Abstract
Since the initial in vitro attempts to more complex models, research on uterine regeneration is moving towards the creation of a functional bioengineered uterus with possible clinical applications. We describe here the most relevant advances in bioengineering of the uterus published in the last decades considering the use of stem cells and biomaterials as well as future developing techniques in Regenerative Medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infertility is defined as the inability to achieve clinical pregnancy after 1 year of regular unprotected intercourse and can affect up to 14% of couples at reproductive age.73 The most common identifiable female causes (an estimated 38% of all cases are female) are ovulatory disorders and endometriosis; many assisted reproductive techniques developed can circumvent these pathologies and great advances are still being made.28,29,46 Nonetheless, a subgroup of patients suffering from uterine-related infertility remains untreatable. This absolute uterine factor infertility (AUFI), described as the absence of a functional uterus, affects 1 in every 500 women of fertile age.48 It can be congenital (examples are Mayer-Rokitansky-Küster-Hauser syndrome, uterine hypoplasia, uterine malformation) or acquired. Acquired AUFI is caused by hysterectomy, benign diseases (including leiomyoma and adenomyosis), postpartum hemorrhage and intrauterine adhesions (Asherman’s Syndrome). These patients can only conceive through gestational surrogacy for now, but personal, legal, and ethical/religious factors may make this option unusable.23,63
Organ transplantation represents the gold standard for the majority of disorders that lead to irreparable organ failure and can hold also the key to treat AUFI. Recently, a proof-of-concept of this treatment was provided by Brännström and collaborators with the first live birth after uterus transplantation.11 But this approach suffers from severe limitations such as the lack of donor organs39 and the need for long-term immunosuppression,3 the recently failed transplantation in the US as a result of infection is a good example of this.56 This situation has originated the necessity for functional tissues or whole organs not suffering from these constraints, a possible solution could be found in the field of Regenerative Medicine (RM).
This novel science using tissue engineering (TE), cell therapy and stem cell technologies was defined for the first time in 1999 as “a new medicine for a new millennium”.53 Today, RM can be divided in two branches separated only by the use or non-use of scaffolds: cell therapy and TE. Cell therapy is based on the application of specific cells to restore or reconstruct damaged tissues or organs; TE, in contrast, is more related to the growth of specific three-dimensional (3D) structures using different supports or scaffolds.7
Bioengineering, as an integral part of TE, could prove to be valuable for the future creation of tissues and organs.26 The word bioengineering is derived from the Greek root word “bio-”meaning “life” and from the Latin words ingeniare “to contrive, devise” and ingenium “cleverness”. As its name suggests, bioengineering pretends to recreate life using advanced methodologies. Applying this definition to organ transplantation we come across a new field of investigation which aims to design tissues and organs similar to their native in origin. Important advances have been demonstrated recently in liver,8 kidney,1 pancreas,10 and heart among others.36,57 Related to the uterus, it is classified as a non-vital organ such as the spleen, appendix, testicles, ovaries, and eyes. Transplantation involving these non-vital organs have been previously performed in humans to improve the quality of life.38 So in this context, the reason to work to obtain an artificial uterus for transplantation is more related to the creation of motherhood options in those patients that cannot conceive.
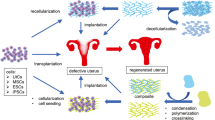
The aim of this review is to provide an overview of the recent advances of RM/TE and its usability in the field of reproductive medicine, from in vitro 3D models to in vivo studies where the ultimate goal will be the transplantation of the bioengineered uterus. Many advances to achieve this are being made: nowadays uterine bioengineering is making headway in animal models,31,32,49 and the surgical knowhow is being established in humans after the first successful uterus transplantation.11,12 Here we summarize the state-of-the-art approaches that are used in uterine bioengineering, as well as the major findings and recent experimental advances in reproductive medicine (a summary showing this is given in Table 1).
Regenerative Medicine: Basic Concepts
Researchers are improving techniques, skills and methodologies based on stem cells, biomaterials and bioengineering in order to obtain biological constructs analogous to native organs and tissues.
