Abstract
Research insights into uterine function and the mechanisms of labour have been hindered by the lack of suitable animal and cellular models. The use of traditional culturing methods limits the exploration of complex uterine functions, such as cell interactions, connectivity and contractile behaviour, as it fails to mimic the three-dimensional (3D) nature of uterine cell interactions in vivo. Animal models are an option, however, use of these models is constrained by ethical considerations as well as translational limitations to humans. Evidence indicates that these limitations can be overcome by using 3D culture systems, or 3D Bioprinters, to model the in vivo cytological architecture of the tissue in an in vitro environment. 3D cultured or 3D printed cells can be used to form an artificial tissue. This artificial tissue can not only be used as an appropriate model in which to study cellular function and organisation, but could also be used for regenerative medicine purposes including organ or tissue transplantation, organ donation and obstetric care. The current review describes recent developments in cell culture that can facilitate the development of myometrial 3D structures and tissue engineering applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
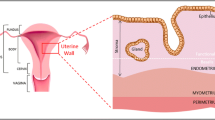
The myometrium is the smooth muscle component of the uterus, and the functioning of myometrial smooth muscle cells (SMCs) plays a key role in human pregnancy and labour.90 The uterus remains quiescent for most of a normal 40-week gestation until coordinated uterine contractions occur at labour.79 The mechanisms that underpin the onset of labour are yet to be fully identified.69 Understanding these mechanisms may provide a means of intervening in major obstetric complications, such as preterm labour, failure of labour to progress and postpartum haemorrhage (where the uterus fails to contract properly after delivery).75 Additionally, uterine function can be compromised in many women as a result of damage caused by previous caesarean section or the removal of uterine fibroids.40 Tissue engineering applied to the myometrium represents a potential means for interrogating uterine function and repairing human uterine defects.17,18,33,75
Myometrial Cells
The myometrium consists of connected collagen fibres supporting bundles of SMCs. When myocytes contract as a result of cross-bridge cycling between actin and myosin filaments,6 these collagen tissue matrices coordinate transmission of the contractile force throughout the bundles. To coordinate their activity, myocytes communicate both metabolically and electrophysiologically.70 Understanding the regulation of genes that permit coordination is essential to understanding parturition. Thus, functional myometrial tissue is more than just individual myometrial SMCs, but rather is a complex interaction between populations of myometrial cells in a three-dimensional (3D) connective tissue matrix. Initial studies have focused on two-dimensional (2D) cell culture systems as well as on intact strips of myometrium,18 however, more recently efforts are being made to recapitulate the 3D structures of intact tissue using tissue engineering. Through reverse engineering of tissue researchers hope to gain a greater understanding of the way in which individual components influence one another to regulate myometrial contractility, and to enable the creation of new pieces of myometrial tissue suitable for use in repairing uterine defects.3
Cell Culture
As a tool for researchers in biotechnology, pharmacology, regenerative medicine, industry, drug discovery, manufacturing and biological research, cell culture is a fundamental and indispensable technique.62 Cell culture requirements can differ between cell lines created for research purposes and those produced for therapies. As such, limitless augmentations of different methods of culturing cells have arisen as more and more cell lines have been produced. Cultured cells obey a regular cycle of proliferation specified by the duration of the cycle, and in the culture environment transition through distinct phases, including the lag, log, plateau and decline phases.62 To maximise health, cells are split during the log phase, which is a period of exponential growth. As such cells in culture typically transition between lag and log phase as they are passaged. Primary myometrial cells, for instance, may require 6–10 days of culture to reach 80–90% confluence, at which point the cells can be subcultivated at a 1:3 split.10
A variety of uterine smooth muscle cell lines have been produced and require different conditions for culture. An immortalised cell line of non-pregnant human myometrial cells was created by transfection with human telomerase reverse transcriptase (hTERT).10 The hTERT cells have been commonly used to study myometrial cell function due to their enhanced survival and proliferative ability because of their increased expression of telomerase.65 A pregnant human myometrial (PHM) cell line, which was produced by infecting human pregnant term myometrium with an adenovirus vector expressing the E6/E7 proteins of human papilloma virus 16, has been used as the cells retain the morphological and proliferating characteristics of uterine SMCs in culture.54 In addition, isolated human primary myometrial cells, obtained from tissue biopsies during caesarean section deliveries, are a popular option for pregnancy investigations in vitro, however, they have a limited life span and decreased contractility in culture.8 In order to be useful in understanding in vivo events during labour, cultured SMCs, including primary myometrial cells, hTERTs and PHMs, must transition from a relaxed phenotype to a contractile phenotype in vitro.67
To achieve a contractile phenotype, the cultured myometrial cells have to be exposed to biochemical signals and a biophysical environment that mimics the environment of myometrial cells in vivo.8 The biophysical environment of cells in vivo encompasses aspects such as exposure to stretch, pressure and shearing forces, while the biochemical environment encompasses aspects such as oxygen tension, exposure to hormones and blood-borne signals.14 Together, exposure to these elements affects cell phenotype and differentiation. To this end, the biophysical and biochemical conditions need to be considered when culturing cells in vitro.
