Abstract
Atherosclerosis is a major risk factor for cardiovascular disease. However, mechanisms of interaction of atherosclerotic plaque development and local stiffness of the lamellar structure of the arterial wall are not well established. In the current study, the local Young’s modulus of the wall and plaque components were determined for three different groups of healthy, mildly diseased and advanced atherosclerotic human abdominal aortas. Histological staining was performed to highlight the atherosclerotic plaque components and lamellar structure of the aortic media, consisting of concentric layers of elastin and interlamellar zones. The force spectroscopy mode of the atomic force microscopy was utilized to determine Young’s moduli of aortic wall lamellae and plaque components at the micron level. The high variability of Young’s moduli (E) at different locations of the atherosclerotic plaque such as the fibrous cap (E = 15.5± 2.6 kPa), calcification zone (E = 103.7±19.5 kPa), and lipid pool (E = 3.5±1.2 kPa) were observed. Reduction of elastin lamellae stiffness (18.6%), as well as stiffening of interlamellar zones (50%), were detected in the diseased portion of the medial layer of abdominal aortic wall compared to the healthy artery. Additionally, significant differences in the stiffness of both elastin lamellae and interlamellar zones were observed between the diseased wall and disease-free wall in incomplete plaques. Our results elucidate the alternation of the stiffness of different lamellae in the human abdominal aortic wall with atherosclerotic plaque development and may provide new insight on the remodeling of the aortic wall during the progression of atherosclerosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aorta is the largest vessel in the human circulatory system. The proper function of the aorta depends directly on its elastic behavior which may change during physiologic and pathologic processes such as aging, hypertension, and atherosclerosis.2,6 It has been noted that for normotensive and hypertensive patients, the abdominal aorta has a greater involvement with the disease of atherosclerosis than the thoracic aorta.19 The existence of complex flow near the aortic bifurcation may trigger atherosclerotic plaque formation in the abdominal aorta.8,46 The correlation between the progression of atherosclerotic plaque in the abdominal aorta and coronary heart disease has been reported.25
The tunica media is the main load-bearing layer of the aortic wall, which is characterized by concentric lamellar structures. Such lamellar structure is built by the repetition of a structural unit through the thickness of the media. Each lamellar unit consists of an elastin layer and an adjacent interlamellar zone in which smooth muscle cells (SMCs) and extracellular matrix (ECM) fibers, mainly collagen type I and III, are found.52 Elastin fibers engage at low blood pressures while collagen fibers bear the load at higher tensions.5 It is demonstrated that elastin constitutes up to 22% of the dry weight of the human aorta.11 This abundant elastin content allows the aorta to stretch when the ventricle contracts and ejects blood during the systolic phase to maintain and deliver blood flow throughout the entire cardiac cycle.
It is well established that arteries are remodeled in response to new circumstances through persistent changes in composition and size, to maintain their proper function and reduce the possibility of pathologic conditions.51 Previous studies suggested that the induction of non-physiologic ranges of wall tensile stress and wall shear stress respectively due to pulsatile blood pressure and pulsatile blood flow may lead to arterial wall remodeling.28,42 It was shown that with thickening and hardening of the arterial wall, circumferential stress within hypertensive aortic wall remains essentially unchanged in comparison with normotensive arteries.34,44 For instance, in hypertensive rats the number of lamellar units remains constant, while the thickness of interlamellar zones increases.34
Although aortic stiffness has been typically examined at the level of the whole vessel using tensile test,18,26 there is a growing interest in characterizing mechanical properties of the aorta at the micron scale.1 Accessing this information is vital because the lamellar units which govern the overall stiffness of the aorta are detectable at this length scale. Moreover, the mechanical characterization of atherosclerotic plaques mostly developed in the abdominal aorta is important. It is well established that micromechanical properties of the arterial wall and atherosclerotic plaque can influence the orientation, migration, proliferation, and differentiation of both endothelial cells and SMCs13,24,29,53 and may play a role in vascular disease development.29 For instance, it has demonstrated that increasing matrix stiffness promote endothelial permeability by destabilizing cell–cell junction and upgrade leukocyte transmigration into the vessel wall which is crucial for atherosclerotic plaques development.24 It is also of special interest to investigate mechanical properties of the lamellar structure of the abdominal aorta during plaque development. Considering the heterogeneous structure of atherosclerotic lesions, it is difficult to measure the mechanical properties of the arterial wall and atherosclerotic plaque components with tensile test.35 Consequently, there is a need to develop reliable, quantitative methods to obtain the mechanical properties of vascular tissue at the micro-scale.1
Atomic force microscopy (AFM) indentation has introduced as a useful technique for the imaging and mechanical characterization of biological tissues in micro and nano-scales.10,12,21,48 AFM utilizes a sharp probe mounted on a compliant cantilever which allows probing of the surface of biological samples at small indentation depths and low forces. The high spatial resolution of AFM can provide a framework to determine local mechanical properties of the arterial wall and plaque components.21,48 Using nano-indentation Ebenstein et al. determined the stiffness of fibrous tissue, calcification and the blood clot in human atherosclerotic carotid arteries and demonstrated that nano-indentation is a suitable method for the characterization of heterogeneous plaque tissue.15 Lundkvist et al. measured the elastic and viscoelastic properties of healthy femoral arteries using AFM indentation.31 AFM test was also used to measure the Young’s modulus of the subendothelial matrix in bovine carotid arteries. The results showed that the Young’s modulus of the endothelium and sub-endothelium layers are very similar.39 Tracqui et al. and Hayenga et al. reported the regional elastic properties of murine aortic plaques using AFM.21,48 Moreover, using frequency-modulated AFM, significant changes were observed in the elastic and viscoelastic properties of both elastic lamellae and adjacent interlamellar spaces in female sheep aorta with aging.2
To the best of our knowledge, the effect of atherosclerosis disease on the lamellar structure of human abdominal aortic wall has not examined. In the present study, using AFM indentation, we quantified the stiffness of elastin lamellae and inter-lamellar zones within the medial layer of abdominal aorta for healthy, mildly diseased and advanced atherosclerotic samples. Moreover, the stiffness of abdominal aortic wall in diseased parts and disease-free segments of incomplete plaques was compared based on its lamellar microstructure. Additionally, precise data relevant to the micromechanical properties of atherosclerotic plaque components within human abdominal aorta presented.
