Abstract
Population structure studies play an increasingly integral role in conservation and management of marine mammal species. Genetic markers are commonly used; however, ecological markers (i.e. chemical compounds) are a fairly recent and useful tool to investigate ecological management units. The objective of this study is to investigate the population structure of the Atlantic spotted dolphin (Stenella frontalis) within its distribution in the Atlantic Ocean using data from stable isotopes of δ13C and δ15N and persistent organic pollutants as ecological markers. Based on previous studies that addressed distribution, morphometric analyses and molecular and ecological markers, we hypothesize that there are several ecological management units within the Atlantic Ocean. Our results confirmed population differentiation previously detected using genetic markers. Additionally, dolphins from the south-eastern coast of Brazil do not show complete ecological segregation from the Caribbean ones, while molecular analyses suggested genetic differentiation between the two regions. In the light of these results, we propose that at least two ecological management units should be considered, east and west of the Atlantic Ocean; however, the presence of one or two management units along the Atlantic coast of Central and South America needs further investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Knowledge of population structure is a prerequisite for any assessment of population abundance and for understanding the impact of anthropogenic pressures. The development of management strategies is based on the definition of intra-specific ecological or evolutionary management units on which surveys and conservation efforts can be conducted. Two major types of management units exist, those reflecting an ecological paradigm and those reflecting an evolutionary paradigm (Andrewartha and Birch 1984; Crawford 1984). In the ecological paradigm, the cohesive forces are mainly demographic, and emphasis is on co-occurrence in space and time such that individuals have an opportunity to interact (Andrewartha and Birch 1984). In the evolutionary paradigm, the cohesive forces are largely genetic, and emphasis is on reproductive interactions between individuals (Crawford 1984). Most scientific studies use the evolutionary paradigm to define wildlife populations. To this aim, neutral molecular markers have been widely applied to identify potential demographically and evolutionarily independent units driven by micro-evolutionary forces (Ballard and Whitlock 2004; Selkoe and Toonen 2006; Xu et al. 2010). Nevertheless, adaptation to environmental and ecological conditions may also lead to discontinuous ecological populations, which can represent uniquely adapted units that may occur even in the absence of genetic differentiation (Coyle 1998). Those conditions include oceanographic characteristics, habitat discontinuities, geographic barriers, social organizations or dietary specializations (e.g. Lyrholm et al. 1999; Fullard et al. 2000; Fontaine et al. 2007, 2010; Foote et al. 2009; Wiszniewski et al. 2010).
Ecological markers are natural or synthetic chemical compounds, such as stable isotopes, fatty acids, trace elements or persistent organic pollutants (POPs). The compounds or elements are incorporated into organism tissues mainly through food providing information of foraging habitat and habits, individual movements or contamination levels. Therefore, they can reveal populations or ecotype differentiation in marine top predators based on the idea that consistent differences in trophic ecology would be sufficient to delimit ecological populations, possibly even in the presence of gene flow (e.g. Born et al. 2003; Caurant et al. 2009; Giménez et al. 2013; Quérouil et al. 2013). Moreover, depending on the tissue turnover and the half-life of the compounds, or elements analysed, the period of integration represented by the specific ecological marker signature or concentration differs. In contrast to genetic data, which reveal information at the generational scale, marker signatures or concentrations may reveal segregations over time scales spanning from weeks to the lifetime of the individuals (e.g. Hobson and Clark 1992; Nordstrom et al. 2008). Thereby, molecular and ecological markers are very complementary tools allowing to better define ecological management units (EMUs) that includes ecologically similar individuals, which are especially proper for short- to medium-term management actions (Borrell et al. 2006; Murphy et al. 2009; Giménez et al. 2017).
The Atlantic spotted dolphin (Stenella frontalis, ASD) is an endemic species from tropical and warm-temperate waters of the Atlantic (Perrin et al. 1994; Perrin 2002). It is widely distributed ranging from the eastern coasts of South and North America (ca. 35°S–45°N) (including the Caribbean Sea) to the west coast of Africa from Mauritania to Angola (e.g. Nieri et al. 1999; Moreno et al. 2005; Jefferson et al. 2008; Weir 2010; Paro et al. 2014). The ASD appears to have a discontinuous distribution along the eastern coast of South America with no records between 6 and 18°S (Moreno et al. 2005). Only a single sighting of ca. 80 individuals exists until now, which suggests a possible northward extension range in 350 km along the coast of Brazil (Danilewicz et al. 2013). In addition, two parapatric morphotypes have been identified in the North Atlantic for ASD. These morphotypes display variation in terms of body size and degree of spotting (Perrin 2002): offshore individuals are generally found beyond the continental shelf break and around oceanic islands. These animals are smaller and have few or no spots. Inshore individuals inhabiting continental shelf waters are bigger and highly spotted (Perrin et al. 1987). The distinct genetic units recently revealed in north-western Atlantic populations most likely correspond to the two described morphotypes (Viricel and Rosel 2014).
Recent studies tried to elucidate how many managements units of ASD exist throughout the species range using distribution, morphometric analyses, molecular and ecological markers (Moreno et al. 2005; Adams and Rosel 2006; Quérouil et al. 2010, 2013; Caballero et al. 2013; Do Amaral et al. 2015). From molecular markers, the number of managements units differed from 0 to 4, while studies using ecological markers mostly found two managements units. However, none of these studies was conducted at the species distribution scale.
In that context, this study aims to elucidate the existence of different EMUs of ASD within the Atlantic Ocean using ecological markers as approach. Specifically, stable isotopes of δ13C and δ15N were analysed in skin samples and POPs in blubber samples.
