Abstract
The adsorption behaviors of CO2 and CH4 on new siliceous zeolites JSR and NanJSR (n = 2, 8, 16) were simulated using the Grand Canonical Monte Carlo method. The adsorption isotherms of CO2 became higher with an increase in the Na+ number at a low pressure range (<150 kPa), whereas the isotherms showed a crossover with increasing pressure and the adsorption amount became smaller at a high pressure range (>850 kPa). With an increase in Na+ number, the pore volume decreased as the pore space was occupied by increasing Na+ ions. Additionally, two energy peaks on the interaction energy curves implied that CO2 was adsorbed on two active sites. On the other hand, the adsorption amount of CH4 decreased with an increase in the Na+ number and only one energy peak was observed. Adsorption isotherms were well fitted with the Langmuir and Freundlich equations up to 1000 kPa and the adsorption affinity of CO2 on Na16JSR zeolite was highest. The adsorption capacities of CO2 in the studied zeolites were up to 38 times higher than those of CH4. Diffusion constants of CO2 and CH4 decreased with an increase in the adsorbed amount and Na+ number. Considering the adsorbed amount, adsorption selectivity and affinity, zeolites JSR with a low Na+ number (JSR and Na2JSR) is a good candidate for a pressure swing adsorption in the separation of CO2/CH4 mixture whereas JSR zeolites with high Na+ ratios (Na16JSR and Na8JSR) may be a better selection for a vacuum swing adsorption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Separation of CH4 and CO2 mixtures is a challenging research topic due to environmental and economic concerns. Several separation technologies have been introduced for CH4 and CO2 mixtures (Singh et al. 2009; Scholes et al. 2012; Kim et al. 2006). Due to excellent separation and energy efficiency, adsorptive processes have been widely studied for post-combustion CO2 capture (Ebner and Ritter 2009), biogas purification (Alonso-Vicario et al. 2010), and the purification of coal mine methane and coal mine ventilation air (Olajossy et al. 2003; Lee et al. 2013). In the adsorptive cyclic processes, the adsorbent is a decisive factor for process feasibility and separation cost. According to many previous reports (Ackley et al. 2003; Yang et al. 2012; Ju et al. 2015; Mofarahi and Gholipour 2014), zeolites are considered as potential adsorbents for the separation of CH4 and CO2 mixtures.
Zeolites are crystalline, microporous materials with a large surface area and a molecular- sized pore structure. To date, more than 200 types of zeolites were reported, and each year a few new zeolites are distributed from IZA-SC (Structure Commission of the International Zeolite Association). The extra-framework cations in zeolites play a significant role in determining their adsorptive behaviors (Sebastian et al. 2007). Therefore, zeolites are commercially and widely applied to the fields of separation, purification, ion exchange and catalysis. However, there are inherent difficulties in quickly and intuitionally evaluating the adsorption behavior and capacity on new-issued zeolites.
In this study, JSR and NanJSR (modified JSR with Na+), the newly issued zeolites, were selected as candidate adsorbents for the separation of CO2 and CH4 mixture. And the Grand Canonical Monte Carlo (GCMC) simulation was employed to understand the adsorption behaviors of CO2 and CH4 on JSR and NanJSR up to 1000 kPa. Then, the influence of Si/Al ratios and the Na+ number on the adsorption behaviors of CO2 and CH4 was determined by four types of zeolite JSR (JSR, Na2JSR, Na8JSR and Na16JSR). The results can contribute to selecting a proper adsorbent for effective gas separation processes.
2 Computational methods
2.1 Zeolite structures
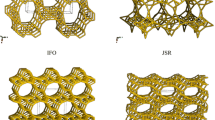
In the study, a model of the silica JSR constructed in Xu’s group was used for the selected JSR zeolites (Xu et al. 2013). The zeolite structure is described in Pa-3 space group. The crystal parameters are a = 1.9845 nm, b = 1.9845 nm, c = 1.9845 nm, α = 90°, β = 90°, and γ = 120°.
The box in the simulation contained 8 unit cells (2 × 2 × 2) of the zeolite JSR. To construct the framework of NanJSR with a certain Si/Al ratio, Si atoms were partially substituted by Al atoms, and Na cations were added into the cell to compensate for the total charge to zero. Framework structures with various Si/Al ratios were obtained by replacing different numbers of Si atoms with Al atoms in the simulation box. For example, the Si/Al molar ratio of Na2JSR with ten Al atoms in a simulation box was about 47. The structures of zeolites NanJSR with various Si/Al ratios were optimized by the energy minimization method to search for a conformational space of low energy structures. This method can provide not only reasonable configurations of NanJSR but also appropriate positions of all Na+ ions. The structural properties for the simulation box of NanJSR are listed in Table 1.
