Abstract
The generation of cellularized bioartificial blood vessels resembling all three layers of the natural vessel wall with physiological morphology and cell alignment is a long pursued goal in vascular tissue engineering. Simultaneous culture of all three layers under physiological mechanical conditions requires highly sophisticated perfusion techniques and still today remains a key challenge. Here, three-layered bioartificial vessels based on fibrin matrices were generated using a stepwise molding technique. Adipose-derived stem cells (ASC) were differentiated to smooth muscle cells (SMC) and integrated in a compacted tubular fibrin matrix to resemble the tunica media. The tunica adventitia-equivalent containing human umbilical vein endothelial cells (HUVEC) and ASC in a low concentration fibrin matrix was molded around it. Luminal seeding with HUVEC resembled the tunica intima. Subsequently, constructs were exposed to physiological mechanical stimulation in a pulsatile bioreactor for 72 h. Compared to statically incubated controls, mechanical stimulation induced physiological cell alignment in each layer: Luminal endothelial cells showed longitudinal alignment, cells in the media-layer were aligned circumferentially and expressed characteristic SMC marker proteins. HUVEC in the adventitia-layer formed longitudinally aligned microvascular tubes resembling vasa vasorum capillaries. Thus, physiologically organized three-layered bioartificial vessels were successfully manufactured by stepwise fibrin molding with subsequent mechanical stimulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular diseases are the number one cause of death worldwide.5 Within this spectrum, vasculopathies of small caliber arteries (< 5 mm in diameter) represent a surgically challenging etiology and cause a decisive part of morbidity and mortality in industrialized countries. In current clinical practice, vascular prostheses made of alloplastic materials are commonly used for replacing or bypassing diseased natural vessels. Although larger diameter synthetic grafts have been used for decades with good clinical results, insufficient biocompatibility of alloplastic graft materials causes complications such as graft occlusion by thrombosis or inflammatory processes, which is especially problematic in small diameter prostheses. Thus, there is an ongoing need for innovations in the field of vascular prostheses especially for uses in small diameter vasculature. For this application, innovative vascular grafts must meet distinctively higher requirements concerning hemo- and biocompatibility than current options. Vascular tissue engineering has emerged as a promising approach to maximize biocompatibility of vascular grafts by creating bioartificial vessels, which mimic the structure of natural blood vessels as accurately as possible. Generation of small caliber bioartificial vessels with physiological morphology and function requires the combination of suitable matrix materials and specific cell types with physiological cell alignment. While promising advances in the generation of functional bioartificial vessels using tissue engineering techniques have been achieved,17 resembling all layers of the physiological arterial wall in an in vitro cellularized tissue engineered vascular prosthesis optimizes the emulation of the natural vessel wall structure and thus, potentially increases biocompatibility.
Human blood vessel walls physiologically consist of three layers: The tunica intima built up by endothelial cells, the tunica media with vascular smooth muscle cells and the tunica adventitia. Besides collagen producing fibroblasts and myofibroblasts, autonomic nerves and lymphatic vessels, this layer contains a dense capillary network emerging from the so called vasa vasorum which facilitate nutrition of the vessel wall.
First prerequisite for the generation of cellularized bioartificial vessels resembling all three layers of the vessel wall is to obtain the different cell types needed and to identify or generate a matrix suitable for luminal and intramural cell seeding and capillary tube formation. In addition to that, the components have to be arranged in a physiologically layered fashion. Within each layer, proper cell morphology and orientation is needed to comply with the physiological arrangement of native arteries. In order to accurately resemble the complex structure of the vessel wall, a tissue engineering strategy combining specific techniques for each layer is needed.
As one of these techniques, mechanical stimulation in pulsatile bioreactor perfusion systems (BPS) has been shown to be of pivotal importance not only for mesenchymal stem cell differentiation towards vascular smooth muscle cells8 but also for proper alignment and function of both, mural smooth muscle cells and luminal endothelial cells.11 Application of physiological mechanical stimuli to multi-layered bioartificial vessels requires highly sophisticated techniques for vascular construct generation and bioreactor design. This represents a limitation that has not yet been sufficiently overcome.
Previous works have focused on generating single layered bioartificial vessels of either the tunica intima or the tunica media or combining both layers to generate bi-layered vessels.22 Recent attempts for the generation of three-layered vessels including a tunica adventitia-equivalent focus on extracellular matrix production by seeding with fibroblasts rather than capillarization of the adventitial layer.18,32 Another approach is based on the generation of acellular three-layered matrices resembling stiffness properties of each layer,33 which are implanted and subsequently cellularized in the target organism.4 Until now, only very few studies have targeted in vitro capillarization of the tunica adventitia.6 Furthermore, a completely cellularized bioartificial vessel that includes a capillarized tunica adventitia-equivalent and resembles the physiological hierarchical arrangement of all three layers of the vascular wall with their specific cell orientation and morphology has not been generated in vitro yet.
Here, we present the generation of a cellularized three-layered bioartificial vessel built up on a fibrin-based matrix and containing a bioengineered tunica intima resembled by a luminal layer of human umbilical vein endothelial cells (HUVEC), a tunica media-equivalent built up by vascular smooth muscle cells (SMC), which are differentiated from adipose-derived stem cells (ASC), and a tunica adventitia-equivalent that contains a capillary network formed by HUVEC and ASC. Subsequently, the role of mechanical stimulation for cell distribution, differentiation, morphology and alignment was evaluated by comparing three-layered vessels stimulated under physiological conditions in a pulsatile BPS to statically incubated controls.