Materials and methods
Samples collection
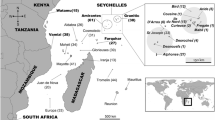
Skin and blubber of ASD were collected from stranded individuals in São Paulo (Southeast Brazil), Canary Islands (Spain) and Caribbean Sea (Guadeloupe Island) from 2000 to 2016. Experienced stranding network personnel from local areas (Table 1) performed complete necropsies, if the condition of the animal permitted. Only samples collected from fresh or moderately decomposed animals were used for stable isotope analyses (SIA) and POP analyses to prevent sampling biases associated with tissue decomposition [decomposition state ≤ 3 by Kuiken and Hartmann (1991) and category 2 using the classification proposed by Geraci and Lounsbury (1993)]. Biopsies of skin and blubber from São Paulo (Brazil) and La Guajira (Colombia) were also collected (Table 1) using a crossbow bolt (BARNETT Veloci-Speed®Class, 68-kg draw weight) with Finn Larsen (Ceta-Dart, Copenhagen, Denmark) and PAXARMS biopsy darts with tips, especially designed for small cetaceans (dart: 9 and 25 mm long and 5 mm diameter) (Krützen et al. 2002). When animals were close to the boat from a distance of approximately 10 m (Fruet et al. 2016), dolphins were hit below the dorsal fin and samples stored on ice from 1 to 8 h at sea. Behavioural responses and physical attempts of biopsies were recorded and reported in previous studies (Oremus 2008; Giménez et al. 2011; Kowarski et al. 2014). Level of short-term responses was low in spotted dolphins and similar to that reported elsewhere (e.g. Krützen et al. 2002).
Biopsies complied with all country’s laws and were carried out under ministries of environment permits. Figure 1 shows the sample locations and the methodology applied to collect them (i.e. biopsy or stranding).
Once in the laboratory, skin and blubber were separated using a scalpel blade, and then, blubber samples were wrapped in aluminium foil and both tissues were preserved frozen (− 20 °C) or in 70% alcohol until SIA and POP analyses.
Stable isotope analyses (SIA)
Skin samples were freeze-dried and then ground into a fine powder before isotopic analyses. Because lipids are highly depleted in 13C relative to other tissue components (DeNiro and Epstein 1978), they were extracted from skin tissues using cyclohexane. To this end, all the skin powder was agitated with 1.5 mL of cyclohexane for 1 h. Next, the sample was centrifuged for 5 min at 4000 g, and the supernatant containing the lipids was discarded. This procedure was repeated 1–3 times depending on sample lipid content. Then, the sample was dried in an oven at 45 °C for 48 h, and 0.35 ± 0.05 mg subsamples of lipid-free dry powder were weighed in tin capsules for SIA.
These analyses were performed with an elemental analyser coupled to an Isoprime (Micromass) continuous-flow isotope ratio mass spectrometer (CF IR-MS). Results are expressed in δ notation relative to PeeDee Belemnite and atmospheric N2 for δ13C and δ15N, respectively, according to the equation:
where X is 13C or 15N and R is the isotope ratio 13C/12C or 15N/14N (Peterson and Fry 1987). Replicate measurements of internal laboratory standards (acetanilide) during each run indicate measurement errors of ± 0.15 and 0.2‰ for δ13C and δ15N, respectively.
Skin samples from Azores previously analysed by Quérouil et al. (2013) were included in this study. These samples followed the same protocol for lipid removal and for δ13C and δ15N analyses.
Persistent organic pollutant (POP) analyses
Blubber samples were previously analysed for POPs and for lipid content by Méndez-Fernandez et al. (2018). However, blubber from Colombia samples was not available for POP analyses. Briefly, approximately 0.2 g of blubber was cut, ground with anhydrous Na2SO4 and extracted using a Soxhlet apparatus for 8 h with 80 mL of n-hexane and methylene chloride (1:1, v:v). The extract was then concentrated (by rotary evaporation) to 2 mL, of which 200 µL was used to determine the amount of lipids by gravimetry. The remaining extract was cleaned up with sulphuric acid, centrifuged, washed twice with pre-cleaned water and subsequently concentrated to 0.9 mL before transferring to amber glass gas chromatography (GC) vials and completed to 1 mL with internal standard 2,4,5,6-tetrachlorometaxylene (TCMX). Then, the concentrations of PCBs (IUPAC Nos. 8, 18, 28, 31, 33, 44, 49, 52, 56/60, 66, 70, 74, 77, 81, 87, 95, 97, 99, 101, 105, 110, 114, 118, 123, 126, 128, 132, 138, 141, 149, 151, 153, 156, 157, 158, 167, 169, 170, 174, 177, 180, 183, 187, 189, 194, 195, 201, 203, 206 and 209), DDTs (op’-DDE, pp’-DDE, op’-DDD, pp’-DDD, op’-DDT, and pp’-DDT), HCB, CHLs (α- and γ-chlordane), Mirex and PBDEs (IUPAC Nos. 28, 47, 99, 100, 153, 154 and 183) were determined by gas chromatography using an electron capture detector (GC-ECD) and gas chromatography coupled to a mass spectrometer (GC–MS) in a selected ion mode.
The methods employed were validated by the replicate analysis of standards and samples, regular blank controls and through spiking experiments or analysis of certified and laboratory reference material (SRM 1945—standard reference material of organics in whale blubber).
Concentrations were expressed in µg g−1 lipid weight (lw) in order to reduce differences on lipid content between stranding and biopsy samples but also between individuals.
Data treatment
Values of δ13C meet the assumptions of parametric tests (i.e. normality and homogeneity of variances) but not δ15N values. Therefore, to compare δ13C and δ15N values among ASD areas nonparametric tests (i.e. Kruskal–Wallis followed by Dunn post hoc tests) were used.
