Abstract
Rainbow trout have been stocked in naturally fishless lakes in the reproductive area of the endangered Hooded Grebe, an endemic aquatic bird of Southern Patagonia. The effects of trout introduction were proposed as one of the potential causes of their critical situation. Trout could modify the trophic structure of the aquatic community by altering the trophic relationships of stocked lakes. Changes in the spatial distribution and/or diet of the Grebe’s main prey, which is dominated by amphipods, could affect their trophic position, and introduce the use of alternative carbon sources. We compared the food webs between fishless and stocked lakes using stable isotope analysis and associated community metrics. Results provided evidence that the presence of fish influences the food web structure of the studied communities. Stocked lakes showed higher values of δ15N compared to fishless ones. Community metrics estimation described an increase in trophic redundancy in stocked lakes, which could be explained by the high degree of trout omnivory. Additionally, the study found changes in use of carbon sources in amphipods between stocked and fishless lakes. The spatial distribution of the amphipods toward littoral zone of the stocked lakes, as an anti-predatory response, could impact the Hooded Grebe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salmonids, in particular, the rainbow trout [Oncorhynchus mykiss (Walbaum 1792)], were widely introduced into lakes and rivers on every continent except Antarctica, causing fast and severe impacts on the environments containing these predatory fish (Townsend, 2003; Arismendi et al., 2019). The introduction of a novel predator into lakes changes the size spectrum and species composition of macroinvertebrates and zooplankton communities (Knapp et al., 2001). Consequently, the introduction of the omnivorous rainbow trout can result in top-down and bottom-up controls that may alter the structuring of aquatic food webs. In top-down controls, fish can reduce the abundance of larger zooplankton by predation thus enhancing phytoplankton biomass (Blanco et al., 2008). With bottom-up controls, enhanced populations of algae can be triggered by an increase in nutrients in the water column, which can occur by direct nutrient excretion from fish (Vanni, 2002) and/or from sediment resuspension generated by fish feeding on benthic organisms (Schauss et al., 2002). Furthermore, top-down and bottom-up processes owing to fish introduction promote the shift to a more turbid state of the water bodies that may become permanent (Blanco, 2001).
Frequently, predation on the native fauna by introduced fishes causes ecological impacts in higher trophic levels, causing the reduction of the abundance or the eradication of the most palatable species for the introduced predator (Tyler et al., 1998; Knapp, 2005). Rainbow trout were intensively introduced into fishless lakes of Southern arid Patagonia and have been proposed as one of the main threats for the Hooded Grebe (Podiceps gallardoi Rumboll 1974) (Roesler et al., 2012), a globally, critically endangered diving bird endemic to this region (BirdLife International, 2021). The Hooded Grebe with a population estimated to be 800 individuals (Roesler et al., 2020) only reproduce in natural fishless shallow lakes located on a collection of basaltic plateaus of Southern Patagonia (Fig. 1). However, more than 40 of these lakes were stocked with rainbow trout for aquaculture.
The effects of trout introduction have been documented for several water bodies of the Patagonian steppe, including changes in the size structure and the species composition of the receiving lacustrine communities (Reissig et al., 2006; Ortubay et al., 2006). In the Strobel Lake Plateau, one of the most important reproductive areas for the Hooded Grebe (Roesler, 2016), trout have caused negative impacts to the abundance and size distribution of organisms that trout feed on (Lancelotti et al., 2017). Likewise, there has been a documented greater phytoplankton biomass and greater abundance of cyanobacteria in lakes containing introduced fish (Izaguirre & Saad, 2014) and changes in the relative proportion of different groups of pleustonic macroinvertebrates (Lopez, 2015).
Trout and Hooded Grebe are both visual predators with diets restricted to a small number of crustacean species (e.g., amphipods, copepods and cladocerans) and insect larvae (Fjeldså, 1986; Beltrán et al., 1992; Lancelotti et al., 2015), which can potentially be predated by both species. In particular, amphipods represent the most dominant prey group within the trout diet, regardless of the size of the fish (Lancelotti et al., 2015). Amphipods also represent a primary prey item for the Hooded Grebe, suggesting a strong dietary overlap with trout. In this context, the reduction in prey availability due to trout introduction could lead to competitive interactions, which could potentially affect the survival of the endangered grebe (Lancelotti et al., 2015; Roesler, 2016). Although these two studies described the trout’s effects on the community structure of the lakes, as well as potential effects on the grebe and its habitat, little is known about the alterations in food webs and in changes in sources of matter and energy as a result of fish introductions. This information is essential to evaluate changes at ecosystem scales and to identify mechanisms and processes that could modify extra-Andean aquatic environments.
Shallow lakes are dynamic environments with a spatial configuration characterized by a deeper pelagic zone and shallower benthic and littoral zones often covered by macrophytes (Scheffer, 1998). Fish, as highly mobile organisms, link both the benthic and the pelagic food webs (Schindler & Scheuerell, 2002). This habitat coupling could have significant consequences for nutrient cycling, predator–prey interactions, and food web structure. In some environments, for example, pelagic zone processes are characterized by interactions that may include both the littoral and benthic zones of the lake (Pace, 1993; France, 1995a; Jeppesen et al., 1997; Covich et al., 1999; Rothhaupt, 2000; Schindler & Scheuerell, 2002; Rautio & Vincent, 2007). For example, nutrient excretion by benthivorous consumers can account for a substantial portion of inputs to pelagic nutrients; benthic/littoral resources also subsidize carnivore populations that have important predatory effects on plankton communities (Vander Zanden et al., 1999b; Schindler & Scheuerell, 2002; Vander Zanden & Vadeboncoeur, 2002). In addition, the complexity of littoral habitats (e.g., the presence of macrophytes, rocks, coarse woody debris) could have substantial effects on predator–prey interactions between fish and their invertebrate prey. Therefore, the magnitude of food web alterations by the presence of fish may depend on multiple factors related to the particular characteristics of each water body (Carpenter & Kitchell, 1993; Pace et al., 1999; Polis, 1999; Polis et al., 2000; Beisner et al., 2003).
The introduction of a generalist predator in a novel habitat, as is the case with rainbow trout, can alter the pathways and rates of recirculation of organic matter and nutrients. In particular, the extensive use of benthic prey could subsidize fish populations, altering trophic dynamics and the ecosystem processes of the pelagic zone. Cross omnivory between benthic and pelagic habitats by trout was observed in numerous studies, highlighting the importance of an integrated view of aquatic ecosystems, taking into account the duality of pathways in benthic and pelagic production (Schindler & Scheuerell, 2002; Vander Zanden & Vadeboncoeur, 2002; Jeppesen et al., 2003).
The presence of predators can also induce anti-predatory responses, altering the morphology, behavior, and spatial distribution of their prey (Ferrari et al., 2010; Lancelotti et al., 2017). On the Strobel Lake Plateau, amphipods are abundant in both the water column and in littoral zones of shallow lakes without fish, while in stocked ones, they are restricted to littoral and benthic areas (Lancelotti, unpublished data). These changes in the spatial distribution of amphipods could induce alterations in the use of resources in the presence of trout, directing the diet of amphipods toward predominantly littoral/benthic resources.
Based on the arguments discussed above, the objective of this study was to analyze comparatively the trophic structure, nutrients, and energy pathways in shallow lakes of the Strobel Lake Plateau, evaluating water bodies with similar limnological characteristics that have been stocked with rainbow trout or environments in their natural fishless state. Stable isotopes at the natural abundance level were used to determine the trophic structure.
Under the cited general conceptual framework, trout effects in stocked lakes could generate changes that alter the trophic position of their prey and also the trophic structure of the entire community. In this context, the following hypotheses were tested: (1) Trout introduction modifies the trophic structure of the community, altering the configuration of trophic relationships of stocked lakes with respect to fishless lakes; (2) Trout introduction generates changes in the spatial distribution and/or diet of its main prey, affecting their trophic position and forcing the use of alternative carbon sources.
