Abstract
Different aquatic organisms have different reactions to environmental variations due to their different ways of reaction traits. Understanding the effect of hydrological disturbance on lake from the perspective of multiple aquatic organisms is important for lake management. Poyang Lake (PYL) experiences severe hydrological disturbance under the effects of flood pulse during the rainy season. In this study, we analyzed the responses of phytoplankton, zooplankton, and macroinvertebrates to hydrological disturbance during the rainy season in PYL. Flood pulse determined the spatial variation in turbidity (Turb), transparency (Trans), dissolved oxygen (DO), total phosphorus (TP), and soluble reactive phosphorus. Physical factors (Turb, Trans, conductivity) explained phytoplankton (9.6%) and zooplankton (15%) community variation, and the combination of physical and nutrient factors explained macroinvertebrate (3.8%) community variation. Cyclotella sp., Attheya zachariasi, and Melosira ambigua were the keystone taxa of phytoplankton, and these taxa were driven by Turb and Trans. Brachionus angularia, Polyarthra vulgaris, Filinia longiseta, and Diffugia globulosa were the keystone taxa of zooplankton and entire aquatic organisms, which contributed a lot to maintain the biological community stability possibly through the food web. These keystone taxa were stimulated by high Trans, DO, dissolved organic carbon (DOC) and nitrate (NO3−). We found habitat conditions (Turb, Trans and DO) were the prominent factors influencing the aquatic organism structure in strong hydrological disturbance environment. For the keystone taxa, both habitat conditions (i.e., DO) and exogenous nutrient inputs (i.e., DOC and NO3−) caused significant effect. This study provides new insights into the holistic response of multiple aquatic communities to flood pulse as well as the role of keystone species in maintaining community stability, which could guide the conservation and management of seasonal lake ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the high flow period, hydrological disturbance could cause sediment suspension, decreasing light availability and photosynthesis, and accelerating the releases of sediments’ nutrients (Ding et al. 2019; Luo et al. 2016; Ni et al. 2015), which could further result in variations in densities and biomass of the aquatic organisms. Understanding the response of aquatic organisms to flood pulse is vital for keeping the seasonal lake ecosystems in balance (Li et al. 2019; Naselli-Flores 2000; Rodrigues et al. 2019; Sevindik et al. 2015; Tavares et al. 2019). In the lentic ecosystems, phytoplankton, zooplankton, and macroinvertebrates are usually the most sensitive to the hydrological fluctuations and flow regimes (Li et al. 2019; Meng et al. 2017; Qu et al. 2019a). These aquatic organisms also have been used widely as important biological indicators for aquatic ecosystem stability (Vezi et al. 2019; Wang et al. 2007; Zhang et al. 2018a).
Phytoplankton, zooplankton, and macroinvertebrates often have different responses to short-term environmental variations due to their different ways of response traits and adaptive mechanisms (Southwood 1977). However, evaluating the effect of hydrological disturbance on lake ecosystems from the perspective of multiple aquatic organisms is rare. The concentration of suspended solid could control the composition of phytoplankton community (David et al. 2005), because the increase of turbidity (Turb) caused by hydrological disturbance could decrease the water light permeability, which would decline the phytoplankton production and change the community structure (Albay and Akçaalan 2003; Atici and Alas 2012). Phytoplankton usually supply the zooplankton with food and nutrients through the food web connections (Su et al. 2017), thus, the changes of phytoplankton could influence on zooplankton. Zooplankton play an important role in transforming energy from lower trophic organisms to higher ones, and their nitrogen excretion is the dominant nutrient source for benthic organisms (Li et al. 2019; Protopapa et al. 2019; Rodrigues et al. 2019; Wang et al. 2007), suggesting that change of zooplankton also have impact on macroinvertebrates. In addition, the suspended solids variation caused by hydrological disturbance could also directly affect macroinvertebrate community structure by changing their habitat conditions (Franco et al. 2012; Zhang et al. 2017b). The mutual interactions between phytoplankton, zooplankton, and macroinvertebrates are complex and important in lake ecosystems (Ger et al. 2016; Li et al. 2019; Tavares et al. 2019; Wang et al. 2007). Therefore, analyzing simultaneous responses of phytoplankton, zooplankton, and macroinvertebrates to environmental variations in disturbance environment is very important for a profound understanding of the function and eventually guiding management of aquatic ecosystems (Li et al. 2019).
Poyang Lake (PYL) is a typical Yangtze-connected and active seasonal lake, subjecting to strong fluctuations of hydrological characteristics that are affected by the regulation of Yangtze River (Ding et al. 2019; Feng et al. 2013; Yao et al. 2015). PYL plays a vital role in supporting and regulating environmental functions of aquatic ecosystems, maintaining ecological security in the Yangtze River watershed, and maintaining regional and global biodiversity (Wang et al. 2019b; Zhang et al. 2017a). During the rainy season, floods from Yangtze River flowing into the lake, which caused extreme hydrological disturbance (Shankman et al. 2006; Zhao et al. 2011). The curve of water level fluctuation is unimodal, and the water level raises regularly from January to August (Ding et al. 2019; Wang and Liang 2015). The process of water level rise usually caused the high concentrations of suspended particular, low level of Trans, and nutrients variations etc., which could influence ecosystem stability (Jiang and Guo 2019; Wang and Liang 2015; Xiang and Zhou 2011). In PYL, the drastic changes of hydrological regimes under flood pulse causing the changes of sediment regimes, which have potential threats to the lake ecological integrity and biodiversity (Wang et al. 2019a). Although there are numerous studies on the response of individual aquatic organisms to water level fluctuations and nutrient enrichment in PYL (Liu et al. 2019; Ni et al. 2015; Ren et al. 2019a, b; Yang et al. 2018), the response of multiple aquatic organisms on hydrological disturbance still unclear. Thus, it is important to fill the knowledge gaps about revealing the simultaneously response of multiple aquatic organisms to environmental variations.
In this study, we examined the responses of the aquatic organisms in terms of phytoplankton, zooplankton, and macroinvertebrates on flood pulse by using ordination and network analyses. The aims of this study are: (1) to illuminate the spatial variations of the aquatic organisms, (2) to illuminate the responses of the aquatic organisms on spatial variations of environmental variables caused by flood pulse, and (3) to identify the key environmental variables influencing the aquatic organism community structure and distribution. This study could provide basic and important information for protection and management of Yangtze-connected shallow lakes.