POPs were summed by groups due to the great number of compounds (i.e. ΣPCBs, ΣDDTs, ΣPBDEs, HCB and mirex). Statistical tests to compare the mean concentrations of these groups among areas were previously performed in Méndez-Fernandez et al. (2018). In these tests, dolphins from Caribbean and Occidental Canary Islands were removed because POP analyses had not been completed or only one sample was available (Table 1).
To investigate the niche region (NR) and its overlap among ASD from the four studied areas, the nicheRover package was used. This free R package is a recently developed ellipsoid probabilistic method (Swanson et al. 2015). The NR was defined as a specific region in which a species or group (in this case population) has a 95% probability of being found in bivariate space using stable isotopes or other indicators of niche (i.e. physical or chemical habitat variables) (Swanson et al. 2015). The niche overlap is calculated as the probability that an individual from species A is found in the NR of species B. Uncertainty is accounted for in a Bayesian framework, and is the only aspect of the methodology that depends on sample size. Then, overlap uncertainty was accounted for by performing 1000 elliptical projections of NR using Bayesian statistics.
The levels of significance for statistical analyses were always set at α = 0.05, and all statistical analyses were performed using R v.3.5.3 (R Core Team 2013).
Results
Stable isotopes
Caribbean dolphins showed the highest δ15N- and δ13C-enriched (i.e. high proportion of the heavy 13C isotope) values followed by individuals from São Paulo (Table 2). Dolphins from Canary Islands and Azores showed the lowest and closer δ13C and δ15N values (Table 2).
Moreover, comparison tests revealed significantly higher values in the Caribbean Sea and São Paulo than in the archipelagos (Azores and Canary Islands) (Kruskal–Wallis followed by Dunn post hoc test, p < 0.05), but no significant differences between São Paulo and Caribbean dolphins, and also between archipelago dolphins (Kruskal–Wallis followed by Dunn post hoc test, p > 0.05). In addition, Caribbean dolphins showed high data variability for both stable isotopes (Fig. 2). In contrast, dolphins from the archipelagos revealed small data variability for δ15N values but high for δ13C values in samples from the Canary Islands (Fig. 2).
Carbon (δ13C) and nitrogen (δ15N) stable isotope ratios in skin samples of Atlantic spotted dolphin (Stenella frontalis) by areas. Data are shown as mean (%) ± SD (standard deviation) and SE (standard error) in grey. Azores δ13C and δ15N values are from Quérouil et al. (2013)
Persistent organic pollutants
POPs data were previously published by Méndez-Fernandez et al. (2018) from a contamination perspective. Authors revealed that among all POP groups analysed, PCBs, DDTs and PBDEs were the predominant accounting for 64.0%, 31.2% and 2.0% of the total of POP compounds. The chlorinated pesticides, Mirex and HCB, were found in much smaller proportions (1.9% and 0.7%, respectively) (data from Méndez-Fernandez et al. 2018).
The different areas of the Atlantic Ocean studied showed different POP concentrations and profiles. São Paulo and Canary dolphins revealed the highest concentrations of PCBs (Median = 10.5 and 8.65 µg g−1 lw, respectively) followed by those from the Azores (Fig. 3). Concerning chlorinated pesticides, DDTs had the highest concentrations in the areas studied, with the highest medians found in dolphins from the Canary Islands (5.13 µg g−1 lw) followed by those from Azores (3.64 µg g−1 lw) (Fig. 3). PBDEs also varied by location, with the highest and significantly different concentrations (pairwise test with Bonferroni correction, p < 0.05) in dolphins from São Paulo (0.41 µg g−1 lw). Among the other chlorinated pesticides, the concentrations of HCB were highest in Azores and significantly different from São Paulo dolphins (pairwise test with Bonferroni correction, p < 0.05); however, Mirex showed the most similar concentrations among the areas (pairwise test with Bonferroni correction, p > 0.05) (Fig. 3).
Box and whisker plots of POP concentrations (in µg g−1 lipid weight, lw) of Atlantic spotted dolphins (Stenella frontalis) in the four areas studied. The bottom and top of the box are the lower and upper quartiles. The band inside the box is the median, and the black dot the mean of the data. The ends of the whiskers represent the lowest datum of the lower quartile and the highest datum of the upper quartile. The open circles represent the outliers. Different letters indicate that concentrations are significantly different (p < 0.05). The number of samples analysed by area is indicated in Table 1. n = number of blubber samples analysed
Ecological niche region
To determine ecological niche region (NR), only stable isotope values were used to represent the four areas studied (Fig. 4). The probability of São Paulo and Caribbean dolphins occurring within the NR of Canary Islands was near 10% each, while only 0.35% and 1% within NR of Azores, respectively. There was a 91% probability of São Paulo dolphins occurring within the NR of Caribbean dolphins. Moreover, when looking at Azores and Canary Islands NR, the probability of Azores dolphins occurring within the NR of Canary Islands was almost complete (99.6%) (Fig. 4).
The niche overlap was also calculated using stable isotope values and the most concentrated groups of POPs in ASDs (i.e. PCBs, DDTs and PBDEs). Thus, only three areas could be represented (i.e. Azores, Canary Islands and São Paulo) (Fig. 5).
We found a high probability (81%) of Azores dolphins overlapping onto the ecological niche of Canary dolphins, similar to what we found using only stable isotopes values (Fig. 4). However, the probability of São Paulo dolphins overlapping onto archipelago niches was very low, 0.01% and 1.6% for Azores and Canary Islands, respectively. Finally, the probability of overlapping of Canary dolphins onto Azores and São Paulo NRs was of 6.2% and 4.7%, respectively (Fig. 5).