Materials and Methods
Three-Layered Fibrin Vessel Fabrication
A total of six three-layered fibrin based small diameter bioartificial vessels were generated using a four step approach (Fig. 1):
Schematic cross sections through the vessel wall showing the steps of the three-layered bioartificial vessel generation. (1) The tunica media-equivalent is molded with a high concentration fibrin gel containing smooth muscle cells. (2) The construct is compacted and strengthened in a custom built rotation unit. (3) The tunica adventitia-equivalent is molded around it using a low concentration fibrin gel containing endothelial cells and adipose-derived stem cells. (4) Endothelial cells are seeded on the luminal surface to generate the tunica intima-equivalent.
In the first step, the tunica media-equivalent was fabricated (Fig. 1-1). Therefore, ASCs were isolated from subcutaneous adipose tissue of a patient scheduled for visceral surgery after informed consent and ethical approval by the ethics commission of Hannover Medical School. Cell isolation and verification of the donor specific differentiation capacity as well as biochemical pre-differentiation towards vascular SMCs was performed as described recently.15 Successful pre-differentiation was verified for each culture prior to seeding by evaluating characteristic morphological changes of the cells indicating SMC-phenotypes using light microscopy (Fig. S1). Fibrinogen was isolated from human plasma obtained from donors at the blood donation service at Hannover Medical School after informed consent (Institute for Transfusion Medicine, Hannover Medical School). After pre-differentiation, 3 × 106 cells were suspended in 600 µL of a solution containing 25 mg × mL−1 cryoprecipitated fibrinogen replenished in human blood serum, 100 µL × mL−1 M199 10X (Sigma Aldrich, Steinheim, Germany) and 100 U × mL−1 Aprotinin (Bayer, Leverkusen, Germany). pH was adapted by titration of 1 N NaOH. Polymerization was initiated by adding 600 µL of a solution containing 2.5 U × mL−1 Thrombin (Vascular Solutions, Mineapolis, USA), 2.5 U × mL−1 Factor XIII (CSL Behring, Marburg, Germany) and 40 mM CaCl2. The solution was filled in a custom-built cylindrical mold with a diameter of 5 mm.
After 30 min of static polymerization, post-polymerization compaction was performed in a custom-built rotation unit (Department of medical device construction, Hannover Medical School) as described previously.8 Here, the media-equivalent was centrifugated at 36×g for 10 min, 145×g for 10 min and 402×g for 15 min, which increased mechanical stability and decreased wall thickness (Fig. 1-2).
In the third step (Fig. 1-3), the media-equivalent was transferred into a second custom built vascular mold with an outer diameter of 7.75 mm and the tunica adventitia-equivalent is molded around the media-equivalent. Here, a low-concentration fibrin gel was produced by using a fibrinogen concentration of 5 mg × mL−1 with a total volume of 2 mL. 3 × 106 red fluorescent protein expressing human umbilical vein endothelial cells (RFP-HUVECs, Pelo Biotech, Planegg/Martinsried, Germany) and 3 × 106 ASCs were suspended in the fibrinogen solution and molding was performed as described for step 1.
In the last step, the tunica intima-equivalent was generated by luminal seeding with endothelial cells (Fig. 1-4). For this, the fibrin vessels were fixed onto hose-nozzles, a solution of 6 × 106 RFP-HUVECs × mL−1 suspended in EGM2 (Lonza, Basel, Switzerland) was filled into the lumen of the vessel and the ends were closed using caps. The vessel was incubated in a horizontal position and cells were allowed to attach for a total of 1 h. Every 15 min, the vessel was turned for 90° to ensure complete endothelialization of the luminal circumference.
When seeding was completed, the caps were opened and the vessels were implemented into a custom built bioreactor (Fig. 2b) and exposed to pulsatile perfusion or incubated statically in cell culture tubes (TPP, Trasadingen, Switzerland) (each n = 3). For both, perfusion and static incubation, a feeding medium based on M199 containing FGF (40 ng × mL−1), VEGF (40 ng × mL−1), l-ascorbic acid-2-phosphate (50 µg × mL−1) and aprotinin (100 U × mL−1; all from Sigma) was used.
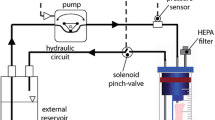
Perfusion system setup. a: schematic illustration of the pulsatile perfusion system. The bioartificial vessels were placed in a custom built bioreactor (b) and pulsatile perfusion was performed using a pulsatile peristaltic pump (p) and an air-compliance chamber (c). Pressure was adjusted with a screwing clamp (sc) and continuously monitored using a pressure sensor (s). Gas exchange was facilitated by an open reservoir (r) with an air filter (f). b: photographic close-up showing a bioartificial vessel in the custom built bioreactor. c: pressure waveform in an 8 s interval as displayed by the monitoring system.
For the characterization of the biomechanical properties, burst pressure, tensile strength and secant elastic modulus were measured as described previously.2
Pulsatile Perfusion
Pulsatile perfusion was performed in a bioreactor perfusion system (BPS, Fig. 2a) derived and modified from a hemodynamic simulator described in detail elsewhere.8
Briefly, a pulsatile peristaltic pump (P; Ismatec MCP Standard, Cole Parmer, Wertheim, Germany) was equipped with two check valves to mimic the discontinuous working pattern of the heart and connected to a compliance chamber (C) mimicking the ‘Windkessel’ effect of the aorta. The bioreactor (B) containing the bioartificial vessel was placed downstream to that, followed by a pressure sensor (S) connected to a monitor system (Datex Ohmeda, Helsinki, Finland) for continuous real-time pressure monitoring. Pressure was adapted using a screwing clamp (SC) and gas exchange was facilitated by an open reservoir (R). The system was placed in an incubator at 37 °C and 5% CO2.