Materials and Methods
Sample Preparation
At autopsy, 20 human aortas were harvested within 5 h postmortem from individuals who died due to post-accident complications (Fig. 1a). The study protocol and the use of material from human subjects were approved by Ethics Committee of Baghiatallah Hospital, Tehran, Iran. Samples were held in ion-free phosphate-buffered saline (PBS) solution at a temperature of 4 °C immediately after removal to minimize tissue degradation. Then, samples were frozen using liquid nitrogen and kept at – 80 ºC. As others have highlighted, freezing does not affect essential mechanical properties of the arterial tissue.38,48 From each sample, two cryo-sections with 20 µm thickness were taken from the distal part of abdominal aortas for indentation test and adsorbed on to glass slides. Moreover, two serial sections with 5 µm thickness were extracted for histological examination from the adjacent tissue of each 20 µm-thick section.48 Figure 1b shows a typical 20 μm-thick aortic section prepared for probing by AFM.
Histological Examination
Histological staining was performed to provide precise information about atherosclerotic plaque composition and arterial wall structure during AFM testing. The extracted 5 µm thickness sections were dehydrated and stained with Hematoxylin and Eosin (H&E) and Verhoeff Van Gieson (VVG). H&E staining was performed to highlight the general morphology and different components of atherosclerotic plaques including fibrous cap, lipid pool, and calcium deposits. On H&E sections, connective tissues and calcified deposits were identified respectively by pink and blue hue and lipid droplets characterized by the area of the residual cholesterol crystals.17 VVG staining was used to distinguish elastin lamellae from interlamellar zones in the abdominal aortic wall.9 After staining, slides were photographed by a transmitted light microscope (Leica DMLB2) equipped with a digital camera (Leica DFC 480).
The type of atherosclerotic plaques was determined according to the published protocol.43 Then samples were categorized into three different groups of healthy, mildly atherosclerotic (Lesion types II, III and IV) and advanced atherosclerotic arteries (Lesion types V, VI). As can be seen in Table 1, there were no significant differences between the average ages of subjects allocated to different groups.
Figure 2a represents a VVG staining of the abdominal aortic wall consists of elastin lamellae (dark areas) and interlamellar zones (light zones). To find the volume fraction of elastin lamellae in the abdominal aortic wall section, resultant images of VVG staining were converted to black and white using ImageJ software (Fig. 2b). After the elimination of plaque section together with small artifacts from the image, the ratio of black pixels number (elastin lamellae) to total number of pixels was calculated as a volume fraction of elastin lamellae (fI). The volume fraction of interlamellar zone (fII) was determined as below:
(a) The VVG Staining of an aortic media. Dark regions in the tissue section represent elastin lamellae and light-colored regions denote interlamellar zones, including collagen fibers and SMCs (Layer II); (b) For volume fraction computation, each image was converted to black and white, the artifacts were removed by filtering and the proportion of number of black pixels (elastin lamellae) to the total number of pixels was defined as the volume fraction. For this typical mildly diseased abdominal aorta, the volume fraction of elastin lamellae is equal to 24%.
To minimize location dependency, the volume fractions were measured in several locations and the average value was used in further calculations.45
AFM Indentation
The force-spectroscopy mode of the AFM (Nanowizard III, JPK Instruments AG, Germany) was utilized to apply nano-indentation. Three hours before the test, prepared slides with 20 µm thickness were transferred to PBS solution for freeze-thawing of samples. Slides were placed on the petri dish and immobilized. Then, PBS solution was added, and the petri dish was placed on the AFM stage. All experiments were made in liquid medium (PBS soluble) at room temperature. Samples were indented by a CSC17/noAl cantilever (MikroMasch, USA) with a nominal spring constant of 0.15 N/m and a conical shaped tip. Based on thermal noise calibration method,23 the spring constant of the cantilever was calculated as 0.15 ± 0.07 N/m.
Before conducting AFM tests, several straight lines were considered as indentation paths in the histological images throughout regions of interest within the samples. As suggested previously,48 AFM tests were performed at different locations by line scan and the results were reported as a function of distance. An area of 10 × 10 µm2 was selected for indentation of 10 points in each site. The Young’s modulus was calculated for all points, and the average value was reported for each site. Then, histological images were compared with data acquired from the indentation tests and used for determination of indentation sites. The average of measurements at several sites within each component of atherosclerotic plaque and arterial wall was reported as the Young’s modulus of each constituent. Due to the correlation between AFM and histological images, an accurate identification of the AFM probed site was made.