Stem Cells
Stem cells are considered one of the most promising tools capable to act in RM.54 Since the first hematopoietic stem cell transplantation in the 1960s,24 cell therapy (only using stem cells of different types like fetal stem cells, adipose stem cells, bone marrow stem cells, mesenchymal stem cells, umbilical cord stem cells etc.…) is showing huge potential in many clinical areas (nowadays more than 5000 trials registered with stem cells in www.clinical-trials-com). Injection of stem cells could be useful in hematopoietic-derived therapies but they can also be used to differentiate into somatic functional cells in different tissues and organs despite of some of their tumorigenic complications.66 On a technical level the recellularization (detailed in next sections) of the matrix in 3D scaffolds could be achieved. These cells represent the ideal source of material thanks to the ability to proliferate and to interplay with the extracellular matrix (ECM) provoking signals into the cell niche.68 Several types of stem cells can be considered for this purpose: embryonic, fetal and adult stem cells. The secretion of specific paracrine factors by several adult stem cells could play a significant function in the activation of the endogenous stem cell niches giving rise to tissue repair, the suppression of inflammatory responses and the modulation of the Immune system.27
In summary, cell-based therapies with different types of stem cells or reprogrammed somatic cells (induced pluripotent stem cells), are an attractive therapeutic option, particularly in autologous transplantation treatments, avoiding intrinsic problems with rejections of the transplant as with ethical and moral objections.
Three-Dimensional (3D) Scaffolds Using Biomaterials
Manufactured artificial scaffolds provide 3D structures for the cells to grown, attach and organize forming new tissues. Different biomaterials can be used to obtain the mechanical support for the maintenance of the new organ. They can come from natural origin (for example naturally derived materials and acellular tissue matrices) or synthetic polymers.45
Naturally derived scaffolds can be classified into many groups including polysaccharides, proteins, and polyester; and also decellularized tissue-derived bioscaffolds.22 All together, they have ability to adequately support cell adhesion, migration, proliferation and differentiation.4 The polysaccharides most widely used in TE consist of cellulose, chitosan, pectins, alginate, agar and dextran. Their uses include encapsulation of cells for delivery to the organism or can be used to from porous 3D scaffolds for TE. Collagen (COL), laminin and elastin, among others, are protein-based biomaterials commonly used in biomedicine mainly used as vehicles for cell delivery and with specific medical applications in eye, skin, cartilage and nerve. Finally, the most degradable biomaterials such as synthetic polyester like poly(l-lactic acid) (PLLA) and poly(l-glycolic acid)(PLGA) are suitable due to their physico-chemical properties, biodegradability rate and different possible application forms: as scaffolds, fibers, hydrogels or microspheres.25
Decellularized tissue-derived bioscaffolds are the original tissues/organs without their cellular components. These cellular components were removed from their extracellular environment by mechanical, enzymatical and chemical means to produce complex collagen-rich matrices. The resulting 3D ECM scaffolds have been shown to provide an excellent environment for the in vitro growth of cells (also called recellularization).26 ECM from a variety of tissues, including heart,50 blood vessels,60 skin,15 small intestinal submucosa (SIS),5 urinary bladder,16 and liver8,41 have been studied for TE and RM applications.
Next Generation: 3D Bioprinting
It is necessary to optimize and enhance the protocols and methods in the field of organ/tissue de- and recellularization. For this purpose, there is a need to develop techniques that mimic the complexity of the native tissues and organs. One of the examples that could provide a solution is the printing of organs with 3D bioprinting.52 Recently, Kang et al. published a system capable to create human-scale tissue constructs with structural integrity.35 They conclude that with this technology they may produce tissues and organs (mandible bone, cartilage and skeletal muscle) similar to native structure and function ready for clinical applications.
Uterine Bioengineering: How Far are We?