Methods
Area of study
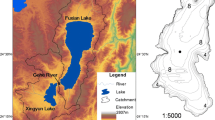
PYL (115°47′–116°45′ E, 28°22′–29°45′ N) is one of the two lakes retaining free connection with Yangtze River. As a shallow seasonal lake, PYL is a water-carrying and throughput lake and its hydrological regimes mainly controlled by Yangtze River and its five tributaries (Feng et al. 2012). The seasonal floods of its tributaries and Yangtze River lead to large seasonal lake hydrological disturbance (Fand et al. 2011; Zhao et al. 2011). From October to March, the water from the lake flow reversely to Yangtze River, causing the lake area to shrink (< 1000 km2), water level to decrease (< 10 m), and form a meandering and narrow channel (Shankman et al. 2006; Zhang et al. 2018b). In the wet seasons from April to September, the floods from Yangtze River flow into PYL, resulting in the water level reach a peak (> 15 m) with the lake surface > 4000 km2 (Liu et al. 2020b; Shankman et al. 2006; Wang and Liang 2015). During the rainy season, the frequent water exchange in PYL could result in hydrological disturbance at water exchange areas (Ding et al. 2019; Lu et al. 2018), and the annual average water exchange cycle is 19d (Fand et al. 2011; Zhao et al. 2011). The spatial variations of hydrological disturbance intensity in different lake areas could result in profound spatial dynamics in biological and physicochemical properties of the lake.
Field sampling and analyzing
In total, 52 sampling sites were selected in the wet seasons (August in 2016) (Fig. 1). YSI multiparameter instrument (professional plus) was used to measure water temperature (WT), pH, conductivity (Cond), and dissolved oxygen (DO) in situ. Van Dorn water sampler was used to collect water samples at the depth of 0.5 m. Water depth (WD) was measured using Sounding Rod with scale, Trans was measuring using Secchi disk, and Turb was measured using Handheld Turbidity Meter (2100Q, Hach). Total nitrogen (TN) and nitrate (NO3−) were measured using chromatography. The indophenol colorimetric method was used to measure ammonium (NH4+). Soluble reactive phosphorus (SRP) and total phosphorus (TP) were measured using the ammonium molybdate method. Shimadzu TOC Analyzer (TOC-VCPH, Shimadzu Scientific Instruments, Columbia, Maryland) was conducted to analyze dissolved organic carbon (DOC).
Phytoplankton were collected at 52 sites. 500 ml water was collected into a plastic jar and fixed by 4% formaldehyde. 1.5% liquid mixture of potassium iodide and iodine were used to dye and diagnose phytoplankton. After the sedimentation in laboratory (72 h), we obtained the residual concentrated sample (35 mL) by syphon off the supernatant using 2-mm-diameter hose. Upright microscope (Olympus BX41) was used to taxonomical determine and count at × 400 magnifications. We classified the phytoplankton base on the lowest taxonomical level. The definition of higher taxonomic species names was determined based on previous literatures (Guiry and Guiry 2016). The density was expressed as number of cells per litter (× 10–6 cells/L), and biomass was estimated on the basis of tables with the standard weight of the phytoplankton organisms (Fedorov 1979). Zooplankton were collected at 52 sites (Fig. 1). 50L water was collected and filtered through a mesh net (25 μm). Zooplankton in the net were carefully rinsed and preserved in a 100 ml bottle with 4% formaldehyde. Based on the concentration of zooplankton samples, the samples were diluted into 1-5L solutions. The dissecting microscope was used to count and identify species at the lowest taxa level based on specific literatures (Shen 1999; Wang 1961; Zhou and Chen 2010). Abundance was estimated by counting the organisms using an optical microscope (Macêdo et al. 2018). Macroinvertebrates were collected at 52 sites (Fig. 1) using a Ponar Grab sampler (1/32 m2) with three replicates. We disturbed the substrate using a shovel to ensure the macroinvertebrates could floating and directed into the net. The 40 mm mesh was used to filter and rinse the samples, and all samples were preserved into a 500 ml jar with 4% formaldehyde. The samples were used to identify macroinvertebrates at the lowest taxa level (mainly to genus) in laboratory based on available references (Brinkhurst 1986; Merritt and Cummins 1996; Morse et al. 1994).
Statistical analyses
The spatial variation of environmental factors and biological parameters of the aquatic organisms was visualized in ArcGIS 10.2. The relationships between environmental factors and biological parameters were analyzed using Spearman rank correlation analyses (SPSS Statistic 23). The UPGMA (Unweighted Pair Group Method with Arithmetic Mean) cluster was used to analyze the relationships within the aquatic organisms (Vegan 2.5–3 in R 3.5.2). Redundancy analyses (RDA) were generated to reveal the aquatic organismal community distributions under the effect of environmental stresses (Vegan 2.5–3 in R 3.5.2). Monte Carlo permutations (p < 0.05) were used to select a set of environmental factors that had significant and independent effects on aquatic organisms, and the not significant factors were eliminated from the final RDA. Variance partitioning analysis (VPA) was applied to calculate the contributions of each environmental variables (Vegan 2.5–3 in R 3.5.2).
The relationships between each pairwise of taxa were calculated using Spearman correlation analyses (Hmisc 4.0–1 in R 3.5.2). Only significant correlations (p < 0.05 adjusted by FDR correction) were used to construct the network. Networks were analyzed in Cytoscape software (version 3.6.1) with the edge-weighted spring-embedded layout. Nodes represent the OTUs and edges represent correlations relationship. The gray edges and red edges represent the positive and negative correlations, respectively. Topological properties (e.g., network density, clustering coefficient, characteristic path length, centralization, and heterogeneity) were calculated using Network Analyzer application 2.6 in Cytoscape. Network Randomizer application in Cytoscape (version 1.1.3) was used to generate the random networks, which have the same number of nodes and edges with the real network. Modular structure and groups of highly interconnected nodes were estimated using MCODE 1.3 in Cytoscape (Banerjee et al. 2016; Halary et al. 2010; Ibekwe et al. 2019). Modularity value was calculated in the Cluster Maker in Cytoscape (Newman 2006). Keystone species were calculated based on high degree, high closeness centrality and low betweenness centrality (85% accuracy) (Banerjee et al. 2018; Berry and Widder 2014).
Results
Hydrochemical variables
Environmental factors were divided into 3 categories including physical factors, nutrients and organic matter, and stoichiometry (Table 1). Turb ranged from 2.47 to 201.67 (CV: 1.73) following Trans (0.15 ~ 2.30, CV: 0.47), WD (2.50 ~ 7.50, CV: 0.20), and DO (3.68 ~ 8.50, CV: 0.15). In nutrients and organic matter category, TP have distinct spatial variability (CV: 1.16) following NH4+ (CV: 0.88) and SRP (CV: 0.63). Turb, TP, and SRP had the similar spatial patterns and peaked in eastern part of the lake (Fig. 2). There were also similar spatial patterns between Trans, DO, TN, NO3−, and DOC, which peaked in the western part of the lake (Fig. 2). Turb positively associated with TP and SRP, while Trans negatively associated with them (Table 2). However, TN, NO3−, and NH4+ were not significantly correlated with Turb and Trans.
Aquatic organism community composition
Totally 101 phytoplankton species affiliated with 7 phylum, 67 zooplankton species belonging to 4 main groups, and 27 macroinvertebrate taxa affiliated with 14 families (Fig. S1). Cyanophyta (89.43%) was the dominant phylum of phytoplankton, following Chlorophyta (6.08%) and Bacillariophyta (3.19%) (Fig. 3a).