Materials and methods
Study area
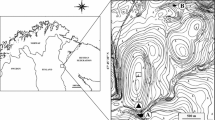
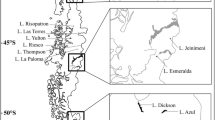
The study was conducted in shallow lakes of the Strobel Lake Plateau (48°50’S, 71°20’W) in the arid Patagonia (Fig. 1) during southern summer, between February 18 and 26, 2017. This region is characterized by its extreme conditions of cold and dry weather. The average annual rainfall is about 300 mm, and the average temperatures are 2.9ºC and 14ºC in winter and summer, respectively.
The tectonic origin of this plateau was formed during the Miocene-Pliocene periods (Panza & Franchi, 2002), where the sinking of the underlying mantle generated a wide diversity of water bodies in terms of size, shape, and configuration (Pereyra et al., 2002). These natural depressions collect snow during autumn and winter (Pereyra et al., 2002). The number of water bodies on the Strobel Plateau varies from 700 to 2000 depending on annual precipitation (Lancelotti et al., 2020). While many water bodies are quite shallow (< 5 m maximum depth) and temporary, numerous lakes are deeper than 10 m and permanent. At these latitudes, the lakes experience high irradiance and strong winds from the west, which generate a continuously mixed full water column preventing the establishment of thermoclines (Lancelotti, 2009).
Sampling and methodology
Samples were collected in four shallow lakes of the Strobel Lake Plateau with similar characteristics of limnological variables (i.e., without vegetation) and morphometry (i.e., area and depth) (Fig. 1; Table 1): two stocked with trout in 2013 (R2 and R3) and two fishless (R22 and R26). These lakes correspond to the organic turbid regimen (Porcel, 2020) from high turbidity levels evaluated through the vertical attenuation coefficient of photosynthetically active radiation (kd) and chlorophyll a (Table 1). The morphometric variables were similar among environments: the lake areas had a range of 0.10 to 0.31 km2, and the mean depth was 5 m. The protocol for measuring limnological variables was fully described in Porcel et al. (2020). Stocked lakes showed high values of conductivity, dissolved organic and inorganic carbon concentrations, and total nitrogen compared to fishless lakes. These differences could be a response to the hydrology of the lakes, as well as the presence of trout (Porcel, 2020).
Samples for stable isotopes analyses were taken from the following groups (Online Resource 1, Table 1_SI): (1) pelagic basal carbon based on particulate organic matter (POM), (2) benthic carbon sources from biofilm associated with coastal rocks, (3) pelagic, and (4) littoral crustaceans (copepods, amphipods, and cladocerans), invertebrates (insect larvae and leeches), and samples of dorsal muscle of trout (at least 4 individuals per lake). POM samples were collected within the euphotic zone at the subsurface layer (~ 30–50 cm depth). For the isotopic determination of POM, 100–500 ml of water was filtered onto GF/F Whatman® filters (47 mm diameter, 0.7 micron nominal pore size), previously muffled (550 °C; 15 h) (Wetzel & Likens, 2000). Biofilm samples were obtained by scraping rocks from the littoral zone of the shallow lakes and also placed onto previously muffled GF/F filters. The filters with samples were wrapped in foil and transported in liquid nitrogen to the laboratory and stored at -20 °C until processing.
Planktonic organisms were collected using conical net sweeps of 45 μm pore size. Each qualitative sample was integrated by ten 5-m horizontal and vertical sweeps that were performed in the center of the lakes. Amphipods and invertebrates of littoral zones were manually collected from 20 rocks selected at random, located on the shore. Each rock was quickly placed in a bucket where it was shaken with lake water for at least 5 min to remove adhering organisms. Fish were caught using a 50 m long and 2 m high gillnet (mesh size was 60 and 40 mm between knots) set for 8 h. Gillnets were set transverse to the shoreline of the lakes. Relative abundance of fish was calculated as catch per unit of effort (CPUE, number of fish per net h−1). The muscle samples were taken following the protocol described in Post (2002) and Sarakinos et al. (2002). Samples were preserved in 96% ethanol within a week of collection and stored at room temperature (Smyntek et al., 2007; Syväranta et al., 2011).
In the laboratory, the specimens were identified and separated under a stereoscopic microscope in the aforementioned categories. From each sample at least 5 subsamples were obtained, when possible, that were composed of several individuals from the corresponding taxonomic group. Once the material was separated, it was dried to a constant weight in an oven (60 °C) and ground using a mortar and pestle. Approximately 1 mg of each sample was weighed and packaged in tin capsules (9 × 5 mm), then analyzed for the isotope ratios (δ13C and δ15N) of the total carbon and nitrogen content via an elemental analyzer linked to an isotope ratio mass spectrometer (Costech ECS 4010-Delta V Advantage IRMS) at the Environmental Dynamics and Geoecology Institute at the University of California (Riverside, USA). The processing was carried out following a standard protocol (Teece & Fogel, 2004). Results are expressed in the delta notation (‰) and calculated using the following equation:
δX = [(Rsample/Rstandard) − 1] × 103, where X is 13C or 15 N, and R is the isotope ratio 13C/12C or 15 N/14 N (Barrie & Prosser, 1996).
The δ13C values in ecosystems are generally indicators of the energy or carbon source, whereas δ15N values reflect a consumer’s trophic level (Post, 2002). In aquatic environments, consumers gain an average of 3.4 ‰ in δ15N and 1 ‰ in δ13C for each increase in trophic level (Vander Zanden et al., 1999a), although these values may vary according to the type of prey or predator identity and the analyzed tissue (Caut et al., 2009; Vander Zanden et al., 1999a). Primary producers show contrasting carbon (δ13C) isotope signals depending on their habitat: organisms inhabiting littoral and benthic zones tend to have more positive δ13C compared to those that inhabit pelagic zones (France, 1995b; Hecky & Hesslein, 1995; Vander Zanden & Rasmussen, 1999). Therefore, these isotopic compositions are used as indicators of the energy flow in aquatic food webs, since they reflect the signature of the energy sources assimilated over time (from days to years), depending on the tissue and organism size (Peterson & Fry, 1987; Fry, 1988; Pinnegar & Polunin, 2000; Vander Zanden et al., 2003, 2015; Thomas & Crowther, 2015).
Statistical analyses to test ecological hypotheses
Changes in trophic structure can be analyzed based on the relative trophic position of the organisms that make up the community, comparing environments with and without predators. In the present work, a community approach was used to comprehensively analyze these changes. The analyses were based on the metrics developed by Layman et al. (2007a), which allow the study of the structure, function (for example, energy flow among species), and food web dynamics using Bayesian statistical tools (Jackson et al., 2011).
Layman’s community-wide metrics were used to describe the trophic structure of the studied shallow lakes (Layman et al., 2007a). These metrics use stable isotope compositions of the food web components to describe their structure. The first four metrics are measures of the total extent of space within the δ13C–δ15N biplot space, providing information about trophic diversity throughout the community. The two final metrics reflect the relative position of the species to each other within the niche space and can be used to estimate the degree of trophic redundancy. The six parameters are described below. Total area (TA) is the area of the convex hull that encompasses all groups within the food web, giving a measure of niche area. The δ13C range (CR) is the distance between the groups with the most positive and most negative δ13C values (i.e., maximum–minimum δ13C). High CR values indicate the existence of basal values with alternative and contrasting carbon sources, which provide niche diversification at the base of the food web. The δ15N range (NR) is the distance between the groups with the most positive and most negative δ15N values (i.e. maximum–minimum δ15N). High NR values suggest more trophic levels and, therefore, a higher degree of trophic diversity. Mean distance to centroid (CD) is the average Euclidian distance of each community component to the centroid, giving an indication of the average trophic diversity. Mean nearest neighbor distance (MNND) measures the density of species packing: small MNND means increased trophic redundancy. Finally, standard deviation of nearest neighbor distance (SDNND) measures the evenness of species packing: lower SDNND means more even distribution of species, suggesting an increased trophic redundancy as different groups have more similar trophic ecologies. In addition, the standard ellipse area (SEA) was calculated as a measure of the "central isotopic niche". SEA provides a more accurate comparison of the isotopic niche of a community than the parameter TA, since it is less sensitive to the effects of extreme values and therefore always increases with sample size (Jackson et al., 2011).