Discussion
Population structure
The importance of defining EMUs has been the subject of much debate in marine mammals’ ecology (Murphy et al. 2007; Banguera-Hinestroza et al. 2010; Evans and Teilmann 2009; ICES 2014). These highly mobile marine species (with continuous distribution) can adapt their temporal distribution according to a number of parameters such as physiography, prey availability, predators’ pressure or localized anthropogenic threats (Cañadas et al. 2002), exhibiting fine-scale population structure. Therefore, delineating EMUs is necessary to achieve site-specific management actions in shorter time frames.
Although the Atlantic spotted dolphin is an endemic species from the Atlantic Ocean, there is a lack of consistent knowledge regarding its population structure and potential EMUs. A geographically and possibly genetically isolated population may occur off southern Brazil from 6° to 18°S, as was previously proposed by Moreno et al. (2005) based on observation and stranding records. Ten years later, Amaral et al. (2015) updated the distribution patterns of Stenella dolphins in the south-western Atlantic Ocean (SWA). Ecological niche modelling using a set of environmental variables as explanatory variables of the location records (i.e. sighting and accidental captures) was used. Stenella frontalis is the only Stenella species that shows strictly coastal habits, occurring over the continental shelf and slope up to 1000 m depth, and with an isolated distribution from 18°S to 35°S in the SWA. Molecular and ecological markers were also used by several authors in order to infer the population structure and morphotypes presence of the species at different spatial scales. First, Adams and Rosel (2006) evidenced significant genetic differentiation between the western North Atlantic and Gulf of Mexico ASD. Later on using a larger data set, Viricel and Rosel (2014) identified a total of four putative populations in the US waters of these areas: one in oceanic waters of the western North Atlantic, one in continental shelf waters of the western North Atlantic, one in the eastern Gulf of Mexico and one in the western Gulf of Mexico. This study also showed that ASD from the Azores and from oceanic waters of the western North Atlantic belong to the same genetic unit, coinciding with the offshore morphotype distribution (Perrin et al. 1987). Quérouil et al. (2010) conducted a molecular study of the population structure of ASD into the waters of Azores and Madeira archipelagos, and their results did not identify any population structure at the scale of the studied areas indicating a single population. However, based on data from stable isotopes and fatty acids (thus on shorter time scales), the same authors revealed differences between archipelagos suggesting two different ecological stocks for ASD despite the existence of gene flow (Quérouil et al. 2013). Finally, on a larger spatial scale and based on molecular markers, Caballero et al. (2013) and Mesa-Gutiérrez et al. (2015) suggested historical or present connectivity between the Azores and Madeira ASDs with the ASDs from Caribbean and south-eastern Brazil. They also observed population differentiation between the Caribbean and south-eastern Brazil dolphins, supporting the notion of two separate stocks in the waters around the Atlantic coast of South America as proposed by Moreno et al. (2005) and Do Amaral et al. (2015). In our study, stable isotope ratios and POP concentrations showed clear differences among the investigated areas. Dolphins from São Paulo (i.e. south-eastern Brazil) and the Caribbean region seem to be highly isolated from Azores and Canary dolphins. However, differences between São Paulo and Caribbean groups are less evident (Figs. 4 and 5) than in previous studies (Moreno et al. 2005; Caballero et al. 2013; Do Amaral et al. 2015; Mesa-Gutiérrez et al. 2015). The absence of significant distinction between the neighbouring archipelagos Azores and Canary Islands (99.6% and 81% overlapping, Figs. 3 and 4) is likely to indicate dolphins’ movements among them (~ 1500 km distance). In this sense, Canary dolphins showed the highest variability of δ13C ratios (Table 2). Previous studies indicate that ASDs in the Azores and Madeira archipelagos are transient and are found around these islands from April to October (Quérouil et al. 2008, 2010; Silva et al. 2014). The offshore morphotype also seems to be mostly present around these oceanic islands (Viricel and Rosel 2014; Perrin et al. 1987). Thereby, our results seem to corroborate the presence of a single population in these archipelagos, which individuals probably belonging to the offshore morphotype as previously reported by Quérouil et al. (2010) and Viricel and Rosel (2014), respectively.
The absence of δ13C and δ15N values differences among Caribbean and São Paulo dolphins together with the high overlap of their ecological niche regions (91%, Table 2 and Fig. 4) may suggest that on a shorter time scale, dolphins from south-eastern Brazil are not completely isolated from the Caribbean as assumed (Moreno et al. 2005; Caballero et al. 2013; Do Amaral et al. 2015; Mesa-Gutiérrez et al. 2015). However, several factors may explain the absence of differences found between these two areas and should be considered.