Tests were conducted on both atherosclerotic plaques and arterial walls. Samples from cross sections of atherosclerotic plaques were divided into eccentric and concentric plaques. Four different areas including calcification zone, fibrous cap, lipid pool and intimal fibrosis were specified in atherosclerotic plaques. To further elaborate, the fibrosis tissue beneath the plaque was distinguished from the fibrous cap and its stiffness was reported under the title of intimal fibrosis region. For eccentric atherosclerotic plaques, the arc of the artery circumference that was free of disease was determined and indentation tests were performed on both disease-free and diseased parts of the abdominal aortic wall to examine effects of plaque formation on the elastic properties of the abdominal aortic wall. Considering the lamellar structure of the aortic media, distinct Young’s moduli were determined for elastin lamellae and interlamellar zones.
Data Analysis and Mechanical Characterization of Atherosclerotic Aortas
In the course of indentation, the cantilever tip interacts with the sample and deflects. This deflection is measured by the positioning of the laser beam which is reflected off the cantilever arm. The cantilever arm is attached to a piezoelectric element able to move in the lateral and vertical directions. The indentation depth (δ) is calculated as:
where (d) and (z) represent deflection of the cantilever tip and position of piezoelectric crystals and d0 and z0 stand for cantilever deflection and the piezoelectric position on the contact point of the tissue surface and tip. For small deflections, the cantilever can be considered as a linear spring. Hence, by knowing the cantilever deflection and spring constant, the applied force can be determined using Hooke’s law.16 A typical force–indentation curve is shown in Fig. 2c. By applying the Hertz model to the force–displacement curves, the Young’s modulus of samples can be determined. The Hertz model assumes the sample to be an isotropic and linear elastic material and neglects the interaction between the indenter and sample.27 The assumption of linear elasticity for biological tissues is only valid for small deformations such as conditions of AFM indentation test. Hence, the Young’s modulus acquired by AFM indentation may be considerably different to those obtained by other compression tests in which the range of strains are considerably higher.10
Considering the geometrical shape of the cantilever tip, different constitutive equations have been suggested for calculation of Young’s modulus based on applied force and indentation depth. The force–indentation equation for conical cantilever tip can be written as:
In this equation, F and δ respectively show force and indentation depth, parameter E represents the Young`s modulus and µ the Poisson’s ratio of the sample.30 Considering the incompressibility of soft biological tissues, the Poisson’s ratio of samples was assumed to be 0.5. Parameter α represents the cone angle half angle, which was 20° for the cantilever used in our experiments.
Statistical Analysis
Considering the normal distribution of the majority of data obtained by AFM test, the results were expressed as mean and standard deviation of Young’s moduli for each specified location. The t test analysis was carried out to compare Young’s modulus values between the paired groups. For conducting statistical analysis to compare three groups of healthy, mildly diseased and advanced atherosclerotic arteries, one-way ANOVA test was performed. The statistical significance level was considered to be P = 0.05.
Results
The Stiffness of Aortic Wall and Plaque Components in Typical Samples
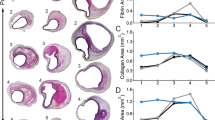
Figure 4 provides histological images (H&E staining) and histograms of Young’s moduli of typical healthy and atherosclerotic abdominal aortas. The indentation test was performed by line scan (dash line in Fig. 4) and the results related to typical locations were represented in the histograms. For a typical healthy abdominal aorta, shown in Fig. 4a, the average Young’s moduli for 5 different sites at the lamellar structure of the arterial wall are presented in the histogram. It was observed that the average Young’s modulus within the areas 1, 3 and 5 located on the elastin sheets was greater than the calculated values within the areas 2 and 4 located on the interlamellar spaces. Consequently, our data on the stiffness of healthy abdominal aorta can be divided into two groups, the upper range related to the stiffness of elastin sheets and the lower range related to the space between them.
For a typical atherosclerotic abdominal aorta with initial plaque shown in Fig. 3b, the calculated average Young’s modulus of interlamellar zones (Locations 2 and 4) was less than 40% of the reported value for the elastin lamellae (Locations 1 and 3). The average Young’s modulus at the thickened intima formed by fibrous tissue, SMCs and lipid droplets (Location 5 and 6) was about half of that of interlamellar zones within the tunica media. Of special interest is that the standard deviation of Young’s modulus at intimal fibrosis of initial abdominal aortic plaques was relatively small, which means this component can be considered generally homogeneous.
Measuring the Young’s modulus of atherosclerotic plaque components based on AFM test. (a) Spatial variables for determination of plaque stiffness. The indentation depth δ is related to the imposed displacement z and cantilever deflection d. (b) A typical force-indentation curve which is related to an elastin sheet within abdominal aorta media.