The analyses to test the first hypothesis were based on the general model proposed by Layman shown in Fig. 2. This model proposes various scenarios of change in the trophic positions of the community through the introduction of a predator, analyzing the redistribution of the relative distances of the community components—in the space defined by the stable isotope values—depending on the relative position that the introduced species will occupy. However, this model does not take into account the possible re-distributions of prey induced by the presence of the predator, although the metrics can capture these changes. Therefore, the present study proposes a complementary scenario focused on prey (Model B, Fig. 2d) and also when the predator is introduced (Model A, Fig. 2c). In the proposed scenario, the presence of the predator induces changes in the spatial distribution and/or diet of the prey, which could be analyzed based on the same metrics proposed by Layman et al. (2007a).
Scenario models of possible changes in a food web due to the species introduction (circles). a the trophic web of a hypothetical community composed of three species. b and c, the resulting configuration is shown after the introduction of a top predator and an omnivorous species, respectively. d an alternative scenario: changes due to the relative position of the species with respect to the introduced species (as in c), the changes are generated by the relocation of one of the prey species (triangles) of the introduced species (circles). a–c scenarios are adaptations of the model proposed by Layman et al. (2007a) (with permission of the author), d scenario is an alternative model proposed in the present study. Legends show the expected changes for every metric proposed by Layman in each of the proposed scenario
Based on Layman's conceptual model (Fig. 2c) and the alternative scenario (Fig. 2d) proposed in the present study, the following predictions were expected.
The presence of the rainbow trout:
(1.a) will increase the trophic niche area (TA/SEA) because in these lakes the trout is omnivorous and has a pelagic-benthic diet. It can force its main prey to inhabit and use resources from benthic/littoral zones (13C enrichment), increasing the range of δ13C values (Fig. 2d).
(1.b) will increase the CR since trout can force its main prey to inhabit and use resources from benthic/littoral zones (13C enrichment, Fig. 2d).
(1.c) will have the same NR because the distance between species with higher or lower values of δ15N will not change, considering that the trout is an omnivorous species and would not increase the number of trophic levels (Fig. 2c).
(1.d) will increase the CD, given the greater use of benthic/littoral resources (Fig. 2d).
(1.e) the potential occurrence of C and D scenarios generates uncertainty about the directionality of MNND and SDNND metrics. Under scenario C, the species will be increasingly grouped within the food web, generating redundancy among the groups given by the trout's omnivory (Fig. 2c). In contrast, under scenario D, increases in these metrics could be observed due to a greater diversity of resource use (Fig. 2d).
While the above-mentioned analyses are focused on evaluating trophic changes at the community level, the occurrence of alterations in the trophic position of a particular prey would provide information about specific processes involved on these community changes. In this context, our second hypothesis postulates that trout introduction generates changes in the spatial distribution and/or diet of its main prey, affecting their trophic position and forcing the use of alternative carbon sources. Therefore, differences in the carbon isotopic compositions of amphipods between stocked and fishless lakes would indicate changes in resource use, for example, pelagic vs. benthic/littoral sources of carbon. By assuming the hypothesis 2, the prediction is that in stocked lakes, amphipods will have isotopic values with greater dependence on littoral resources (higher values of δ13C and lower values of δ15N) than in fishless lakes, since this prey could increase the use of benthic/littoral zones as an anti-predatory response. On the other hand, significant changes in δ13C values are expected for other collected groups that represent secondary prey of trout, although occasionally cladocerans and copepods are intensely preyed by trout (Lancelotti et al., 2015).
General description of the food webs
Biplots (δ13C and δ15N) were examined to determine the relative position of the organisms on the graph and to evaluate the occurrence of feeding pathways that might be differentiated with isotopic analyses. In lakes of the basaltic plateaus, POM is an adequate indicator of the δ13C of the pelagic pathway (Lancelotti, 2009), while snails (Lymnaea) have been identified as reliable indicators of the δ13C of the benthic/littoral zone (Adami, 2016). For instance, the biofilm that also is a potential candidate integrates organic matter from both benthic and pelagic origin, presenting great variability in these aquatic environments (Lancelotti, 2009). Although snails were not present in our samples, our interpretations of the trophic structure were based on the relative position of the organisms only in relation to the pelagic pathway, considering POM as the basal level for this zone, with the theoretical expected trophic enrichment (3.4 ‰ δ15N and 1.0 ‰ δ13C). The raw δ15N values were used to compare the trophic position of the studied groups of organisms.
In order to interpret the results in the context of pelagic and littoral/benthic food webs, we used an arbitrary threshold enrichment value to define the benthic pathways from the pelagic pathways. Stable isotopes analyses conducted in 12 lakes of the Strobel (Lancelotti, 2009), and one lake of the Buenos Aires Plateau (47°09ʹ S, 71°14ʹ W; Adami, 2016), indicated a difference of at least 10 ‰ in δ13C between benthic and pelagic pathways. This value was arbitrarily used in this study to determine the placement of organisms to one of these zones.
Data analysis
Six metrics (TA, CR, NR, CD, MNND and SDNND) proposed by Layman et al. (2007a) were calculated for the studied lakes and compared between lakes using the package SIBER (Parnell & Jackson, 2013) in R (R Core Team, 2016). This analysis is adequate to compare communities that differ in δ13C range and in sample sizes (Jackson et al., 2011). The results of these tests were a posteriori distribution of the estimates of each metric, providing a measure of uncertainty and allowing comparisons with statistical validation. These results were graphically compared between lakes based on the visual analysis of the confidence intervals (CIs), where the degree of overlap between the Bayesian distributions was used as an indication of similarities/dissimilarities among lakes (Abrantes et al. 2014). Based on the aforementioned distributions, the corrected standard ellipse area (SEAc) was also calculated using the package SIBER (Jackson et al., 2011) in R (R Core Team, 2016). It allows bias corrections due to small sample sizes and differences in the groups analyzed number (Jackson et al., 2011). Finally, the overlap between the SEA among lakes was calculated as a quantitative measure of the trophic similarity between the communities.
To test hypothesis 2, we generated CIs of the linear distance of the average value of δ13C of amphipods with respect to the average value of δ13C obtained for POM, and for the best represented organisms in the samples. The null hypothesis was that there are no significant differences in the mean distance between amphipods and the groups analyzed between fishless and stocked shallow lakes. The analyses were also performed to evaluate variations in δ15N values. The CIs for each isotope separately were generated from Bootstrap resampling (Efron & Tibshirani, 1993).
Results
Food webs
The aquatic communities of the four analyzed lakes were dominated by three groups of crustaceans: amphipods (Hyalella sp. of different sizes), copepods of the species Parabroteas sarsi (Daday 1901) and Boeckella spp. (two size classes), and cladocerans, which were represented by several species of Daphnia and Alona (only in stocked lakes). Leeches (Hirudinea) were also relatively abundant in these environments. Insect larvae of the Leptophlebiidae family (Order Ephemeroptera) and Chironomidae family (Order Diptera) were only collected in fishless lakes, with an average of 18 individuals per sample.
The food webs of the four lakes showed two groups of organisms clearly differentiated based on stable isotope compositions: a strictly pelagic group (P. sarsi and POM) and a group of organisms more related to littoral resources (amphipods, Alona, Ephemeroptera and Chironomidae larvae). Meanwhile, Boeckella, Daphnia and Hirudinea were associated with both groups (Fig. 3).