The first factor is the seasonality, an important issue because of the seasonal variations in water temperature, prey availability and composition, dolphin behaviour (migration/movements) and nutritional condition. Additionally, the time scale represented by the ecological markers depends on the integration time and turnover of the tissues and elements (or compounds) analysed. Dolphin skin is a metabolically active tissue with a medium isotopic turnover [~ 30 days of half-life (Giménez et al. 2016)]. Therefore, isotopic information in skin provides insights into habitat use and diet during the previous few months. Contaminant tracers, such as POP concentrations and profiles, provide information over a longer time scale (from few years to the entire life of animals) than few months as for stable isotopes. This is owing to the different bioaccumulation processes of molecules, the large fat deposits that marine mammals have and a mean lifetime for some small cetacean species of about 20 years (Weijs et al. 2010, 2011). In addition to season, another factor to consider is the geographic variation in isotope and element abundances across marine environments (Hobson 1999). The increase in population and community ecology studies using these ecogeochemistry approaches allowed researchers to create robust isoscapes of the Atlantic Ocean using meta-analyses of δ13C and δ15N values at the base of the food web (i.e. organic zooplankton) (McMahon et al. 2013). Based on these isoscapes, zooplankton δ13C baseline values showed no differences among Azores, Canary Islands and south-eastern Brazil (− 22.5‰ to all the locations). However, there was approximately 2.5‰ difference between the Caribbean region and south-eastern coast of Brazil (− 20 and − 22.5‰, respectively) (McMahon et al. 2013). This suggests that if the Caribbean dolphins δ13C values (i.e. 15.9‰) are corrected with the 2.5‰ baseline difference, values from both areas are meaningfully different (i.e. − 13.4 and − 16.8 adjusted δ13C values for Caribbean and São Paulo, respectively). For δ15N values, differences at the base of the food webs are higher than for δ13C values and, especially, for the Caribbean region (6, 8, 6 and 2‰ for Canary Islands, Azores, São Paulo and Caribbean, respectively). The Caribbean region and surrounding Sargasso Sea are the most oligotrophic waters of the Atlantic Ocean; thus, this low value (2‰) is not surprising. However, it suggests that Caribbean dolphins that showed the highest δ15N ratios (14.5‰) of our study may effectively have fed at a higher trophic position than dolphins from the other areas. The diet of ASD is mainly based on cephalopods and fish that prey on pelagic and demersal items (Di Beneditto et al. 2001; Fernandez et al. 2009; Melo et al. 2010; Lopes et al. 2012; Herzing and Elliser 2013). The prey species targeted by ASDs differs between the areas where diet studies have been performed (south-eastern Brazil, Canary Islands and Bahama islands); however, these studies listed species that are generally situated at comparable trophic levels. Caribbean dolphins also showed the highest variability on δ15N data (Table 2) and 10% ecological niche region overlap with the Canary Islands (Fig. 4). To our knowledge, there are no diet studies conducted in ASDs of this region. Therefore, different feeding habits in the Caribbean dolphins cannot be ignored.
Finally, blubber samples for POP analyses were not available in the Caribbean region apart for one individual (from Guadeloupe Island), restricting the comparison of ecological niche regions to three rather than four areas using POPs besides stable isotopes (Fig. 5). Atlantic spotted dolphins are subjected to different forms of contamination as reflected by the significant differences found for most of the POP groups analysed in the geographical areas studied (Fig. 3). This was also confirmed by the DDT-PCB ratio calculated by Méndez-Fernandez et al. (2018). This ratio is considered a proxy of agricultural and/or public health origins vs. industrial origins (Aguilar et al. 1999; Borrell and Aguilar 2005). São Paulo dolphins had low and significantly different DDT-PCB ratios (0.23) in comparison with those from the Azores, Canary Islands and the Caribbean (0.76, 0.65 and 0.42, respectively). This quotient reflects the important industrialization of the south-eastern coast of Brazil, compared to the effects of intensive agricultural activities and control programs for diseases (e.g. malaria, yellow fever and dengue fever) in the other areas.
Identifying the limitations of the study is necessary to delineate its range of validity. Sampling from wildlife fauna and stranded individuals does not often allow examination of interannual or seasonal variation since it is very unlikely that sufficient individuals were available. Moreover, the different integration time of elements and compounds in organisms and the different tissues turnover makes this task very complex. However, and despite the potential biases of the sampling regime, this study provides a unique possibility to compare potential populations of ASD at its distribution scale and at a time-frame that properly allows identifying ecologically different units.
Contribution to Atlantic spotted dolphin conservation
This study contributes to the understanding of ASD population structure and helps define EMUs at a shorter time scale (months to years) and larger spatial scale that what has been done so far. In the light of the above findings, there is a population differentiation for ASD that should be structured in at least two different EMUs: the Northeast Atlantic Ocean including Azores and Canary dolphins, which moreover may belong to the offshore morphotype, and the South/Central West Atlantic Ocean including Caribbean and São Paulo dolphins. This structuration may reflect a morphotype division; however, further investigations using morphometric data should be done in order to validate this hypothesis. The potential isolation of south-eastern Brazil dolphins that was previously proposed by several authors is less evident in our study. However, POP concentrations (a longer time-scale marker) show a quite clear different pattern of São Paulo dolphins (Fig. 3). Thereby, considering the lack of blubber samples from Caribbean dolphins this isolation cannot be discarded.
The Atlantic spotted dolphin faces different threats over its distribution range. Incidental catches in fisheries are known for several areas of the range (Brazil, the Caribbean, off the east coast of the USA, and in Mauritania). Some are probably also taken incidentally in tuna purse seines off the West African coast and Azores (Van Waerebeek et al. 2000; Cruz et al. 2018). The ASD carcasses are also used for shark bait and human consumption in Caribbean Islands (St. Vincent and the Grenadines) and Venezuela (Perrin et al. 1994), and potential impact of the gas and oil exploration and exploitation in the Gulf of Mexico should not be overlooked (Viricel and Rosel 2014). Added to all this, the POP concentrations obtained by Méndez-Fernandez et al. (2018) demonstrate that dolphins from Canary Islands and São Paulo have the highest concentrations of PCBs, DDTs and PBDEs. This implies that, regardless of whether São Paulo dolphins are an isolated population, their vulnerability to POP concentrations is greater and should be considered in future local management actions.
Finally, and in the light of the results presented, authors emphasize the need for an integrated approach coupling ecological and molecular markers together with a high-density sampling, including animals that are north of 18°S. This multi-approach study could clarify the presence of one or two management units along the coasts of Central and South America and on an evolutionary and ecological time scale.