Two-Dimensional (2D) Culture
2D or classical cell culture methods involve growing cells at the bottom of a culture dish or flask inside an incubator under controlled conditions of temperature, typically 37°C, in a humidified atmosphere containing 5% CO2.29 Utilising a layer of cells attached to the surface of a culture dish that has been modified with optically clear polystyrene has improved screening assays of cells and has been in use for decades. Improvement of cell culturing techniques has increased our understanding of the regulation of 2D architectures. Controlling the function, proliferation, and adhesion of cells onto the basic matrix is affected by the interactions between the matrix and cells in the culture.39 Although in vitro 2D cultures are currently in common use for experiments, they do not mimic the 3D nature of cell interactions within tissues.57 Traditional 2D culture restricts cell attachment and spread to the flat surface of the plastic or glass culture dish.89 These limitations of the 2D system in studying cell function and responses to drug treatment indicate the need for in vitro culture systems that more closely mimic the in vivo environment of the cell. As such, researchers are increasingly exploring options for 3D culture.62
3D Culture Models
Overview and Goals of 3D Culture
There are many challenges associated with studying the functioning of mammalian organs due to their inaccessibility for experimental manipulation, which renders it difficult to make optical observations.64 Furthermore, the use of animal models is limited by ethical considerations,15 as well as concerns regarding their translational applicability to humans.52 Together, these necessitate the development of models that more accurately capture critical aspects of the in vivo environment.34 Creation of a 3D model in vitro, that can mimic physiological function in a simple environment, is one of the main goals of 3D culture modelling. 3D culture more closely resembles the in vivo environment than that of 2D culture, and as such, is more effective in maintaining cellular differentiation in vitro (Table 1).39
Benefits of 3D Culture
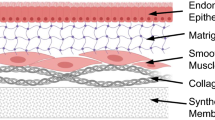
3D culturing systems have been developed for the purposes of mimicking the in vivo structure and condition of cells.23 These artificial 3D environments permit the proliferation of cells in different layers by seeding them onto a scaffold, and for the purposes of regenerative medicine and tissue engineering, can provide a model system for studies into cellular proliferation, metabolism, structural aspects, aging and disease.54 Within these 3D systems, cell function and adhesion may change in terms of their responses to treatments and interactions with neighbouring cells compared to that seen in 2D culture.48 Cells in 3D cultures can behave differently to 2D cultures, and there are multiple possible approaches to the development of 3D cultures.63 For example, evidence indicates that epithelial cells from the female reproductive system including the uterus, the mammary glands and the ovaries grown in 3D culture are more similar to in vivo cells than that of 2D cultured cells. The 3D epithelial cell structure establishes a basoapical polarity axis according to the distribution of cytoplasmic and membrane proteins, and is a critical aspect in the control of survival, proliferation, and the functional differentiation of the cells. Use of collagen, laminin, and mixtures of extracellular components such as Matrigel permitted Adissu et al. to make a 3D cell culture model for regenerative medicine laboratory investigations in vitro. 1
Developing 3D cell models in vitro may enhance the reproducibility of experiments, improve the ability to investigate molecular aspects of cell function, and allow more accurate testing of therapeutics.47 3D models are relevant to drug screening, disease discovery, regenerative medicine and the study of physiological mechanisms in vitro.15,93 The optimal 3D culturing system and conditions differ between cell types and cell lines.62
Different cell lines within 3D models can be maintained in a similar condition to the in vivo situation and yield more homogenous materials for study. So far more than 380 cell lines have been used in different approaches to 3D culture. Studies on 3D culturing systems can probe the influence of selective manipulations on cell and tissue functions.1
Downside of 3D Culture
Analysing, imaging and optimising 3D systems are key challenges associated with 3D culturing systems.87 The fact that 3D cultures can have substantial depth to their structure means that traditional bright field/confocal microscopy often cannot be used to visualise cell layers thicker than 100 µm due to the focal depth and lack of light penetration.22 3D cultures are also more complex to set up than traditional 2D culture. Furthermore, different cells grow better in different types of 3D culture. As such, investigation into different 3D culturing methods is needed to determine appropriate and optimal 3D culturing conditions for different cell lines.87 A variety of 3D models have been developed, and these can be categorised into scaffolding and non-scaffolding (scaffold-free) methods.
3D Culture Methods
Recent studies in 3D culture have been performed using either the ‘top-down’ or ‘bottom-up’ approaches, both of which are fundamental for tissue engineering and cell biology. The top-down approach involves utilising a biomaterial scaffold, and then seeding cells into the scaffold for macroscopic tissue creation.21 In contrast, the bottom-up approach involves the sequential application of small blocks, in order to build the microscale tissue and recreate the physical and chemical aspects of the original tissue.21 Mammalian cell assembly into 3D cultured constructs can be achieved by taking advantage of either of these approaches.21,29
Scaffolding Methods
Tissue engineering has faced various challenges amidst the development of 3D culture. These challenges include forming the tissue architecture, vascularisation patterns and the seeding of different types of cells.76 In terms of the top-down approach, these issues can be addressed by varying the properties of the scaffold material. This involves introducing differences in porosity and shape of the scaffold, as well as surface morphology and scaffold size.16 To cater for various 3D culture applications there are a variety of commercially available manufactured scaffolds, in addition to different materials and methods for de novo scaffold creation (Table 2). Gelatine, collagen, hydrogel and poly(lactic-co-glycolic acid) (PLGA) are examples of material choices used for de novo scaffold creation.87 PLGA is a synthetic polymer whereas collagen, gelatine and hydrogel are natural materials, however, all are commonly used as scaffolds due to their varying pore size, structural stiffness, manipulation simplicity and low cost.62
Organic matrices, including collagen and Matrigel (BD Biosciences, US), are the groups most commonly used in 3D cultures.1 Collagen and Matrigel have typically been used in top-down 3D cultures by providing a matrix for cells to be seeded on or into.47 The use of Matrigel to assess differentiation and proliferation of muscle progenitor cells is more common than collagen.23 The presence of biochemical factors including growth factors and extracellular matrix (ECM) factors within Matrigel may overlay higher proendocrine effects and more appropriate morphogenesis than collagen in epithelial cells from adult mouse pancreas.5 Nevertheless collagen has also been widely used in tissue engineering, however, standardising culture conditions between different batches of collagen has proved extremely difficult. A 3D model of epithelial cells in collagen gel has proved to be a useful tool for cell biology and cancer analysis.83 To date, 3D collagen gels mainly fulfil a supportive role as scaffolds in biological investigations. Collagen-like nanofiber hydrogels have been used effectively for the 3D culture of human embryonic stem cell-derived cardio myocytes (hESC-CMs) to investigate their function, viability and the effect of growth factors.31
As regenerative medicine moves toward the recreation of tissues or organs in vitro, numerous 3D culture methods for different cell types and functions have been developed. In a study, cardiac stem cells were used in a long term 3D-nanoculture system based on a collagen scaffold. This model can not only be used to build an artificial heart, but can also be used to evaluate stem cell differentiation into desired cell lineages by growth factor manipulation.94 Several scaffolds have been developed that are comprised of self-assembling peptides, a class of biological material, including EAK16-II and RADA16-I, which consist of a peptide content of 1–10 mg/mL and more than 99% water.28,31 Peptide scaffolds have proven their effectiveness in promoting cell proliferation and adhesion, in which they support the differentiation of a variety of mammalian cell lines in different culture environments.93 These peptides have been constructed to contain repeating hydrophobic and hydrophilic amino acids which gives alternating polar and non-polar surfaces. Temperature, pH or concentrated denaturing agents do not influence the stability of sheet structures formed by the self-assembling peptides.28 The production of macroscopic scaffolds can originate from peptide self-assembly in physiological salt-containing solutions (PBS, 5 mM Na3-PO4/150 mM NaCl), which results in highly stable structures, however, they do not form fibres longer than 20 cm for strings or sheets up to several cm2 in constructs.93 In some methods, combinations of materials have also been used to make 3D culturing systems. Cells-in-Gels-in-Mesh (CiGiM) was created to support seeded MDA-MB-231 breast cancer cells in gel by utilising stacked sheets of polyethylene-based mesh made by placing them inside a SPI Plasma Prep II Chamber (Structure Probe Inc., West Chester, PA) to promote a tissue-like structure.