δ13C and δ15N values (mean and standard deviation) of the most abundant groups in the four studied lakes. The dotted lines indicate the expected enrichment under the hypothetical model (3.4 ‰ δ15N and 1.0 ‰ δ13C), with the particulate organic matter (POM) considered as the basal level of the pelagic pathway. Amph: Amphipods; BIOF: Biofilm; Ephem: Ephemeroptera; sizes: large (L), medium (M), and small (S)
In stocked lakes, the communities had more positive δ15N (4.7–16.9 ‰) than in fishless lakes (0.6 – 11.7 ‰) and also showed greater ranges of δ13C values (− 26.7 ‰ to − 14.4 ‰), with respect to fishless lakes (-30 ‰ to -18.1 ‰) (Fig. 3 and Online Resource 1, Table 1_ SI). The POM δ13C values did not show substantial variations between the two groups of lakes, ranging between − 27.5 ‰ and − 26.2 ‰ in fishless lakes, and between − 26.6 and − 25.9 ‰ in stocked lakes. The values of δ13C of biofilm in fishless lakes ranging from − 25.7 ‰ to − 23.9 ‰, whereas in the stocked lake R2 was -26.3 ‰. Unfortunately, carbonate contaminated the single biofilm sample from the stocked lake R3.
We estimated qualitative trophic positions based on nitrogen isotopic compositions of an organism group relative to other organisms in the lakes. For example, in the pelagic environment, P. sarsi occupied the highest trophic position with δ15N values of 11.5 ± 3.0 ‰ with more negative values of δ13C with respect to the rest of the organisms (Fig. 3 and Online Resource 1, Table 1_ SI). Both large and small sized Boeckella spp. were at intermediate trophic positions with δ15N of 9.3 ± 3.7 and 8.4 ± 3.9 ‰, respectively, although the smaller species were more positive in δ13C than the larger ones. Daphnia spp. δ15N values suggested a lower trophic position: 7.2 ± 3.0 ‰ with relatively low δ13C values. Hirudinea were located at 10.2 ± 2.2 ‰ δ15N, with δ13C values more positive with respect to the pelagic pathway. Within the fishless lakes, the insect larvae (both Ephemeroptera and Diptera) δ15N were 3.2 ± 1.4 ‰, having more positive δ13C with respect to POM, indicating these organisms were at a very low trophic level. Alona species, which appeared only in one stocked lake (R2), were located at the same trophic level as Boeckella (small size) and close to the Hirudinea group (Fig. 3 and Online Resource 1, Table 1_ SI). Littoral and pelagic amphipods occupied the lowest trophic position with respect to the rest of the organisms and in some cases had more negative δ15N than the POM sample we measured. Amphipods collected from pelagic and littoral zones showed similar δ13C values, related to the littoral zone in all four lakes (Fig. 3 and Online Resource 1, Table 1_SI).
Although size and sex can affect stable isotope values in fish, no relationship between size and δ15N or δ13C was found for rainbow trout measuring 39.4 to 66.7 cm (Online Resource 1, Table 2_SI). Trout were located at a trophic level higher than that of P. sarsi based on nitrogen isotope compositions. The values of δ13C and δ15N varied from -24.2 to -18.1 ‰ and 14.5 to 17.2 ‰, respectively (Online Resource 1, Table 2_SI), showing an intermediate location between the pelagic and littoral pathway. In lake R3, the CPUE for trout was twice (1 fish per net h−1) than in lake R2 (Online Resource 1, Table 2_SI).
Community metrics
Isotope ratios of organisms from these lakes showed substantial differences in the position of the isotopic niche between fishless and stocked lakes (Fig. 4). These two groups were clearly separated and did not spatially overlap. The areas calculated as proxy for the total isotopic niche size (TA), and the standard ellipse areas (SEA), showed a clear shift regarding the isotopic values of the stocked lakes, toward more positive values of δ13C and δ15N. R3 (with fish) and R26 (without fish) lakes presented the highest values of niche areas 43.7 ‰2 and 56.7 ‰2, respectively (Fig. 4 and 5a), and corrected SEA (SEAc), 21.5 ‰2 and 17.4 ‰2, respectively (Fig. 5b).
Density plots showing confidence intervals of the: a areas of the total isotopic niche (TA), b standard ellipse areas (SEA) occupied by the species in each lake, c mean distance of the nearest neighbor (MNND) and d standard deviation of nearest neighbor distance (SDNND). Black circles are TA and SEA means, the boxes indicate 50%, 75% and 95% of confidence intervals, from wider to thinner. Red crosses are the sample size-corrected SEA size (SEAc)
Although TA/SEA values did not show significant differences among the studied lakes, R22 (fishless lake) presented the smallest total occupied niche area compared to the rest of the water bodies. The TA and SEA overlapped at 95% CIs between lakes with and without fish (Fig. 5.a, 5.b and Online Resource 3), indicating that the community responded with the type A model, which is focused on the novel species added to the system (Table 2). The standard ellipses only overlapped between the two defined groups of lakes, accounting for 5.19 ‰2 and 7.66 ‰2 for fishless and stocked lakes, respectively (Fig. 4).
All other metrics analyzed did not show substantial differences between fishless and stocked lakes (Table 2), as shown by the large overlaps in CIs (Online Resource 3). While the highest CD was obtained in one of the stocked lakes (R3 = 3.8 ‰) (Online Resource 2, Fig. 1a_SI), this value was not significantly different to the other lakes, and no differences were observed among the rest of the water bodies either. Likewise, the δ13C ranges showed greater variation within the groups defined by the presence/absence of the trout than between them (Online Resource 2, Fig. 1.b_SI). Lakes R3 (stocked) and R26 (fishless) showed the greatest range of δ13C, 9.4 ‰ and 10.1 ‰, respectively. There were no significant differences in the δ15N ranges among any of the studied lakes (Online Resource 2, Fig. 1.c_SI). Finally, the MNND and SDNND metrics showed higher values in fishless lakes (Fig. 5.c and 5.d), although only significant differences were observed between R26 (fishless) and the rest of the lakes. These metrics supported model A, which is focused on the introduced trout to the system (Table 2).
Amphipod analysis
In fishless lakes, amphipods were collected from both littoral and pelagic zones. In the stocked lake R2, no amphipods were found in the water column, and only six individuals of different sizes were found in the stocked lake R3 (Online Resource 1, Table 1_SI). Amphipods collected in fishless and stocked lakes occupied the first trophic position (Online Resource 1, Table 1_SI). Amphipods collected in pelagic and littoral zones had δ13C values related to the littoral zone in the four analyzed lakes (Fig. 3). However, in fishless lakes, large amphipods had more negative δ13C than the small and medium size individuals (closer to the pelagic zone; Figs. 3 and 6). In contrast, in stocked lakes the δ13C values of the amphipods of all sizes clearly were more positive than fishless lakes (Figs. 3 and 6).
δ13C and δ15N values of small (crosses), medium (triangles) and large (circles) amphipods collected in the pelagic (red) and littoral (black) zones of lakes with fish (R2, R3) and without fish introduction (R22, R26). Dashed, dotted and solid red lines correspond, respectively, to large, medium, and small sizes of pelagic amphipods of R22. Notice that the x axes are no alienated and represent different range of values
Large amphipods showed shorter distances from the mean value of δ13C relative to every other compared organism (Daphnia, Boeckella, and P. sarsi) and POM, than medium size amphipods (Online Resource 2, Fig. 2a_SI), indicating higher dependence on pelagic resources for larger sized amphipods. Additionally, these distances, for large and medium size amphipods, were significantly higher in stocked lakes than in fishless lakes (Online Resource 2, Fig. 2a_SI), indicating that amphipods in stocked lakes comparatively depend more on littoral resources.
A clear pattern has not been found among lakes with and without fish in relation to the δ15N distances values, when amphipods were compared with the other analyzed groups (Online Resource 2, Fig. 2b_SI). In general, shorter distances between the δ15N means were observed among larger amphipods vs. the selected groups (POM, Daphnia, Boeckella, and P. sarsi). Particularly, a smaller δ15N distance between amphipods (large and medium sizes) vs. POM, Daphnia and P. sarsi was found in the R2 lake (stocked).