References
Adams LD, Rosel PE (2006) Population differentiation of the Atlantic spotted dolphin (Stenella frontalis) in the western North Atlantic, including the Gulf of Mexico. Mar Biol 148:671–681
Aguilar A, Borrell A, Pastor T (1999) Biological factors affecting variability of persistent pollutant levels in cetaceans. J Cetacean Res Manag 1:83–116
Andrewartha HG, Birch LC (1984) The ecological web. University Chicago Press, Chicago
Ballard JWO, Whitlock MC (2004) The incomplete natural history of mitochondria. Mol Ecol 13(4):729–744
Banguera-Hinestroza E, Bjørge A, Reid RJ, Jepson P, Hoelzel AR (2010) The influence of glacial epochs and habitat dependence on the diversity and phylogeography of a coastal dolphin species: Lagenorhynchus albirostris. Conserv Genet 11(5):1823–1836
Born EW, Outridge P, Riget FF, Hobson KA, Dietz R et al (2003) Population substructure of North Atlantic minke whales (Balaenoptera acutorostrata) inferred from regional variation of elemental and stable isotopic signatures in tissues. J Mar Syst 43(1–2):1–17
Borrell A, Aguilar A (2005) Differences in DDT and PCB residues between common and striped dolphins from the southwestern Mediterranean. Arch Environ Contam Toxicol 48(4):501–508
Borrell A, Aguilar A, Tornero V, Sequeira M, Fernandez G, Alis S (2006) Organochlorine compounds and stable isotopes indicate bottlenose dolphin subpopulation structure around the Iberian Peninsula. Environ Intern 32:516–523
Caballero S, Santos MCdO, Sanches A, Mignucci-Giannoni AA (2013) Initial description of the phylogeography, population structure and genetic diversity of Atlantic spotted dolphins from Brazil and the Caribbean, inferred from analyses of mitochondrial and nuclear DNA. Biochem Syst Ecol 48:263–270
Cañadas A, Sagarminaga R, Garcia-Tiscar S (2002) Cetacean distribution related with depth and slope in the Mediterranean waters off southern Spain. Deep Sea Res 49:2053–2073
Caurant F, Chouvelon T, Lahaye V, Méndez-Fernandez P, Rogan E et al (2009) The use of ecological tracers for discriminating populations: the case of the short-beaked common dolphin Delphinus delphis in the European Atlantic waters. Rep Int Whal Comm Spec Issue, Madeira
Coyle T (1998) Stock identification and fisheries management: the importance of using several methods in a stock identification study. In: Hancock DA (ed) Taking stock: defining and managing shared resources. Australian Society for Fishery Biology, Sydney, pp 173–182
Crawford TJ (1984) What is a population? In: Shorrocks B (ed) Evolutionary ecology. Blackwell, Oxford, pp 135–173
Cruz MJ, Machete M, Menezes G, Rogan E, Silva MA (2018) Estimating common dolphin bycatch in the pole-and-line tuna fishery in the Azores. PeerJ 6:4285
Danilewicz D, Ott PH, Secchi ER, Andriolo A, Zerbini AN (2013) Occurrence of the Atlantic spotted dolphin, Stenella frontalis, in southern Abrolhos Bank, Brazil. MB Records 6
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506
Di Beneditto AM, Ramos RMA, Siciliano S, Santos RA, Bastos G, Fagundesnetto E (2001) Stomach contents of delphinids from Rio de Janeiro, southeastern Brazil. Aquat Mamm 27:24–28
Do Amaral KB, Alvares DJ, Heinzelmann L, Borges-Martins M, Siciliano S, Moreno IB (2015) Ecological niche modeling of Stenella dolphins (Cetartiodactyla: Delphinidae) in the southwestern Atlantic Ocean. J Exp Mar Biol Ecol 472:166–179
Evans PGH, Teilmann J (2009) Report of ASCOBANS/HELCOM small cetacean population structure workshop. ASCOBANS, Bonn, Germany
Fernandez R, Santos MB, Carrilo M, Tejedor M, Pierce GJ (2009) Stomach contents of cetaceans stranded in the Canary Islands 1996–2006. J Mar Biol Assoc UK 89(5):873–883
Fontaine MC, Baird SJ, Piry S, Ray N, Tolley KA, Duke S et al (2007) Rise of oceanographic barriers in continuous populations of a cetacean: the genetic structure of harbour porpoises in Old World waters. BMC Biol 5(1):30
Fontaine MC, Tolley KA, Michaux JR, Birkun A, Ferreira M et al (2010) Genetic and historic evidence for climate-driven population fragmentation in a top cetacean predator: the harbour porpoises in European water. Proc R Soc Lond B Biol Sci 277(1695):2829–2837
Foote AD, Newton J, Piertney SB, Willerslev E, Gilbert MTP (2009) Ecological, morphological and genetic divergence of sympatric North Atlantic killer whale populations. Mol Ecol 18(24):5207–5217
Fruet PF, Dalla Rosa L, Genoves RC, Valiati VH, De Freitas TRO, Möller LM (2016) Biopsy darting of common bottlenose dolphins (Tursiops truncatus) in southern Brazil: evaluating effectiveness, short-term responses and wound healing. Lat Am J Aquat Mamm 11(1–2):121–132
Fullard KJ, Early G, Heide-Jørgensen MP, Bloch D, Rosing-Asvid A, Amos W (2000) Population structure of long-finned pilot whales in the North Atlantic: a correlation with sea surface temperature? Mol Ecol 9(7):949–958
Geraci JR, Lounsbury VJ (1993) Marine mammals ashore: a field guide for strandings. Texas A and M Sea Grant College Program, pp 304
