explain this difference from exclosures. Firstly, angling at the whole lake scale reduced trout biomass less dramatically than exclosures, being reduction rather than exclusion, and with angling tending not to catch the smaller trout size classes present. Fish reductions may need to reach a certain threshold, before strong overall macro-invertebrate responses can be detected (Holomuzki et al. 2010). Secondly, because angling began later in the study, there were only three (rather than five, for exclosures) post-treatment years of invertebrate data. Sometimes invertebrate responses to fish reduction can be lagged by a few or several years (Knapp et al. 2001; Schilling et al. 2009; Pope and Hannelly 2013). Thirdly, the precision with which invertebrate biomass was measured was higher in exclosures, due to the higher sampling rate (more samples per unit area). Finally, the greater size and complexity of lakes compared to quadrats might result in greater variation between taxonomic groups in their response to trout reduction, giving a less clear overall response at the lake level by combined macro-invertebrates. Such between-group variability was indeed revealed by group-specific analyses (below), showing a greater spread of means among groups at the lake level, than at the quadrat level.
Trout reduction should affect different invertebrate groups differently. Active and exposed taxa, perhaps most vulnerable to fish predation, might show strongest effects (Schilling et al. 2009; Martin-Sanz et al. 2010; Jonsson and Jonsson 2011; Tiberti et al. 2014). In this study, the clearest taxon-specific association with trout reduction was the ~ 6-fold increase in shrimp biomass at the whole lake level, when angling was introduced. Brown trout strongly select gammarid prey, developing red spotting when feeding on shrimps (Frost and Brown 1967), and this is linked to better body condition (Parolini et al. 2018). Gammarids responded positively to reductions in related fish species (Leavitt et al. 1994; Milardi et al. 2016b). Many waterbirds also prey heavily on gammarids, including the same (or closely related) bird species as those studied here (MacNeil et al. 1999), including common scoter (Stein Byrkjeland pers. comm.).
The number of insects sampled at the water’s surface (e.g. adults of aquatic groups like mayflies and caddisflies; terrestrial insects) also increased following trout reduction by angling. Surface feeding is important to brown trout in lakes (Jonsson and Jonsson 2011; Sanchez-Hernandez and Amundsen 2015), as is well known to anglers (Headley 2005), underpinning the effectiveness of fly fishing. Surface food is particularly important in small oligotrophic lakes like those studied here (Frost and Brown 1967; Carpenter et al. 2010; Milardi et al. 2016a). Aquatic insects like mayflies and caddisflies may be most vulnerable to trout predation as they pass through the surface layer to emerge as adults (Pope et al. 2009).
Both exposed and concealed larvae (mainly mayflies and caddisflies) also showed positive responses to trout exclusion by exclosures. The lack of a clear relationship with degree of exposure, as assigned here, implied that other factors affected trout influences on these groups. Trout predation might have most impact at more vulnerable parts of the life-cycle, for example when emerging (see above) or ovipositing. Variability in fitted effects for these groups implied much intra-group variation, perhaps reflecting variation within these groups in anti-predator strategies or effectiveness. For example, some species reduce investment in predator avoidance/defence, allowing increased resource acquisition rates (Johansson 1991; Peckarsky 1996).
Only one macro-invertebrate group declined in association with trout reduction: pea mussels, a result found elsewhere (Thorp and Bergy 1981; Tiberti et al. 2014). Perhaps fish indirectly benefit pea mussels and other filter-feeders by enhancing nutrient flows, such as algae falling to lake-beds (Leavitt et al. 1994).
Three-spined sticklebacks can be important alternative prey for trout (e.g. L’Abée-Lund et al. 1992) but results suggested they were very rare at these lakes. They were unrecorded at two of the four lakes, and, with only 38 records overall, had a mean trap rate of only about 0.08 per trap-hour. This contrasts with more neutral lakes in the same region inhabited by stickleback predators, like those studied by Perkins et al. (2005), where stickleback trap rates (in the same trap type, in one study year) averaged around two orders of magnitude higher (unpublished data). Lakes in the current study may have low stickleback abundance due to their low pH: 87% of pH readings for this study fell below pH 6.5; such values are associated with stickleback egg hatch rates lower than 20% (Faris and Wootton 1987).