Discussion
Comparative studies of fishless and stocked lakes of the Strobel Plateau have shown the consequences at several organizational levels in the aquatic communities, due to rainbow trout introductions. Stocked lakes revealed changes in the planktonic communities: higher phytoplankton biomass, greater cyanobacteria abundances (Izaguirre & Saad, 2014), and also the reduction of the abundance and size spectrum of zooplankton, with smaller Daphnia spp. and Boeckella spp. (Lancelotti et al., 2017). Alternatively, higher total zooplankton and macrozooplankton biomass were registered in fishless lakes (Izaguirre et al., 2018). Additionally, shifts in the community macroinvertebrate structure were observed with differences mainly driven by the relative abundance of Ostracoda, Hyalella, Chironomidae, and Haliplus (Lopez et al., 2021). In particular, Lancelotti et al. (2017) showed the absence of pelagic amphipods in stocked lakes and high abundance of these organisms in fishless ones. In line with these results, the findings presented here provide evidence that the presence of fish exerts a marked influence on the food web structure and nutrient cycles of the analyzed lakes, which could have significant consequences for the receiving communities, as it is discussed below.
Food webs and community metrics
Insect larvae appeared only in fishless lakes, indicating a direct effect of trout on the community composition. Similar results were reported for the pelagic organisms in this lake system, in which trout were responsible for the reduction of abundance and prey size of their main prey (Lancelotti et al., 2017). The extinction and reductions of aquatic insects due to fish introduction have been extensively covered in literature, as is the case of Ephemeroptera populations (Luecke, 1990; Knapp et al., 2001; Caudill, 2003; Finlay & Vredenburg, 2007; Epanchin et al., 2010). Additionally, the reduction of the abundance of insect larvae could suppress the grazing pressure on the periphytic community, enhancing the development of algal biomass, and changing algal species composition (Townsend, 2003).
The differences in δ13C and δ15N values between fishless and stocked lakes can be explained by multiple factors related to natural variations among the analyzed environments and by the presence of trout. Our results are in line with bottom-up processes in which the regulation of food web components occurs via the type of primary producer or by limiting primary producers’ nutrient resources (Pace et al., 1999). The higher concentrations of total nutrients, DOC, and higher conductivity, in addition to high basal δ15N values observed in the stocked lakes, could be related to the presence of fish that would increase organic matter resuspension and nutrient recycling, as well as natural processes related to water levels, drainage patterns, and bottom substrate composition (Vanni, 1996; Schindler & Scheuerell, 2002; Feniova et al., 2016). In shallow systems, fish are known to increase turbidity significantly by bioturbation of the sediment, as well as by indirect mechanisms that influence algal biomass and phosphorus concentrations (Jeppesen, 1998). The microbial decomposition of organic matter results in the initial release of isotopically light nitrogen, such as ammonia, and the residual organic matter becomes isotopically heavier (Macko & Estep, 1985). Decomposition results in an accumulation of isotopically heavy organic nitrogen at the deeper depths, which can then be incorporated by the periphyton, grazing invertebrates and finally their predators (i.e., fish) (Estep & Vigg, 1985).
Trout modify the trophic structure of the aquatic community of stocked lakes in the Strobel Plateau. The strong overlap of the standard ellipses occupied by the species of each lake within each set of lakes (fishless and stocked) denoted the isotopic niches superposition that reveal the similarity of the trophic structure among the studied lakes with a clear shift in values of δ15N and δ13C between stocked vs. fishless lakes. The variations in some metrics between the two stocked lakes could be related with trout density and predation pressure and limnological differences between lakes.
Among the Layman’s community metrics, the MNND and SDNND were the most informative about the trout’s effects, showing lower values on lakes with trout, meaning that the organisms belonging to lakes with O. mykiss had a more uniform species distribution, which would indicate greater trophic redundancy. This finding could be related to the fact that trout and P. sarsi, a pelagic predatory/omnivorous copepod (Hansson & Tranvic, 2003), occupied a similar trophic role, resulting in an unaltered trophic dimension but with higher number of components. These results are coherent with model A, which is focused on the introduced species, and not on the main prey (amphipods). Similar results were obtained in studies based on these metrics. For example, Layman et al. (2007b) reported that the introduction of a new predator caused a reduction of prey species, because of a homogenization of energy flow pathways to top predators. Vidal et al. (2020) suggested that fish feeding in the pelagic zone in Tasmanian lakes caused the reduction of trophic diversity of zooplankton.
Amphipod analysis
In the absence of trout, amphipods depend on both littoral and pelagic resources, as previously reported by Lancelotti (2009), based on stable isotopes analyses in more than 12 water bodies. Additionally, the available information indicates that in this lake system, amphipods feed mainly on diatoms and bacteria from periphyton and detritus (Lucchini, 1975; Casset et al., 2001). In the present study, we infer that the diet of amphipods changes throughout their life cycle, with a greater use of pelagic resources in the larger size classes and a greater dependence on littoral/benthic resources in the smaller sizes.
Our study showed that trout generate changes in the spatial distribution and/or diet of its main prey, including the use of alternative carbon sources. In stocked lakes amphipods showed higher dependency on benthic/littoral resources, which could respond to anti-predatory responses to the presence of trout. Similar trophic changes in prey were observed in other aquatic systems with fish introduction (Vander Zanden et al., 1999b). The most noticeable changes in the relative position of amphipods, between fishless and stocked lakes, were observed in relation to values of POM and organisms at the most basal levels (Daphnia and small Boeckella), which are secondary prey of trout (Bandieri, 2011; Lancelotti et al., 2015). This result also supports the hypothesis of changes in spatial distribution of amphipods as an anti-predatory response, considering that the secondary prey did not change the spatial distribution in comparison with amphipods.
In contrast, the δ15N distances between amphipods and the analyzed groups did not produce conclusive results regarding the presence/absence of trout. The statistical distances calculated between amphipods vs. large Boeckella and vs. P. sarsi showed higher variations when fishless and stocked lakes were compared. This is an expected result, considering that these latter groups are secondary prey for trout and belong to higher trophic levels than Daphnia and small Boeckella.
Changes in spatial distribution of prey following the introduction of exotic fish have been extensively reported in the literature (Gliwicz & Rowan, 1984; Fraser & Huntingford, 1986; Stuart-Smith et al., 2008; Martínez-Sanz et al., 2010; Milano et al., 2010; Vidal et al., 2020). Like amphipods, invertebrate communities typically use macrophyte beds or rocks as refuges against predators (McIntosh, 2000; Stuart-Smith et al., 2008). Behavioral research in New Zealand streams addressed the effect of Salmo trutta Linnaeus 1758 introductions on grazing invertebrates, confirming that they spend less time out in the open and feed closer to refuges when trout are present (Townsend, 2003). Experimental studies demonstrated that galaxiids, a group of fish that inhabit most aquatic systems of Patagonia, change their habitat use in the presence of non-native predatory fish, tending to spend less time in open waters, shifting to macrophyte or rock beds while simultaneously reducing their swimming activity (Stuart-Smith et al., 2008; Milano et al., 2010).
Potential impact on the Hooded Grebe
The fishless and stocked lakes of the Strobel Lake Plateau provided an opportunity to carry out this “natural experiment” elucidating essential ecological questions about rainbow trout introductions and to provide basic information to support management actions. The results of the present investigation reinforce the concerns about the effects of trout on primary reproductive habitat and on the survival of the Hooded Grebe. In particular, amphipods are among the most abundant and largest prey in these environments, and among the most important food items recorded for this endangered species (Fjeldså, 1986). The lack of amphipod availability in the water column could involve a higher cost to obtain alternative prey and also could drive grebes to use less nutritive prey, affecting their performance from a bioenergetic point of view. Besides, the reduction of prey availability has been proposed as a potential cause of the abandonment of an active colony of these birds (Fjeldså, 1986). In this context, the effects of trout introductions could be particularly drastic in lakes with Hooded Grebe colonies, where the survival of chicks strongly depends on food availability.