Moreover, Phormidium tenuis (33.69%), Pseudanabaenaceae sp. (32.14%), and Aphanizomenon sp. (5.53%) were the dominant species of phytoplankton. Cladocera (60.74%) was the dominant group of zooplankton following Rotifera (17.99%), and Copepoda (13.77%) (Fig. 3b). Bosmina longirostris (56.89%), Cyclops larva (10.54%), and Polyarthra vulgaris (6.22%) were the dominant species of zooplankton. Corbicula fluminea (38.46%), Parafossarulus striatulus (22.45%), and Limnodrilus hoffmeisteri (16.63%) dominated the macroinvertebrates (Fig. 3c).
Spatial distribution of environmental factors and aquatic organisms
Phytoplankton, zooplankton, and macroinvertebrates showed distinct spatial variations (Figs. 3 and 4). Spatial patterns of phytoplankton and zooplankton (density, biomass, and biodiversity) were opposite to that of Turb, TP, and SRP. High density and biomass of phytoplankton and zooplankton were mainly distributed at the areas with low Turb and low content of TP and SRP. While the high density and biomass of macroinvertebrates mainly distributed at the areas with high Turb (Figs. 2 and 4).
The biological parameters (density, biomass, richness, and diversity) of phytoplankton were positively correlated with Cond, DO, Trans, C/P, and N/P, while negatively correlated with Turb, TP, and SRP (Table 3). Zooplankton biological parameters also were positively associated with DO, Trans, TN, NO3−, DOC, C/P, and N/P, and negatively correlated with Turb, TP, and SRP (Table 3). Macroinvertebrate biological parameters were positively associated with WD, while negatively associated with C/P and N/P (Table 3).
Environmental factors related to variation of the aquatic community composition
For phytoplankton communities, the first two axes accounted for 73.76% (axis 1: 45.11%; axis 2: 28.65%). Trans, Cond, Turb, TP, SRP, WD, C/P, and N/P were the significant factors influenced phytoplankton community distribution (p < 0.05, Fig. 5a). For zooplankton communities, the first two axes accounted for 71.67% (axis 1: 58.42%; axis 2: 13.25%, Fig. 5b). Zooplankton community distribution was influenced by pH, Turb, DO, Cond, Trans, WD, TN, DOC, TP, SRP, and C/P (p < 0.05). Macroinvertebrate communities at first axis (38.86%) were mainly influenced by Cond, Trans, C/P, N/P, Turb, WD, TP and SRP (axis 1: 38.86%; axis 2: 23.72%, Fig. 5c). The first two axes accounted for 72.51% of entire aquatic organisms (axis 1: 56.93%; axis 2: 15.58%, Fig. S2). Trans, Cond, DO, C/P, N/P Turb, TP and SRP defined the aquatic organism distribution at the first axis. Physical factors (9.6%, 15%, 1.8%, and 15.3%) driven the distribution of phytoplankton, zooplankton, macroinvertebrate, and entire aquatic organisms (Fig. 5 a, b and Fig. S2b). The combination of physical factors, nutrients, and stoichiometry driven the macroinvertebrate community distribution (3.8%) (Fig. 5c).
Networks and connectedness
Topological parameters of networks were calculated to analyze the interrelationships between species (Table S1). Clustering coefficient, clustering centralization, and average path length of the real networks were higher than that of random networks (Fig. 6 and Table S1). The positive edges accounted for 90.8% of total edges in phytoplankton network (Fig. 6a and Table S1). The clustering coefficient, centralization, and heterogeneity of phytoplankton network were 0.305, 0.136, and 0.813, respectively (Table S1). Based on the values of degree, centrality and betweenness centrality, Bacillariophyta (Cyclotella sp., Attheya zachariasi, Melosira ambigua and Rhizosolenia sp.) were defined as keystone taxa of the phytoplankton (Table 4). Trans and Turb were closely related with the keystone taxa (p < 0.05, Table S2). MCODE structured five highly interconnected sub-modules with scores ranged from 2.7 to 3.5 (Fig. 6a). Trans and Turb influenced the MCODE1 which mainly composed by the keystone taxa (Rhizosolenia sp. and Staurastrum gracile).
Network analyses showing connectedness among taxa of (a) phytoplankton and (b) zooplankton communities. Each node represents unique taxa, gray solid line represents positive correlation, and the red solid lines represent negative relationship. Variables in gray boxes show various environmental factors. Macroinvertebrate communities did not construct a community network due to low clustering coefficient
Zooplankton network comprised 70 nodes (61 species and 9 environmental factors) and 203 edges (positive correlation accounted for 86.2%) (Fig. 6b and Table S1). Network clustering coefficient, network centralization and heterogeneity were 0.363, 0.272, and 0.992, respectively (Table S1). Brachionus angularia, Polyarthra vulgaris, Pedalia mira, Filinia longiseta, and Difflugia globulosa were the keystone taxa of zooplankton communities (Table 4). DO, Turb, DOC, and Trans were the key environmental factors influencing zooplankton communities (Table S2). MCODE algorithm generated five highly interconnected sub-modules with scores ranged from 3 to 9 (Fig. 6b and Table 4). DO driven the MCODE1 that consisted with 9 taxa and 43 edges, and DOC driven the MCODE3 that consisted with 4 taxa and 5 edges (Table 4).
Although macroinvertebrate species not construct the effective co-occurrence network in the present study, the entire aquatic organism network was analyzed to explore the interaction among phytoplankton, zooplankton, and macroinvertebrates (Fig. 7). This network was made of 178 nodes (167 taxa and 11 environmental factors) and 398 edges (positive correlation accounted for 93.97%). Clustering coefficient, centralization and heterogeneity of network were 0.482, 0.083, and 0.814, respectively (Table S1). Moreover, Polyarthra vulgaris, Brachionus angularia, Filinia longiseta and Trichocerca pusilla also were the keystone taxa of the aquatic entire organisms. MCODE analyses structured nine highly interconnected sub-modules with scores ranged from 2.7 to 8 (Fig. 7). Turb, Trans, DO, and DOC also were the dominant environmental factors driven the entire aquatic organism communities (Table S2), and DO mainly driven the MCODE1 (Fig. 7).
Network analyses showing connectedness among taxa of three aquatic organism communities and environmental factors. Each node represents species, gray solid lines represent positive linear relationship, and the red solid lines represent negative linear relationship. Environmental parameters are presented by gray boxes
Discussion
The influence of hydrological disturbance on nutrients
PYL is an “active seasonal lake”, the frequent water exchange largely affect lake sediments suspension (Baldwin and Mitchell 2000) and lake material transformation processes (Feng et al. 2013; Yao et al. 2015). Suspended sediments are the primary factor affecting the Turb and Trans of water (Li et al. 2009). In recent years, Turb has been increasingly used as a surrogate for suspended sediments concentration (Marchant et al. 2015; Minella et al. 2008). Thus, in the present study, Turb and Trans were used as the representative parameters which indirectly reflect hydrological disturbance condition in PYL. Yangtze River inflow into the PYL during the flood season, which could cause the strong hydrological disturbance at water exchanged area in the lake (Shankman et al. 2006; Zhao et al. 2011). In the present study, most of sampling sites at the eastern region were located in the water exchange area, which resulted in the high Turb of these sampling sites. There were positive relationships between Turb, WD, TP, and SRP, which indicated that the release of phosphorus from the sediments could influence the phosphorus in PYL under the strong flood pulse during rainy season.