2.2 Simulation techniques
The Grand Canonical Monte Carlo (GCMC) method was employed in this work, which is widely applied in the field of adsorption (Metropolis et al. 1953). The GCMC method used a grand canonical ensemble, in which the chemical potential of each species, the volume of system and the temperature were fixed (Agnihotri et al. 2008; Biswas and Cagin 2010). It was reported that the simulation reasonably predicted the experimental data (Rahmati and Modarress 2013; Nugent et al. 2013). In the present work, each zeolite framework was considered to be rigid. All the interactions of gas-zeolite and gas–gas were modeled using the PCFF force field (Hill and Sauer 1994; Sun 1995), which had been proved as a powerful force field to simulate adsorption in various zeolite systems (Song et al. 2002; Yang et al. 2008; Zhao et al. 2012).
The cut-off distance for the calculation of van der Waals potential energy was taken as 1.3 nm based on the atom-based technique. The partial charges of atoms were estimated by the charge-equilibration method (Rappe and Goddard 1991), and the electrostatic energy was calculated using the Ewald summation method (Karasawa and Goddard 1989) with an accuracy of 0.0001 kcal mol−1. The partial pressure for describing the chemical potential of adsorbed molecules was calculated by using an ideal gas law.
In the GCMC simulation, adsorbate molecules in the zeolites were translated, rotated, created and deleted from the zeolite framework at a random with a probability of 0.225, 0.225, 0.275 and 0.275, respectively (Rahmati and Modarress 2009; Rahmati et al. 2012). For every state, 1 × 107 configurations were generated. The first 5 × 106 steps were used to achieve equilibration, and the last 5 × 106 steps were utilized to calculate the required adsorption properties. Periodic boundary conditions were applied to three-dimensions in order to simulate a definite system. Further details of the GCMC method can be found in the previous study (Dubbeldam et al. 2004a, b).
In this study, the diffusion of CO2 and CH4 on each JSR was also simulated using Newton’s equations of motion until the average properties of the system did not change with time. Molecular dynamics (MD) simulations with the canonical (NVT) ensemble and periodic boundary conditions were performed to study the diffusion behaviors of CO2 and CH4 in the selected zeolites using a Forcite module in the Materials Studio. During the initializing period, an NVT MC simulation was utilized to rapidly achieve an equilibrium molecular arrangement. After the initialization step, the velocities of pseudo-atoms from the Boltzmann distribution were assigned at the desired average temperature. The total momentum of the system was set to zero. Then, the NVT MD simulation was further carried out for the system using the Nose–Hoover thermostat (Martyna et al. 1996). In the MD simulation, a simulation time step of 1.0 fs was employed, which was sufficiently small to ensure good energy conservation. 200 ps was initially allowed for the equilibrium of system and was followed by 400 ps in NVT-MD simulation. And the trajectories were saved every 1000 steps for further analysis.
The mean square displacement (MSD) obtained from the simulation works, the self- diffusivities (D), was applied to the analysis with the following Einstein equation:
In this equation, N a represents the number of molecules of species i and r i (t) is the position of species i at a time t.
3 Results and discussion
3.1 Comparisons with experimental data
For the verification of the simulation techniques used, the adsorption isotherms of CO2 and CH4 in the zeolite MFI were compared with the experimental data (Babarao et al. 2007; Krishna and van Baten 2007; Zhu et al. 2006; Golden and Sircar 1994). As shown in Fig. 1, a good agreement between the simulation results and experimental isotherms was observed. It implied that the simulation method in the study was feasible for evaluating and estimating the adsorption behaviors of CO2 and CH4 on purely siliceous zeolites. Therefore, the simulation method was applied to the CO2 and CH4 adsorption in the zeolites JSR and NanJSR in the study.