The BPS was set to mimic the physiological flow- and pressure environment blood vessels are exposed to in the human body. Prior to starting the perfusion, the vessels where incubated in the bioreactor without flow for 12 h to facilitate complete attachment of all cell types. Subsequently, perfusion was started with half of the target flow (30 bpm, 22 mL × min−1) for 10 h to avoid directly exposing luminal RFP-HUVEC to full shear stress. Afterwards, full flow (60 bpm, 44 mL × min−1) was initiated and the vessels were stimulated for 72 h in the BPS under the mechanical conditions shown in Table 1. Cyclic stretch was quantified by obtaining video clips of the segments during perfusion and measuring the maximal (“systolic”) and minimal (“diastolic”) outer vessel diameter as described previously.8 Shear stress (τ) was calculated using the following formula:
with µ ≙ viscosity of the media, Q ≙ volume flow and r ≙ baseline radius of the segment.20
Pulse pressure was defined as follows:
Vessel Characterization
After pulsatile perfusion or static incubation, the fibrin vessels where fixed with 4% paraformaldehyde. Vessel dimensions were assessed on cross sections using light-microscopy and layer-specific analysis was performed using fluorescent microscopy (Nikon Eclipse TE300, Düsseldorf, Germany) and the software ImageJ (NIH, Bethesda, USA).
For morphological analysis of the tunica intima-equivalent, 5 mm long segments were excised and cut longitudinally to obtain two half-cylinders, which were placed on a microscope slide with the luminal circumference facing towards the objective without additional staining. Thus, only RFP-expressing HUVECs were visualized with fluorescent microscopy for this analysis. Endothelial cell coverage was assessed by marking the cell-covered luminal area of 10 fields in 10-fold primary magnification and using the following formula:
Cell orientation was analyzed by marking the main axis of each cell in 5 fields in 20-fold primary magnification for each vessel and comparing them to the longitudinal axis of the vessel, which was defined as 0°.
Evaluation of the tunica media was performed on 6 µm thick cryosections, which were stained for SMC-specific cell markers. Therefore, sections were fixed with 4% paraformaldehyde, permeabilized using 0.1% Triton-X-100 and blocked with PBS containing 10% fetal calf serum, 0.1% bovine serum albumin and 0.05% Tween-20 (all from Sigma-Aldrich). Immunostaining was performed using the antibodies ‘Mouse alpha smooth muscle actin’ (1:100; DM001, Acris Antibodies, Herford, Germany) and ‘rabbit calponin’ (1:100; ab46794; Abcam, Cambridge, UK). Alexa Fluor 555 coupled anti rabbit IgG and Alexa Fluor 488 coupled anti mouse IgG were used as secondary antibodies. To counterstain nuclei, sections were mounted with medium containing 4,6-diamidino-2-phenylindole (DAPI, Carl Roth GmbH, Karlsruhe, Germany).
The tunica adventitia-equivalent was assessed on 5 mm long segments, which were excised, opened longitudinally and placed on a microscope slide with the outer circumference facing towards the objective. Vascular tube formation was quantified using the software “AngioTool”35 on 5 fields in 10-fold primary magnification for each vessel. Additionally, 12 µm thick longitudinal cryosections were obtained and nuclei were again counterstained with DAPI. To confirm presence of a lumen in the microvascular structures, cross-sections of the adventitia were analyzed by laser scanning fluorescent microscopy using the “Olympus FluoView 1000” confocal laser scanning microscope (Olympus Europe, Hamburg, Germany) with a 555 nm excitation wave length and an emission wave length ranging from 564 to 620 nm.
Statistics
Statistical analyses were performed by using the software “Graphpad Prism 6.04” (Graphpad software, San Diego, USA). Normal distribution was tested with D’Agostino and Pearsons omnibus normality test. Unpaired Student’s t-test was used to compare two groups. Differences were considered significant at p < 0.05.
Results
Technical Aspects
The bioreactor system developed in this study was characterized with respect to its accuracy for in vitro simulation of the physiological mechanical environment that blood vessels are exposed to in the human body. The mechanical conditions obtained in the bioreactor perfusion system are shown in Table 1 and Fig. 2c. While shear stress, pulse pressure, pulse frequency and cyclic distension were well within the physiological range of the human vasculature, slightly lower systolic and diastolic pressures were applied due to the relative higher compliance of fibrin vessels compared to native arteries as indicated by the secant elastic modulus. The pressure-waveform shows a dicrotic morphology indicating accurate simulation of the physiological arterial pressure curve in small diameter vessels.10
Overall Vessel Wall Architecture
The manufacturing process resulted in stable and homogeneous fibrin vessels with a burst pressure of 191.78 ± 24.64 mmHg, tensile strength of 20.11 kPa ± 4.90 kPa with a secant elastic modulus of 3.74 kPa × mm−1 ± 0.56 kPa × mm−1 and an initial wall thickness of 3.05 mm (Fig. 3a). Both static and dynamic incubation reduced the thickness of the vessel wall after 72 h, which was slightly more pronounced with static incubation (75.3% vs 72.2%, Table 2). This can also be appreciated qualitatively in Fig. 3. Moreover, the reduction of the luminal diameter was more pronounced in the statically incubated group.
To evaluate the overall structure of the bioartificial vessel wall, cross sections were stained and analyzed by fluorescence microscopy (Fig. 4). In both groups, the vessel was built up by three clearly distinct layers seeded with the respective layer-specific cell types. On the luminal surface, a closed endothelial cell layer formed by RFP-HUVEC was observed. Within the fibrin matrix, two additional layers were present: The middle one contained aSMA-positive cells and the outer layer was interwoven with RFP-HUVEC in a scattered distribution. Equal cellularity was observed in both groups. Thus, the manufacturing technique was successful in generating a stable three-layered vessel construct. Each layer was further characterized after incubation with or without mechanical stimulation.