The uterus is a pear-shaped muscular female reproductive organ and is divided into a fundus, body, and cervix. It consists out of three layers: an outer layer of connective tissue (perimetrium), a middle layer of smooth muscle (myometrium), and an inner epithelial layer (the endometrium). The endometrium is the mucosal layer lining the uterine cavity and exists out of two regions: a stratum basalis and stratum functionalis, the latter transforms, sheds and regenerates every month (Fig. 1).
A good example for uterine bio-engineering to follow is the successful ex vivo creation of neovaginal constructs and its implantation into patients suffering from vaginal aplasia.55 Such a study done by Raya-Rivera et al. used autologous cells of the patient and a 50:50 copolymer of poly(dl-lactide-co-glycolide) to produce an engineered vaginal organ in 5–6 weeks. These engineered organs were transplanted and reported to be functional in a clinical setting up to 8 years after surgery, its functional variables were in the normal range for desire or arousal, lubrication, orgasm, satisfaction, and absence of pain. These results were only possible because they build upon the experience obtained from in vitro testing, mouse19 and rabbit18 animal models and from other organs such as the bladder.2 This is a demonstration of the great potential hidden within bioengineering and its putative role in translational medicine.
To follow this success story, we provide an overview of the recent advances made in the uterus. Here a division into the three possible applications we can appreciate during the pursuit of the bioengineered uterus is made: in vitro testing, partly repairing the uterus and whole organ bioengineering with the ultimate goal of treating patients suffering from AUFI.
Uterine Tissue Engineering: In Vitro Models
The lack of a comparable menstrual cycle limits the usability of most common animal models and explains the pursuit of a viable alternative. TE in human in vitro models could prove to be useful in developing disease models,21,51 by investigating interactions between human embryo and endometrium70 and between the different cell layers of the endometrium,37 drug testing47 and could even play a role in personalized medicine.59
One of the first steps made concerning the endometrium was done by Bentin-Ley et al. A cell culture system was made with the stromal and epithelial fractions obtained from human endometrial biopsies, the stromal cells were imbedded in a COL1 enriched Matrigel and subsequently covered by an epithelial monolayer resulting in a 3D structure. It was the first in vitro system attempting to imitate the normal endometrium.9
Schutte et al., amongst others, built further upon this concept, creating a tissue engineered model using telomerase immortalized human endometrial stromal cells in a collagen-Matrigel hydrogel.62 It resulted in an engineered stroma able to react to cues for decidualization and menstruation. In an effort to study cell–cell interactions they added adenocarcinoma epithelial cells to this model. The cells were mixed in the hydrogel with the stromal cells or used as a monolayer covering the engineered stroma.61 A possible limitation in the translation of the results in these studies was that they only used immortalized cells instead of primary cells for the sake of reproducibility and decreasing variability. These primary endometrial bovine and human cells were previously used in other 3D cell culture models published.44,51,69,71
In a study done by MacKintosh et al., polyglycolide (PGA) electrospun scaffolds were seeded with primary endometrial bovine cells. These synthetic constructs could mimic the complex architecture of the endometrium but does not make for an ideal substrate for in vivo use.44 Park et al. on the other hand, used human primary cells in a hydrogel mixed with COL1 to investigate its applicability in studies concerning endometrial cancer invasion.51 Even though these studies made many advancements, the presence of the most abundant structure in the endometrium was not observed, this was the case in a study of Wang et al. They used a fibrin-agarose matrix derived from human plasma and primary cells in an effort to examine the attachment of human trophoblast cell spheroids.69 In this 3D culture model spontaneous formation of glands was reported.