Trout reduction and waterbirds
This study showed that a guild of invertivorous waterbird species occurred more frequently at peatland lakes following trout reduction by angling, but this was only apparent from 3 years after trout reduction. Bird responses to changes in fish abundance are sometimes rapid (e.g. within a season: Haas et al. 2007). However, more often, studies report lagged responses, e.g. following a 1-year delay (Eriksson 1979) or growing markedly between years one and three after fish reduction (2.3-fold: Hanson and Butler 1994; 50-fold: Giles 1994). In another study, following a sudden drop in fish abundance, there was no first-year response by breeding adult goldeneye Bucephela glangula, but duckling numbers increased (Pöysä et al. 1994; Nummi et al. 2012). A delayed response would be consistent with the high site fidelity commonly shown by adult ducks and waders at their breeding sites (Methods), and the one or more years taken for young birds to join the breeding population. Longer-term studies would enable better understanding of long-term waterbird responses, including species level responses; such follow-up work is recommended (see below).
While many studies have investigated interactions between fish and ducks (reviewed in Bouffard and Hanson 1997; Nummi et al. 2016), we could find no similar studies of fish-wader interactions in freshwater habitats. Waders like dunlins and greenshanks associate strongly with pools and small lakes in their breeding grounds (Thompson and Thompson 1991; Lavers et al. 1996; Hancock et al. 2009), feeding on similar macro-invertebrates to ducks (Cramp and Simmonds 1977; 1983) in shallow littoral zones. Hence, they could be just as vulnerable as ducks to competition with brown trout.
Among piscivorous birds, there was no evidence that angling reduced the occurrence of grey herons. While grey herons likely feed on trout at these lakes, they can also take eels, amphibians and insects, which sometimes make up significant proportions of their freshwater diet (Cramp and Simmons 1977). Although angling decreased trout biomass density, there may have been some increases in mean trout size, which could benefit a large piscivore like grey heron. Other piscivorous birds were very rare: most such species in the region are pursuit predators, a strategy more typical of lakes with much higher water clarity (Methods: Study area).
Caveats and potential future work
This study suggests that trout and waterbirds compete for macro-invertebrate prey. However, this study has not elucidated the process by (for example) demonstrating overlap in prey base or fine-scale (within-lake) foraging habitat use (Eadie and Keast 1982). These would be highly worthwhile subjects for future studies, to enrich and inform management trials like this one. Diet could be investigated using molecular methods, e.g. metabarcoding the stomach contents of culled trout (Hoenig et al. 2021), and the faeces of waterbirds (e.g. Rytkönen et al. 2019), the latter potentially gathered at loafing sites (e.g. islets). Stable isotope methods can also be valuable for dietary studies (Antón-Tello et al. 2021). Fine-scale habitat use by waterbirds could be quantified using observational methods, as done for common scoters (Hancock et al. 2019). Parallel studies could quantify fine-scale habitat use by trout, using tagging approaches (such as the use of Passive Integrated Transponders) to identify the position of marked fish, or the movement of fish within spawning streams. Radio or acoustic tagging approaches can also be used to actively track individual fish within lakes (e.g. Skov et al. 2008; Cook et al. 2014). Such approaches have already been used to identify key fish habitats in many lakes, including small systems similar to those used in this study.
Although this study extended over 7 years, waterbird responses were still developing in the final years of the study. Although some studies have found rapid ecosystem responses to fish reduction, others suggest they may develop slowly. For example, Knapp et al. (2001), investigating complete removal of stocked fish, found that it took over 10 years for invertebrate communities to align with naturally fishless lakes. It would be valuable to maintain the contrasting angling treatments at the study lakes for several more years, monitoring trout using catch-returns, and waterbirds using regular surveys.
At many lakes in the Flow Country, the origin of their brown trout population is uncertain. Lake stocking with brown trout descended from either local or distant populations was widely practised in Scotland until recently (Bridgett 1924; Maitland and Campbell 1992) including within our study region (Frost and Brown 1967; reports from local anglers and land managers). Such stocking is now considered highly inadvisable, both in terms of angling management (Headley 2005) and maintenance of genetic diversity (Lewin et al. 2006; Ferguson 2007). Stocking may have taken place in lakes that were naturally fishless, as studied in North America (Knapp et al. 2001; Schilling et al. 2009). In the Flow Country, while some lakes may hold trout of stocked origin, others may hold native trout populations of high nature conservation importance, descended from post-glacial colonizations (McKeown et al. 2010). To help manage trout populations appropriately, future studies could usefully clarify, for example using genetic methods (Klütsch et al. 2019), which populations are native, and which have been affected by stocking.