Giménez J, De Stephanis R, Gauffier P, Esteban R, Verborgh P (2011) Biopsy wound healing in long-finned pilot whales (Globicephala melas). Vet Record 168(4):101
Giménez J, Gómez-Campos E, Borrell A, Cardona L, Aguilar A (2013) Isotopic evidence of limited exchange between Mediterranean and eastern North Atlantic fin whales. Rapid Com Mass Spectrosc 27(15):1801–1806
Giménez J, Ramírez F, Almunia J, Forero MG, De Stephanis R (2016) From the pool to the sea: applicable isotope turnover rates and diet to skin discrimination factors for bottlenose dolphins (Tursiops truncatus). J Exp Mar Biol Ecol 475:54–61
Giménez J, Louis M, Barón E, Ramírez F, Verborgh P et al (2017) Towards the identification of ecological management units: a multidisciplinary approach for the effective management of bottlenose dolphins in the southern Iberian Peninsula. Aquat Cons Mar Fresh Ecosyst 28(1):205–215
Herzing DL, Elliser CR (2013) Nocturnal feeding of Atlantic spotted dolphins (Stenella frontalis) in the Bahamas. Mar Mammal Sci 30:367–373
Hobson KA (1998) Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120:314–326
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes I: turnover of 13C in tissues. Condor 94(1):181–188
ICES (2014) Report of the working group on marine mammals (WGMME), 10–13, Woods Hole, Massachusetts, USA. ICES CM 2014/ACOM:27, 234 pp
Jefferson TA, Webber MA, Pitman RL (eds) (2008) Marine mammals of the world. A comprehensive guide to their identification. Academic Press, London, p 73
Kowarski KA, Augusto JF, Frasier TR, Whitehead H (2014) Effects of remote biopsy sampling on long-finned pilot whales (Globicephala melas) in Nova Scotia. Aquat Mamm 40(2):117–125
Krützen M, Barre LM, Möller LM, Heithaus MR, Simms C, Sherwin WB (2002) A biopsy system for small cetaceans: darting success and wound healing in Tursiops spp. Mar Mamm Sci 18:863–878
Kuiken T, Hartmann M (1991) Proceedings of the first European Cetacean Society workshop on ‘Cetacean pathology: dissection techniques and tissue sampling’. ECS Newslett 17:1–39
Lopes XM, Santos MCdO, Silva ED, Bassoi M, Santos RAD (2012) Feeding habits of the atlantic spotted dolphin, Stenella frontalis, in southeastern Brazil. Braz J Oceanogr 60(2):189–198
Lyrholm T, Leimar O, Johanneson B, Gyllensten U (1999) Sex–biased dispersal in sperm whales: contrasting mitochondrial and nuclear genetic structure of global populations. Proc R Soc Lond B Biol Sci 266(1417):347–354
McMahon KW, Hamady LL, Thorrold SR (2013) A review of ecogeochemistry approaches to estimating movements of marine animals. Limnol Oceanogr 58(2):697–714
Melo CLL, Santos RA, Bassoi M, Araújo AC, Lailson-Brito J, Dorneles PR, Azevedo AF (2010) Feeding habits of delphinids (Mammalia: Cetacea) from Rio de Janeiro State, Brazil. J Mar Biol Assoc UK 90(8):1509–1515
Méndez-Fernandez P, Taniguchi S, Santos MCdO, Cascão I, Quérouil S et al (2018) Contamination status by persistent organic pollutants of the Atlantic spotted dolphin (Stenella frontalis) at the metapopulation level. Environ Poll 236:785–794
Mesa-Gutiérrez R, Barragán-Barrera D, Chávez-Carreño D, Farías-Curtidor N, Caballero S (2015) Population structure of the Atlantic spotted dolphin (Stenella frontalis) in the Caribbean. MsC thesis, Universidad de los andes, Colombia
Moreno IB, Zerbini A, Danilewicz D, Santos MCdO, Simões-Lopes PC et al (2005) Distribution and habitat characteristics of dolphins of the genus Stenella (Cetacea: Delphinidae) in the Southwest Atlantic Ocean. Mar Ecol Prog Ser 300:229–240
Murphy S, Mirimin L, Rogan E (2007) Stock structure reports for bottlenose dolphins Tursiops truncatus, striped dolphins Stenella coeruleoalba, and white-sided dolphins Lagenorhynchus acutus in the Northeast Atlantic. NECESSITY Contract 501605 Periodic Activity Report No 2
Murphy S, Natoli A, Amaral AR, Mirimin L, Viricel A et al (2009) Short-beaked common dolphin Delphinus delphis. In: ASCOBANS/HELCOM Small cetacean population structure workshop. 8–10 October 2007 at UN Campus, Hermann-Ehlers-Str. 10, 53113 Bonn, Germany 111–130
Nieri M, Grau E, Lamarche B, Aguilar A (1999) Mass mortality of Atlantic spotted dolphins (Stenella frontalis) caused by a fishing interaction in Mauritania. Mar Mamm Sci 15:847–854
Nordstrom CA, Wilson LJ, Sara IJ, Tollit DJ (2008) Evaluating quantitative fatty acid signature analysis (QFASA) using harbour seals Phoca vitulina richardsi in captive feeding studies. Mar Ecol Prog Ser 360:245–263
Oremus M (2008) Genetic and demographic investigation of population structure and social system in four delphinid species. PhD thesis, University of Auckland
Paro AD, Rojas E, Wedekin LL (2014) Southernmost record of the Atlantic spotted dolphin, Stenella frontalis in the south-west Atlantic Ocean. Mar Biol Assoc 7:e78
Perrin WF (2002) Stenella frontalis. Mamm Species 702:1–6
Perrin WF, Mitchell ED, Mead JG, Caldwell DK, Caldwell MC et al (1987) Revision of the spotted dolphins, Stenella spp. Mar Mamm Sci 3:99–170
Perrin WF, Caldwell DK, Caldwell MC (1994) Atlantic spotted dolphin Stenella frontalis (Cuvier G 1829). In: Ridgway SH, Harrison R (eds) Handbook of marine mammals, vol 5. The first book of Dolphins. Academic Press, New York, pp 173–190