The uterine wall is more than just the endometrium, also the fabrication of a neo-myometrium made from a decellularized pregnant myometrium was reported.72 A more elaborate reconstruction of this tissue was also attempted by Lü et al. in vitro, differentiating itself by the inclusion of a smooth muscle layer in their model to mimic the normal uterine wall even more closely.43
Lastly, tackling the last main anatomical structure of the uterus, House et al. created three-dimensional cervical-like tissue constructs for studies related to cervical remodeling. They used cervical cells obtained from two menopausal women and a silk protein sponge scaffold. After culturing under static or dynamic culture conditions for 8 weeks they could appreciate cervical cells proliferating in three-dimensions. An ECM with biochemical constituents and morphology similar to native tissue was synthesized, but was better formed under dynamic conditions.33 The biomaterial was evaluated for its possible further use in subsequent studies.17,30
Despite the efforts in in vitro uterine TE, it remains challenging to initiate the research while encompassing the complexity of the endometrial and uterine structure creating the necessity to develop new methodologies focused on whole organ reconstruction.
Repairing Sections of the Uterus: Patch Studies
In almost all of the aforementioned studies, TE was mainly used to create models for in vitro studies without the explicit desire to use in the (partial) regeneration of uteri. One of the first attempts in regenerating uterine wall tissues was done by Taveau et al. using porcine SIS. In this proof-of-concept study, fecundity and tube patency were tested; half of the rabbits (three out of six) conceived by natural mating but there were some issues concerning the structural integrity of the grafts.67 Synthetic materials, such as polytetrafluoroethylene (PTFE), polyetherurethane (PU/PLLA) were also used as grafts to assess their applicability.34
More interestingly a first real bioengineering approach was done by Campbell et al., where they used the peritoneal cavity of rats or rabbits as a bioreactor. Myofibroblast-rich tissue capsules were produced to be used as autologous grafts for hollow, smooth muscle-walled visceral organs such as the bladder, vas deferens and uterus. They demonstrated that in 2–3 weeks myofibroblast tissue formed after implanting foreign objects, in the case of the uterus made out of boiled blood clots molded into tubular shapes. 12 weeks after grafting said tissues, it was reported that the graft thickened to a size comparable to that of the control horn. A structural complex tissue was formed, showing a well-defined layer of columnar epithelium at the endometrium and different muscle layers interspersed with collagen fibers. In contrast to the 4 and 8 weeks grafts it was also able to stretch to support a near-term embryo.13
Several studies from Hu’s group have been published using collagen scaffold in different ways in order to improve or restore endometrial function for adhesion and implantation whilst providing structural stability for carrying pregnancy.20,40,42,65 Collagen scaffolds functionalized with basic fibroblast growth factor (bFGF) were used and characterized in other studies64 and used by Li et al. as a bFGF delivery system in a rat severe uterine damage model.40 The effectiveness of the collagen based delivery system was evaluated by partial uterine horn excision/reconstruction. It showed better integration and improved regeneration of endometrium and partial organization of muscle bundles, also postoperative pregnancy rate showed to be comparable to the sham operated group (86.67 vs. 100%). In following studies bone marrow mesenchymal stem cells and human embryonic stem cell were also used on this collagen scaffold delivery system, the same uterine damage model as described above was used. In both cases the cells/collagen group preformed markedly better than the collagen group, with higher pregnancy rates and even implantation on the grafted tissues.20,65
Even though collagen and other naturally derived materials could have the potential advantage of biologic recognition, they only don’t fully mimic the complexity of the natural tissues; 3D scaffolds created from decellularization (DC) could provide a promising alternative. In a first study of its type, Santoso et al. studied the effectiveness of three different DC methods of segments of the rat uterine horn and the usability of the acellular tissue in vivo.58 The integration of decellularized tissue graft took place within 30 days, showing more structural integrity than previously used scaffold material such as SIS.67 They suggested that, even though sodium dodecyl sulfate (SDS) decellularized tissues presented thicker and faster regeneration, samples prepared by HPP or high hydrostatic pressure were favorable due to less protein denaturation and no residual SDS or Triton X-100. Also mentioned was that cell migration towards the graft was less than optimal due to the absence of connected microvasculature to supply nutrients, this lack of vasculature can be circumvented by applying DC techniques to entire organs.58