Management implications
This study supports using traditional, low-intensity fly fishing to further nature conservation objectives of increased biomass of freshwater macro-invertebrates and the occurrence of waterbirds that feed on them. Any such management should take place alongside future research to better understand the origins (native or stocked) and connectivity of these fish populations, to properly inform their management. Emerging aquatic insects could also enhance habitat quality for terrestrial species like land birds and bats (Pope et al. 2009). Angling return forms completed by anglers (standard practice in our study area) provide useful information to help manage trout populations (Frost and Brown 1967). Careful monitoring of angling returns, perhaps supported by fisheries models, should help reveal any signs of over-exploitation, such as reduced fish sizes (Almodóvar and Nicola 2004). A response to this might be for anglers to release larger fish (e.g. Olin et al. 2017). Regulating the number of angling excursions and taking fish at all sizes, as done here, can prevent over-exploitation or excessive disruption of population structure (Lewin et al. 2006). Monitoring of trout angling returns should go along with waterbird monitoring, consistent with recommendations to monitor more than one element in lake food webs (McParland and Pazcowski 2007; Carpenter et al. 2010).
If trout populations derived from past stocking were identified in lakes that were naturally fishless, then more rigorous management options to restore these lakes could be considered (Schilling et al. 2009; Nummi et al. 2016), although stocked populations can also die out naturally (Pope et al. 2009).
Anglers can be effective supporters of nature conservation, both of native fish populations, and of wider conservation interests like habitat quality, waterbirds and macro-invertebrates (Cooke et al. 2016; Williams and Moss 2001). This study also supports a common interest between well-regulated, low-intensity traditional brown trout angling, and nature conservation at small peatland lakes.
The current study aimed to inform decisions around fish management, to support aquatic conservation by maintaining invertebrate biomass and waterbird habitat quality. Similar issues are widely relevant around the world, as shown by reviews like Bouffard and Hanson (1997), Nummi et al. (2016) and Britton (2022). While these illustrate the concentration of studies in North America and Europe, there are also related examples from elsewhere, such as South America (Hurlbert et al. 1986; Ortubay et al. 2006; Porcel et al. 2022), Australia (Smith et al. 2009) and Africa (Kadye et al. 2013). More broadly, fisheries management in lakes with important invertebrate and waterbird populations is a key issue in freshwater conservation, highlighted by Dudgeon et al. (2006) in their global review. They emphasized the need for reconciliation between biodiversity conservation and human uses of freshwaters. This study supports that approach, by showing that good alignment can be achieved between carefully managed trout angling and aquatic conservation objectives.
Data availability
Data are available from the authors upon reasonable request.
References
Adams WA (1889) Twenty-six years’ reminiscences of Scotch grouse moors. [Facsimile]. Lightning Source, Milton Keynes
Almodóvar A, Nicola GG (2004) Angling impact on conservation of Spanish stream-dwelling brown trout Salmo trutta. Fisheries Manag Ecol 11:173–182. https://doi.org/10.1111/j.1365-2400.2004.00402.x
Antón-Tello M, Britto VO, Gil-Delgado JA, Rico E, Dies JI, Monrós JS, Vera P (2021) Unravelling diet composition of colonial waterbirds in a Mediterranean wetland using stable isotopes. Ibis 163:913–927. https://doi.org/10.1111/ibi.12928