Although this system brings simplicity into the generation and analysis of cells that are cultured layer-by-layer (9 layers; 1.6 mm per layer) under uniform pressure (~5000 psi), cells can die within lower layers due to lack of oxygen.68 To prevent the non-equivalency of layers, porous structures have been considered for 3D culturing purposes. In this regard, nano-culture plates made of transparent cycloolefin resin can permit the 3D culture of cells in vitro.89 Depending on the application, types of nanoscale cultures differ from nano-culture plates to nanofiber scaffolding. The benefits of nanofiber scaffolds include increased stress resistance and better elasticity, however, the nanofiber scaffolds have high production costs. According to the ultimate stress and strain test, Young’s moduli of elasticity, 2D and 3D nanofiber samples were loaded with a 1 kN load as well as being tested with a loading velocity of 10 mm/min until composite rupture. Both mechanical properties were found to be significantly higher in nanofiber 3D structures.61 More cost-effective 3D culturing systems such as polyglactin-910 (Vicryl) mesh have been developed for studying human uterine cell proliferation and contractile behaviour. This model has been demonstrated to support 3D culture of human uterine myocytes, and can be used as a model for cell-to-cell interactions in myometrium, however, low transparency of the scaffold restricts microscopic analysis.91
Scaffold culturing techniques are of interest in cancer research due to the benefits of these techniques. The study of drug effects on targets and biomarker profiles in 3D cultured cells has introduced new strategies for improved cancer treatment and has furthered our understanding of signalling networks.32 A polymer based 3D model established to study the effect of tamoxifen on MCF-7 human breast cancer cells has shown that when grown in 3D culture, production of lactate by MCF-7 cells is more similar to in vivo cells than that of 2D cultured cells.13 In addition, 3D culture of lung carcinoma cell lines HCC827 (activating EGFR mutation) and A549 (EGFR wild-type) is helping to elucidate how tumours integrate into the small intestinal submucosa microenvironment, whilst more accurately reflecting the clinical situation in vitro.72
3D cell droplet is another important development of 3D culture that has made the in vitro assessment of in vivo processes more meaningful.68 A plastic pillar insert has been developed for 3D hydrogel droplets, and involves placing eight pillars together, with each pillar being 2 mm in diameter, 9 mm in height and 9 mm between pillar distance. The hydrogel droplets contain approximately 1x106 cells/mL. This has made it possible to fit 3D cultured cells into 96-well plates. This technique allows simple changing of the growth media or the treatment of different wells in the 96-well plate by immersing the pillar insert into any well.43 The usefulness of hydrogels in 3D culture models in mimicking tissue-like properties has been demonstrated. To maximise cytocompatibility and minimise hydrogel processing, synthetic hydrogels with polymeric networks have been used.35 In addition, advanced cancer cell 3D culturing methods have used macro porous hydrogel (Cryogel) (Protista Biotechnology AB, Sweden), which provides an interconnected structure for the cells and tissue-like elasticity characteristics in vitro, which maintains fluids inside the macropores.11
Utilising a biodegradable, synthetic hydrogel scaffold is another method of forming a 3D cell culture environment.58 Combinations of fibrinogen and cross-linked polyethylene glycol (PEG) with 10, 15, and 20% w/v amount of polymer to construct 1 mm thick scaffolds with varying molecular weights (4, 6 and 20 kDa) have been investigated. This type of scaffold was shown to be biodegradable and suitable for cell adhesion of SMCs. Moreover, it allows SMCs to form connected networks, which is important for studying synchronicity of contractions.2 Cell migration in 3D hydrogels can be monitored by utilising nuclear fluorescent staining with diamidino-2-phenylindole (DAPI) at 5 and 24 h after cell seeding. This study showed that algorithms for image processing facilitated quantitative measurements of migration in large-scale drug screening on 3D matrix structures in which the ratio of the area of cell population (~6.2 mm) over the gel total height (∼8.3 mm) was calculated.78
The use of easy to obtain, inexpensive materials to make cost-effective 3D culture is a key approach in scaffold and material preparation for 3D models. In an effort to streamline 3D scaffold fabrication, we utilised glass wool fibres due to their surface properties to investigate the feasibility, proliferation properties and efficiency of 3D culturing of uterine SMCs, as well as to study their cellular responses in three dimensions.33 hTERT-immortalised myometrial cells were seeded onto glass wool scaffolding fibres placed in culture plates and were maintained for a period of one month. While glass wool fibre is not an ideal choice for engineered tissue implantation, hTERT myometrial cells were found to readily attach to the surface of glass wool. The absolute structural stability of glass wool fibres enabled long-term investigations of cell-scaffold interactions.33
Polyvinyl alcohol (PVA) is another inexpensive material that is biocompatible and highly biodegradable, like natural polymer starch.7 By means of a simple method of chemical cross-linking with the absorption peaks associated with C=O, CH2 and OH groups, PVA-g-starch has been made as result of reaction of formaldehyde cross-linking agent (aldehyde group) with PVA and starch in an acidic catalytic environment, and has been used to form 3D scaffolds capable of supporting cells. Fibroblasts were cultured in DMEM (Dulbecco’s Modified Eagle’s Medium) supplied with 10% bovine calf serum (FBS) and 1% penicillin, at 37°C with 5% CO2 and 95% relative humidity. In this culture, to simulate cell growth and scaffold degradation in presence of implant materials in the body, lipase, α-amylase, and lysozyme enzyme were utilised. 3D scaffold biodegradation was 30–60% after 28 days of culture. Enzymatic degradation of the scaffold by fibroblasts demonstrates that the scaffold is biocompatible and biodegradable.29
A critical aspect of 3D scaffolds to mimic vascularization patterns in vivo is porosity, and research into controlling porosity of 3D scaffolds is ongoing. However, the issues can be solved by utilizing hydrogels and polymers with differentiation in porosity that provides an environment for cells to proliferate and function in vitro in an accurate model of tissue repair.16,71 Understanding cell dynamics is a considerable challenge in designing an active 3D material scaffold.94 This is because migration, orientation and morphology of cells has been shown to influence their response to treatments on nanoscale structure surfaces.24
Some of the challenges of tissue engineering, such as achieving tissue architecture and cell seeding, have been resolved by controlling scaffold shape, surface morphology, size and porosity.81 To highlight this, tissue decellularization has been reported as a successful means of developing appropriate 3D architectures in vitro. 72 Recellularization of the decellularized scaffolds can be used to produce models that mimic tissue structure and organ formation. Tissue reconstruction of organs has been explored using tissue decellularization to produce hearts,19 blood vessels,55 uterus26 or kidney73 replacements. In consideration of transplantation applications, patches of bioengineered uterine tissue have been reconstructed using decellularized tissue as a scaffold. The bioengineered tissue can then be used for wound repair, such as repairing scar tissue, defects or even a portion of the uterus.26 Decellularization of rat uterine tissue has been achieved by whole organ perfusion through uterine arteries with dimethyl sulfoxide (DMSO; 4%) and/or 1% Triton-X100 and SDS.26,53 The decellularized scaffold was recellularized by injection with primary uterine cells and green fluorescent protein–labelled bone marrow–derived mesenchymal stem cells (GFP-MSCs). The patches of bioengineered tissue were later transplanted into rats to repair uterine defects. While cell distribution within the scaffolds was limited, the study was successful in restoring functionality of the uterus, and therefore provides evidence that bioengineered tissues are a feasible option for clinical use and further development is warranted.27
As mentioned previously, an important concern for 3D culture models is imaging. Conventional bright field microscopy imaging techniques are poorly suited for imaging 3D models as the thick, tissue-like structures can prevent light penetration and cause distortions.9 Imaging of 3D culture models therefore requires high resolution imaging techniques, including confocal microscopy (CM) with penetration depth up to 100 µm, optical coherent tomography with penetration depth twofold deeper than CM, and multi-photon microscopy, which provides a penetration depth of up to several millimetres.22 When performing scaffold-based 3D culturing, it is therefore important to consider not only the type of scaffold to use, but also the techniques that can be used to image cells as they are cultured in their 3D environment.