Overall vessel wall morphology. The three-layered wall of the bioartificial vessels after 72 h of static incubation (a) or pulsatile perfusion (b) is shown in cross sections of the vessel wall. RFP-HUVEC (red) indicate the tunica intima-equivalent on the luminal surface (I) and capillary tubes of the tunica adventitia-equivalent in the abluminal part of the vessel wall (a). Smooth muscle cells of the tunica media-equivalent (m) and adipose-derived stem cells in the bioartificial tunica adventitia are visualized by immunofluorescence staining of α smooth muscle actin (green). Nuclei were counterstained using 4,6-diamidino-2-phenylindole (DAPI). Scale bar = 100 µm, RFP-HUVEC Red-fluorescent protein expressing human umbilical vein endothelial cells.
Effect of Pulsatile Perfusion
Pulsatile perfusion influenced the morphology of each layer. At first, the inner layer was examined under fluorescence microscopy of the luminal surface of the bioartificial vessels cultivated under static and dynamic conditions (Fig. 5). Cell coverage was equal for both groups with 86.8% ± 1.56% in the static group and 86.7% ± 0.78% in the mechanically stimulated group (p = 0.91). However, endothelial cells showed a longitudinal alignment parallel to the vessel axis and flow direction when exposed to pulsatile perfusion (Figs. 5a and 5b). Quantitative analysis of the cell orientation confirmed this orientation of the luminal HUVEC monolayers by showing a gaussian distribution with the highest number of cells in the range of − 5° to + 5° relative to the vessel axis (Figs. 5c and 5d). In contrast to that, random orientation was observed in the static control group. In both groups, no vascular tube formation was present on the luminal surface. Additionally, no evidence for unintended SMC-cellularization of the intima-layer was found (Fig. S2).
Evaluation of the tunica intima-equivalent. Endothelial coverage by RFP-HUVEC (red) was assessed on fluorescence microscopy images of the luminal surface of the bioartificial vessels after 72 h static incubation (a) or pulsatile perfusion (b). Cell alignment was quantified on 5 microscopic fields in 20-fold primary magnification for each vessel. Frequency distribution of the main axis of the cells is shown as deviation from the longitudinal vessel axis, which was defined as 0° (c static incubation: total cell number: 560; d pulsatile perfusion, total cell number: 549). Scale bar = 100 µm, RFP-HUVEC Red-fluorescent protein expressing human umbilical vein endothelial cells.
In the middle layer, SMC alignment and marker expression were analyzed immunohistochemically on cross sections of the vessel wall (Fig. 6). Cells in statically and dynamically incubated vessels showed co-expression of the early marker αSMA and the more specific SMC marker protein Calponin confirming successful myogenic differentiation from ASC. SMC in the static control showed a random cell orientation. In contrast, as shown recently,8 pulsatile perfusion resulted in a circular SMC alignment perpendicular to the vessel axis similar to the tunica media of natural arterial vessels.
Evaluation of the tunica media-equivalent. Cross sections of the vessel wall (same orientation as in Fig. 4) after 72 h static incubation (a, c) or pulsatile perfusion (b, d) were stained for the smooth muscle cell marker proteins α smooth muscle actin (a, b; green) and calponin (c, d, red). Nuclei were counterstained using 4,6-diamidino-2-phenylindole (DAPI). Scale bar = 100 µm.
Microscopic investigation of the abluminal surface of the bioartificial vessels demonstrated a complex network of tubular structures under static conditions with a random orientation (Fig. 7a). Interestingly, pulsatile perfusion induced an oriented structure of this capillary network. Similar to the luminal endothelial cells, also the vascular tubes aligned strictly parallel to the longitudinal vessel axis after pulsatile perfusion (Fig. 7b). Network analysis by ‘AngioTool’ software revealed that in these longitudinal tubes, branching existed exclusively in form of bifurcations (Fig. 7d). In contrast, randomly orientated tubes on the abluminal surface of the statically incubated vessels showed a branching pattern with bi-, tri- and quadrifurcations (Fig. 7c). ‘Angio-Tool’ analysis also revealed a higher number of junctions per microscopic field in the statically incubated group (138.93 ± 16.88 vs. 78.13 ± 3.43, p < 0.01; Fig. 8). Additionally, tube density was slightly but significantly higher compared to the pulsatile perfusion group whereas the total vessel length did not change (Fig. 8).
Evaluation of the tunica adventitia-equivalent. Tube formation by RFP-HUVEC was assessed on fluorescence microscopy images of the abluminal surface of the bioartificial vessels after 72 h static incubation (a) or pulsatile perfusion (b). (c) and (d) show images a and b after analysis by the ‘Angiotool’-software, which was used for quantification of the vascular network properties. (e) and (f) show cryosections of the vessel wall parallel to the longitudinal vessel axis. Nuclei were counterstained with DAPI. The arrow indicates an exemplary tube formed by at least 4 cells. Scale bar = 100 µm, RFP-HUVEC Red-fluorescent protein expressing human umbilical vein endothelial cells. (g) Shows a laser-scanning-microscopic image of a cross section of a microvascular tube indicating the presence of an open lumen. (i) Laser scanning image with excitation wave length of 555 nm and emission wave length ranging from 568 to 620 nm (ii) Respective bright field image, Scale bar = 10 µm.