Barrett JH, Nicholson RA, Cerón-Carrasco R (1999) Archaeo-ichthyological evidence for long-term socioeconomic trends in northern Scotland: 3500 BC to AD 1500. J Archaeol Sci 26:353–388. https://doi.org/10.1006/jasc.1998.0336
Bouffard SH, Hanson MA (1997) Fish in waterfowl marshes: waterfowl managers’ perspective. Wildl Soc Bull 25:146–157
Bridgett RC (1924) Loch-fishing in theory and practice. Herbert Jenkins, London
Britton JB (2022) Contemporary perspectives on the ecological impacts of invasive freshwater fish. J Fish Biol. https://doi.org/10.1111/jfb.15240
Carpenter SR, Cole JJ, Kitchell JF, Pace ML (2010) Trophic Cascades in lakes: lessons and prospects. In: Terborgh J, Estes JA (eds) Trophic Cascades: predators, prey and the changing dynamics of nature. Island Press, Washington, pp 55–70
Christie AP, Amano T, Martin PA, Shackleford GE, Simmons BI, Sutherland W (2019) Simple study designs in ecology produce inaccurate estimates of biodiversity responses. J Appl Ecol 56:2742–2754. https://doi.org/10.1111/1365-2664.13499
Cook AM, Bradford RG, Bentze P (2014) Hydroacoustic tracking of the endangered Atlantic whitefish (Coregonus huntsmani); comparative analysis from wild and hatchery reared populations. Environ Biol Fish 97:955–964. https://doi.org/10.1007/s10641-013-0197-4
Cooke SJ, Hogan ZS, Butcher PA, Stokesbury MJW, Raghavan R, Gallagher AJ et al (2016) Angling for endangered fish: conservation problem or conservation action? Fish Fish 17:249–265. https://doi.org/10.1111/faf.12076
Cramp S, Simmons KEL (eds) (1977) The birds of the western Palearctic, vol 1. Oxford University Press, Oxford
Cramp S, Simmons KEL (eds) (1983) The birds of the western Palearctic, vol 3. Oxford University Press, Oxford
Croft PS (1986) A key to the major groups of British freshwater invertebrates. Field Stud 6:531–579
Downing JA, Plante C (1993) Fish production in lakes. Can J Fish Aquat Sci 50:110–120. https://doi.org/10.1139/f93-013
Dudgeon D, Arthington AH, Gessner MO, Kawabata Z-I, Knowler DJ, Lévêque C et al (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81:163–182
Eadie JM, Keast A (1982) Do goldeneye and perch compete for food? Oecol 55:225–230. https://doi.org/10.1007/BF00384491
Eriksson MOG (1979) Competition between freshwater fish and goldeneyes Bucephala clangula (L.) for common prey. Oecol 41:99–107. https://doi.org/10.1007/BF00344840
Faris AA, Wootton RJ (1987) Effect of water pH and salinity on the survival of eggs and larvae of the euryhaline teleost, Gasterosteus aculeatus L. Environ Pollut 48:49–59. https://doi.org/10.1016/0269-7491(87)90085-6
Ferguson, A. (2007) Genetic impacts of stocking on indigenous brown trout populations. Environment Agency science report: SC040071/SR. Environment Agency, Bristol. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/291703/scho0707bmzi-e-e.pdf
Frost WE, Brown ME (1967) The trout. Collins, London
Furness RW, Galbraith H, Gibson IP, Metcalfe NB (1986) Recent changes in numbers of waders on the Clyde Estuary, and their significance for conservation. Proc Roy Soc Edinb B Biol Sci 90:171–184. https://doi.org/10.1017/S0269727000004978
Gaffney PPJ, Hancock MH, Taggart MA, Andersen R (2021) Catchment water quality in the year preceding and immediately following restoration of a drained afforested blanket bog. Biogeochemistry 153:243–262. https://doi.org/10.1007/s10533-021-00782-y
Giles N (1994) Tufted duck (Aythya fuligula) habitat use and brood survival increases after fish removal from gravel pit lakes. Hydrobiologia 279(280):387–392. https://doi.org/10.1007/978-94-011-1128-7_35
Gurevitch J, Morrison JA, Hedges LV (2000) The interaction between competition and predation: a meta-analysis of field experiments. Am Nat 155:435–453. https://doi.org/10.1086/303337
Haas K, Köhler U, Diehl S, Köhler P, Dietrich S, Holler S et al (2007) Influence of fish on habitat choice in water birds: a whole system experiment. Ecol 88:2915–2925. https://doi.org/10.1890/06-1981.1