Previous studies suggested that endogenous replenishment was the main source of phosphorus in PYL during rainy season (Ding et al. 2019; Liu et al. 2020a; Wang and Liang 2015), the strong agitation of water exchange on sediment could promote the endogenous release of phosphorus in summer (Deng et al. 2011; Ni et al. 2015; Song et al. 2007; Xiang and Zhou 2011). Previous studies also indicated that there were high concentration of phosphorous in eastern tributaries of PYL (Rao River and Xinjiang River) (Wang and Liang 2015), which indicated that the inflow of these tributaries also increased the content of phosphorus at lake eastern region. DO negatively associated with Turb and WD, and positively correlated with Trans in PYL. In shallow lakes, many processes could control the variability of DO contents including net primary production, suspended particulate concentration, and gaseous oxygen dissolution (Tan et al. 2018; Yin et al. 2014). In general, the photosynthesis of phytoplankton is the major source of DO, and the process of water diffusivity, mixing and overturning could influence DO concentration in water through limiting phytoplankton photosynthesis. In addition, there were weak hydrological disturbance at the western part of the lake caused low Turb and high Trans. Moreover, most of the western tributaries were urban rivers that flows through Xichang city, which caused these tributaries might contained more nutrients (Ding et al. 2019). In rainy season, these western tributaries could carry large amounts of nutrients into the lake, which caused high content of nitrogen and DOC in lake western region. Moreover, no significant relationships between Turb vs. nitrogen and Turb vs. DOC indicated that hydrological disturbance not influenced nitrogen and DOC in the lake, which could further imply that the high nitrogen and DOC in the western part of lake was mainly from the tributaries and lakeshore exogenous. Some studies also confirmed that anthropogenic activities and external input from tributaries and soil were the key source of nitrogen in PYL (Deng et al. 2011; Liang et al. 2018).
Response of aquatic organisms to hydrological disturbance
There are distinct seasonal and spatial variations of the aquatic organism communities in PYL (Hu 2014; Huang et al. 2018; Nie et al. 2018). In our study, Turb was the determinant factor negatively influencing phytoplankton community composition and distribution (Table 3). Previous study on Yangtze-connected lakes also confirmed that underwater light penetration was the crucial factor affecting the growth of phytoplankton (Liu et al. 2019, 2017). Density and biomass of phytoplankton are mainly depending on temperature, light permeability, and available of nutrients (Albay and Akçaalan 2003; Atici and Alas 2012; Celik and Ongun 2008), and high Turb could suppress the growth of phytoplankton by reducing the light penetration (Liu et al. 2017). In addition, it has been demonstrated that available nutrients play important roles in increasing phytoplankton communities (Carpenter et al. 1998; Li et al. 2019; Liu et al. 2017; Wang et al. 2007). In the present study, phosphorus negatively associated with the density, biomass, and richness of phytoplankton in PYL (Table 3), which implied that the phosphorus (TP and SRP) under the hydrological the disturbance was the key factors regulated phytoplankton communities. Thus, in a lake with frequent hydrological disturbance, the environment change caused by the disturbance contributed more to the phytoplankton community than the pure nutrient.
DO, Turb, phosphorus, and nitrogen also were the key factors affecting zooplankton communities in PYL. Estuary areas also have strong hydrological disturbance, and Turb and DO in the areas determine the zooplankton community structure (David et al. 2005; Farhadian and Pouladi 2014; Vezi et al. 2019). In general, phosphorus, nitrogen, and DOC are major environmental factors determining the structure variations of zooplankton communities in lake ecosystems (Degerman et al. 2018; Gorokhova et al. 2016; Li et al. 2019; Lorenz et al. 2019). Based on the food web structure, these environmental factors usually bottom-up influence zooplankton by regulating phytoplankton growth (Li et al. 2019; Vezi et al. 2019). Besides the direct effect, Turb, DO, and phosphorus could indirectly affect zooplankton community composition by restricting phytoplankton growth in PYL. Although DOC and nitrogen (TN and NO3−) were not significantly associated with phytoplankton communities, these nutrients were the key factors impacted on zooplankton growth (Degerman et al. 2018; Faithfull et al. 2012). There was positively relationship between DO and pH, and the appropriate concentration of DO and pH is favorable for the growth of zooplankton (Wang et al. 2018). In the present study, pH was the key factor affected the zooplankton communities, which was consistent with that pH could regulated rotifers community composition (Akin et al. 2010; Atici and Obali 2006, 2010; Wang et al. 2018; Zhuang 1993). Nevertheless, both physical factors and exogenous nutrients (including nitrogen and organic matters) determined the zooplankton community structures.
In the present study, Corbicula fluminea, Parafossarulus striatulus, and Limnodrilus hoffmeisteri dominated the macroinvertebrate assemblages. In general, Corbicula fluminea usually was the dominant species in the areas with high hydrological disturbance (Cai et al. 2016; Franco et al. 2012; Yuan et al. 2002), because these areas with sand substrates and high disturbance environment favor for these species growth (Cai et al. 2010; Yang et al. 2018). In our study, Corbicula fluminea mainly located in the eastern part of PYL, where have frequent water exchange and strong hydrological disturbance. In addition, in our study, Parafossarulus striatulu, Bellamya aeruginosa and Limnodrilus hoffmeisteri mainly lived in the western part of PYL, where have weak hydrology disturbance and high contents of DOC and nitrogen. Parafossarulus striatulu and Bellamya aeruginosa usually prefer to live in stable environment with mud substrates (Gong and Xie 2001; Yang et al. 2018), and Limnodrilus hoffmeisteri mainly survive in stable water with low Turb and high nutrient contents (Gong and Xie 2001; Zhang et al. 2017b). However, different to phytoplankton and zooplankton, WD, C/P and N/P were the key factors influencing density, biomass, richness, and diversity of macroinvertebrates (Table 3), furthermore, these variable were closely associated with Turb. Previous studies also indicated that sediment properties and WD are determinant factors influencing macroinvertebrate communities (Cai et al. 2016; Meng et al. 2017; Qu et al. 2019a; Wang et al. 2011).
Response of connectedness aquatic organisms to hydrological disturbance
Network analyses can provide unique insights into highly complex aquatic organisms interactions, such as the correlations of different taxa within the assemblages (Qu et al. 2019b; Ren et al. 2019a), the associations between species and environmental variables (Banerjee et al. 2016), as well as identify the keystone taxa of aquatic biota communities (Banerjee et al. 2018; Qu et al. 2019a). The modular structures of phytoplankton and zooplankton network did not follow taxonomic classification (Table S1), which indicated that these communities were mainly driven by environmental factors not phylogeny (Banerjee et al. 2016; Burke et al. 2011). In each network, nearly 90% positive associations between the organisms, which also indicated that these communities have potential functional interdependencies among taxa under disturbance environmental stresses (Mandakovic et al. 2018).