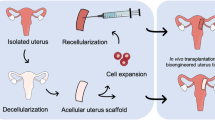
Whole Organ Tissue-Engineering: Uterus Decellularization
This technique uses perfusion-based DC to achieve entire acellular organs for use in recellularization. Its successful use has been published in many organs.6 In 2014, two groups published their results concerning the DC of the rat uterus very closely after each other.31,49 Maruyama et al. used a perfusion system with SDS and Triton-X100 and investigated the resulting decellularized uterine matrix for its potential for in vitro recellularization and in vivo regeneration of the uterus. In the in vitro study, primary uterine cells and mesenchymal stem cells were injected in the uterine wall. After 3 days of perfusion culture, endometrial-like tissue but no robust smooth muscle layer was observed. The regenerative ability was demonstrated in vivo by using an excision/replacement model, a drop of pregnancy rates to 75% occurred.49 Meanwhile, Hellström et al. compared three different decellularization protocols, based on the use of different detergents. The best results were achieved using a freeze–thaw step, with repeated perfusion cycles of Triton X-100 and DMSO (dimethyl sulfoxide). The successful removal of major histocompatibility complex (MHC) class I and II was also demonstrated.31 Patches obtained from uteri subjected to these three previously mentioned protocols were recellularized with green fluorescent protein-labeled bone marrow-derived mesenchymal stem cells (GFP-MSCs) by injection. After 3 days of cultivation, the regenerative capacity of matured constructs were assessed by patch transplantation. The results obtained in the previous study were corroborated in that the Triton X-100/DMSO protocol produced patches performed better, they were able to support near term foetuses located at the transplantation sites.32
This field of investigation was also of interest to the authors’ own group. Building upon the progress made by other groups we were able to scale up and developed a DC protocol of the entire porcine uterus. Figure 1 shows the successful DC of a uterine horn vs. a non-perfused one, and the techniques used to demonstrate the appropriate balance between DC and its detrimental effects on the ECM.14 We made use of perfusion cycles of SDS and Triton X-100 and assessed the usability of a previous freeze–thaw step.The preservation of a reusable ECM while maintaining the vascular network until the capillary level was demonstrated, the feasibility of recellularization was also shown using human endometrial stem cells on small decellularized ECM discs. This is the first work describing a desirable protocol for the DC of a large reproductive organ.
Conclusion and Future Perspectives
Despite the massive leaps being made in the treatment of patients in the field of reproductive medicine, one group of infertile female patients remained untreated. For women suffering from AUFI, gestational surrogacy was, next to adoption, the only solution available. This comes with its own set of economical, legal and ethical problems.63 Only 2 years ago, the first successful live birth after uterus transplantation was published by Brännström et al. proving a proof-of-concept for a viable alternative.11,12 RM can contribute providing a bioengineering based answer not only for the issues found in transplantation technologies, but also for the field of reproductive medicine in general.
The knowledge and expertise in this area is still advancing, from tissue and bioengineering (culture and behavior of uterine cells and creation of novel 3D scaffolds) to the chirurgical know-how to perform in vivo patch studies and whole organ transplantations. While this field of investigation is starting to take off it should strive towards the expanding the animal model based knowledge, developing new techniques and making headway in clinical trials.
References
Arenas-Herrera, J. E., I. K. Ko, A. Atala, and J. J. Yoo. Decellularization for whole organ bioengineering. Biomed. Mater. 8:14106, 2013.
Atala, A., S. B. Bauer, S. Soker, J. J. Yoo, and A. B. Retik. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet Lond. Engl. 367:1241–1246, 2006.
Azimzadeh, A., J. Lees, Y. Ding, and J. Bromberg. Immunobiology of transplantation: impact on targets for large and small molecules. Clin. Pharmacol. Ther. 90:229–242, 2011.
Bačáková, L., K. Novotná, and M. Pařízek. Polysaccharides as cell carriers for tissue engineering: the use of cellulose in vascular wall reconstruction. Physiol. Res. Acad. Sci. Bohemoslov. 63(Suppl 1):S29–S47, 2014.