Hancock MH, Grant MC, Wilson JD (2009) Associations between distance to forest and spatial and temporal variation in abundance of key peatland breeding bird species. Bird Study 55:53–64. https://doi.org/10.1080/00063650802648176
Hancock MH, Robson HJ, Smith TD, Douse A (2016) Correlates of lake use by breeding common scoters in Scotland. Aquat Conserv 26:749–760. https://doi.org/10.1002/aqc.2606
Hancock MH, Robson HJ, Smith TD, Douse A (2019) Spatial and temporal patterns of foraging activity by breeding common scoters (Melanitta nigra) in Scotland. Ornis Fenn 96:124–141
Hancock MH, Robson HJ, Smith TD, Stephen A, Byrne P, MacLennan A et al (2020) From a research study to a conservation partnership: developing approaches to restoring common scoter populations. Aquat Conserv 30:1770–1774. https://doi.org/10.1002/aqc.3414
Hanson MA, Butler MG (1994) Responses to food web manipulation in a shallow waterfowl lake. Hydrobiol 279(280):457–466. https://doi.org/10.1007/BF00027877
Headley S (2005) The loch fisher’s bible. Robert Hale, London
Hoenig BD, Trevelline BK, Nuttle T, Porter BA (2021) Dietary DNA metabarcoding reveals seasonal trophic changes among three syntopic freshwater trout species. Freshw Biol 66:509–523. https://doi.org/10.1111/fwb.13656
Holomuzki JR, Feminella JW, Power ME (2010) Biotic interactions in freshwater benthic habitats. J North Am Benthol Soc 29:220–244. https://doi.org/10.1899/08-044.1
Hornung JP, Foote AL (2006) Aquatic invertebrate responses to fish presence and vegetation complexity in Western Boreal wetlands, with implications for waterbird productivity. Wetlands 26:1–12. https://doi.org/10.1672/0277-5212(2006)26[1:AIRTFP]2.0.CO;2
Hurlbert SH, Loayza W, Moreno T (1986) Fish-flamingo-plankton interactions in the Peruvian Andes. Limnol Oceanogr 31:457–468. https://doi.org/10.4319/lo.1986.31.3.0457
Jackson DB (1994) Breeding dispersal and site-fidelity in three monogamous wader species in the Western Isles, U.K. Ibis 136:463–473. https://doi.org/10.1111/j.1474-919X.1994.tb01123.x
Johansson A (1991) Caddis larvae cases (Trichoptera, Limnephilidae) as anti-predatory devices against brown trout and sculpin. Hydrobiol 211:185–194. https://doi.org/10.1007/BF00008534
Johnson DH, Grier JW (1988) Determinants of breeding distribution of ducks. Lincoln, US Geological Survey
Jonsson B, Jonsson N (2011) Ecology of Atlantic salmon and brown trout. Habitat as a template for life histories. Springer, London
Joosten H, Szallies I, Tegetmeyer C (2016) The Flow Country (Scotland) as a blanket bog landscape—A global evaluation. International Mire Conservation Group, Greifswald. https://www.theflowcountry.org.uk/assets/Uploads/171108-Flow-Country-WHS-Comparative-Study-A2454376.pdf. Accessed July 2020
Kadye WT, Chakona A, Marufu LT, Samukange T (2013) The impact of non-native rainbow trout within Afro-montane streams in eastern Zimbabwe. Hydrobiol 720:75–88. https://doi.org/10.1007/s10750-013-1624-4
Kloskowski J, Nieoczym M, Polak M, Pitucha P (2010) Habitat selection by breeding waterbirds at ponds with size-structured fish populations. Naturwissenschaften 97:673–682. https://doi.org/10.1007/s00114-010-0684-9
Klütsch CFC, Maduna SN, Polikarpova N, Forfang K, Aspholm PE, Nyman T et al (2019) Genetic changes caused by restocking and hydroelectric dams in demographically bottlenecked brown trout in a transnational subarctic riverine system. Ecol Evol 9:6068–6081. https://doi.org/10.1002/ece3.5191
Knapp RA, Matthews KR, Sarnelle O (2001) Resistance and resilience of alpine lake fauna to fish introductions. Ecol Monogr 71:401–421. https://doi.org/10.1890/0012-9615(2001)071[0401:RAROAL]2.0.CO;2
L’Abée-Lund JH, Langeland A, Sægrov H (1992) Piscivory by brown trout Salmo trutta L. and Arctic char Salvelinus alpinus (L.) in Norwegian lakes. J Fish Biol 41:91–101. https://doi.org/10.1111/j.1095-8649.1992.tb03172.x