Although Cyanophyta was the dominant phylum of phytoplankton, Bacillariophyta (Cyclotella sp., Attheya zachariasi, Melosira ambigua and Rhizosolenia sp.) was the keystone taxa. These keystone taxa could play crucial roles in maintaining biological community stability, and their disappearance may cause break apart of modules and networks (Banerjee et al. 2018; Berry and Widder 2014; Xue et al. 2018). The close relationship between the keystones taxa and Trans suggesting that underwater penetration light intensity was the limited factor for the growth of keystone taxa (Furusato et al. 2004; Ozkan and Rorrer 2017; Yang and Jin 2008). High Turb could limit the growth of phytoplankton by declining underwater light penetration (Liu et al. 2017; Ni et al. 2015). All of these keystone phytoplankton species mainly lived in the western part of PYL, where have weak hydrological disturbance and high Trans. There were strong underwater light penetration at the areas with high Trans, which could promote the growth of phytoplankton communities (Yang and Jin 2008). In addition, rotifer was the keystone taxa of zooplankton and entire aquatic organisms, which suggested that rotifer play a vital role in maintaining the connectedness of the aquatic organisms in PYL. Rotifer could be affected by multiple factors including N and P (Liang et al. 2017), available carbon (DOC) (Degerman et al. 2018; Schälicke et al. 2018), food quantity and quality (Schälicke et al. 2018; Seymour et al. 2017; Souza et al. 2019). In PYL, rotifer positively related with NO3− and DOC, and previous study also confirmed that rotifer favor to live in high nutrient environment (Sha et al. 2018; Snell and Joaquim-Justo 2007). Moreover, pH was the key factor regulated zooplankton communities, and rotifer usually were the dominant species when water pH above 7 (Atici and Obali 2010; Zhuang 1993). Based on food web structure, previous study demonstrated that phytoplankton could bottom-up regulate rotifer community structure (Rougier et al. 2005).
In conclusion, we have examined the effects of hydrological disturbances on phytoplankton, zooplankton, and macroinvertebrate communities in PYL. Hydrological disturbance was strong in the eastern part of the PYL and weak in the western part of the lake. The spatial variations in Turb, Trans, Cond, DO, P, C/P and N/P were regulated by flood pulse. Turb, Trans, and DO were the key factors influencing the plankton community composition and distribution. In addition, Bacillariophyta (Cyclotella sp., Attheya zachariasi, Melosira ambigua and Rhizosolenia sp.) was the keystone taxa of phytoplankton. Rotifera (Brachionus angularia, Polyarthra vulgaris, Pedalia mira and Filinia longiseta) was the keystone taxa of both zooplankton and entire aquatic organisms. Most of these keystone taxa favor to live in the western part of the lake with low hydrological disturbance area, and high Trans and DO promoted the growth of these taxa. The macroinvertebrate data not construct effective co-occurrence network. The spatial distribution of macroinvertebrate dominant taxa that depends on WD, C/P and N/P indicated that the dominant taxa have adapted to live at sandy substrates areas with strong hydrological disturbance. Habitat conditions (Turb, Trans, and DO) and concomitant change of phosphorus as well as the exogenous nutrient inputs (i.e., DOC and NO3−) were the crucial factors determining the aquatic organism structures and spatial distribution in the strong hydrological disturbance environment. This study provided the understanding of the holist response of aquatic organisms on flood pulse and the role of keystone species in maintaining community stability, which could be used to protect and manage seasonal lake ecosystems under strong hydrological disturbance.
References
Akin BS, Atıci T, Katircioglu H, Keskin F (2010) Investigation of water quality on Gökçekaya dam lake using multivariate statistical analysis, in Eskişehir. Turk Environ Earth Sci 63:1251–1261. https://doi.org/10.1007/s12665-010-0798-6
Albay M, Akçaalan R (2003) Factors influencing the phytoplankton steady state assemblages in a drinking water reservoir (Ömerli reservoir, Istanbul). Hydrobiologia 502:85–95
Atici T, Alas A (2012) A study on the trophic status and Phytoplanktonic Algae of Mamasin Dam Lake (Aksaray-Turkey). Turk J Fish Aquat Sci 12:595–601. https://doi.org/10.4194/1303-2712-v12_3_06
Atici T, Obali O (2006) Seasonal variation of phytoplankton and value of chlorophyll a in the Saryar Dam Reservoir (Ankara, Turkey). Turk J Bot 30:349–357
Atici T, Obali O (2010) The diatoms of Asartepe Dam Lake (Ankara), with environmental and some physicochemical properties. Turk J Bot 34:541–548
Baldwin DS, Mitchell AM (2000) The effects of drying and re-flooding on the sediment and soil nutrient dynamics of lowland river–floodplain systems: a synthesis. Regul Rivers Res Manag 16:457–467
Banerjee S et al (2016) Determinants of bacterial communities in Canadian agroforestry systems. Environ Microbiol 18:1805–1816. https://doi.org/10.1111/1462-2920.12986
Banerjee S, Schlaeppi K, van der Heijden MGA (2018) Keystone taxa as drivers of microbiome structure and functioning. Nat Rev Microbiol 16:567–576. https://doi.org/10.1038/s41579-018-0024-1
Berry D, Widder S (2014) Deciphering microbial interactions and detecting keystone species with co-occurrence networks. Front Microbiol 5:219. https://doi.org/10.3389/fmicb.2014.00219
Brinkhurst RO (1986) Guide to the freshwater aquatic Microdrile Oligochaetes of North America. Department of Fisheries and Oceans, Ottawa, Canada
Burke C, Steinberg P, Rusch D, Kjelleberg S, Thomas T (2011) Bacterial community assembly based on functional genes rather than species. Proc Natl Acad Sci USA 108:14288–14293. https://doi.org/10.1073/pnas.1101591108
Cai W, Cai Y, Gong Z, Qin B (2010) Temporal and spatial patterns of Corbicula fluminea in Lake Taihu. J Lake Sci 22:714–722
Cai Y, Zhang Y, Wu Z, Chen Y, Xu J, Gong Z (2016) Composition, diversity, and environmental correlates of benthic macroinvertebrate communities in the five largest freshwater lakes of China. Hydrobiologia 788:85–98
Carpenter SR, Cole JJ, Kitchell JF, Pace ML (1998) Impact of dissolved organic carbon, phosphorus, and grazing on phytoplankton biomass and production in experimental lakes. Limnol Oceanogr 43:73–80