Quantitative comparison of the microvascular network parameters. Tube networks of the outer layer of independent manufactured vessels (n = 3 for each group) were analyzed by ‘Angiotool’ software. For the indicated tube parameters, means ± SEM of 5 images per vessel are shown. Tube density was defined as the fraction of each field covered by microvascular structures (= relative tube area), total vessel length is the sum of the lengths of all vessels within one analysis field and number of junctions represents the total number of branching points per analysis field **p < 0.01 by unpaired Student’s t-test.
The morphology of the vascular networks was further assessed on longitudinal cryosections (Figs. 7e and 7f) confirming that the respective tube orientation was present throughout all zones of the outer layer. Moreover, it was observed that under dynamic conditions tubes consisted of several aligned endothelial cells, whereas under static conditions no similar structures were observed. The arrow in Fig. 7f marks an exemplary longitudinally oriented tube that consists of at least four different HUVECs as indicated by counterstaining of the nuclei.
Finally, it was evaluated whether the longitudinal tubes contained a lumen. Laser scanning microscopy clearly showed a lumen in the cross section of a microvascular tube and thus, confirmed it as a capillary-like structure (Fig. 7g).
Discussion
In this study, we developed a tissue engineering technique for the generation of three layered fibrin-based bioartificial blood vessels by combining a stepwise molding technique and physiological mechanical stimulation. The insights resulting from this in vitro proof-of-concept study can be summarized as follows: (i) Fibrin-based matrices of different concentrations and mechanical properties can be combined to a three layered bioartificial vessel wall facilitating seeding with the cell types relevant for in vitro vascular tissue engineering. (ii) All three layers of natural blood vessels were emulated within the physiologically structured bioartificial vessel wall after short-term culture of 72 h. (iii) Physiological mechanical stimulation in a sophisticated BPS is of pivotal importance to facilitate proper cell alignment in all three layers of the vascular wall. (iv) In this context, it was shown for the first time that biomechanical stimulation induced a parallel alignment of hollow capillary structures in an artificial tunica adventitia-equivalent.
Stepwise Fibrinogen Molding Technique
The need to resemble the physiological architecture of the vascular wall in bioartificial vessels has been recognized for a long time, and first attempts to generate three-layered cellularized vascular constructs have been made in different fields of tissue engineering. A very popular approach is the use of 3D-bioprinting, which allows for the generation of complex three-dimensional constructs.7 One example of a bioprinting approach targeting the vascular wall was provided by Schöneberg et al., who generated three-layered vascular channels in a fibrin/collagen matrix with subsequent fluid perfusion.28 However, the maximal fibrinogen concentration applicable for the bioprinting technique was limited to only 2.5% and the generated constructs were not suitable for regenerative approaches including in vitro mechanical conditioning. Opposed to that, mechanical stability of the fibrin matrix generated by our molding technique with subsequent centrifugation was sufficient to generate self-supporting vessels suitable for mechanical stimulation under pulsatile pressure conditions.
As an alternative technique, Iwasaki et al. generated mechanically robust three-layered bioartificial vessels using cellularized polyglycolic acid (PGA)- and polygalactone-sheets and subsequent pulsatile perfusion in a BPS.12 While this study underlines the importance of mechanical stimulation for generating the physiological morphology of the vascular wall, the applicability of these bioartificial vessels for regenerative approaches is limited due to the use of bovine cells and biodegradable scaffolds, which potentially produce cytotoxic degradation products.29 In our study, we used HUVECs and human ASCs as cell sources. ASCs are known to be an easily accessible and thus, potentially autologous cell source for vascular tissue engineering approaches3 and respond well to both biochemical and biomechanical differentiation factors.8 Moreover, fibrinogen can be easily precipitated in large amounts from peripheral blood making fibrin an ideal biological matrix material for bioartificial vessels. It allows seeding of multiple cell types29 and its degradation products are not toxic. Its high biocompatibility has been studied and proven extensively since fibrin glue is used frequently as a sealant for adjuvant hemostasis in cardiovascular surgery for many years.26 On the other hand, the mechanical stability of fibrin based vascular grafts is considerably lower than that of natural vessels or synthetic polymers. The burst pressure and tensile strength observed in this study was sufficient for in vitro pulsatile stimulation, but higher safety margins have to be achieved when clinical application is targeted in the future.
The decrease in wall thickness of cellularized fibrin constructs during the culture period is a known phenomenon,8 which appears to be even more pronounced in this study due to the low fibrinogen concentration of the tunica adventitia. Comparison of the relative media- and adventitia-thickness of the graft with physiological values of the carotid artery of healthy individuals with low cardiovascular risk30 shows similarity for both groups with a lower absolute wall thickness (Table 2).
Generation and Mechanical Stimulation of the Tunica Intima-Equivalent
A confluent endothelial cell layer in the tunica intima is of pivotal importance for hemocompatibility of bioartificial vessels.21 In this study, the percentage area covered with endothelial cells was high in both, the static and biomechanically stimulated three-layered vessels. This shows that endothelial cell attachment was sufficient to withstand physiological shear rates and stretch amplitudes. This strong cell attachment to the fibrin matrix, that has also been shown by Isenberg et al.11 again underlines suitability of fibrin as a matrix material in vascular tissue engineering.
Mechanical stimulation in the BPS induced endothelial cell elongation and alignment parallel to the vessel axis, which resembles the physiological alignment in natural blood vessels. On the contrary, no elongation or directed alignment was observed on the luminal surface of statically incubated three-layered vessels. It long has been known that endothelial cells respond to shear stress with a variety of effects including alignment parallel to flow.24 Moreover, cyclic stretch13 and potentially also luminal pressure19 have been shown to influence endothelial cell alignment and connectivity. Since the BPS used here exerted flow, stretch and pressure on the luminal surface, most likely all three mechanical stimuli influenced endothelial cell alignment synergistically and thus induced the strict longitudinal cell orientation in this tunica intima-like layer.