The adsorption isotherms of CO2 and CH4 in purely siliceous zeolite MFI: a CO2 (square), literature data from (Babarao et al. 2007), 300 K (circle), literature data from (Krishna and van Baten 2007), 303 K (triangle), this work, 298 K. b CH4 (square), literature data from (Zhu et al. 2006), 303 K (circle), literature data from (Golden and Sircar 1994), 304 K (triangle), this work, 300 K
3.2 Adsorption of CO2 and CH4
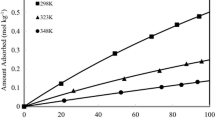
Adsorption isotherms of CO2 and CH4 in JSR, Na2JSR, Na8JSR and Na16JSR at 298 K were presented in Fig. 2. After the CO2 adsorption isotherms of the zeolites NanJSR with high Na+ ratios (Na8JSR and Na16JSR) increased steeply at a low pressure range (<150 kPa), it approached asymptotically equilibrium saturation. On the other hand, the adsorption isotherms of JSR and Na2JSR were much lower than those of Na8JSR and Na16JSR at a low pressure range, showing small curvature in an isotherm shape.
At a low pressure range (<150 kPa), the adsorption amount became higher with an increase in Na+ ratio. However, the difference between Na8JSR and Na16JSR was minute. Furthermore, the isotherms of both adsorbents showed a crossover with further increase in pressure. The crossover of isotherms between JSR and Na2JSR was also observed at approximately 500 kPa. In addition, the adsorption amount of CO2 at a high pressure range (>850 kPa) became higher in the JSRs with a low Na+ number due to the crossover of both isotherms.
As shown in Fig. 2b, the isotherms of CH4 was much lower than the corresponding isotherms of CO2, showing almost a linear shape. The adsorption amount became smaller in the whole pressure range as the Na+ number increased. And the difference in the CH4 isotherms between Na8JSR and Na16JSR was relatively larger than that of CO2.
CO2 molecules yield a large quadrupole. Moreover, the polarity of C–O bond and lone pairs of electrons of oxygen atoms produce strong electrostatic interaction with Na+ in the zeolite framework. In addition, the two energy peaks of the adsorption interaction curves between CO2 and NanJSR in Fig. 3 imply that Na+ ions in NanJSR also creates extra active adsorption sites at a low pressure. Therefore, the adsorbed amount increased with the Na+ number in the CO2 adsorption.
However, it is noted that the sequence of the CO2 amount adsorbed at a high pressure range (>850 kPa) was opposite to those at a low pressure range due to the steric effect of the Na+ ions occupied in the zeolite structural space (Siriwardane et al. 2003; Zhang et al. 2014). The volume effect of Na+ on adsorption could be quantified by the free volume characterization of the zeolites, which were analyzed by the Connolly surface method (Connolly 1985). The probe molecule selected was the hard spheres with a radius of 0.1 nm and the simulated results for the free volume are summarized in Table 2. The free volume became small with a decrease in the Si/Al ratio or an increase in the Na+ numbers
In the case of CH4 adsorption, CH4 molecules with a tetrahedral structure have a weak electrostatic interaction with Na+ in a zeolite framework (Qian et al. 2011), and the steric effect of Na+ on CH4 adsorption caused a decrease in the adsorbed amount. No extra active adsorption sites between CH4 and NanJSR appeared in Fig. 4. It implies that the contribution of Na+ ions to CH4 adsorption is not significant. According to the comparison of the adsorption energy between CH4 and CO2 in different zeolites NanJSR as shown in Figs. 3 and 4, the adsorption process is exothermic, but the adsorption affinity of CO2 is much higher than that of CH4. And the extra peak intensity of the adsorption energy turned weaker when the pressure became higher.
Table 3 shows that the adsorption capacities of CO2 in these zeolites are up to 38 times higher than those of CH4 in terms of the ratios of equilibrium adsorption capacity. Equilibrium selectivity between CO2 and CH4 increased with an increase in Na+ numbers, while it decreased with an increase in the adsorption pressure. Considering the adsorption capacity, selectivity and regeneration, the zeolites Na8JSR and Na16JSR are good candidates for the separation of the CO2/CH4 mixture at vacuum swing adsorption processes because the interaction energy between CO2 and Na16JSR is quite strong at a low pressure range. On the other hand, zeolites JSR and Na2JSR are recommended for pressure swing adsorption processes when the feed pressure is relatively high due to the high adsorption capacity and relatively easy regeneration.
3.3 Prediction of adsorption isotherms
Adsorption isotherms of CO2 and CH4 on zeolites JSR and NanJSR were fitted with the Langmuir Eq. (1) and Freundlich Eq. (2), respectively.