Lavers CP, Haines-Young RH, Avery MI (1996) Habitat associations of dunlin (Calidris alpina) in the Flow Country of northern Scotland. J Appl Ecol 33:279–290. https://doi.org/10.2307/2404750
Leavitt PR, Schindler DE, Paul AJ, Hardie AK, Schindler DW (1994) Fossil pigment records of phytoplankton in trout-stocked alpine lakes. Can J Fish Aquat Sci 51:2411–2423. https://doi.org/10.1139/f94-241
LeBourdais SV, Ydenberg RC, Eslera D (2009) Fish and harlequin ducks compete on breeding streams. Can J Zool 87:31–40. https://doi.org/10.1139/Z08-135
Lewin W-C, Arlinghaus R, Mehner T (2006) Documented and potential biological impacts of recreational fishing: insights for management and conservation. Rev Fish Sci 14:305–367. https://doi.org/10.1080/10641260600886455
Lindsay RA, Charman DJ, Everingham F, O’Reilly RM, Palmer MA, Rowell TA et al (1988) The Flow Country. The peatlands of Caithness and Sutherland. Nature Conservancy Council, Peterborough
MacNeil C, Dick JTA, Elwood RW (1999) The dynamics of predation on Gammarus spp. (Crustacea: Amphipoda). Biol Rev 74:375–395. https://doi.org/10.1111/j.1469-185X.1999.tb00035.x
Maitland PS, Campbell RN (1992) Freshwater fishes of the British Isles. HarperCollins, London
Marklund O, Sandsten H, Hansson L-A, Blinkow I (2002) Effects of waterfowl and fish on submerged vegetation and macroinvertebrates. Freshw Biol 47:2049–2059. https://doi.org/10.1046/j.1365-2427.2002.00949.x
Martínez-Sanz C, García-Criado F, Fernández-Aláez F (2010) Effects of introduced salmonids on macroinvertebrate communities of mountain ponds in the Iberian system of Spain. Limnetica 29:221–232. https://doi.org/10.23818/limn.29.18
McKeown NJ, Hynes RA, Duguid RA, Ferguson A, Prodöhl PA (2010) Phylogeographic structure of brown trout Salmo trutta in Britain and Ireland: glacial refugia, postglacial colonization and origins of sympatric populations. J Fish Biol 76:319–347. https://doi.org/10.1111/j.1095-8649.2009.02490.x
McParland CE, Paszkowski CA (2007) Waterbird assemblages in the Aspen Parkland of western Canada: the influence of fishes, invertebrates, and the environment on species composition. Ornithol Sci 6:53–65. https://doi.org/10.2326/1347-0558(2007)6[53:WAITAP]2.0.CO:2
Milardi M, Käkelä R, Weckström J, Kahilainen KK (2016a) Terrestrial prey fuels the fish population of a small, high-latitude lake. Aquat Sci 78:695–706. https://doi.org/10.1007/s00027-015-0460-1
Milardi M, Siitonen S, Lappalainen J, Liljendahl A, Weckström J (2016b) The impact of trout introductions on macro- and microinvertebrate communities of fishless boreal lakes. J Paleolimnol 55:273–287. https://doi.org/10.1007/s10933-016-9879-1
Nethersole-Thompson D, Nethersole-Thompson M (1986) Waders: their breeding, haunts and watchers. Poyser, Calton
Nummi P, Väänänen V-M, Malinen J (2006) Alien grazing: indirect effect of muskrats on invertebrates. Biol Invasions 8:993–999. https://doi.org/10.1007/s10530-005-1197-x
Nummi P, Väänänen V-M, Rask M, Nyberg K, Taskinen K (2012) Competitive effects of fish in structurally simple habitats: perch, invertebrates, and goldeneye in small boreal lakes. Aquat Sci 74:343–350. https://doi.org/10.1007/s00027-011-0225-4
Nummi P, Väänänen V-M, Holopainen S, Pöysä H (2016) Duck–fish competition in boreal lakes—a review. Ornis Fenn 93:67–76
Ockendon N, Amano T, Cadotte M, Downey H, Hancock MH, Thornton A et al (2021) Effectively integrating experiments into conservation practice. Ecol Solut Evid 2:e12069. https://doi.org/10.1002/2688-8319.12069
Met Office. (2021) Climate summaries: Data tables of UK and regional monthly series. https://www.metoffice.gov.uk/research/climate/maps-and-data/uk-and-regional-series. Accessed July 2021
Olin M, Tiainen J, Rask M, Vinni M, Nyberg K, Lehtonen H (2017) Effects of non-selective and size-selective fishing on perch populations in a small lake. Boreal Environ Res 22:137–155