Non-Scaffold Methods
Non-scaffolding methods refer to achieving 3D cell culture in a scaffold-free environment, and are often based on the production of spheroids or 3D formations of cells on gels.87 Spheroid formation is a self-assembly process that can be achieved by seeding and culturing cells on a plate with very low adhesion to form spheroids of 200 µm diameter.31 The spheroid itself consists of a cellular aggregation in a small area, which attempts to reflect the natural structure and physiology of cells through mimicking in vivo tissue conditions.89 Rotating-well vessel,45 dynamic cell cultivation in spinner flasks,20 and hanging drop assembly20 are all examples of spheroid culture techniques.45 Recent work on spheroidal 3D cultures has shown phenotypic changes of immortalised mouse dental papilla cells, promotion of angiogenesis, as well as osteoblastic gene expression when compared to 2D cultures.87 The high permeability of spheroids makes them suitable for studying cell aggregations without the need for scaffold 3D modelling and this facilitates mass-production.31 Spheroids can also be applied to studies of interactions between cells, tissue engineering and for studying tumour biology. However, spheroid cultures do have some limitations. The number of seeded cells should be less than saturation level (7000–10,000 cells/well, 96 well plate, well area 0.316 cm2) in order to maintain cell viability, while a lack of control over the organisation and size of the spheroids may also arise during culture.42
Apart from spheroids, cell sheet engineering has been introduced as a scaffold-free method of tissue construction, and a solution for membrane tissue regeneration and organ transplantation.80 Cell sheet engineering can be used to fabricate multi-layered tissues with a thickness of 50–100 µm.48 In a study on cardiac tissue engineering, cell sheet engineering was used as a scaffold-free approach to generate layered cardiac cell sheets. Using poly(N-isopropylacrylamide)-based hydrogel to create a temperature-responsive culture surface, confluent cultured cells were harvested as intact cell sheets and transplanted onto damaged hearts in several animal models.48,71 Similar approaches have been used to study smooth muscle regeneration of the bladder. Multilayered PCL/collagen nanofiber sheet have been fabricated and seeded with human muscle-derived stem cells (h-MDSCs) and implanted in the bladder of a mucosa preserving partial cystectomy (MPPC) rat model.66 Development of these micro-engineering approaches has led to the creation of in vitro bio-printed models that reconstitute more complex 3D organ-level structures, and integrate crucial dynamic mechanical cues as well as chemical signals.37
Three-Dimensional Bioprinting
Bioprinting is the process of transferring materials in vitro to assemble patterns of cells and tissues relevant to the in vivo situation.51 Bioprinters have already been considered in many areas of biological research, and have been commonly used to create 3D scaffolds and cell constructs to mimic in vivo formation of cells, tissues or organs.50 Variations of 3D printing include 3D hybrid bioprinting,37 Extrusion 3D printing,41 Nozzle-based 3D printing,77 Inkjet 3D printing,85 and Laser-assisted 3D printing (Table 3).74 Development of this fabrication technology has made the creation of living tissues and organs from the artificial manipulation of cells and materials much easier.86 The manufacturing of complex organs by bioprinting continues to be developed in the hope that bioprinting may one day become a replacement for insufficient supply of donor organs.94
3D bioprinters need to combine tissue engineering approaches and developmental biology concepts to print reliable 3D models.49 For tissue engineering and regenerative medicine purposes, tissue fabrication methods have been developed to exert control over the placement of various cells and matrices in three dimensions, with the ability to recreate native tissue architecture and complexity.86 The tissue microstructure is a key factor in bioprinting, which is related to matrix materials and dispensing methods to form 3D cell-laden structures.60 These 3D structures exhibit increased levels of cell differentiation and tissue organisation compared to traditional 2D culture systems.30 There have been numerous recent studies using both scaffold and scaffold-free bio-printing tissue engineering strategies reviewed by Norotte et al. 56 Printed cells in combination with hydrogel have been studied. During the study it was observed that the hydrogel material was gradually degraded while the cells remained in their desired 3D structures. Scaffold degradation is one of the key problems affecting 3D printed cell structure in vitro, however, it can also be a necessity.77 Printed scaffolds, like other 3D scaffolds, can provide a suitable environment for cells to attach to and differentiate, and promote the formation of desired structures by seeding them into porous structure of scaffolds.28 However, to be useful in tissue engineering the printed scaffold must degrade away once the cells have assumed the necessary 3D structure and formed attachments with adjacent cells.94
Cell Viability in 3D Culture
The purpose of tissue regeneration is to create an engineered tissue with high cellular viability that is suitable for transplantation. In vivo, complex vascularisation ensures adequate nutrient supply, gas exchange and waste removal to and from cells. In vitro, scaffold material and matrix porosity affect nutrient supply and gas exchange for cells embedded deep within scaffolds, and therefore affect cell survival.87 When designing a bioengineered tissue for transplantation, consideration must be given to the original tissue to ensure adequate complexity is engineered into the replacement tissue.