Celik K, Ongun T (2008) Spatial and temporal dynamics of the steady-state phytoplankton assemblages in a temperate shallow hypertrophic lake (Lake Manyas, Turkey). Limnology 9:115–123
David V, Sautour B, Chardy P, Leconte M (2005) Long-term changes of the zooplankton variability in a turbid environment: the Gironde estuary (France). Estuar Coast Shelf Sci 64:171–184. https://doi.org/10.1016/j.ecss.2005.01.014
Degerman R, Lefébure R, Byström P, Båmstedt U, Larsson S, Andersson A (2018) Food web interactions determine energy transfer efficiency and top consumer responses to inputs of dissolved organic carbon. Hydrobiologia 805:131–146. https://doi.org/10.1007/s10750-017-3298-9
Deng X, Zhao Y, Wu F, Lin Y, Qi Lua JD (2011) Analysis of the trade-off between economic growth and the reduction of nitrogen and phosphorus emissions in the Poyang Lake Watershed, China. Ecol Model 222:330–336
Ding Y, Wang S, Zhang W, Feng M, Yu R (2019) A rule of hydrological regulating on nutritional status of Poyang Lake, since the operation of the Three Gorges Dam. Ecol Indic 104:535–542. https://doi.org/10.1016/j.ecolind.2019.04.054
Faithfull C, Huss M, Vrede T, Karlsson J, Bergström A-K (2012) Transfer of bacterial production based on labile carbon to higher trophic levels in an oligotrophic pelagic system Canadian. J Fish Aquat Sci 69:5–93
Fand C, Lai Z, Yang J, Xu Y (2011) Study on the nonuniform spatial distribution of water level in Poyang Lake based on ASAR images and DEM procedia. Environ Sci 10:2540–2546. https://doi.org/10.1016/j.proenv.2011.09.395
Farhadian O, Pouladi M (2014) Seasonal changes in the abundance and biomass of zooplankton from shallow mudflat river-estuarine system in Persian Gulf Brazilian. J Aquat Sci Technol 18:19–29. https://doi.org/10.14210/bjast.v18n2.p19-29
Fedorov VD (1979) On the methods of study of phytoplankton and its activity. Publishing Institute in Moscow, Moscow
Feng L, Hu C, Chen X, Cai X, Tian L, Gan W (2012) Assessment of inundation changes of Poyang Lake using MODIS observations between 2000 and 2010. Remote Sens Environ 121:80–92. https://doi.org/10.1016/j.rse.2012.01.014
Feng L, Hu C, Chen X, Zhao X (2013) Dramatic inundation changes of China’s two largest freshwater lakes linked to the Three Gorges Dam. Environ Sci Technol 47:9628–9634. https://doi.org/10.1021/es4009618
Franco JN, Ceia FR, Patrício J, Modesto V, Thompson J, Marques JC, Neto JM (2012) Population dynamics of Corbicula fluminea (Müller, 1774) in mesohaline and oligohaline habitats: invasion success in a Southern Europe estuary. Estuar Coast Shelf Sci 112:31–39. https://doi.org/10.1016/j.ecss.2011.07.014
Furusato E, Asaeda T, Manatunge J (2004) Tolerance for prolonged darkness of three phytoplankton species, Microcystis aeruginosa (Cyanophyceae), Scenedesmus quadricauda (Chlorophyceae), and Melosira ambigua (Bacillariophyceae). Hydrobiologia 527:153–162
Ger KA, Urrutia-Cordero P, Frost PC, Hansson LA, Sarnelle O, Wilson AE, Lurling M (2016) The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 54:128–144. https://doi.org/10.1016/j.hal.2015.12.005
Gong Z, Xie P (2001) Impact of eutrophication on biodiversity of the Macrozoobenthos community in a Chinese Shallow Lake. J Freshwater Ecol 16:171–178. https://doi.org/10.1080/02705060.2001.9663802
Gorokhova E et al (2016) Indicator properties of Baltic zooplankton for classification of environmental status within marine strategy framework directive. PLoS ONE 11:e0158326. https://doi.org/10.1371/journal.pone.0158326
Guiry M, Guiry G (2016) AlgaeBase. World-wide electronic publication: National University of Ireland, Galway
Halary S, Leigh JW, Cheaib B, Lopez P, Bapteste E (2010) Network analyses structure genetic diversity in independent genetic worlds. Proc Natl Acad Sci USA 107:127–132. https://doi.org/10.1073/pnas.0908978107
Hu C (2014) Study on the community structure of macrozoobenthos in Poyang Lake. Dissertation, East China University of Science annd Technology
Huang X, Chen C, Yuan Y, Yu Z, Jin W (2018) Analysis on Phytoplankton community characteristics of Poyang Lake. Jiangxi Chemical Industry (02):180–184
Ibekwe AM, Jordaan K, Comeau AM, Khasa DP, Bezuidenhout CC (2019) An integrated insight into the response of bacterial communities to anthropogenic contaminants in a river: a case study of the Wonderfonteinspruit catchment area. S Afr PlOS ONE 14:e0216758. https://doi.org/10.1371/journal.pone.0216758
Jiang Y, Guo X (2019) Stoichiometric patterns of soil carbon, nitrogen, and phosphorus in farmland of the Poyang Lake region in Southern China. J Soil Sediment 19:3476–3488. https://doi.org/10.1007/s11368-019-02317-3
Li Y, Pang Y, Xu Q (2009) Simulation of transparency and its factors in Taihu Lake under hydrodynamic conditions. J Hohai Univ 37:625–630
Li C et al (2019) Temporal variation in zooplankton and phytoplankton community species composition and the affecting factors in Lake Taihu-a large freshwater lake in China. Environ Pollut 245:1050–1057. https://doi.org/10.1016/j.envpol.2018.11.007
Liang D, Wang Q, Wei N, Miloslav D, Yang Y (2017) Spatial and temporal variation in rotifer community structure and the response to environmental factors among different water bodies in Guangzhou City. J Lake Sci 29:1433–1443
Liang Y, Xiao H, Liu X, Hu Q, Xiong J, Li W, Tang C (2018) Variation in sources of inorganic nitrogen under different hydrological conditions in a floodplain lake: a case study of Bang Lake (Poyang Lake, Jiangxi Province, China). Inland Waters 8:176–185. https://doi.org/10.1080/20442041.2018.1457854
Liu X, Qian K, Chen Y, Gao J (2017) A comparison of factors influencing the summer phytoplankton biomass in China’s three largest freshwater lakes: Poyang, Dongting, and Taihu. Hydrobiogia 792:283–302
Liu J et al (2019) Water-level fluctuations are key for phytoplankton taxonomic communities and functional groups in Poyang Lake. Ecol Indic 104:470–478. https://doi.org/10.1016/j.ecolind.2019.05.021
Liu J, Wen C, Han L, Dai G, Wang F, Hu F, Lou Q (2020a) Study on the response characteristics of nitrogen and phosphorus to water level change of Poyang Lake during 2012–2017. Environ Pollut Ctrl 42:1274–1279
Liu Y, Ren Z, Qu X, Zhang M, Yu Y, Zhang Y, Peng W (2020b) Microbial community structure and functional properties in permanently and seasonally flooded areas in Poyang Lake. Sci Rep 10:4819. https://doi.org/10.1038/s41598-020-61569-z