Figure 4c represents histological images of a typical advanced atherosclerotic lesion within the abdominal aorta and the related histograms which display the Young’s moduli of specific regions of the arterial wall and atherosclerotic plaque. The fracture of elastin membranes, as well as the suppression of the media layer underneath the atherosclerotic plaque, was observed in the histological images. Large amounts of collagen fibers were detected in the diseased intima; however, elastin fibers were scarce in the atherosclerotic intima. The average Young’s modulus at interlamellar spaces (Location 1 and 3) reached to nearly half of Young’s modulus of elastin lamellae within the media layer (Location 2). The calculated Young’s modulus at the fibrous tissue beyond the lipid pool (Location 4) was higher than the Young’s modulus at the fibrous cap (Location 9). The minimum and maximum values of Young’s moduli were those of the lipid pool (Location 5, 8) and calcification zone (Location 6) respectively.
The location of AFM test specified on the stained abdominal aorta arteries. Related histograms represented the calculated Young’s modulus in specified locations. The horizontal axis of histograms represents the indentation location number and the vertical axis indicates the average calculated Young’s modulus within the zone. (a) The H&E staining of a cross section of healthy abdominal aorta and the related histogram. (b) The H&E staining of a cross section of mildly atherosclerotic lesion within abdominal aorta and the related histogram. (c) The H&E staining of a cross section of an advanced atherosclerotic plaque within abdominal aorta and the related histogram.
The Stiffness of Healthy, Mildly Diseased and Advanced Atherosclerotic Arteries
Figure 4 shows results of Young’s moduli of typical abdominal aortas and the overall results of all subjects are presented in Table 2. The average and standard deviation values of calculated Young’s moduli at different locations of the arterial wall and plaque components for all examined subjects are shown in Table 2 together with statistical analyses to compare Young’s moduli of three groups of subjects. Considering the large number of probed sites which were chosen randomly, the presented results were independent of locations of indentation. Except for intimal fibrosis in the mildly diseased samples, results not only indicated a significant heterogeneity in mechanical properties of atherosclerotic plaques, but also showed large variations in Young’s moduli within plaque components. It was found that the lipid pool and calcification zone are the softest and stiffest components of atherosclerotic plaques. Moreover, the fibrosis tissue within atherosclerotic plaques became significantly stiffer with plaque progression. In addition, a significant difference was found between the stiffness of fibrous cap and the fibrosis tissues beneath the lipid pool in the advanced atherosclerotic plaques (P < 0.05).
The stiffness of elastic lamellae and interlamellar zones within the diseased parts of media layer of initial atherosclerotic lesions in the abdominal aorta was comparable with that in normal arteries. However, there were significant differences between Young’s moduli of elastin lamellae and interlamellar zones of mildly atherosclerotic and advanced atherosclerotic abdominal aortas (P < 0.05). With the formation and development of atherosclerotic plaques, the stiffness of elastin sheets of the aortic wall quantified by their Young’s modulus decreased almost 18.6% and conversely, the stiffness of interlamellar zones increased up to 50%, which might be respectively related to the disruption/degeneration of elastin fibers and collagen deposition. Moreover, the average volume fraction of elastin lamellae was determined for healthy arteries, mildly diseased arteries, and advanced atherosclerotic lesions (Table 2). A gradual reduction of volume fraction of elastic lamellae (P > 0.05) with atherosclerotic plaque development was observed.
Comparing the Stiffness of Diseased and Disease-Free Portions of the Aortic Wall
Of particular interest is to investigate effects of plaque development on the mechanical properties of media layers both in diseased and disease-free portions of the arteries. Table 3 compares the average Young’s modulus of disease-free segments and diseased parts in mildly diseased and advanced atherosclerotic abdominal aortic samples. In aortas with advanced plaques, a significant increase in the stiffness of the interlamellar zones (corresponding to higher Young’s modulus) and a significant decrease in the stiffness of elastin lamellae were observed in diseased parts of the arterial wall compared to disease-free segments (P < 0.05). Statistical analysis did not show significant alteration in the Young’s moduli of elastin lamellae and interlamellar spaces between healthy arteries (Table 2) and disease-free segments of atherosclerotic lesions (P > 0.05). However, a gradual increase of Young’s modulus of interlamellar zones and a decrease of elastin lamellae stiffness in disease-free segments of abdominal aortic media can be observed in comparison with healthy arteries. Hence, it can be concluded that the formation and progression of atherosclerotic plaques not only affects the stiffness of media layer behind the plaque but also influence the stiffness of disease-free parts of abdominal aortic media to a lesser extent.