Assessing Contractility in the 3D Structure
Over the past decades, researchers have focused on methods to understand and measure smooth muscle cell activity and contractile behaviour. The design of biosensors for the detection of cell activity has been an area of increasing attention. In bioanalysis applications, it is necessary to utilise a simple and cost-effective tool for investigating 3D cultured models.44 Electrochemical biosensors that are cell-based and non-destructive have remarkable benefits and features, such as being low-cost and convenient to operate, while still managing to provide rapid detection and good sensitivity for measuring smooth muscle cell contractile behaviour.92 In most studies, both treatments and electrodes have been placed in a culture plate together for simultaneous treatment and signal measurement. The impediametric measuring of cell proliferation is possible non-invasively alongside real-time monitoring of the cells.44
Field potentials can be recorded extracellularly by placing cells on a patterned multi-electrode array (MEA). Cells have been cultured on the MEA devices consisting 60 titanium-nitride electrodes (30 µm diameter, 200 µm pitch size), which have been patterned on a glass substrate (8 × 8 square lattice). When performing MEA, the propagation of electrical excitation along the length of a strip of cells is affected by defects in cell coupling. Propagation speed of ~0.30 ± 0.01 m/s has been measured from central difference signal of each spike.82 As such, extracellular recordings with patterned MEAs can be a reliable measure for quantitative analyses of guided excitation of cardiomyocytes, and could be applied to the study of artificial tissue strips printed from uterine myocytes. Conventional MEA has been used for neurons, muscle cells and cardiomyocytes, which were seeded onto the electrode array. The action potentials generated by the cells were measured or stimulated through the electrodes.36 The current methods have not yet achieved a unified system for measuring cell function and their interactions with the nanostructures.38 Stretchable MEAs have been used to achieve stretchability with minimal effect of electrical interconnection on surface topological properties, while catering for the alignment of the sensors and directional stretching of the cells.82
In SMCs, measuring cell contraction requires a system for distinguishing between phasic contractions, and tension transduced by cell interactions. As a factor in 3D modelling design, it should be considered in addition to requiring techniques for protein and gene analysis to create a valid model.
Assessing Stem Cell Differentiation into Smooth Muscle Cells
The 3D culture and 3D printing of vascular smooth muscle cell structures require an unlimited source of cells to provide an appropriate vascular environment for cell adhesion, proliferation, migration and differentiation within the culture.77 Primary myometrial SMCs obtained from uterine biopsies have limited proliferative capacities, which slow the process of culturing these cells.12 Preparing and developing 3D models of myometrial tissue may therefore necessitate the use of alternative cell sources in order to provide sufficient numbers of functional SMCs that can be cultured in a short time. In recent years, researchers have achieved progress by investigating the use of stem cells within 3D cultures.84
Different types of stem cells are able to differentiate into different cell lineages under appropriate stimuli. Previous studies have demonstrated the ability of human adipose tissue-derived mesenchymal stem cells (hASCs),59 muscle-derived stem cells,4 bone marrow mesenchymal stem cells (BMSC)88 and hair-follicle smooth muscle progenitor cells (HF-SMPCs)46 to differentiate into SMCs.95 Reactive oxygen species (ROS), histone deacetylases (HDAC), microRNAs, ECM collagen, integrins and serum response factors (SRF)-Myocardins have been shown to affect SMC differentiation.84 The effects of transforming growth factor β-1 (TGFβ1) and angiotensin II (ANG) on SMC differentiation have been published,25 while the inhibitory role of Vitamin C in SMC growth in culture has been reported.32 Moreover, differentiation of hASCs into contractile SMCs and expression of SMC-like ion channels have been induced in presence of TGFβ1.59 To assess differentiation of stem cells into SMCs, several molecular markers such as α-smooth muscle actin (ASMA), SM22, calponin, caldesmon, smoothelin and myosin heavy chain (MHC) have been monitored.25 The contractile ability of differentiated SMCs has been reported in functional assessments, which highlights differentiated stem cells as an alternative source for uterine myometrial cells for study within 3D culture systems.95
Conclusion
Although there remain challenges to overcome, 3D culture represents the exciting next step in the development of in vitro cell culture systems. These systems encompass a variety of scaffold-dependent and scaffold-free approaches. Scaffold-dependent approaches can be based on synthetic or naturally occurring compounds, and have been adapted for the printing of live cells. Regardless of the 3D culture system employed, the aim is to better mimic the in vivo, 3D environment of the cell, and in doing so enhance the capacity of researchers to better understand the complex interactions and cellular processes as they occur in vivo. Ongoing efforts to reconstruct the 3D in vivo environment have also opened the door to tissue engineering. Developments in reproductive medicine have facilitated the construction of artificial uterine tissues, which can be used as in vitro models for investigations into pregnancy, as well as tissue transplantation. Once the dream of abstract science fiction, 3D culture and tissue engineering is now paving the way for whole organ generation in vitro, followed by transplantation into living individuals. This new approach toward organ transplantation has the capacity to reduce the need for organ donation.
References
Adissu, H. A., E. K. Asem, and S. A. Lelievre. Three-dimensional cell culture to model epithelia in the female reproductive system. Reprod. Sci. 14:11–19, 2007.
Almany, L., and D. Seliktar. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials 26:2467–2477, 2005.
Astashkina, A., B. Mann, and D. W. Grainger. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol. Ther. 134:82–106, 2012.
Bajpai, V. K., P. Mistriotis, Y. H. Loh, G. Q. Daley, and S. T. Andreadis. Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates. Cardiovasc. Res. 96:391–400, 2012.
Boretti, M. I., and K. J. Gooch. Effect of extracellular matrix and 3D morphogenesis on islet hormone gene expression by Ngn3-infected mouse pancreatic ductal epithelial cells. Tissue Eng. A 14:1927–1937, 2008.
Bursztyn, L., O. Eytan, A. J. Jaffa, and D. Elad. Mathematical model of excitation-contraction in a uterine smooth muscle cell. Am. J. Physiol. Cell Physiol. 292:C1816–C1829, 2007.
Castillejo, M., E. Rebollar, M. Oujja, M. Sanz, A. Selimis, M. Sigletou, S. Psycharakis, A. Ranella, and C. Fotakis. Fabrication of porous biopolymer substrates for cell growth by UV laser: The role of pulse duration. Appl. Surf. Sci. 258:8919–8927, 2012.
Chamley-Campbell, J., G. R. Campbell, and R. Ross. The smooth muscle cell in culture. Physiol. Rev. 59:1–61, 1979.
Charwat, V., K. Schütze, W. Holnthoner, A. Lavrentieva, R. Gangnus, P. Hofbauer, C. Hoffmann, B. Angres, and C. Kasper. Potential and limitations of microscopy and Raman spectroscopy for live-cell analysis of 3D cell cultures. J. Biotechnol. 205:70–81, 2015.
Condon, J., S. Yin, B. Mayhew, R. A. Word, W. E. Wright, J. W. Shay, and W. E. Rainey. Telomerase immortalization of human myometrial cells. Biol. Reprod. 67(2):506–514, 2002.