Lorenz P, Trommer G, Stibor H (2019) Impacts of increasing nitrogen:phosphorus ratios on zooplankton community composition and whitefish (Coregonus macrophthalmus) growth in a pre-alpine lake. Freshwater Biol 64:1210–1225
Lu J, Chen X, Zhang L, Sauvage S, Sánchez-Pérez J-M (2018) Water balance assessment of an ungauged area in Poyang Lake watershed using a spatially distributed runoff coefficient model. J Hydroinform 20:1009–1024. https://doi.org/10.2166/hydro.2018.017
Luo F-L, Jiang X-X, Li H-L, Yu F-H (2016) Does hydrological fluctuation alter impacts of species richness on biomass in wetland plant communities? J Plant Ecol 9:434–441. https://doi.org/10.1093/jpe/rtv065
Macêdo RL, Lopes VG, Kozlowskysuzuki B, Branco CWC (2018) Zooplankton community attributes in an oligo-mesotrophic reservoir: A comparative study of two sampling strategies. Ann Braz Acad Sci 91:e20170807
Mandakovic D et al (2018) Structure and co-occurrence patterns in microbial communities under acute environmental stress reveal ecological factors fostering resilience. Sci Rep 8:8575. https://doi.org/10.1038/s41598-018-23931-0
Marchant R, Reading D, Ridd J, Campbell S, Ridd P (2015) A drifter for measuring water turbidity in rivers and coastal oceans. Mar Pollut Bull 91:102–106. https://doi.org/10.1016/j.marpolbul.2014.12.021
Meng J, Yu Z, Miao M, Kong Q, Zhang Y, Liu J (2017) Differentiated responses of Plankton and Zoobenthos to water quality based on annual and seasonal analysis in a freshwater lake. Polut J Environ Stud 26:755–764. https://doi.org/10.15244/pjoes/66713
Merritt RW, Cummins KW (1996) An introduction to the aquatic insects of North America. Kendall Hunt, Dubuque
Minella JPG, Merten GH, JeM R, Clarke RT (2008) Estimating suspended sediment concentrations from turbidity measurements and the calibration problem. Hydrol Process 22:1819–1830
Morse JC, Yang L, Tian L (1994) Aquatic insects of China useful for monitoring water quality. Hohai University Press, Nanjing
Naselli-Flores L (2000) Phytoplankton assemblages in twenty-one Sicilian reservoirs: relationships between species composition and environmental factors. Hydrobiologia 424:1–11
Newman MEJ (2006) Modularity and community structure in networks. Proc Natl Acad Sci USA 103:8577–8582
Ni Z, Wang S, Zhang L, Wu Z (2015) Role of hydrological conditions on organic phosphorus forms and their availability in sediments from Poyang Lake, China. Environ Sci Pollut Res Int 22:10116–10129. https://doi.org/10.1007/s11356-015-4198-z
Nie X, Hu X, Liu G, Qin H (2018) Species diversity of zooplankton and water quality biological assessment in a sub-lake of Poyang Lake. J Nanchang Univ 42:161–167
Ozkan A, Rorrer GL (2017) Effects of light intensity on the selectivity of lipid and chitin nanofiber production during photobioreactor cultivation of the marine diatom Cyclotella sp. Algal Res 25:216–227
Protopapa M, Zervoudaki S, Tsangaris C, Velaoras D, Koppelmann R, Psarra S, Möllmann C (2019) Zooplankton distribution, growth and respiration in the Cretan Passage. Topical Studies in Oceanography, Eastern Mediterranean Deep Sea Research Part II. https://doi.org/10.1016/j.dsr2.2019.03.001
Qu X, Peng W, Liu Y, Zhang M, Ren Z, Wu N, Liu X (2019a) Networks and ordination analyses reveal the stream community structures of fish, macroinvertebrate and benthic algae, and their responses to nutrient enrichment. Ecol Indic 101:501–511. https://doi.org/10.1016/j.ecolind.2019.01.030
Qu X, Zhang M, Yang Y, Xie Y, Ren Z, Peng W, Du X (2019b) Taxonomic structure and potential nitrogen metabolism of microbial assemblage in a large hypereutrophic steppe lake. Environ Sci Pollut Res Int 26:21151–21160. https://doi.org/10.1007/s11356-019-05411-8
Ren Z, Qu X, Peng W, Yu Y, Zhang M (2019a) Nutrients drive the structures of bacterial communities in sediments and surface waters in the river-lake system of Poyang Lake. Water 11:930. https://doi.org/10.3390/w11050930
Ren Z, Qu X, Zhang M, Yu Y, Peng W (2019b) Distinct bacterial communities in wet and dry seasons during a seasonal water level fluctuation in the largest Freshwater Lake (Poyang Lake) in China. Front Microbiol 10:1167. https://doi.org/10.3389/fmicb.2019.01167
Rodrigues MA, Agostini VO, Ozorio CP (2019) Short-term temporal variations in the zooplankton community of the surf zone influenced by estuarine discharge. Reg Stud Mar Sci 29:100687. https://doi.org/10.1016/j.rsma.2019.100687
Rougier C, Pourriot R, Lam-Hoai T, Guiral D (2005) Ecological patterns of the rotifer communities in the Kaw River estuary (French Guiana). Estuar Coast Shelf Sci 63:83–91. https://doi.org/10.1016/j.ecss.2004.08.023
Schälicke S, Sobisch L-Y, Martin-Creuzburg D, Wacker A (2018) Food quantity-quality co-limitation: interactive effects of dietary carbon and essential lipid supply on population growth of a freshwater rotifer. Freshwater Biol 64:903–912
Sevindik T, Altundal E, Kucuk F (2015) The Seasonal and spatial distribution of the Phytoplankton of a Mesotrophic Lake related to certain physical and chemical parameters. Ekoloji 24:14–23. https://doi.org/10.5053/ekoloji.2015.09
Seymour JR, Amin SA, Raina JB, Stocker R (2017) Zooming in on the phycosphere: the ecological interface for phytoplankton-bacteria relationships. Nat Microbiol 2:17065. https://doi.org/10.1038/nmicrobiol.2017.65
Sha J, Dai Y, Pan Y, Li J, Xu Z, Zhang J (2018) Research progress in using rotifers in ecotoxicological studies Asian. J Ecotox 13:56–70
Shankman D, Keim BD, Song J (2006) Flood frequency in China’s Poyang Lake region: trends and teleconnections. Int J Climatol 26:1255–1266. https://doi.org/10.1002/joc.1307
Shen W (1999) Protozoology. Science press, Beijing
Snell TW, Cl J-J (2007) Workshop on rotifers in ecotoxicology. Hydrobiologia 593:227–232
Song KY, Zoh KD, Kang H (2007) Release of phosphate in a wetland by changes in hydrological regime. Sci Total Environ 380:13–18. https://doi.org/10.1016/j.scitotenv.2006.11.035
Southwood TRE (1977) Habitat, the template for ecological strategies. J Anim Ecol 46:337–365
Souza CAd, Vieira LCG, Legendre P, Carvalho Pd, Velho LFM, Beisner BE (2019) Damming interacts with the flood pulse to alter zooplankton communities in an Amazonian river. Freshwater Biol 64:1040–1053