Badylak, S. F., G. C. Lantz, A. Coffey, and L. A. Geddes. Small intestinal submucosa as a large diameter vascular graft in the dog. J. Surg. Res. 47:74–80, 1989.
Badylak, S. F., D. Taylor, and K. Uygun. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 13:27–53, 2011.
Badylak, S. F., D. J. Weiss, A. Caplan, and P. Macchiarini. Engineered whole organs and complex tissues. Lancet 379:943–952, 2012.
Baptista, P. M., M. M. Siddiqui, G. Lozier, S. R. Rodriguez, A. Atala, and S. Soker. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 53:604–617, 2011.
Bentin-Ley, U., B. Pedersen, S. Lindenberg, J. F. Larsen, L. Hamberger, and T. Horn. Isolation and culture of human endometrial cells in a three-dimensional culture system. J. Reprod. Fertil. 101:327–332, 1994.
Berney, T., and E. Berishvili. Toward clinical application of the bioartificial pancreas. Transplantation 99:2241–2242, 2015.
Brännström, M., H. Bokström, P. Dahm-Kähler, C. Diaz-Garcia, J. Ekberg, A. Enskog, H. Hagberg, L. Johannesson, N. Kvarnström, J. Mölne, M. Olausson, J. I. Olofsson, and K. Rodriguez-Wallberg. One uterus bridging three generations: first live birth after mother-to-daughter uterus transplantation. Fertil. Steril. 2016. doi:10.1016/j.fertnstert.2016.04.001.
Brännström, M., L. Johannesson, H. Bokström, N. Kvarnström, J. Mölne, P. Dahm-Kähler, A. Enskog, M. Milenkovic, J. Ekberg, C. Diaz-Garcia, M. Gäbel, A. Hanafy, H. Hagberg, M. Olausson, and L. Nilsson. Livebirth after uterus transplantation. Lancet 385:607–616, 2015.
Campbell, G. R., G. Turnbull, L. Xiang, M. Haines, S. Armstrong, B. E. Rolfe, and J. H. Campbell. The peritoneal cavity as a bioreactor for tissue engineering visceral organs: bladder, uterus and vas deferens. J. Tissue Eng. Regen. Med. 2:50–60, 2008.
Campo, H., P. M. Baptista, N. López-Pérez, A. Faus, I. Cervelló, and C. Simón. De- and recellularization of the pig uterus: a bioengineering pilot study. Biol. Reprod., 2016 (in press).
Chen, R.-N., H.-O. Ho, Y.-T. Tsai, and M.-T. Sheu. Process development of an acellular dermal matrix (ADM) for biomedical applications. Biomaterials 25:2679–2686, 2004.
Chen, F., J. J. Yoo, and A. Atala. Acellular collagen matrix as a possible “off the shelf” biomaterial for urethral repair. Urology 54:407–410, 1999.
Critchfield, A. S., R. Mccabe, N. Klebanov, L. Richey, S. Socrate, E. R. Norwitz, D. L. Kaplan, and M. House. Biocompatibility of a sonicated silk gel for cervical injection during pregnancy in vivo and in vitro study. Reprod. Sci. 21:1266–1273, 2014.
De Filippo, R. E., R. E. De Philippo, C. E. Bishop, L. F. Filho, J. J. Yoo, and A. Atala. Tissue engineering a complete vaginal replacement from a small biopsy of autologous tissue. Transplantation 86:208–214, 2008.
de Filippo, R. E., J. J. Yoo, and A. Atala. Engineering of vaginal tissue in vivo. Tissue Eng. 9:301–306, 2003.
Ding, L., X. Li, H. Sun, J. Su, N. Lin, B. Péault, T. Song, J. Yang, J. Dai, and Y. Hu. Transplantation of bone marrow mesenchymal stem cells on collagen scaffolds for the functional regeneration of injured rat uterus. Biomaterials 35:4888–4900, 2014.