The macrophytes, strongly dominated by Myriophyllum quitense Kunth in this aquatic system, are the building material and platform for the nests of Hooded Grebe, representing also their main foraging area (Roesler, 2016). The increase of nutrient recirculation caused by trout could modify the limnological characteristics of lakes, changing from a clear water/macrophyte dominated state to a turbid condition (Scheffer et al., 1993; Scheffer and van Nes, 2007), favoring the development of phytoplankton and adversely affect the growth of aquatic vegetation. The importance of these conditions is supported by the higher values of total nitrogen and δ15N observed in the studied stocked lakes compared to fishless ones. In addition, the decrease in water level on lakes may also enhance the adverse effects of fish introduction in this lake system (Izaguirre et al., 2018).
Based on the available evidence of the effects of trout on this aquatic system, and their potential consequences at community and ecosystem level, it is recommended that non-native trout populations be removed to guarantee the conservation of Hooded Grebe. Furthermore, it is necessary to prevent new fish introductions. There is an ongoing initiative of trout eradication in several lakes of the Lake Strobel Plateau, which involve non-governmental organizations, aquaculture producers, researchers, governmental authorities, and FAO/UN, which could represent a first significant step to change the productive matrix of this aquatic system, to a more sustainable scheme in terms of habitat conservation.
Data availability
Not applicable.
Code availability
Not applicable.
References
Abrantes, K. G., A. Barnett & S. Bouillon, 2014. Stable isotope based community metrics as a tool to identify patterns in food web structure in east African estuaries. Functional Ecology 28: 270–282.
Adami, M. A., 2016. Estructura trófica de una laguna clave para la reproducción del Macá Tobiano (Podiceps gallardoi). Graduate Thesis. Universidad Nacional de la Patagonia San Juan Bosco.
Arismendi, I., B. Penaluna, D. Gomez-Uchida, C. Di Prinzio, D. Rodríguez-Olarte, F. M. Carvajal Vallejos & P. Savaria, 2019. Trout and char of South America. Trout and char of the world 279–311.
Bandieri, L., 2011. Dieta y crecimiento de la trucha arco iris (Oncorhynchus mykiss) en lagunas de la meseta del Lago Strobel, Santa Cruz. Graduate Thesis. Universidad Nacional de la Patagonia.
Barrie, A. & S. J. Prosser, 1996. In Barrie, A. & S. J. Prosser (eds), Mass Spectrometry of Soils Marcel Dekker Inc, New York: 1–46.
Beisner, B., D. Haydon & K. Cuddington, 2003. Alternative stable states in ecology. Frontiers in Ecology and the Environment 1: 376–382.
Beltrán, J., C. Bertonatti, A. Johnson, A. Serret & P. Sutton, 1992. Actualizaciones sobre la distribución, biología y estado de conservación del Macá Tobiano (Podiceps gallardoi). Hornero 13: 193–199.
BirdLife International, 2021. Species factsheet: Podiceps gallarhttp://www.birdlife.org. 17 Feb 2021.
Blanco, S., 2001. Estudio Experimental sobre la Influencia de los Nutrientes en la Ecología Trófica de los Peces de una Laguna Somera. Master Thesis. Universidad de León, España.
Blanco, S., M. Fernández-Aláez & E. Bécares, 2008. Efficiency of top-down control depends on nutrient concentration in a Mediterranean shallow lake: a mesocosm study. Marine and Freshwater Research 59: 922–930.
Carpenter, S. R. & J. F. Kitchell, 1993. The Trophic Cascade in Lakes, Cambridge University Press, Cambridge, England:
Casset, M., F. Momo & A. D. N. Giorgi, 2001. Dinámica poblacional de dos especies de anfípodos y su relación con la vegetación acuática en un microambiente de la cuenca del río Luján (Argentina). Ecología Austral 11: 79–85.
Caudill, C. C., 2003. Empirical evidence for nonselective recruitment and a source-sink dynamic in a mayfly meta population. Ecology 84: 2119–2132.
Caut, S., E. Angulo & F. Courchamp, 2009. Variation in discrimination factors (δ15N and δ13C): the effect of diet isotopic values and applications for diet reconstruction. Journal of Applied Ecology 46: 443–453.
Covich, A. P., M. A. Palmer & T. A. Crowl, 1999. The role of benthic invertebrate species in freshwater ecosystems. BioScience 49: 119–127.
Efron, B. & R. Tibshirani, 1993. An introduction to the Bootstrap, Chapman & Hall, New York:
Epanchin, P. N., R. A. Knapp & S. P. Lawler, 2010. Nonnative trout impact an alpine-nesting bird by altering aquatic-insect subsidies. Ecology 91: 2406–2415.
Estep, M. L. & S. Vigg, 1985. Stable carbon and nitrogen isotope tracers of trophic dynamics in natural populations and fisheries of the Lahontan Lake System, Nevada. Canadian Journal of Fisheries and Aquatic Sciences 42: 1712–1719.
Feniova, I. Y., V. G. Petrosyan, M. Rzepecki, I. Kostrzewska- Szlakowska, N. S. Zilitinkevicz, A. V. Krylov, N. N. Majsak, V. I. Razlutskij & A. R. Dzialowski, 2016. Experimental impacts of fish on small and large Cladocerans under eutrophic conditions. Inland Water Biology 9: 375–381.
Ferrari, M. C. O., B. D. Wisenden & D. P. Chivers, 2010. Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. Canadian Journal of Zoology 88: 698–724.
Finlay, J. C. & V. T. Vredenburg, 2007. Introduced trout sever trophic connections in watersheds: consequences for a declining amphibian. Ecology 88: 2187–2198.
Fjeldså, J., 1986. Feeding ecology and possible life history tactics of the Hooded Grebe Podiceps Gallardoi. Ardea 74: 40e58.
France, R. L., 1995a. Carbon-13 enrichment in benthic compared to planktonic algae: food web implications. Marine Ecology Progress Series 115: 205–207.
France, R. L., 1995b. Differentiation between littoral and pelagic food webs in lakes using stable carbon isotopes. Limnology and Oceanography 40: 1310–1313.
Fraser, D. E. & F. A. Huntingford, 1986. Feeding and avoiding predation hazard: the behavioral response of the prey. Ethology 73: 56–68.
Fry, B., 1988. Food web structure on Georges Bank from stable C, N and S isotopic compositions. Limnology and Oceanography 33: 1182–1190.
Gliwicz, Z. M. & M. G. Rowan, 1984. Survival of Cyclops abyssorum tatricus (Copepoda, Crustacea) in alpine lakes stocked with planktivorous fish. Limnology and Oceanography 29: 1290–1299.
Hansson, L.- A. & L. J. Tranvik, 2003. Food webs in sub-Antarctic lakes: a stable isotope approach. Polar Biology 26: 783–788.
Hecky, R. E. & R. H. Hesslein, 1995. Contributions of benthic algae to lake food webs as revealed by stable isotope analysis. Journal of the North American Benthological Society 14: 631–653.
Izaguirre, I. & J. F. Saad, 2014. Phytoplankton from natural water bodies of the Patagonian Plateau. Advances in Limnology 309–319.
Izaguirre, I., J. Lancelotti, J. F. Saad, S. Porcel, M. C. Marinone, I. Roesler & M. C. Dieguez, 2018. Influence of fish introduction and water level decrease on lakes of the arid Patagonian plateaus with importance for biodiversity conservation. Global Ecology and Conservation 14: e00391. https://doi.org/10.1016/j.gecco.2018.e00391.
Jackson, A. L., R. Inger, A. C. Parnell & S. Bearhop, 2011. Comparing isotopic niche widths among and within communities: SIBER - Stable Isotope Bayesian Ellipses in R. Journal of Animal Ecology 80: 595–602.