Discussion
It has been demonstrated that the AFM technique is a powerful tool for quantitatively assessing the local stiffness differences in soft tissues.2,21,39,48 Using AFM, we provided new insights into the behavior of the abdominal aorta in response to formation and progression of atherosclerotic plaque. To the best of our knowledge, this is the first study which shows how the stiffness of both elastin lamellae and interlamellar zones within human abdominal aortic media change during atherosclerotic plaque development. Moreover, the Young’s moduli of different components of atherosclerotic plaques within the abdominal aorta were determined. The range of data obtained regarding the Young’s moduli of human abdominal aortic wall and plaque components is comparable with the results of others achieved by indentation tests on animal models of atherosclerosis.21,33,48 For instance, using a scaled-up version of an AFM instrument, Matsumoto et al. demonstrated that in compression the elastin lamellae are approximately 2.5 times stiffer than the interlamellar regions in porcine thoracic aortae.33 The higher stiffness of elastin lamellae compared to interlamellar zones under AFM tests can be related to the presence of collagen fibers at interlamellar zones which can tolerate tension but are unable to bear loads under compression.21 In fact, although it is accepted that collagen fibers are stiffer than elastin fibers in tension, they are not stiffer in compression or at low strains at which AFM indentation is performed.21
While AFM indentation provides local and compressive properties of the arterial wall and atherosclerotic plaque components, the tensile test measures the bulk and tensile properties of the tissue. Consequently, the presented Young’s modulus in this study cannot describe the elastic response of the arterial tissue to the physiological range of blood pressure and is consistently lower than those obtained by tensile tests due to inherent differences between methods.35 However, our results are in agreement with those obtained by tensile tests qualitatively.22,32,47 For instance, although using the presented results in a finite element model lead to the overestimation of stress distribution within atherosclerotic arteries, it can introduce the locations of stress concentration accurately. It was found that with the development of the atherosclerotic plaque, the homogeneity of the atherosclerotic intima fade and the consequent stiffness gradient in advanced atherosclerotic plaques can lead to stress concentration. The Young’s modulus of the lipid pool in comparison with the other components is markedly lower, which may result in a stress concentration in the neighboring stiff fibrous tissue. This corresponds to the formation of cracks in the fibrous cap and further propagation towards the lipid pool,40 a phenomenon that causes vulnerability of the plaque and the subsequent critical conditions. The existence of the calcification zone, as the stiffest component of atherosclerotic plaques, intensifies stress concentration and elevates the risk of plaque rupture.40,50
Considering the high heterogeneity of atherosclerotic lesions, the main practical implication of AFM indentation is obtaining data regarding the local stiffness variation in the lamellar structure of the arterial wall and atherosclerotic lesion components with plaque development. To further elaborate, for determination of mechanical properties of plaque components using tensile test, it is required to separate different components of atherosclerotic plaques which can be challenging by considering the heterogeneous structure of atherosclerotic plaques.22 Moreover, there is no investigative technique at present to measure the Young’s modulus of different lamellae of abdominal aortic media using tensile test. However, the tensile test might have an advantage in determining tensile properties of the arterial wall and plaque tissue in a broad range of loading up to rupture35 compared to AFM indentation, which is limited in compressive properties in low load range.10,35 Hence, while elastin fibers are highly deformable and show lower Young’s modulus in tensile tests compared to collagen fibers, they appear stiffer under indentation tests.21 Consequently, the interlamellar zone of the arterial wall with high collagen content appears softer than elastic lamellae as in previous findings achieved by compressive test on animal samples.21,33,48 This is due to the mesh-like structure of interlamellar zones with collagen fibers that deflect under compression3,21 compared to the dense sheets of elastin fibers in the axial direction of arteries. Using tensile tests on whole aortic segments and continuum based modeling, the higher contribution of elastic lamellae in lower pressures and the gradual engagement of collagen fibers was shown before.44
A significant finding in our study was that the formation and progression of atherosclerotic plaques affect mechanical properties of the aortic wall. This alternation was characterized by considering the lamellar structure of the aortic media. It was found that the Young’s modulus of elastin lamellae within diseased segments of the abdominal aortic wall decreased considerably with atherosclerotic plaque development. It is well known that elastin sheets in the aortic wall play an important role in its elastic behavior in response to physiological ranges of blood pressure.5 Hence, considering the role of elastin lamellae in the aortic wall distensibility and capacitive effects, the degeneration of elastin lamellae can influence the proper function of the abdominal aorta.
Furthermore, it was found that the stiffness of interlamellar zones within diseased parts of abdominal aortic media increased significantly with the formation and development of atherosclerotic plaques. Stiffening of interlamellar zones might be due to the incremental build-up of collagen fibers synthesized by SMCs.7 The alternation of wall tensile stress during atherosclerotic plaque development may trigger stiffening of interlamellar zones.44 The overall state of stress across the arterial wall, obtained by the superposition of circumferential stress caused by blood pressure and the inherent residual stress, might adjust the production of ECM by SMCs. It is well established that the profile of stress across the arterial wall is approximately uniform.20 This uniform profile ensures that SMCs across the healthy arterial wall experience almost similar tension.20,44 However, with formation and progression of the atherosclerotic plaque, SMCs of different parts of arterial wall may experience a wide range of stresses. For instance, with the eccentric progression of atherosclerotic plaques, due to the unsymmetrical geometry, the circumferential stress induced in the diseased wall and the diseased-free wall may be significantly different. The non-physiological ranges of stress and strain in different parts of aortic media may trigger migration, proliferation, and phenotype changing of SMCs from contractile to proliferative14 leading to more synthesis of collagen fibers (especially type I and V36) by SMCs and subsequent stiffening of interlamellar zones of aortic media.37
With the histological examination of the abdominal aortic wall, the average volume fractions of elastin lamellae and interlamellar zones in healthy, mildly diseased and advanced atherosclerotic abdominal aortas were determined. The gradual reduction of the volume fraction of elastin lamellae with atherosclerotic plaque development was detected. Considering the greater volume fraction of interlamellar zones compared to elastin lamellae (about 4 times) in both healthy and atherosclerotic abdominal aortas, it can be suggested that interstitial layers have a major contribution to the overall stiffness of the abdominal aorta. Furthermore, it was observed that the percentage of stiffness alternation in the interlamellar regions is higher than elastin lamellae during atherosclerotic plaque development. The greater sensitivity of interlamellar zones may be related to the existence of SMCs which sense the alternation of mechanical stresses and regulate the synthesis of the extra-cellar matrix to return their mechanical environment to the physiological range.