Where q is adsorption amount, mmol/g; q m denotes saturated adsorption amount, mmol/g; p represents adsorption pressure,kPa; b and n are Langmuir constant and Freundlich constant, respectively; k is a constant correlated with the characteristics between adsorbents and adsorbates.
The predicted results are shown in Figs. 5 and 6, and the fitting parameters are listed in Table 4. The experimental adsorption isotherms were well fitted with the Langmuir and Freundlich isotherms, but the Freundlich isotherms were slightly better than the Langmuir isotherms. As can be expected from Figs. 3 and 4, the affinity constant, b or n, between CO2 and zeolite NanJSR increased with the number of Na+ in Table 4, which indicated increased adsorption affinity with an increase in the Na+ number. However, the difference of affinity constant, b or n, between CH4 and zeolite NanJSR was small, which demonstrated a minute contribution of Na+ in zeolite NanJSR to the adsorption of CH4.
3.4 Diffusion constants
According to the simulation method mentioned above, the diffusion constants of CO2 and CH4 on JSR, Na2JSR, Na8JSR and Na16JSR zeolites at 298 K are presented in Fig. 7. The results denoted that their diffusion constants decreased with an increase in the adsorption amount and the Na+ number. Furthermore, the diffusion of CH4 was faster than that of CO2 in the same zeolite. The variation of the diffusion constant of CO2 on Na8JSR and Na16JSR zeolites with a loading amount was minute. However, the small variation of the diffusion constant was only observed at Na16JSR in CH4 adsorption, and the difference of diffusion between JSR and Na16JSR zeolites was much higher than that in the CO2 adsorption. The phenomena stemmed from the relatively high interaction energy of adsorbate molecules and steric effect of Na+ in the CO2 adsorption.
4 Conclusions
The GCMC techniques were employed to simulate adsorption behaviors of CO2 and CH4 in zeolites JSR and NanJSR. The adsorption isotherms of CO2 and CH4 in zeolites JSR and NanJSR were obtained from the constructed frameworks of the zeolites with various Si/Al ratios.
The pore volume of the zeolites played important roles in the adsorption behaviors, and they decreased with an increase in the Na+ number. The adsorption amount of CO2 was higher with the Na+ number at a low pressure range (<150 kPa). However, the result became opposite as in the Na+ number increased at a high pressure range (>850 kPa). CO2 was adsorbed on two active sites in the zeolites because two energy peaks were observed from the interaction energy curves. CO2 isotherms on Na8JSR and Na16JSR reached adsorption saturation at a relatively low pressure range after showing a steep increase of the isotherms with pressure. On the other hand, the adsorption amount of CH4 became smaller with an increase in the Na+ number and only one energy peak was observed. It implied that introduction of more Na+ was not beneficial to CH4 adsorption. Adsorption energy indicated that the adsorption process was exothermic, but extra peak intensity of the CO2 adsorption energy turned weak with increasing pressure.
Adsorption isotherms of CO2 and CH4 were well fitted with the Langmuir and Freundlich equations, showing slightly better results in the Freundlich isotherms. The adsorption capacities of CO2 in these zeolites were up to 38 times larger than those of CH4 based on the ratios of the equilibrium adsorption capacity. Diffusion constants of CO2 and CH4 in the zeolites decreased with an increase in the adsorption amount and the Na+ number. Considering the adsorption affinity, capacity and selectivity, zeolites JSR with a low Na+ number (JSR and Na2JSR) can be regenerated by a pressure swing in a cyclic adsorptive process for the separation of CO2/CH4 mixture. And JSR zeolites with a high Na+ number (Na16JSR and Na8JSR) may need a vacuum for regeneration because of the strong adsorption affinity of CO2 under high CO2/CH4 selectivity.