Ortubay S, Cussac V, Battini M, Barriga J, Aigo J, Alonso M et al (2006) Is the decline of birds and amphibians in a steppe lake of northern Patagonia a consequence of limnological changes following fish introduction? Aquat Conserv 16:93–105. https://doi.org/10.1002/aqc.696
Parker BR, Schindler DW, Wilhelm FM, Donald DB (2007) Bull trout population responses to reductions in angler effort and retention limits. N Am J Fish Manag 27:848–859. https://doi.org/10.1577/M06-051.1
Parolini M, Iacobuzio R, Possenti CD, Bassano B, Pennati R, Saino N (2018) Carotenoid-based skin coloration signals antioxidant defenses in the brown trout (Salmo trutta). Hydrobiologia 815:267–280. https://doi.org/10.1007/s10750-018-3571-6
Peckarsky BL (1996) Alternative predator avoidance syndromes of stream-dwelling mayfly larvae. Ecology 77:1888–1905. https://doi.org/10.2307/2265793
Perkins AJ, Hancock MH, Butcher N, Summers RW (2005) Use of time-lapse video cameras to determine causes of nest failure of Slavonian Grebes Podiceps auritus. Bird Study 52:159–165. https://doi.org/10.1080/00063650509461386
Pope KL, Hannelly EC (2013) Response of benthic macroinvertebrates to whole-lake, non-native fish treatments in mid-elevation lakes of the Trinity Alps, California. Hydrobiol 714:201–215. https://doi.org/10.1007/s10750-013-1537-2
Pope KL, Piovia-Scott J, Lawler SP (2009) Changes in aquatic insect emergence in response to whole-lake experimental manipulations of introduced trout. Freshw Biol 54:982–993. https://doi.org/10.1111/j.1365-2427.2008.02145.x
Porcel S, Fogel ML, Izaguirre I, Roesler I, Lancelotti JL (2022) Effect of rainbow trout introductions on food webs in the lakes of arid Patagonia. Hydrobiologia 849:2057–2075. https://doi.org/10.1007/s10750-022-04848-2
Pöysä H, Rask M, Nummi P (1994) Acidification and ecological interactions at higher trophic levels in small forest lakes: the perch and the common goldeneye. Ann Zool Fenn 31:317–404
Quinn GP, Keogh MJ (2002) Experimental design & data analysis for biologists. Cambridge University Press, Cambridge
Rich LN, Miller DA, Robinson HS, McNutt JW (2016) Using camera trapping and hierarchical occupancy modelling to evaluate the spatial ecology of an African mammal community. J Appl Ecol 53:1225–1235. https://doi.org/10.1111/1365-2664.12650
Robson HJ, Jones VJ, Hilton GM, Brooks S, Sayer CD, Douse A (2019) Combined palaeolimnological and ecological approach provides added value for understanding the character and drivers of recent environmental change in Flow Country lakes. Mires Peat. https://doi.org/10.19189/MaP.2018.OMB.386
Rytkönen S, Vesterinen EJ, Westerduin C, Leviäkangas T, Vatka E, Mutanen M et al (2019) From feces to data: a metabarcoding method for analyzing consumed and available prey in a bird-insect food web. Ecol Evol 9:631–639. https://doi.org/10.1002/ece3.4787
Sanchez-Hernandez S, Amundsen P-A (2015) Trophic ecology of brown trout (Salmo trutta L.) in subarctic lakes. Ecol Freshw Fish 24:148–161. https://doi.org/10.1111/eff.12139
Sandison B (1992) Trout lochs of Scotland, 3rd edn. HarperCollins, London
SAS (2014) SAS/STAT version 9. SAS Institute, Cary
Schilling EG, Loftin CS, Huryn AD (2009) Effects of introduced fish on macroinvertebrate communities in historically fishless headwater and kettle lakes. Biol Conserv 142:3030–3038. https://doi.org/10.1016/j.biocon.2009.08.003
Skov C, Brodersen J, Nilsson PA, Hansson L-A, Brönmark C (2008) Inter- and size-specific patterns of fish seasonal migration between a shallow lake and its streams. Ecol Freshw Fish 17:406–415. https://doi.org/10.1111/j.1600-0633.2008.00291.x
Smith K, Norriss J, Brown J (2009) Population growth and mass mortality of an estuarine fish, Acanthopagrus butcheri, unlawfully introduced into an inland lake. Aquat Conserv 19:4–13. https://doi.org/10.1002/aqc.962
Southwood TRE, Henderson RA (2000) Ecological methods, 3rd edn. Blackwell, Oxford