Dainiak, M. B., I. N. Savina, I. Musolino, A. Kumar, B. Mattiasson, and I. Y. Galaev. Biomimetic macroporous hydrogel scaffolds in a high-throughput screening format for cell-based assays. Biotechnol. Prog. 24:1373–1383, 2008.
Dallot, E., M. Pouchelet, N. Gouhier, D. Cabrol, F. Ferre, and M. Breuiller-Fouche. Contraction of cultured human uterine smooth muscle cells after stimulation with endothelin-1. Biol. Reprod. 68:937–942, 2003.
Dhiman, H. K., A. R. Ray, and A. K. Panda. Three-dimensional chitosan scaffold-based MCF-7 cell culture for the determination of the cytotoxicity of tamoxifen. Biomaterials 26:979–986, 2005.
Drover, J. W., and R. F. Casper. Initiation of parturition in humans. Can. Med. Assoc. J. 128:387–392, 1983.
Elliott, N. T., and F. Yuan. A review of three-dimensional in vitro tissue models for drug discovery and transport studies. J. Pharm. Sci. 100:59–74, 2011.
El-Sherbiny, I. M., and M. H. Yacoub. Hydrogel scaffolds for tissue engineering: Progress and challenges. Global Cardiol. Sci. Pract. 2013:38, 2013.
Equils, O., P. Nambiar, C. J. Hobel, R. Smith, C. F. Simmons, and S. Vali. A computer simulation of progesterone and Cox2 inhibitor treatment for preterm labor. PLoS ONE 5:e8502, 2010.
Fitzgibbon, J., J. J. Morrison, T. J. Smith, and M. O’Brien. Modulation of human uterine smooth muscle cell collagen contractility by thrombin, Y-27632, TNF alpha and indomethacin. Reprod. Biol. Endocrinol. 7:2, 2009.
Fleischer, S., J. Miller, H. Hurowitz, A. Shapira, and T. Dvir. Effect of fiber diameter on the assembly of functional 3D cardiac patches. Nanotechnology 26(29):291002, 2015.
Foty, R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J. Vis. Exp. 51:2720, 2011.
Gong, P. Y., W. Zheng, D. Xiao, and X. Y. Jiang. Microscale methods to assemble mammalian cells into tissue-like structures. Sci. China Life Sci. 55:862–871, 2012.
Graf, B. W., and S. A. Boppart. Imaging and analysis of three-dimensional cell culture models. Methods Mol. Biol. 591:211–227, 2010.
Grefte, S., S. Vullinghs, A. M. Kuijpers-Jagtman, R. Torensma, and J. W. Von den Hoff. Matrigel, but not collagen I, maintains the differentiation capacity of muscle derived cells in vitro. Biomed. Mater. 7:055004, 2012.
Grima, R. Directed cell migration in the presence of obstacles. Theor. Biol. Med. Model. 4:2, 2007.
Harris, L. J., H. Abdollahi, P. Zhang, S. McIlhenny, T. N. Tulenko, and P. J. DiMuzio. Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. J. Surg. Res. 168:306–314, 2011.
Hellström, M., R. R. El-Akouri, C. Sihlbom, B. M. Olsson, J. Lengqvist, H. Bäckdahl, B. R. Johansson, M. Olausson, S. Sumitran-Holgersson, and M. Brännström. Towards the development of a bioengineered uterus: Comparison of different protocols for rat uterus decellularization. Acta Biomater. 10:5034–5042, 2014.
Hellström, M., J. M. Moreno-Moya, S. Bandstein, E. Bom, R. R. Akouri, K. Miyazaki, T. Maruyama, and M. Brännström. Bioengineered uterine tissue supports pregnancy in a rat model. Fertil. Steril. 106(2):487–496, 2016.
Holmes, T. C., S. de Lacalle, X. Su, G. Liu, A. Rich, and S. Zhang. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc. Natl Acad. Sci. USA 97:6728–6733, 2000.
Hsieh, W.-C., and J.-J. Liau. Cell culture and characterization of cross-linked poly(vinyl alcohol)-g-starch 3D scaffold for tissue engineering. Carbohydr. Polym. 98:574–580, 2013.
Huh, D., G. A. Hamilton, and D. E. Ingber. From 3D cell culture to organs-on-chips. Trends Cell Biol. 21:745–754, 2011.
Ikonen, L., E. Kerkelä, G. Metselaar, M. C. A. Stuart, M. R. de Jong, and K. Aalto-Setälä. 2D and 3D self-assembling nanofiber hydrogels for cardiomyocyte culture. BioMed. Res. Int. 2013. doi:10.1155/2013/285678.
Kakade, S., and G. Mani. A comparative study of the effects of vitamin C, sirolimus, and paclitaxel on the growth of endothelial and smooth muscle cells for cardiovascular medical device applications. Drug Des. Dev. Ther. 7:529–544, 2013.
Heidari Kani, M., R. Smith, T. Butler, C. Chan, and R. Young. Glass wool as a model scaffold for 3D culture of uterine smooth muscle cells. Front. Bioeng. Biotechnol. Conference Abstract: 10th World Biomaterials Congress. 2016. doi:10.3389/conf.FBIOE.2016.01.02608.
Khait, L., C. J. Hodonsky, and R. K. Birla. Variable optimization for the formation of three-dimensional self-organized heart muscle. Vitro Cell. Dev. Biol. 45:592–601, 2009.
Khetan, S., and J. Burdick. Cellular encapsulation in 3D hydrogels for tissue engineering. J. Vis. Exp. 2009. doi:10.3791/1590.
Khoshfetrat, Pakazad S. A. Savov, A. Van De Stolpe and R. Dekker. A novel stretchable micro-electrode array (SMEA) design for directional stretching of cells. J. Micromech. Microeng. 24(3):034003, 2014.
Kucukgul, C., B. Ozler, H. E. Karakas, D. Gozuacik, and B. Koc. 3D hybrid bioprinting of macrovascular structures. Procedia Eng. 59:183–192, 2013.
Kuo, C. W., D. Y. Chueh, and P. Chen. Investigation of size-dependent cell adhesion on nanostructured interfaces. J. Nanobiotechnol. 12:54, 2014.
Lawrence, B. J., and S. V. Madihally. Cell colonization in degradable 3D porous matrices. Cell Adhens. Migr. 2:9–16, 2008.
Lee, H. J., E. R. Norwitz, and J. Shaw. Contemporary management of fibroids in pregnancy. Rev. Obstet. Gynecol. 3:20–27, 2010.