Generation and Mechanical Stimulation of the Tunica Media-Equivalent
Biochemical differentiation of ASC to multiple mesenchymal cell types has been studied extensively in the past.36 Additionally, the response of ASCs to mechanical differentiation factors is well known.8 This analysis also revealed that differentiation capacity of ASC is donor dependent and thus requires sound characterization as described previously.15 This also required selection of a suitable donor for SMC differentiation in this study. Here, the relatively short mechanical stimulation time of 72 h was sufficient to induce circular orientation of the SMCs around the vessel lumen resembling the natural arrangement of the tunica media while random orientation of the cells was observed in the statically incubated vessels. However, much longer culture times are usually required to facilitate extracellular matrix production by seeded cells.8 Circular alignment is of pivotal importance to accomplish contraction of the vessel diameter, which represents the main function of the tunica media in natural blood vessels. The suitability of mechanically stimulated SMC to induce contractility in fibrin-based grafts is described extensively elsewhere.8 Moreover, SMCs of the perfused vessels showed a spindle-like morphology, which is characteristic for the desired contractile phenotype of SMCs.23
Expression of the SMC marker proteins alpha smooth muscle actin (αSMA) and Calponin was assessed qualitatively by immunohistochemical staining. Despite deprivation from biochemical growth factors during the cultivation period both, the static and dynamic group where stained positive for these marker proteins. This confirms successful biochemical differentiation towards SMC lineage prior to cell seeding without markable de-differentiation during culture in the bioartificial vessels.
Generation and Mechanical Stimulation of the Tunica Adventitia-Equivalent
To generate a vascular network in the tunica adventitia, RFP-HUVECs where co-seeded with ASCs in a low concentration fibrin matrix and extensive tube formation was observed in both groups. We have previously shown that fibrin gels with a fibrinogen concentration of 5 mg × mL−1 are suitable for vascular tube formation by cell self-assembly but provide only low mechanical strength.34 In the three-layered bioengineered vessel, the compacted and highly concentrated fibrin matrix of the tunica media provided sufficient mechanical strength for pulsatile perfusion. This is in consistence with natural arteries, in which the tunica media contributes most to mechanical stability. Thus, the tunica adventitia-equivalent was not directly exposed to the intraluminal pressure facilitating the use of a low concentration fibrin matrix. The co-culture technique with ASCs has been shown to improve capillary tube formation in fibrin matrices by us and others.9,34 As potential mechanisms for the induction and stabilization of tube formation by ASC, Rohringer et al. showed induction of genes relevant for tube formation in HUVEC in co-culture with ASC.25 Furthermore, we previously described that ASC take on a pericyte-like function in co-cultures with endothelial cells and thus, stabilize the vascular network.16
In contrast to this study, the vast majority of tissue engineering approaches targeting the tunica adventitia was focused on integrating fibroblasts producing extracellular matrix proteins like collagen.12,18,28,32 Though this is certainly an important feature, it should be noted that the natural adventitia in human blood vessels is far more complex than a simple collagen layer as it includes multiple structures and has a variety of functions crucial for vessel biology.31 A proper nutrient and oxygen supply facilitated by the vascular capillary structures of the tunica adventitia is essential for the entire vessel wall. Thus, vascularization of the tunica adventitia should be considered a key feature in vascular tissue engineering approaches as well. In this context, ASC represent suitable stromal supporting cells for in vitro vascular tissue engineering because they facilitate tube formation and can be differentiated to SMC for the use in the media-equivalent as well.
Specifically for fibrin-based bioartificial vessels, limited cellularization in deeper layers of the vessel wall due to insufficient vascularization and thus, nutrition, is a long known limitation.1
One of the very few tissue engineering approaches targeting the vasa vasorum of the adventitia was published by Guillemette et al.6 They showed that vascularization of adventitia-equivalents generated by cell self-assembly facilitated graft integration and perfusion after subcutaneous implantation into mice. Importantly, only minimal in vivo vascularization was observed in adventitia equivalents that were not pre-vascularized in vitro. This again underlines the importance of generating a properly vascularized adventitial tissue as presented here in form of the three-layered vessel with regards to regenerative approaches.
Considering these findings, generating three-layered bioartificial vessels with a pre-vascularized adventitia is a promising strategy to overcome the limitation concerning cellularization of thicker vessel walls in fibrin-based bioartificial vessels. In our study, complete cellularization of the full thickness of the vessel wall was observed in both the statically and the dynamically cultured three-layered vessels indicating sufficient nutrient or oxygen supply throughout the vessel wall.
Although capillary-like tube formation was observed in both groups, the overall structure of the tubes differed distinctively. While a random tubular network with multiple branching points was observed in the static group, the tubes in the adventitia of the dynamic group were aligned strictly parallel to the vessel axis with fewer branching points. Until now, only very little is known about the impact of mechanical stretch on vascular tube formation. Russo et al. describe an amplitude dependent increase or decrease in angiogenesis by endothelial cells subjected to stretch in a 2-dimensional vacuum stretching system without any noticeable influence on tube alignment or direction.27 When comparing these results to our findings, it should be noted that endothelial cells in the three-layered vessel are seeded into a 3-dimensional biological matrix and exposed to a complex multiaxial stretch induced by pulsatile perfusion instead of a 2-dimensional silicon membrane facilitating only uniaxial stretch.
The longitudinal alignment of vascular tubes parallel to the vessel axis with bifurcating branches resembles the alignment of vasa vasorum capillaries in the adventitia of natural vessels, that has been studied and described extensively by Lametschwandtner et al. on human saphenous veins.14 To our knowledge, vascular tube alignment induced by mechanical stimulation has not been reported yet and will be further investigated in subsequent studies targeting this phenomenon specifically.