Stroup WW (2013) Generalized linear mixed models. Modern concepts, methods and applications. CRC Press, Boca Raton
Thompson PS, Thompson DBA (1991) Greenshanks Tringa nebularia and long-term studies of breeding waders. Ibis 133(Suppl. 1):99–112. https://doi.org/10.1111/j.1474-919X.1991.tb07673.x
Thorp JH, Bergey EA (1981) Field experiments on responses of a freshwater, benthic macroinvertebrate community to vertebrate predators. Ecol 62:365–375. https://doi.org/10.2307/1936711
Tiberti R, von Hardenberg A, Bogliani G (2014) Ecological impact of introduced fish in high altitude lakes: a case of study from the European Alps. Hydrobiol 724:1–19. https://doi.org/10.1007/s10750-013-1696-1
Toge T, Yamashita R, Kazama K, Fukuwaka M, Yamamura O, Watanuki Y (2011) The relationship between pink salmon biomass and the body condition of short-tailed shearwaters in the Bering Sea: can fish compete with seabirds? Proc Roy Soc B 278:2584–2590. https://doi.org/10.1098/rspb.2010.2345
Williams AE, Moss B (2001) Angling and conservation at sites of special scientific interest in England economics, attitudes and impacts. Aquat Conserv 11:357–372. https://doi.org/10.1002/aqc.466
Winfield DK, Winfield IJ (1994) Possible competitive interactions between overwintering tufted duck (Aythya fuligula (L.)) and fish populations of Lough Neagh, Northern Ireland: evidence from diet studies. Hydrobiologia 279:377–386. https://doi.org/10.1007/BF00027869
Winfield IJ, Winfield DK, Tobin CM (1992) Interactions between the roach, Rutilus rutilus, and waterfowl populations of Lough Neagh, Northern Ireland. Environ Biol Fish 33:207–214. https://doi.org/10.1007/BF00002565
Youngson AF, Jordan WC, Verspoor E, McGinnity P, Cross T, Ferguson A (2003) Management of salmonid fisheries in the British Isles: towards a practical approach based on population genetics. Fish Res 62:193–209. https://doi.org/10.1016/S0165-7836(02)00162-5
Acknowledgements
We gratefully acknowledge the key roles of N. Russell, R.W. Summers, D. Stevens, J.D. Wilson, C. Eccles and N.R. Cowie, RSPB, and Andy Douse, NatureScot, in setting up and supporting this study. The main funders were RSPB and NatureScot. Major support with fieldwork was provided by RSPB staff and volunteers H. Bernie, I. Cameron, J. Cooke, N. Crutchley. C. Foot-Turner, N. Greaves, C. Michael, S. Ruesch, R. Seddon, F. Sutherland, G. Thompson, P. Turner and S. Udale-Smith. P. Stagg was supported by a Natural Talent Apprenticeship managed by J. MacFarlane of The Conservation Volunteers. The Heritage Lottery Fund’s Flows to the Future project supported volunteers’ costs. R. Andersen, B. Bremner, D. Svobodova and M.A. Taggart of the Environmental Research Institute assisted with water chemistry testing and laboratory space for invertebrate work. C. Adams and S. Wilson of Glasgow University assisted with equipment loans and advice. R. Sweeting of Forsinard Flyfishers’ Club helped set up and carry out the angling treatments. A. Ball and C. Playfair-Hannay of RSPB organized the angling leases. J. Plowman of NatureScot assisted with nature conservation consents. H.J. Robson of the Wildfowl and Wetlands Trust advised on invertebrate laboratory methods. We thank five anonymous reviewers for their useful feedback which helped us improve an earlier version of this manuscript. We gratefully acknowledge the help of many others, who assisted with fieldwork as reserve volunteers or visiting staff and students (Supporting Information).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Handling editor: Dr. Sébastien Villeger
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hancock, M.H., Klein, D., Hughes, R. et al. Testing whether reducing brown trout biomass in peatland lakes increases macro-invertebrate biomass and invertivorous waterbird occurrence. Aquat Ecol 57, 217–240 (2023). https://doi.org/10.1007/s10452-022-10000-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-022-10000-y