Ideally, to survey alterations in the stiffness of plaque components and arterial wall during atherosclerosis progression, in vivo follow-up of the subject would be necessary. New techniques have proposed for measuring the global stiffness of atherosclerotic arteries in vivo4; however, these techniques cannot provide data regarding the alteration of the local stiffness of different constituents of the arterial wall and atherosclerotic plaque. Additionally, AFM test can only be performed at limited indentation depths and is unable to determine the mechanical properties of the arterial wall and atherosclerotic plaques at physiological strains. Hence, the non-linear mechanical behavior of the plaque tissue may not be captured. Moreover, measuring at small strains might lead to underestimation of the tissue stiffness, as the collagenous fibrous tissue often displays strain-stiffening behavior in tension through uncrimping and gradual engagement with an elevation of the strain. Despite limitations, AFM indentation is highly accurate in determining very local properties of highly inhomogeneous atherosclerotic plaques which is almost impossible to be obtained by tensile tests. This allows a detailed study of mechanical properties in different locations of plaque components during plaque development which are under impression of cellular events such as smooth muscle migration, alterations in the phenotypes of endothelial and smooth muscle cells due to change in stress distribution within the diseased arterial wall and the consequent ECM synthesis/degradation with further plaque progression.
In healthy arteries, intima is a thin layer of endothelial cells attached to a basal membrane. With formation and progression of atherosclerotic plaques, the thickness and stiffness of intimal layer increase.49 The main structural components of atherosclerotic intima are fibrous tissue, calcification and lipid pool. Collagen types I, III, IV, and V can be found in the diseased intima. Although collagen type I is dominant, deposits of collagen type IV are frequently observed in the fibrous cap of plaques.3 We measure the stiffness of this constitutes in the mildly diseased and advanced atherosclerotic arteries. It can be a good idea to compare the stiffness of the basal membrane of intima among healthy, mildly diseased and advanced atherosclerotic arteries. However, the basal membrane of intima is hard to locate and this measurement was beyond the scope of this paper. Moreover, for anisotropic materials such as arterial tissue, the Young’ modulus obtained by the indentation test is a composite quantity and can be affected by the loading direction.41 However, like others,15,48 we measured Young’s modulus of aortic tissue in just circumferential planes of the vessel and indented the samples in the perpendicular direction. Indentation of the aortic tissue from different planes and directions and considering the anisotropy of the vessel wall might be examined in further studies.
Conclusion
In the current study, by the micromechanical characterization of the abdominal aortic wall in healthy, mildly diseased and advanced atherosclerotic arteries, it was demonstrated that atherosclerotic plaque development results in the stiffening of interlamellar zones and softening of elastin lamellae in diseased portions of the abdominal aortic wall. Moreover, the stiffness of different components of the atherosclerotic plaque such as lipid pool, calcification zone, and fibrous cap was determined. In conclusion, the mechanical evaluation of the lamellar structure of atherosclerotic abdominal aortic media in diseased parts and plaque-free segments elucidates the mechanobiological responses of the abdominal aorta and may provide new insight in the remodeling of the abdominal aortic wall with atherosclerotic plaque development.
References
Akhtar, R. In vitro characterisation of arterial stiffening: from the macro-to the nano-scale. Artery Res. 8(1):1–8, 2014.
Akhtar, R., H. Graham, B. Derby, M. Sherratt, A. Trafford, R. Chadwick, et al. Frequency-modulated atomic force microscopy localises viscoelastic remodelling in the ageing sheep aorta. J. Mech. Behav. Biomed. Mater. 64:10–17, 2016.
Akyildiz, A. C., L. Speelman, and F. J. Gijsen. Mechanical properties of human atherosclerotic intima tissue. J. Biomech. 47(4):773–783, 2014.
Apostolakis, I. Z., S. D. Nandlall, and E. E. Konofagou. Piecewise pulse wave imaging (pPWI) for detection and monitoring of focal vascular disease in murine aortas and carotids in vivo. IEEE Trans. Med. Imaging 35(1):13–28, 2016.
Avolio, A. Arterial stiffness. Pulse 1(1):14–28, 2013.
Avolio, A., D. Jones, and M. Tafazzoli-Shadpour. Quantification of alterations in structure and function of elastin in the arterial media. Hypertension 32(1):170–175, 1998.
Brüel, A., G. Ørtoft, and H. Oxlund. Inhibition of cross-links in collagen is associated with reduced stiffness of the aorta in young rats. Atherosclerosis. 140(1):135–145, 1998.
Buchanan, J., C. Kleinstreuer, S. Hyun, and G. Truskey. Hemodynamics simulation and identification of susceptible sites of atherosclerotic lesion formation in a model abdominal aorta. J. Biomech. 36(8):1185–1196, 2003.
Burke, A. P., and F. Tavora. Practical cardiovascular pathology. Philadelphia: Lippincott Williams & Wilkins, 2010.