Lee, V., G. Singh, J. P. Trasatti, C. Bjornsson, X. Xu, T. N. Tran, S. S. Yoo, G. Dai, and P. Karande. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. C Methods 20:473–484, 2014.
Lee, J. B., S. H. Son, M. C. Park, T. H. Kim, M. G. Kim, S. D. Yoo, and S. Kim. A novel in vitro permeability assay using three-dimensional cell culture system. J. Biotechnol. 205:93–100, 2015.
Lee, D. W., S. H. Yi, S. H. Jeong, B. Ku, J. Kim, and M.-Y. Lee. Plastic pillar inserts for three-dimensional (3D) cell cultures in 96-well plates. Sens. Actuators B 177:78–85, 2013.
Lei, K. F., M. H. Wu, C. W. Hsu, and Y. D. Chen. Real-time and non-invasive impedimetric monitoring of cell proliferation and chemosensitivity in a perfusion 3D cell culture microfluidic chip. Biosens. Bioelectron. 51:16–21, 2013.
Li, Z., and Z. Cui. Three-dimensional perfused cell culture. Biotechnol. Adv. 32:243–254, 2014.
Liu, J. Y., H. F. Peng, and S. T. Andreadis. Contractile smooth muscle cells derived from hair-follicle stem cells. Cardiovasc. Res. 79:24–33, 2008.
Lovitt, C. J., T. B. Shelper, and V. M. Avery. Advanced cell culture techniques for cancer drug discovery. Biology 3:345–367, 2014.
Masuda, S., and T. Shimizu. Three-dimensional cardiac tissue fabrication based on cell sheet technology. Adv. Drug Deliv. Rev. 96:103–109, 2016.
Mironov, V., T. Boland, T. Trusk, G. Forgacs, and R. R. Markwald. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 21:157–161, 2003.
Mironov, V., V. Kasyanov, and R. R. Markwald. Organ printing: from bioprinter to organ biofabrication line. Curr. Opin. Biotechnol. 22:667–673, 2011.
Mironov, V., N. Reis, and B. Derby. Review: bioprinting: a beginning. Tissue Eng. 12:631–634, 2006.
Mitchell, B. F., and M. J. Taggart. Are animal models relevant to key aspects of human parturition? Am. J. Physiol. 297:R525–R545, 2009.
Miyazaki, K., and T. Maruyama. Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials 35:8791–8800, 2014.
Monga, M., C. Y. Ku, K. Dodge, and B. M. Sanborn. Oxytocin-stimulated responses in a pregnant human immortalized myometrial cell line. Biol. Reprod. 55:427–432, 1996.
Negishi, J., S. Funamoto, T. Kimura, K. Nam, T. Higami, and A. Kishida. Effect of treatment temperature on collagen structures of the decellularized carotid artery using high hydrostatic pressure. J. Artif. Organs 14:223–231, 2011.
Norotte, C., F. S. Marga, L. E. Niklason, and G. Forgacs. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30:5910–5917, 2009.
Ong, S.-M., C. Zhang, Y.-C. Toh, S. H. Kim, H. L. Foo, C. H. Tan, D. van Noort, S. Park, and H. Yu. A gel-free 3D microfluidic cell culture system. Biomaterials 29:3237–3244, 2008.
Palumbo, F. S., G. Pitarresi, C. Fiorica, S. Rigogliuso, G. Ghersi, and G. Giammona. Chemical hydrogels based on a hyaluronic acid-graft-α-elastin derivative as potential scaffolds for tissue engineering. Mater. Sci. Eng. C 33:2541–2549, 2013.
Park, W. S., S. C. Heo, E. S. Jeon, D. H. Hong, Y. K. Son, J. H. Ko, H. K. Kim, S. Y. Lee, J. H. Kim, and J. Han. Functional expression of smooth muscle-specific ion channels in TGF-β1-treated human adipose-derived mesenchymal stem cells. Am. J. Physiol. 305:C377–C391, 2013.
Pati, F., J.-H. Shim, J.-S. Lee, and D.-W. Cho. 3D printing of cell-laden constructs for heterogeneous tissue regeneration. Manuf. Lett. 1:49–53, 2013.
Rampichová, M., J. Chvojka, M. Buzgo, E. Prosecká, P. Mikeš, L. Vysloužilová, D. Tvrdík, P. Kochová, T. Gregor, D. Lukáš, and E. Amler. Elastic three-dimensional poly (ε-caprolactone) nanofibre scaffold enhances migration, proliferation and osteogenic differentiation of mesenchymal stem cells. Cell Prolif. 46:23–37, 2013.
Ravi, M., V. Paramesh, S. R. Kaviya, E. Anuradha, and F. D. Paul Solomon. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 230:16–26, 2015.
Schindler, M., A. Nur-E-Kamal, I. Ahmed, J. Kamal, H. Y. Liu, N. Amor, A. S. Ponery, D. P. Crockett, T. H. Grafe, H. Y. Chung, T. Weik, E. Jones, and S. Meiners. Living in three dimensions: 3D nanostructured environments for cell culture and regenerative medicine. Cell Biochem. Biophys. 45:215–227, 2006.
Shamir, E. R., and A. J. Ewald. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat. Rev. Mol. Cell Biol. 15:647–664, 2014.
Shay, J. W., and W. E. Wright. Use of telomerase to create bioengineered tissues. Ann. N. Y. Acad. Sci. 479–491:2005, 1057.
Shrestha, K. R., Y. H. Park, Y. S. Choi, I. G. Kim, S. Piao, A. R. Jung, S. H. Jeon, S. H. Oh, J. H. Lee, and J. Y. Lee. Bladder reconstruction using stem cells seeded on multilayered scaffolds in a mucosa preserving partial cystectomy model. Tissue Eng. Regener. Med. 12:427–434, 2015.
Shynlova, O., P. Tsui, S. Jaffer, and S. J. Lye. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur. J. Obstet. Gynecol. Reprod. Biol. 144(Supplement 1):S2–S10, 2009.
Simon, K. A., K. M. Park, B. Mosadegh, A. B. Subramaniam, A. D. Mazzeo, P. M. Ngo, and G. M. Whitesides. Polymer-based mesh as supports for multi-layered 3D cell culture and assays. Biomaterials 35:259–268, 2014.
Sims, S. M., E. E. Daniel, and R. E. Garfield. Improved electrical coupling in uterine smooth muscle is associated with increased numbers of gap junctions at parturition. J. Gen. Physiol. 80:353–375, 1982.
Sokolowski, P., F. Saison, W. Giles, S. McGrath, D. Smith, J. Smith, and R. Smith. Human uterine wall tension trajectories and the onset of parturition. PLoS ONE 5:e11037, 2010.