References
Ackley, M.W., Rege, S.U., Saxena, H.: Application of natural zeolites in the purification and separation of gases. Microporous Mesoporous Mater. 61, 25–42 (2003)
Agnihotri, S., Kim, P., Zheng, Y., Mota, J.P.B., Yang, L.: Regio selective competitive adsorption of water and organic vapor mixtures on pristine single-walled carbon nanotube bundles. Langmuir 24, 5746–5754 (2008)
Alonso-Vicario, A., Ochoa-Gómez, J.R., Gil-Río, S., Gómez-Jiménez-Aberasturi, O., Ramírez-López, C.A., Torrecilla-Soria, J., Domínguez, A.: Purification and upgrading of biogas by pressure swing adsorption on synthetic and natural zeolites. Microporous Mesoporous Mater. 134, 100–107 (2010)
Babarao, R., Hu, Z., Jiang, J., Chempath, S., Sandler, S.I.: Storage and separation of CO2 and CH4 in silicalite, C168 schwarzite, and IRMOF-1: a comparative study from Monte Carlo simulation. Langmuir 23, 659–666 (2007)
Biswas, M.M., Cagin, T.: Simulation studies on hydrogen sorption and its thermodynamics in covalently linked carbon nanotube scaffold. J. Phys. Chem. B 114, 13752–13763 (2010)
Connolly, M.L.: Computation of molecular volume. J. Am. Chem. Soc. 107, 1118–1124 (1985)
Dubbeldam, D., Calero, S., Vlugt, T.J.H., Krishna, R., Maesen, T.L.M., Smit, B.: United atom force field for alkanes in nanoporous materials. J. Phys. Chem. B 108, 12301–12313 (2004a)
Dubbeldam, D., Calero, S., Vlugt, T.J.H., Krishna, R., Maesen, T.L.M., Beerdsen, E., Smit, B.: Force field parametrization through fitting on inflection points in isotherms. Phys. Rev. Lett. 93, 088302 (2004b)
Ebner, A.D., Ritter, J.A.: State-of-the-art adsorption and membrane separation processes for carbon dioxide production from carbon dioxide emitting industries. Sep. Sci. Technol. 44, 1273–1421 (2009)
Golden, T.C., Sircar, S.: Gas adsorption on silicalite. J. Colloid Interface Sci. 162, 182–188 (1994)
Hill, J.R., Sauer, J.: Molecular mechanics potential for silica and zeolite catalysts based on Ab initio calculations. 1. Dense and microporous silica. J. Phys. Chem. 98, 1238–1244 (1994)
Ju, Y.S., Park, Y.H., Park, D.Y., Kim, J.J., Lee, C.H.: Adsorption kinetics of CO2, CO, N2 and CH4 on zeolite LiX pellet and activated carbon granule. Adsorption 21, 419–432 (2015)
Karasawa, N., Goddard, W.A.: Acceleration of convergence for lattice sums. J. Phys. Chem. 93, 7320–7327 (1989)
Kim, M.B., Bae, Y.S., Choi, D.K., Lee, C.H.: Kinetic separation of landfill gas by a two-bed pressure swing adsorption process packed with carbon molecular sieve: nonisothermal operation. Ind. Eng. Chem. Res. 45, 5050–5058 (2006)
Krishna, R., van Baten, J.M.: Using molecular simulations for screening of zeolites for separation of CO2/CH4 mixtures. Chem. Eng. J. 133(1–3), 121–131 (2007)
Lee, H.H., Kim, H.J., Shi, Y., Keffer, D., Lee, C.H.: Competitive adsorption of CO2/CH4 mixture on dry and wet coal from subcritical to supercritical conditions. Chem. Eng. J. 230, 93–101 (2013)
Martyna, G., Tuckerman, M., Tobias, D., Klein, M.: Explicit reversible integrators for extended systems dynamics. Mol. Phys. 87, 1117–1157 (1996)
Metropolis, N., Rosenbluth, A.W., Rosenbluth, M.N., Teller, A.H., Teller, E.: Equation of state calculations by fast computing machines. J. Chem. Phys. 21, 1087–1092 (1953)
Mofarahi, M., Gholipour, F.: Gas adsorption separation of CO2/CH4 system using zeolite 5A. Microporous Mesoporous Mater. 200, 1–10 (2014)
Nugent, P., Belmabkhout, Y., Burd, S.D., Cairns, A.J., Luebke, R., Forrest, K., Pham, T., Ma, S., Space, B., Wojtas, L., Eddaoudi, M., Zaworotko, M.J.: Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 495, 80–84 (2013)
Olajossy, A., Gawdzik, A., Budner, Z., Dula, J.: Methane separation from coal mine methane gas by vacuum pressure swing adsorption. Chem. Eng. Res. Des. 81, 474–482 (2003)