Butt, H.-J., B. Cappella, and M. Kappl. Force measurements with the atomic force microscope: technique, interpretation and applications. Surf. Sci. Rep. 59(1–6):1–152, 2005.
Cattell, M. A., J. C. Anderson, and P. S. Hasleton. Age-related changes in amounts and concentrations of collagen and elastin in normotensive human thoracic aorta. Clin. Chim. Acta 245(1):73–84, 1996.
Chai, C.-K., L. Speelman, C. W. Oomens, and F. P. Baaijens. Compressive mechanical properties of atherosclerotic plaques—indentation test to characterise the local anisotropic behaviour. J. Biomech. 47(4):784–792, 2014.
Discher, D. E., P. Janmey, and Y.-L. Wang. Tissue cells feel and respond to the stiffness of their substrate. Science 310(5751):1139–1143, 2005.
Doran, A. C., N. Meller, and C. A. McNamara. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 28(5):812–819, 2008.
Ebenstein, D. M., D. Coughlin, J. Chapman, C. Li, and L. A. Pruitt. Nanomechanical properties of calcification, fibrous tissue, and hematoma from atherosclerotic plaques. J. Biomed. Mater. Res. Part A 91(4):1028–1037, 2009.
Ethier, C. R., and C. A. Simmons. Introductory biomechanics: from cells to organisms. Cambridge: Cambridge University Press, 2007.
Fischer, A. H., K. A. Jacobson, J. Rose, and R. Zeller. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008. https://doi.org/10.1101/pdb.prot4986.
Fukui, T., T. Matsumoto, T. Tanaka, T. Ohashi, K. Kumagai, H. Akimoto, et al. In vivo mechanical properties of thoracic aortic aneurysmal wall estimated from in vitro biaxial tensile test. Biomed. Mater. Eng. 15(4):295–305, 2004.
Glagov, S., D. Rowley, and R. Kohut. Atherosclerosis of human aorta and its coronary and renal arteries. A consideration of some hemodynamic factors which may be related to the marked differences in atherosclerotic involvement of the coronary and renal arteries. Arch Pathol. 72:558, 1961.
Haghighipour, N., M. Tafazzoli-Shadpour, and A. Avolio. Residual stress distribution in a lamellar model of the arterial wall. J. Med. Eng. Technol. 34(7–8):422–428, 2010.
Hayenga, H., A. Trache, J. Trzeciakowski, and J. Humphrey. Regional atherosclerotic plaque properties in ApoE−/− mice quantified by atomic force, immunofluorescence, and light microscopy. J. Vasc. Res. 48(6):495–504, 2011.
Holzapfel, G. A., G. Sommer, and P. Regitnig. Anisotropic mechanical properties of tissue components in human atherosclerotic plaques. J. Biomech. Eng. 126(5):657–665, 2004.
Hutter, J. L., and J. Bechhoefer. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 64(7):1868–1873, 1993.
Huynh, J., N. Nishimura, K. Rana, J. M. Peloquin, J. P. Califano, C. R. Montague, et al. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci. Transl. Med. 3(112):112ra122, 2011.
Imura, T., K. Yamamoto, T. Satoh, T. Mikami, and H. Yasuda. Arteriosclerotic change in the human abdominal aorta in vivo in relation to coronary heart disease and risk factors. Atherosclerosis. 73(2):149–155, 1988.
Kamenskiy, A. V., Y. A. Dzenis, S. A. J. Kazmi, M. A. Pemberton, I. I. Pipinos, N. Y. Phillips, et al. Biaxial mechanical properties of the human thoracic and abdominal aorta, common carotid, subclavian, renal and common iliac arteries. Biomech. Model. Mechanobiol. 13(6):1341–1359, 2014.
Kasas, S., G. Longo, and G. Dietler. Mechanical properties of biological specimens explored by atomic force microscopy. J. Phys. D Appl. Phys. 46(13):133001, 2013.
Korshunov, V. A., S. M. Schwartz, and B. C. Berk. Vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 27(8):1722–1728, 2007.
Last, J. A., S. J. Liliensiek, P. F. Nealey, and C. J. Murphy. Determining the mechanical properties of human corneal basement membranes with atomic force microscopy. J. Struct. Biol. 167(1):19–24, 2009.
Lekka, M., D. Gil, K. Pogoda, J. Dulińska-Litewka, R. Jach, J. Gostek, et al. Cancer cell detection in tissue sections using AFM. Arch. Biochem. Biophys. 518(2):151–156, 2012.
Lundkvist, A., E. Lilleodden, W. Siekhaus, J. Kinney, L. Pruitt, and M. Balooch. Viscoelastic properties of healthy human artery measured in saline solution by AFM-based indentation technique. MRS Online Proc. Library Arch. 436:353, 1996.
Maher, E., A. Creane, S. Sultan, N. Hynes, C. Lally, and D. J. Kelly. Tensile and compressive properties of fresh human carotid atherosclerotic plaques. J. Biomech. 42(16):2760–2767, 2009.
Matsumoto, T., T. Goto, T. Furukawa, and M. Sato. Residual stress and strain in the lamellar unit of the porcine aorta: experiment and analysis. J. Biomech. 37(6):807–815, 2004.
Matsumoto, T., and K. Hayashi. Mechanical and dimensional adaptation of rat aorta to hypertension. J. Biomech. Eng. 116(3):278–283, 1994.