While this study focused on the emulation of the microvascular components of the adventitia, using longer culture times to facilitate extracellular matrix construction by ASC or adding collagen producing cells such as fibroblasts could potentially facilitate an even more accurate emulation of this complex layer.
Moreover, studies are needed to investigate the long term impact of cellularization on mechanical and functional properties of three-layered grafts. Additionally, the generation of the inner and outer elastic membrane, which are characteristic for larger arteries, was not targeted in this study and represents another goal to potentially facilitate even more accurate emulation of the natural vessel wall architecture in bioartificial vascular grafts.
References
Aper, T., O. E. Teebken, G. Steinhoff, and A. Haverich. Use of a fibrin preparation in the engineering of a vascular graft model. Eur. J. Vasc. Endovasc Surg. 28(3):296–302, 2004.
Aper, T., M. Wilhelmi, C. Gebhardt, K. Hoeffler, N. Benecke, A. Hilfiker, et al. Novel method for the generation of tissue-engineered vascular grafts based on a highly compacted fibrin matrix. Acta Biomater. 29:21–32, 2016.
Bajpai, V. K., and S. T. Andreadis. Stem cell sources for vascular tissue engineering and regeneration. Tissue Eng. Part B Rev. 18(5):405–425, 2012.
Enomoto, S., M. Sumi, K. Kajimoto, Y. Nakazawa, R. Takahashi, C. Takabayashi, et al. Long-term patency of small-diameter vascular graft made from fibroin, a silk-based biodegradable material. J. Vasc. Surg. 51(1):155–164, 2010.
GBD. Mortality and causes of death collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 385(9963):117–171, 2013.
Guillemette, M. D., R. Gauvin, C. Perron, R. Labbé, L. Germain, and F. A. Auger. Tissue-engineered vascular adventitia with vasa vasorum improves graft integration and vascularization through inosculation. Tissue Eng Part A. 16(8):2617–2626, 2010.
Hann, S. Y., H. Cui, T. Esworthy, S. Miao, X. Zhou, S. Lee, et al. Recent advances in 3D printing: vascular network for tissue and organ regeneration. Transl Res. 09(211):46–63, 2019.
Helms, F., S. Lau, M. Klingenberg, T. Aper, A. Haverich, M. Wilhelmi, et al. complete myogenic differentiation of adipogenic stem cells requires both biochemical and mechanical stimulation. Ann Biomed Eng. 48:913–926, 2019.
Holnthoner, W., K. Hohenegger, A. Husa, S. Muehleder, A. Meinl, A. Peterbauer-Scherb, et al. Adipose-derived stem cells induce vascular tube formation of outgrowth endothelial cells in a fibrin matrix. J Tissue Eng Regen Med. 9(2):127–136, 2015.
Huang, C., D. Chambers, and G. Metthews. Arterial Pressure Waveforms. In: Basic Physiology for Anaesthetists2nd, edited by C. Huang, D. Chambers, and G. Matthews. Cambridge: Cambridge University Press, 2019, pp. 155–157.
Isenberg, B. C., C. Williams, and R. T. Tranquillo. Endothelialization and flow conditioning of fibrin-based media-equivalents. Ann Biomed Eng. 34(6):971–985, 2006.
Iwasaki, K., K. Kojima, S. Kodama, A. C. Paz, M. Chambers, M. Umezu, et al. Bioengineered three-layered robust and elastic artery using hemodynamically-equivalent pulsatile bioreactor. Circulation. 118(14):52, 2008.
Jufri, N. F., A. Mohamedali, A. Avolio, and M. S. Baker. Mechanical stretch: physiological and pathological implications for human vascular endothelial cells. Vasc. Cell. 7:8, 2015.
Lametschwandtner, A., B. Minnich, D. Kachlik, M. Setina, and J. Stingl. Three-dimensional arrangement of the vasa vasorum in explanted segments of the aged human great saphenous vein: scanning electron microscopy and three-dimensional morphometry of vascular corrosion casts. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 281(2):1372–1382, 2004.
Lau, S., M. Klingenberg, A. Mrugalla, F. Helms, D. Sedding, A. Haverich, et al. Biochemical myogenic differentiation of adipogenic stem cells is donor dependent and requires sound characterization. Tissue Eng. Part A. 25(13–14):936–948, 2019.
Lau, S., C. Schrimpf, M. Klingenberg, F. Helfritz, T. Aper, A. Haverich, et al. Evaluation of autologous tissue sources for the isolation of endothelial cells and adipose tissue-derived mesenchymal stem cells to pre-vascularize tissue-engineered vascular grafts. BioNanoMaterials. 16(4):309–321, 2015.
Lawson, J. H., M. H. Glickman, M. Ilzecki, T. Jakimowicz, A. Jaroszynski, E. K. Peden, et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet. 387(10032):2026–2034, 2016.
L’Heureux, N., S. Pâquet, R. Labbé, L. Germain, and F. A. Auger. A completely biological tissue-engineered human blood vessel. FASEB J. 12(1):47–56, 1998.
Müller-Marschhausen, K., J. Waschke, and D. Drenckhahn. Physiological hydrostatic pressure protects endothelial monolayer integrity. Am. J. Physiol. Cell Physiol. 294(1):324, 2008.
Papaioannou, T. G., and C. Stefanadis. Vascular wall shear stress: basic principles and methods. Hellenic J Cardiol. 46(1):9–15, 2005.
Pawlowski, K. J., S. E. Rittgers, S. P. Schmidt, and G. L. Bowlin. Endothelial cell seeding of polymeric vascular grafts. Front. Biosci. 9:1412–1421, 2004.