Jeppesen, E., 1998. The ecology of shallow lakes - Trophic interactions in the pelagial. PhD Thesis. Silkeborg: National Environmental Research Institute.
Jeppesen, E., J. P. Jensen, M. Søndergaard, T. Lauridsen, L. J. Pedersen & L. Jensen, 1997. Top-down control in freshwater lakes: the role of nutrient state, submerged macrophytes and water depth. Hydrobiologia 342(343): 151–164.
Jeppesen, E., J. P. Jensen, C. Jensen, B. Faafeng, P. Brettum, D. Hessen, M. Søndergaard, T. Lauridsen & K. Christoffersen, 2003. The impact of nutrient state and lake depth on top-down control in the pelagic zone of lakes: study of 466 lakes from the temperate zone to the Arctic. Ecosystems 6: 313–325.
Knapp, R. A., 2005. Effects of nonnative fish and habitat characteristics on lentic herpetofauna in Yosemite National Park, USA. Biology Conservation 121: 265–279.
Knapp, R. A., R. Matthews & O. Sarnelle, 2001. Resistance and resilience of alpine lake fauna to fish introductions. Ecological Monographs 71: 401–421.
Lancelotti, J. L., 2009. Caracterización limnológica de lagunas de la Provincia de Santa Cruz y efectos de la introducción de Trucha Arco iris (Oncorhynchus mykiss) sobre las comunidades receptoras. PhD Thesis. Universidad Nacional del Comahue.
Lancelotti, J. L., L. M. B. Bandieri & M. A. Pascual, 2015. Diet of the exotic rainbow trout in the critical habitat of the threatened Hooded Grebe. Knowledge and Management of Aquatic Ecosystems 416: 1e11.
Lancelotti, J. L., M. C. Marinone & I. Roesler, 2017. Rainbow Trout effects on zooplankton in the reproductive area of the critically endangered Hooded Grebe. Aquatic Conservation: Marine and Freshwater Ecosystems 27: 128–136. https://doi.org/10.1002/aqc.2629.
Lancelotti, J. L., N. L. Pessacg, I. C. Roesler & M. A. Pascual, 2020. Climate variability and trends in the reproductive habitat of the critically endangered Hooded Grebe. Aquatic Conservation: Marine and Freshwater Ecosystems 30: 554–564. https://doi.org/10.1002/aqc.3240.
Layman, C. A., D. A. Arrington, C. G. Montaña & D. M. Post, 2007a. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 88: 42–48.
Layman, C. A., J. P. Quattrochi, C. M. Peyer & J. E. Allgeier, 2007b. Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecology Letters 10: 937–944. https://doi.org/10.1111/j.1461-0248.2007.01087.x.
Lopez, M. E., 2015. Estructura de las comunidades pleustonicas de macroinvertebrados en lagunas de la Patagonia extra-andina (Provincia de Santa Cruz) con y sin introducción de peces. Graduate Thesis. Universidad de Buenos Aires, Buenos Aires.
Lopez, M. E., J. Saad, I. Izaguirre, M. C. Marinone & M. S. Fontanarrosa, 2021. Aquatic macroinvertebrates from littoral vegetated lakes in arid Patagonia: The influence of fish introduction on community structure. Aquatic Conservation: Marine and Freshwater Ecosystems. https://doi.org/10.1002/aqc.3522.
Lucchini, L., 1975. Estudio ecológico preliminar de las diatomeas perifíticas y bentónicas como alimento de anfípodos lacustres (Lago Cardiel Provincia de Santa Cruz). PHYSIS Buenos Aires 34: 85–97.
Luecke, C., 1990. Changes in abundance and distribution of benthic macroinvertebrates after introduction of cutthroat trout into a previously fishless lake. Transactions of the American Fisheries Society 119: 1010–1021.
Macko, S. A. & M. L. F. Estep, 1985. Microbial alteration of stable nitrogen and carbon isotopic compositions of organic matter. Organic Geochemistry 6: 787–790.
Martínez-Sanz, C., F. García-Criado, F. Aláez & M. del Camino, 2010. Effects of introduced salmonids on macroinvertebrate communities of mountain ponds in the Iberian system of Spain. Limnetica 29(2): 221–232.
McIntosh, A. R., 2000. Habitat- and size-related variations in exotic trout impacts on native galaxiid fishes in New Zealand streams. Canadian Journal of Fisheries and Aquatic Sciences Sci 57: 2140–2151.
Milano, D., M. Lozada & H. E. Zagarese, 2010. Predator-induced reaction patterns of landlocked Galaxias maculatus to visual and chemical cues. Aquatic Ecology 44: 741–748.
Ortubay, S., V. E. Cussac, M. Battini, J. Barriga, J. Aigo, M. Alonso, P. J. Macchi, M. Reissig, J. Yoshioka & S. F. Fox, 2006. Is the decline of birds and amphibians in a steppe lake of northern Patagonia a consequence of limnological changes following fish introduction? Aquatic Conservation: Marine and Freshwater Ecosystems 16: 93–105.
Pace, M. L., 1993. Heterotrophic microbial processes. In Carpenter, S. R. & J. F. Kitchell (eds), The Trophic Cascade in Lakes Cambridge University Press, Cambridge, U.K.: 252–277.
Pace, M. L., J. J. Cole, S. R. Carpenter & J. F. Kitchell, 1999. Trophic cascades revealed in diverse ecosystems. Trends in Ecology and Evolution 14: 483–488.
Panza, J. L. & M. R. Franchi, 2002. Magmatismo Basáltico Cenozoico Extrandino. In Geología y Recursos Naturales de Santa Cruz. Relatorio del XV congreso Geológico Argentino, Haller, M.J. (ed). El Calafate 201–236.
Parnell, A. C. & A. L. Jackson, 2013. SIAR: Stable Isotope Analysis in R (Package version 4.2).
Pereyra, F. X., L. Fauqué & E. F. González Díaz, 2002. Geomorfología. In Geología y Recursos Naturales de Santa Cruz. Relatorio del XV Congreso Geológico Argentino, Haller, M.J. (ed). El Calafate 325–352.
Peterson, B. J. & B. Fry, 1987. Stable isotopes in ecosystem studies. Annual Review of Ecology and Systematics 18: 293–320.
Pinnegar, J. K. & N. V. C. Polunin, 2000. Differential fractionation of δ13C and δ15N among fish tissues: implications for the study of trophic interactions. Functional Ecology 13: 225–231.
Polis, G. A., 1999. Why are parts of the world green? Multiple factors control productivity and distribution of biomass. Oikos 83: 3–15.
Polis, G. A., A. L. W. Sears, G. R. Huxel, D. R. Strong & J. Maron, 2000. When is a trophic cascade a trophic cascade? Trends in Ecology & Evolution 15: 473–475.
Porcel, S., 2020. Estructura de las comunidades planctónicas en lagos de mesetas basálticas de Patagonia: influencia de variables ambientales y la introducción de peces exóticos. PhD Thesis. Universidad de Buenos Aires, Buenos Aires.
Porcel, S., G. Chaparro, M. C. Marinone, J. F. Saad, J. Lancelotti & I. Izaguirre, 2020. The role of environmental, geographical, morphometric and spatial variables on plankton communities in lakes of the arid Patagonian plateaus. Journal of Plankton Research 42(2): 173–187.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: models, methods, and asumptions. Ecology 83: 703–718.
R Core Team, 2016. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.Rproject.org/.
Rautio, M. & W. F. Vincent, 2007. Isotopic analysis of the sources of organic carbon for zooplankton in shallow subarctic and artic waters. Ecography 30: 77–87.
Reissig, M., C. Trochine, C. Queimaliños, E. Balseiro & B. Modenutti, 2006. Impacts of fish introduction on planktonic food webs in lakes of the Patagonian Plateau. Biological Conservation 132: 437e447.