Su X, Steinman AD, Xue Q, Zhao Y, Tang X, Xie L (2017) Temporal patterns of phyto- and bacterioplankton and their relationships with environmental factors in Lake Taihu, China. Chemosphere 184:299–308. https://doi.org/10.1016/j.chemosphere.2017.06.003
Tan Z, Yao H, Zhuang Q (2018) A small temperate lake in the 21st Century: dynamics of water temperature, ice phenology, dissolved oxygen, and chlorophyll a. Water Resour Res 54:4681–4699. https://doi.org/10.1029/2017wr022334
Tavares DA, Lambrecht RW, Castilho MCdA, Henry R, Ferragut C (2019) Epipelon responses to N and P enrichment and the relationships with phytoplankton and zooplankton in a mesotrophic reservoir. Aquat Ecol 53:303–314
Vezi M, Downs CT, Wepener V, O’Brien G (2019) Response of zooplankton communities to altered water quality and seasonal flow changes in selected river dominated estuaries in KwaZulu-Natal, South Africa. Ecohydrol Hydrobiol 19:393–406. https://doi.org/10.1016/j.ecohyd.2019.01.005
Wang J (1961) Freshwater rotifers in China. Science Press, Beijing
Wang L, Liang T (2015) Distribution characteristics of phosphorus in the sediments and overlying water of Poyang lake. PLoS ONE 10:e0125859. https://doi.org/10.1371/journal.pone.0125859
Wang S, Xie P, Wu S, Liang X (2007) Phytoplankton biomass in relation to nutrients and zooplankton in thirty subtropical lakes adjacent to the Yangtze River, China. Fund Appl Limnol 169:49–55. https://doi.org/10.1127/1863-9135/2007/0169-0049
Wang Y-S, Sun C-C, Lou Z-P, Wang H, Mitchell BG, Wu M-L, Sun Z-X (2011) Identification of water quality and benthos characteristics in Daya Bay, China, from 2001 to 2004. Oceanol Hydrobiol St 40:82–95
Wang L, Chen J, Li Y, Zhang W, Cao T, Xie P (2018) Effects of light intensity on the epiphytic crustacean zooplankton community. J Hydroecol 39:44–51
Wang Y, Ma J, Xiao X, Wang X, Dai S, Zhao B (2019a) Long-term dynamic of poyang lake surface water: a mapping work based on the google earth engine cloud platform. Remote Sens 11:313. https://doi.org/10.3390/rs11030313
Wang Y, Molinos JG, Shi L, Zhang M, Wu Z, Zhang H, Xu J (2019b) Drivers and changes of the Poyang Lake Wetland ecosystem. Wetlands 39:313. https://doi.org/10.1007/s13157-019-01180-9
Xiang S, Zhou W (2011) Phosphorus forms and distribution in the sediments of Poyang Lake, China. Int J Sediment Res 26:230–238
Xue Y, Chen H, Yang JR, Liu M, Huang B, Yang J (2018) Distinct patterns and processes of abundant and rare eukaryotic plankton communities following a reservoir cyanobacterial bloom. ISME J 12:2263–2277. https://doi.org/10.1038/s41396-018-0159-0
Yang S, Jin X (2008) Critical light intensities for Microcystis aeruginosa, Scenedesmus quadricauda and Cyclotella sp. and competitive growth patterns under different light: N: P ratios. J Freshwater Ecol 23:387–396
Yang C, Zhang M, Qu X, Peng W, Yu Y, Zhu X (2018) Patterns of the macroinvertebrate community in Poyang Lake and related environmental factors Asian. J Ecotox 13:91–100
Yao X, Wang S, Ni Z, Jiao L (2015) The response of water quality variation in Poyang Lake (Jiangxi, People’s Republic of China) to hydrological changes using historical data and DOM fluorescence. Environ Sci Pollut Res Int 22:3032–3042. https://doi.org/10.1007/s11356-014-3579-z
Yin Y, Wu Z, Liu M, He J, Yu Z (2014) Dynamic distributions of dissolved oxygen in lake Qiandaohu and its environmental influence factors. Environ Sci 35:2539–2546
Yuan X, Lu J, Liu H (2002) Distribution pattern and variat ion in the functional groups of Zoobenthos in the Changjiang Estuary. Acta Ecol Sin 22:2054–2062
Zhang L, Liu J, Zhang D, Luo L, Liao Q, Yuan L, Wu N (2017a) Seasonal and spatial variations of microcystins in Poyang Lake, the largest freshwater lake in China. Environ Sci Pollut Res 25:6300–6307. https://doi.org/10.1007/s11356-017-0967-1
Zhang M, Cai Q, Zhang X (2017b) Impacts of flood-driven water level fluctuations on macroinvertebrate assemblages in different zones of a long and narrow subtropical reservoir-bay. Quat Int 440:111–118
Zhang M, Munoz-Mas R, Martinez-Capel F, Qu X, Zhang H, Peng W, Liu X (2018a) Determining the macroinvertebrate community indicators and relevant environmental predictors of the Hun-Tai River Basin (Northeast China): a study based on community patterning. Sci Total Environ 634:749–759. https://doi.org/10.1016/j.scitotenv.2018.04.021
Zhang X, Qin H, Wang H, Wan A, Liu G (2018b) Effects of water level fluctuations on root architectural and morphological traits of plants in lakeshore areas of three subtropical floodplain lakes in China. Environ Sci Pollut Res 25:34583–34594. https://doi.org/10.1007/s11356-018-3429-5
Zhao J, Li J, Yan H, Zheng L, Dai Z (2011) Analysis on the water exchange between the main stream of the Yangtze River and the Poyang Lake. Proc Environ Sci 10:2256–2264. https://doi.org/10.1016/j.proenv.2011.09.353
Zhou F, Chen J (2010) Freshwater microbiological atlas. Chemical Industry Press, Beijing
Zhuang D (1993) Effect of water quality acidification and simulated acid rain on zooplankton Chinese. J Appl Ecol 4:187–191
Acknowledgements
This study was supported by the Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment, China (2019HJ2096001006), and the National Natural Science Foundation of China (No. 51779275 and No. 41671048), and the IWHR Research and Development Support Program (WE0145B532017). Authors wish to thank the anonymous reviewers for their comments.
Author information
Authors and Affiliations
Contributions
The conception and design of the study were carried out by YL under the direction of MZ, WP, and XQ. YL, MZ and XQ processed the data. YL performed the statistical analyses. YY, YZ, and CY performed the field work and laboratory analysis. WP and NW gave suggestions during the whole work. The first draft of the manuscript was written by YL, and all authors commented on previous versions of the manuscript. All authors reviewed the manuscript and gave their final approval for publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests of financial or non-financial nature.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Handling Editor: Télesphore Sime-Ngando.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, Y., Zhang, M., Peng, W. et al. The effects of flood pulse on multiple aquatic organisms in a seasonal shallow lake. Aquat Ecol 55, 379–399 (2021). https://doi.org/10.1007/s10452-020-09829-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-020-09829-y