Stile, R. A., W. R. Burghardt, and K. E. Healy. Synthesis and characterization of injectable poly(N-isopropylacrylamide)-based hydrogels that support tissue formation in vitro. Macromolecules 32:7370–7379, 1999.
Stratmann, A. T., D. Fecher, G. Wangorsch, C. Göttlich, T. Walles, H. Walles, T. Dandekar, G. Dandekar, and S. L. Nietzer. Establishment of a human 3D lung cancer model based on a biological tissue matrix combined with a Boolean in silico model. Mol. Oncol. 8:351–365, 2014.
Sullivan, D. C., S. H. Mirmalek-Sani, D. B. Deegan, P. M. Baptista, T. Aboushwareb, A. Atala, and J. J. Yoo. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials 33:7756–7764, 2012.
Sylvain, C., F. Jean-Christophe, G. Bertrand, P. Benjamin, B. Reine, R. Murielle, L. Eric, D. Bernard, A. Joëlle, and G. Fabien. Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication 3:025001, 2011.
Taggart, M. J., A. Blanks, S. Kharche, A. Holden, B. Wang, and H. Zhang. Towards understanding the myometrial physiome: approaches for the construction of a virtual physiological uterus. BMC Pregnancy Childbirth 7(Suppl 1):S3, 2007.
Tamayol, A., M. Akbari, N. Annabi, A. Paul, A. Khademhosseini, and D. Juncker. Fiber-based tissue engineering: Progress, challenges, and opportunities. Biotechnol. Adv. 31:669–687, 2013.
Tan, Y., D. J. Richards, T. C. Trusk, R. P. Visconti, M. J. Yost, M. S. Kindy, C. J. Drake, W. S. Argraves, R. R. Markwald, and Y. Mei. 3D printing facilitated scaffold-free tissue unit fabrication. Biofabrication 6:024111, 2014.
Topman, G., N. Shoham, O. Sharabani-Yosef, F. H. Lin, and A. Gefen. A new technique for studying directional cell migration in a hydrogel-based three-dimensional matrix for tissue engineering model systems. Micron 51:9–12, 2013.
Turnbull, A. C. Myometrial contractility in pregnancy and its regulation. Proc R. Soc. Med. 64:1015–1017, 1971.
Turner, W. S., N. Sandhu, and K. E. McCloskey. Tissue engineering: Construction of a multicellular 3D scaffold for the delivery of layered cell sheets. J. Visualized Exp. 92:e51044–e51044, 2014.
Wang, Z., Y. Cui, J. Wang, X. Yang, Y. Wu, K. Wang, X. Gao, D. Li, Y. Li, X. L. Zheng, Y. Zhu, D. Kong, and Q. Zhao. The effect of thick fibers and large pores of electrospun poly(ε-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials 35:5700–5710, 2014.
Wang, L., L. Liu, X. Li, N. Magome, K. Agladze, and Y. Chen. Multi-electrode monitoring of guided excitation in patterned cardiomyocytes. Microelectron. Eng. 111:267–271, 2013.
Wozniak, M. A., and P. J. Keely. Use of three-dimensional collagen gels to study mechanotransduction in T47D breast epithelial cells. Biol. Proced. Online 7:144–161, 2005.
Xiao, Q., G. Wang, Z. Luo, and Q. Xu. The mechanism of stem cell differentiation into smooth muscle cells. Thromb. Haemost. 104:440–448, 2010.
Xu, T., H. Kincaid, A. Atala, and J. J. Yoo. High-throughput production of single-cell microparticles using an inkjet printing technology. Trans. ASME Ser. B 130:210171–210175, 2008.
Xu, T., J. I. Rodriguez-Devora, D. Reyna-Soriano, M. Bhuyan, L. Zhu, K. Wang, and Y. Yuan. Chapter 6 - Principles of Bioprinting Technology. In: Regenerative medicine applications in organ transplantation, edited by G. Orlando, J. Lerut, S. Soker, and R. J. Stratta. Boston: Academic Press, 2014, pp. 67–79.
Yamamoto, M., N. Kawashima, N. Takashino, Y. Koizumi, K. Takimoto, N. Suzuki, M. Saito, and H. Suda. Three-dimensional spheroid culture promotes odonto/osteoblastic differentiation of dental pulp cells. Arch. Oral Biol. 59:310–317, 2014.
Yan, P., C. Xia, C. Duan, S. Li, and Z. Mei. Biological characteristics of foam cell formation in smooth muscle cells derived from Bone Marrow stem cells. Int. J. Biol. Sci. 7:937–946, 2011.
Yoshii, Y., A. Waki, K. Yoshida, A. Kakezuka, M. Kobayashi, H. Namiki, Y. Kuroda, Y. Kiyono, H. Yoshii, T. Furukawa, T. Asai, H. Okazawa, J. G. Gelovani, and Y. Fujibayashi. The use of nanoimprinted scaffolds as 3D culture models to facilitate spontaneous tumor cell migration and well-regulated spheroid formation. Biomaterials 32:6052–6058, 2011.
Young, R. C. Myocytes, myometrium, and uterine contractions. Ann. N. Y. Acad. Sci. 1101:72–84, 2007.
Young, R. C., R. Schumann, and P. Zhang. Three-dimensional culture of human uterine smooth muscle myocytes on a resorbable scaffolding. Tissue Eng. 9:451–459, 2003.
Yu, C., Z. Zhu, L. Wang, Q. Wang, N. Bao, and H. Gu. A new disposable electrode for electrochemical study of leukemia K562 cells and anticancer drug sensitivity test. Biosens. Bioelectron. 53:142–147, 2014.
Zhang, S., F. Gelain, and X. Zhao. Designer self-assembling peptide nanofiber scaffolds for 3D tissue cell cultures. Semin. Cancer Biol. 15:413–420, 2005.
Zhao, X., L. Liu, J. Wang, Y. Xu, W. Zhang, G. Khang, and X. Wang. In vitro vascularization of a combined system based on a 3D printing technique. J Tissue Eng Regen Med. 2014. doi:10.1002/term.1863.
Zhao, Z. K., H. L. Yu, F. Xiao, S. W. Li, W. B. Liao, and K. L. Zhao. Muscle-derived stem cells differentiate into functional smooth muscle cells for ureter tissue engineering: An experimental study. Biotechnol. Bioprocess Eng. 17:456–464, 2012.
Acknowledgements
This work was conducted with the support of an Australian NHMRC grant to Roger Smith (G1200367).
Conflict of Interest
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Christiani Amorim oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Heidari Kani, M., Chan, EC., Young, R.C. et al. 3D Cell Culturing and Possibilities for Myometrial Tissue Engineering. Ann Biomed Eng 45, 1746–1757 (2017). https://doi.org/10.1007/s10439-016-1749-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-016-1749-5