Roesler, I., 2016. Conservación del Macá Tobiano (Podiceps gallardoi): factores que afectan la viabilidad de sus poblaciones. PhD Thesis, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires.
Roesler, I., S. Imberti, H. Casañas, B. Mahler & J. C. Reboreda, 2012. Hooded Grebe Podiceps gallardoi population decreased by eighty per cent in the last twenty-five years. Bird Conservation International 22: 371–382.
Roesler, I., L. Fasola, L. Martín & F. González Táboas, 2020. Proyecto Macá Tobiano: Informe de temporada 2018–2019. Buenos Aires, Aves Argentinas & Ambiente Sur. https://www.avesargentinas.org.ar/noticia/proyecto-macá-tobiano-informe-final-2018-2019
Rothhaupt, K. O., 2000. Plankton population dynamics: food web interactions and abiotic constraints. Freshwater Biology 45: 105–109.
Sarakinos, H. C., M. L. Johnson & J. M. Vander Zanden, 2002. A synthesis of tissue-preservation effects on carbon and nitrogen stable isotope signatures. Canadian Journal of Zoology 80: 381–387.
Schaus, M. H., M. J. Vanni & T. E. Wissing, 2002. Biomass dependent diet shifts in omnivorous gizzard shad: implications for growth, food web, and ecosystem effects. Transactions of the American Fisheries Society 131: 40–54.
Scheffer, M., 1998. Ecology of Shallow Lakes, Chapman & Hall, New York:
Scheffer, M. & E. van Nes, 2007. Shallow lakes theory revisited: various alternative regimes driven by climate, nutrients, depth and lake size. Hydrobiologia 584: 455–466.
Scheffer, M., S. H. Hosper, M. L. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology and Evolution 8: 275–279.
Schindler, D. E. & M. D. Scheuerell, 2002. Habitat coupling in lake ecosystems. Oikos 98: 177–189.
Smyntek, P. M., M. A. Teece, K. L. Schulz & S. J. Thackeray, 2007. A standard protocol for stable isotope analysis of zooplankton in aquatic food web research using mass balance correction models. Limnology and Oceanography 52: 2135–2146.
Stuart-Smith, R. D., R. W. G. White & L. A. Barmuta, 2008. A shift in the habitat use pattern of a lentic galaxiid fish: an acute behavioural response to an introduced predator. Environmental Biology of Fishes 82: 93–100.
Syväranta, J., A. Martino & D. Kopp, 2011. Freezing and chemical preservatives alter the stable isotope values of carbon and nitrogen of the Asiatic clam (Corbicula fluminea). Hydrobiologia 658: 383–388.
Teece, M. A. & M. L. Fogel, 2004. Preparation of ecological and biochemical samples for isotope analysis. In Handbook of Stable Isotope Analytical Techniques, Volume-I, pp. 177–202. Amsterdam, The Netherlands: Elsevier
Thomas, S. M. & T. W. Crowther, 2015. Predicting rates of isotopic turnover across the animal kingdom: a synthesis of existing data. Journal of Animal Ecology 84(3): 861–870.
Townsend, C. R., 2003. Individual, population, community, and ecosystem consequences of a fish invader in New Zealand streams. Conservation Biology 17: 38–47.
Tyler, T., W. J. Liss, L. M. Ganio, G. L. Larson, R. Hoffman, E. Deimling & G. Lomnicky, 1998. Interaction between introduced trout and larval salamanders (Ambystoma macrodactylum) in high-elevation lakes. Conservation Biology 12: 94–105.
Vander Zanden, J. M. & G. Rasmussen, 1999. Primary consumer 13C and 15N and the trophic position of aquatic consumers. Ecology 80: 1395–1404.
Vander Zanden, M. J. & Y. Vadeboncoeur, 2002. Fishes as integrators of benthic and pelagic food webs in lakes. Ecology 83: 2152–2161.
Vander Zanden, M. J., B. Shuter, N. Lester & J. Rasmussen, 1999a. Patterns of food chain length in lakes: a stable isotope study. The American Naturalist 154: 406–416.
Vander Zanden, M. J., J. M. Casselman & J. B. Rasmussen, 1999b. Stable isotope evidence for the food web consequences of species invasions in lakes. Nature 401: 464–467.
Vander Zanden, M. J., S. Chandra, B. C. Allen, J. E. Reuter & C. R. Goldman, 2003. Historical food web structure and restoration of native aquatic communities in the Lake Tahoe (California–Nevada) Basin. Ecosystems 6: 274–288.
Vander Zanden, M. J., M. K. Clayton, E. K. Moody, C. T. Solomon & B. C. Weidel, 2015. Stable isotope turnover and half-life in animal tissues: a literature synthesis. PLoS ONE 10(1): 1–16.
Vanni, M. J., 1996. Nutrient transport and recycling by consumers in lake food webs: implications for algal communities. In Polis, G. A. & K. O. Winemiller (eds), FoodWebs: Integration of Patterns and Dynamics Chapman & Hall, New York: 81–95.
Vanni, M. J., 2002. Nutrient cycling by animals in freshwater ecosystems. Annual Review of Ecology and Systematics 33: 341–370.
Vidal, N., C. Trochine, S. L. Amsinck, L. A. Barmuta, K. S. Christoffersen, M. Ventura, T. Buchaca, F. Landkildehus, S. A. Hardie, M. Meerhoff & E. Jeppesen, 2020. Interaction between non-native predatory fishes and native galaxiids (Pisces: Galaxiidae) shapes food web structure in Tasmanian lakes. Inland Waters. https://doi.org/10.1080/20442041.2020.1724047.
Wetzel, R. G. & G. E. Likens, 2000. Limnological Analyses, Springer-Verlag, New York:, 429.
Acknowledgements
We thank the logistic support and the facilities provided by the Rodriguez family (Ea. Lago Strobel) for allowing us to survey lakes and for providing us logistical support in the Strobel Plateau. We thank the collaboration of Dr. C. Sabio y García, Dr. M. C Dieguez, and the technicians and volunteers of the Proyecto Macá Tobiano/Programa Patagonia (Aves Argentinas) during the 2017 field trip to the Strobel Plateau. We also thank the EDGE institute (UC Riverside) for their support of the project that allowed this research to be possible, particularly we thank Bobby Nakamoto for his advice in isotopic analysis and Dr. Ying Lin for assisting with isotopic analyses. We are grateful to the anonymous reviewers for their constructive comments, which greatly improved the article.
Funding
Argentinean Funds for Scientific and Technical Investigations of Argentina (FONCYT PICT 2013-0794); Proyecto Macá Tobiano (Aves Argentinas/ Ambiente Sur) – Programa Patagonia Aves Argentinas (by Toyota Argentina, PAE, BirdLife International Preventing Extinction Programme (B. Olewine and S. & B. Thal), ICFC Canada, Toyota Environmental Activities Grant Programme, ZSL-EDGE, Secretaría de Ambiente de Santa Cruz and Patagonia Inc.); Technical Advisory Agreement IEASA – CONICET – Macá Tobiano, and the Tonolli Award 2017 awarded by the International Society of Limnology (SIL). The isotope analyses were funded by the University of California’s EDGE Institute.
Author information
Authors and Affiliations
Contributions
All authors contributed to the manuscript preparation. SP collected samples in the field; she was responsible for preparing and weighing samples for isotopes analyses, performed statistical analyses, and wrote the manuscript. MLF provided significant methodological suggestions and analyzed isotope data. II and JL were the directors of the project. JL and IR were responsible for the study design. JL also participated in the field trip and contributed to the statistical analyses.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest. All authors have approved the manuscript and agree with its submission to Hydrobiologia.
Additional information
Handling Editor: Pauliina Louhi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Porcel, S., Fogel, M.L., Izaguirre, I. et al. Effect of rainbow trout introductions on food webs in lakes of the arid Patagonia. Hydrobiologia 849, 2057–2075 (2022). https://doi.org/10.1007/s10750-022-04848-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04848-2