Qian, H.Q., Li, B.R., Zhang, L.H.: The molecular simulation of CO2 and CH4 adsorption in siliceous MFI and MFI(2Na+) zelolites. Ion. Exch. Adsorpt. 27, 546–554 (2011)
Rahmati, M., Modarress, H.: Selectivity of new siliceous zeolites for separation of methane and carbon dioxide by monte carlo simulation. Microporous Mesoporous Mater. 176, 168–177 (2013)
Rahmati, M., Modarress, H.: Nitrogen adsorption on nanoporous zeolites studied by grand canonical Monte Carlo simulation. J. Mol. Struct. Theochem. 901, 110–116 (2009)
Rahmati, M., Modarress, H., Gooya, R.: Molecular simulation study of polyurethane membranes. Polymer 53, 1939–1950 (2012)
Rappe, A.K., Goddard, W.A.: Charge equilibration for molecular dynamics simulations. J. Phys. Chem. 95, 3358–3363 (1991)
Scholes, C.A., Stevens, G.W., Kentish, S.E.: Membrane gas separation applications in natural gas processing. Fuel 96, 15–28 (2012)
Sebastian, J., Pillai, R.S., Peter, S.A., Jasra, R.V.: Sorption of N2, O2, and Ar in Mn(II)- exchanged Zeolites A and X using volumetric measurements and Grand Canonical Monte Carlo simulation. Ind. Eng. Chem. Res. 46, 6293–6302 (2007)
Singh, P., Niederer, J.P.M., Versteeg, G.F.: Structure and activity relationships for amine-based CO2 absorbents-II. Chem. Eng. Res. Des. 87, 135–144 (2009)
Siriwardane, R.V., Shen, M.S., Fisher, E.P.: Adsorption of CO2, N2, and O2 on natural zeolites. Energy Fuels 17, 571–576 (2003)
Song, L., Sun, Z.L., Rees, L.V.C.: Experimental and molecular simulation studies of adsorption and diffusion of cyclic hydrocarbons in silicalite-1. Microporous Mesoporous Mater. 55, 31–49 (2002)
Sun, H.: Ab initio calculations and force field development for computer simulation of polysilanes. Macromolecules 28, 701–712 (1995)
Xu, Y., Li, Y., Han, Y., Song, X.W., Yu, J.H.: A gallogermanate zeolite with eleven-membered -ring channels. Angew. Chem. Int. Ed. 52, 5611–5613 (2013)
Yang, J.Z., Chen, Y., Zhu, A.M., Liu, Q.L., Wu, J.Y.: Analyzing diffusion behaviors of methanol/water through MFI membranes by molecular simulation. J. Membr. Sci. 318, 327–333 (2008)
Yang, J., Zhao, Q., Xu, H., Li, L., Dong, J., Li, J.: Adsorption of CO2, CH4, and N2 on gas diameter grade ion-exchange small pore zeolites. J. Chem. Eng. Data 57, 3701–3709 (2012)
Zhang, J.F., Burke, N., Zhang, S.C., Liu, K.Y., Pervukhina, M.: Thermodynamic analysis of molecular simulations of CO2 and CH4 adsorption in FAU zeolites. Chem. Eng. Sci. 113, 54–61 (2014)
Zhao, L., Zhai, D., Liu, B., Liu, Z., Xu, C., Wei, W., Chen, Y., Gao, J.: Grand canonical monte carlo simulations for energy gases on PIM-1 polymer and silicalite-1. Chem. Eng. Sci. 68, 101–107 (2012)
Zhu, W., Hrabanek, P., Gora, L., Kapteijn, F., Moulijn, J.A.: Role of adsorption in the permeation of CH4 and CO2 through a silicalite-1 membrane. Ind. Eng. Chem. Res. 45, 767–776 (2006)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21276101, 21106051), the Jiangsu Program for Production, Teaching, and Research (BY2015052-01), the Natural Science Foundation of Jiangsu Universities (16KJA530001), the Foundation of Jiangsu Key Laboratory for Chemistry of Low-Dimensional Materials (JSKC13132), the Program for Excellent Youth of Jiangsu Qing-Lan Project, and the Huaian Program for Production, Teaching, and Research (HAC2015028).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chu, Xz., Liu, Ss., Zhou, Sy. et al. Adsorption behaviors of CO2 and CH4 on zeolites JSR and NanJSR using the GCMC simulations. Adsorption 22, 1065–1073 (2016). https://doi.org/10.1007/s10450-016-9816-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-016-9816-7