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Ann Rev Ecol Syst 18(1):293–320
Quérouil S, Silva MA, Cascão I, Magalhães S, Seabra MI et al (2008) Why do dolphins form mixed-species associations in the Azores? Ethology 114(12):1183–1194
Quérouil S, Freitas L, Cascão I, Alves F, Dinis A, Almeida JR et al (2010) Molecular insight into the population structure of common and spotted dolphins inhabiting the pelagic waters of the Northeast Atlantic. Mar Biol 157(11):2567–2580
Quérouil S, Kiszka J, Cordeiro AR, Cascão I, Freitas L et al (2013) Investigating stock structure and trophic relationships among island-associated dolphins in the oceanic waters of the North Atlantic using fatty acid and stable isotope analyses. Mar Biol 160(6):1325–1337
R Core Team (2013) R: A Language and Environment for Statistical Computing. RFoundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Selkoe KA, Toonen RJ (2006) Microsatellites for ecologists: a practical guide to using and evaluating microsatellite markers. Ecol Lett 9(5):615–629
Silva MA, Prieto R, Cascão I, Seabra MI, Machete M et al (2014) Spatial and temporal distribution of cetaceans in the mid-Atlantic waters around the Azores. Mar Biol Res 10(2):123–137
Swanson HK, Lysy M, Power M, Stasko AD, Johnson JD, Reist JD (2015) A new probabilistic method for quantifying n-dimensional ecological niches and niche overlap. Ecology 96:318–324
Van Waerebeek K, Ndiaye E, Djiba A, Diallo M (2000) A survey of the conservation status of cetaceans in Senegal, the Gambia and Guinea-Bissau WAFCET-I Report. Convention on the Conservation of Migratory Species (CMS); UNEP
Viricel A, Rosel PE (2014) Hierarchical population structure and habitat differences in a highly mobile marine species: the Atlantic spotted dolphin. Mol Ecol 23(20):5018–5035
Weijs L, Covaci A, Das K, Blust R (2010) Physiologically based pharmacokinetic (PBPK) models for the bioaccumulation of PBDEs in male harbour porpoises. Organohalogen Compd 72:672–675
Weijs L, Covaci A, Yang RSH, Das K, Blust R (2011) A non-invasive approach to study lifetime exposure and bioaccumulation of PCBs in protected marine mammals: PBPK modeling in harbor porpoises. Toxicol Appl Pharmacol 256:136–145
Weir CR (2010) A review of cetacean occurence in West African waters from the Gulf of Guinea to Angola. Mamm Rev 40:2–39
Wiszniewski J, Lusseau D, Möller LM (2010) Female bisexual kinship ties maintain social cohesion in a dolphin network. Anim Behav 80(5):895–904
Xu S, Ren W, Zhou X, Zhou K, Yang G (2010) Sequence polymorphism and geographical variation at a positively selected MHC-DRB gene in the finless porpoise (Neophocaena phocaenoides): implication for recent differentiation of the Yangtze finless porpoise? J Mol Evol 71:6–22
Acknowledgements
This work has been made possible thanks to all members of the institutions and organizations that assist with field and data collection. We also gratefully acknowledge B. Lebreton and G. Guillou from Littoral Environnement et Sociétés (LIENSs) of the University of La Rochelle as well as the personnel of the Organic Marine Chemistry Laboratory (LabQOM) of the Instituto Oceanográfico da Universidade de São Paulo for their help on chemical analyses. We also wish to thank J. Kiszka for his help on data collection. This work was supported through the project “Population structure and contamination status of Atlantic spotted dolphin (Stenella frontalis)” funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 406814/2013-9). PMF was supported by a post-doctoral grant from the CNPq under the program “Ciências sem Fronteiras”, and by a fieldwork grant of the Society for Marine Mammalogy (SMM). Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) funded research on mapping cetacean occurrence and biopsy sampling along the coast of São Paulo state from 2013 to 2015 (Auxílio à Pesquisa, process # 2011/51543-9). MCOS was sponsored by the CNPq under the program Produtividade em Pesquisa from 2011 to 2014 (process # 308331/2010-9). Fieldwork in Colombia was supported by the Rufford Foundation, the Society for Marine Mammalogy, and Sciences Faculty of Universidad de los Andes (“Proyecto Semilla” – Calls 2013-3/2015-1, projects: “Diversidad genética del delfín nariz de botella en la Guajira, Caribe colombiano” and “Ocurrencia, distribución y estatus genético preliminar de delfínidos en la Guajira, Caribe colombiano”). Finally, authors kindly wish to thanks the two anonymous reviewers for their great and constructive revision.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Telesphore Sime-Ngando
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Méndez-Fernandez, P., Taniguchi, S., Santos, M.C.O. et al. Population structure of the Atlantic spotted dolphin (Stenella frontalis) inferred through ecological markers. Aquat Ecol 54, 21–34 (2020). https://doi.org/10.1007/s10452-019-09722-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-019-09722-3