Pennings, I., E. E. van Haaften, T. Jungst, J. A. Bulsink, A. J. W. P. Rosenberg, J. Groll, et al. Layer-specific cell differentiation in bi-layered vascular grafts under flow perfusion. Biofabrication. 12(1):015009, 2019.
Rensen, S. S., P. A. Doevendans, and G. J. van Eys. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 15(3):100–108, 2007.
Resnick, N., H. Yahav, A. Shay-Salit, M. Shushy, S. Schubert, L. C. M. Zilberman, et al. Fluid shear stress and the vascular endothelium: for better and for worse. Prog. Biophys. Mol. Biol. 81(3):177–199, 2003.
Rohringer, S., P. Hofbauer, K. H. Schneider, A. Husa, G. Feichtinger, A. Peterbauer-Scherb, et al. Mechanisms of vasculogenesis in 3D fibrin matrices mediated by the interaction of adipose-derived stem cells and endothelial cells. Angiogenesis. 17(4):921–933, 2014.
Rousou, J. A. Use of fibrin sealants in cardiovascular surgery: a systematic review. J. Card. Surg. 28(3):238–247, 2013.
Russo, T. A., D. Stoll, H. B. Nader, and J. L. Dreyfuss. Mechanical stretch implications for vascular endothelial cells: altered extracellular matrix synthesis and remodeling in pathological conditions. Life Sci. 213:214–225, 2018.
Schöneberg, J., F. De Lorenzi, B. Theek, A. Blaeser, D. Rommel, A. J. C. Kuehne, et al. Engineering biofunctional in vitro vessel models using a multilayer bioprinting technique. Sci Rep. 8(1):10430, 2018.
Shaikh, F. M., A. Callanan, E. G. Kavanagh, P. E. Burke, P. A. Grace, and T. M. McGloughlin. Fibrin: a natural biodegradable scaffold in vascular tissue engineering. Cells Tissues Organs. 188(4):333–346, 2008.
Skilton, M. R., L. Boussel, F. Bonnet, S. Bernard, P. C. Douek, P. Moulin, et al. Carotid intima-media and adventitial thickening: comparison of new and established ultrasound and magnetic resonance imaging techniques. Atherosclerosis. 215(2):405–410, 2011.
Stenmark, K. R., M. E. Yeager, K. C. El Kasmi, E. Nozik-Grayck, E. V. Gerasimovskaya, M. Li, et al. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol. 75:23–47, 2013.
Xu, Y., Y. Hu, C. Liu, H. Yao, B. Liu, and S. Mi. A novel strategy for creating tissue-engineered biomimetic blood vessels using 3D bioprinting technology. Materials (Basel). 11(9):1581, 2018.
Zhang, Y., X. S. Li, A. G. Guex, S. S. Liu, E. Müller, R. I. Malini, et al. A compliant and biomimetic three-layered vascular graft for small blood vessels. Biofabrication. 9(2):025010, 2017.
Zippusch, S., F. Helms, S. Lau, M. Klingenberg, C. Schrimpf, A. Haverich, et al. Perfusion promotes endothelialized pore formation in high concentration fibrin gels otherwise unsuitable for tube development. Int J Artif Organs. 2020. https://doi.org/10.1177/0391398820936700.
Zudaire, E., L. Gambardella, C. Kurcz, and S. Vermeren. A computational tool for quantitative analysis of vascular networks. PLoS ONE. 6(11):e27385, 2011.
Zuk, P. Adipose-derived stem cells in tissue regeneration: a review. ISRN Stem Cells. 2013:713959, 2013.
Acknowledgments
The work was funded by the German Society for Implant Research and Development (funding title: “Vascularization of bioartificial implants 2017–2020”).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Smadar Cohen oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10439_2021_2728_MOESM1_ESM.tif
Supplemental figure S1: Morphological changes during pre-differentiation. Prior to seeding, successful pre-differentiation of ASC was confirmed by evaluating characteristic changes in cell morphology that are associated with differentiation towards a SMC-phenotype. (A) shows light microscopic morphology of cultured ASC expressing multiple cell extensions per cell. In contrast to that, a spindle-like appearance that is characteristic for SMC is observed after 8 days of biochemical pre-differentiation (B). ASC = adipose-derived stem cells, SMC = smooth muscle cells, Scale bar = 100µm. (TIFF 3950 kb)
10439_2021_2728_MOESM2_ESM.tif
Supplemental figure S2: Distribution of αSMA-expressing cells. To exclude unintended cellularization of the luminal surface by SMC or ASC, cross sections were stained green for αSMA and analyzed regarding the presence of αSMA-positive cells in the intima-layer. The luminal surface (arrow) was covered with RFP-expressing HUVEC shown in (A). When removing the red color channel (B), potential green signals on the intima layer are uncovered. As shown in this exemplary representative cross-section of a statically incubated bioartificial vessel, no signals indicating the presence of SMC or ASC were noted on the luminal surface. Nuclei were counterstained using 4,6-diamidino-2-phenylindole (DAPI). αSMA = alpha smooth muscle actin, SMC = smooth muscle cells, ASC = adipose-derived stem cells, RFP-HUVEC = red-fluorescent protein expressing human umbilical vein endothelial cells, scale bar = 100 µm. (TIFF 3147 kb)
Rights and permissions
About this article
Cite this article
Helms, F., Lau, S., Aper, T. et al. A 3-Layered Bioartificial Blood Vessel with Physiological Wall Architecture Generated by Mechanical Stimulation. Ann Biomed Eng 49, 2066–2079 (2021). https://doi.org/10.1007/s10439-021-02728-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-021-02728-9