McKee, C. T., J. A. Last, P. Russell, and C. J. Murphy. Indentation versus tensile measurements of Young’s modulus for soft biological tissues. Tissue Eng. Part B Rev. 17(3):155–164, 2011.
Murata, K., T. Motayama, and C. Kotake. Collagen types in various layers of the human aorta and their changes with the atherosclerotic process. Atherosclerosis 60(3):251–262, 1986.
Ocallaghan, C. J., and B. Williams. Mechanical strain–induced extracellular matrix production by human vascular smooth muscle cells. Hypertension 36(3):319–324, 2000.
O’Leary, S. A., J. J. Mulvihill, H. E. Barrett, E. G. Kavanagh, M. T. Walsh, T. M. McGloughlin, et al. Determining the influence of calcification on the failure properties of abdominal aortic aneurysm (AAA) tissue. J. Mech. Behav. Biomed. Mater. 42:154–167, 2015.
Peloquin, J., J. Huynh, R. M. Williams, and C. A. Reinhart-King. Indentation measurements of the subendothelial matrix in bovine carotid arteries. J. Biomech. 44(5):815–821, 2011.
Rezvani-Sharif, A., M. Tafazzoli-Shadpour, D. Kazemi-Saleh, and M. Sotoudeh-Anvari. Stress analysis of fracture of atherosclerotic plaques: crack propagation modeling. Med Biol Eng Comput. 55:1389–1400, 2016.
Rho, J. Y., M. E. Roy, T. Y. Tsui, and G. M. Pharr. Elastic properties of microstructural components of human bone tissue as measured by nanoindentation. J. Biomed. Mater. Res. 45(1):48–54, 1999.
Schiffrin, E. L., A. Tedgui, and S. Lehoux. mechanical stress and the arterial wall. Blood pressure and arterial wall mechanics in cardiovascular diseases. New York: Springer, pp. 97–106, 2014.
Stary, H. C., A. B. Chandler, R. E. Dinsmore, V. Fuster, S. Glagov, W. Insull, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis a report from the committee on vascular lesions of the council on arteriosclerosis. Am. Heart Assoc. Circ. 92(5):1355–1374, 1995.
Taghizadeh, H., M. Tafazzoli-Shadpour, and M. B. Shadmehr. Analysis of arterial wall remodeling in hypertension based on lamellar modeling. J. Am. Soc. Hypertens. 9(9):735–744, 2015.
Taghizadeh, H., M. Tafazzoli-Shadpour, M. B. Shadmehr, and N. Fatouraee. Evaluation of biaxial mechanical properties of aortic media based on the lamellar microstructure. Materials 8(1):302–316, 2015.
Taylor, C. A., T. J. Hughes, and C. K. Zarins. Finite element modeling of three-dimensional pulsatile flow in the abdominal aorta: relevance to atherosclerosis. Ann. Biomed. Eng. 26(6):975–987, 1998.
Teng, Z., Y. Zhang, Y. Huang, J. Feng, J. Yuan, Q. Lu, et al. Material properties of components in human carotid atherosclerotic plaques: a uniaxial extension study. Acta Biomater. 10(12):5055–5063, 2014.
Tracqui, P., A. Broisat, J. Toczek, N. Mesnier, J. Ohayon, and L. Riou. Mapping elasticity moduli of atherosclerotic plaque in situ via atomic force microscopy. J. Struct. Biol. 174(1):115–123, 2011.
VanderBurgh, J. A., and C. A. Reinhart-King. The role of age-related intimal remodeling and stiffening in atherosclerosis. Adv. Pharmacol. 81:365–391, 2018.
Vengrenyuk, Y., S. Carlier, S. Xanthos, L. Cardoso, P. Ganatos, R. Virmani, et al. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc. Natl. Acad. Sci. 103(40):14678–14683, 2006.
Wayman, B. H., W. R. Taylor, A. Rachev, and R. P. Vito. Arteries respond to independent control of circumferential and shear stress in organ culture. Ann. Biomed. Eng. 36(5):673–684, 2008.
Wolinsky, H., and S. Glagov. A lamellar unit of aortic medial structure and function in mammals. Circ. Res. 20(1):99–111, 1967.
Zieman, S. J., V. Melenovsky, and D. A. Kass. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 25(5):932–943, 2005.
Acknowledgments
Authors thank Dr. Davood Kazemi-Saleh and Dr. Zahra Pourjafar at Baghiatallah Hospital for providing tissue specimens. Authors also thank Dr. Amirnader Emami Razavi at Tehran University of Medical Sciences for assistance in the preparation of samples for AFM test and determination of different components of atherosclerotic plaques and arterial wall layers in specimens.
Conflict of interest
Alireza Rezvani-Sharif, Mohammad Tafazzoli-Shadpour and Alberto Avolio declare that they have no conflict of interest.
Ethical Approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study. No animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Wei Sun and Ajit P. Yoganathan oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Rezvani-Sharif, A., Tafazzoli-Shadpour, M. & Avolio, A. Mechanical Characterization of the Lamellar Structure of Human Abdominal Aorta in the Development of Atherosclerosis: An Atomic Force Microscopy Study. Cardiovasc Eng Tech 10, 181–192 (2019). https://doi.org/10.1007/s13239-018-0370-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-018-0370-1