Fasciani, A., G. Bocci, J. Xu, R. Bielecki, E. Greenblatt, N. Leyland, and R. F. Casper. Three-dimensional in vitro culture of endometrial explants mimics the early stages of endometriosis. Fertil. Steril. 80:1137–1143, 2003.
Furth, M. E., A. Atala, and M. E. Van Dyke. Smart biomaterials design for tissue engineering and regenerative medicine. Biomaterials 28:5068–5073, 2007.
Gardner, D. K., A. Weissman, C. M. Howles, and Z. Shoham. The impact of legislation and socioeconomic factors in the access tp and global practice of ART. In: Textbook of Assisted Reproductive Techniques: Laboratory and Clinical Perspectives, edited by D. Gardner, and et al. Boca Raton: CRC Press, 2001.
Gatti, R. A., H. J. Meuwissen, H. D. Allen, R. Hong, and R. A. Good. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet Lond. Engl. 2:1366–1369, 1968.
Gentile, P., V. Chiono, I. Carmagnola, and P. V. Hatton. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 15:3640–3659, 2014.
Gilbert, T., T. Sellaro, and S. Badylak. Decellularization of tissues and organs. Biomaterials 2006. doi:10.1016/j.biomaterials.2006.02.014.
Gnecchi, M., H. He, N. Noiseux, O. D. Liang, L. Zhang, F. Morello, H. Mu, L. G. Melo, R. E. Pratt, J. S. Ingwall, and V. J. Dzau. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 20:661–669, 2006.
Gurunath, S., Z. Pandian, R. A. Anderson, and S. Bhattacharya. Defining infertility—a systematic review of prevalence studies. Hum. Reprod. Update 17:575–588, 2011.
Healy, D. L., A. O. Trounson, and A. N. Andersen. Female infertility: causes and treatment. Lancet 343:1539–1544, 1994.
Heard, A. J., S. Socrate, K. A. Burke, E. R. Norwitz, D. L. Kaplan, and M. D. House. Silk-based injectable biomaterial as an alternative to cervical cerclage. Reprod. Sci. 20:929–936, 2013.
Hellström, M., R. R. El-Akouri, C. Sihlbom, B. M. Olsson, J. Lengqvist, H. Bäckdahl, B. R. Johansson, M. Olausson, S. Sumitran-Holgersson, and M. Brännström. Towards the development of a bioengineered uterus: comparison of different protocols for rat uterus decellularization. Acta Biomater. 10:5034–5042, 2014.
Hellström, M., J. M. Moreno-Moya, S. Bandstein, E. Bom, R. R. Akouri, K. Miyazaki, T. Maruyama, and M. Brännström. Bioengineered uterine tissue supports pregnancy in a rat model. Fertil. Steril. 106:487–496, 2016.
House, M., C. C. Sanchez, W. L. Rice, S. Socrate, and D. L. Kaplan. Cervical tissue engineering using silk scaffolds and human cervical cells. Tissue Eng. Part A 16:2101–2112, 2010.
Jonkman, M. F., F. M. Kauer, P. Nieuwenhuis, and I. Molenaar. Segmental uterine horn replacement in the rat using a biodegradable microporous synthetic tube. Artif. Organs 10:475–480, 1986.
Kang, H.-W., S. J. Lee, I. K. Ko, C. Kengla, J. J. Yoo, and A. Atala. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 34:312–319, 2016.
Kim, J. J., L. Hou, and N. F. Huang. Vascularization of three-dimensional engineered tissues for regenerative medicine applications. Acta Biomater. 2016. doi:10.1016/j.actbio.2016.06.001.
Kim, M. R., D. W. Park, J. H. Lee, D. S. Choi, K. J. Hwang, H. S. Ryu, and C. K. Min. Progesterone-dependent release of transforming growth factor-beta1 from epithelial cells enhances the endometrial decidualization by turning on the Smad signalling in stromal cells. Mol. Hum. Reprod. 11:801–808, 2005.
Kisu, I., K. Banno, M. Mihara, N. Suganuma, and D. Aoki. Current status of uterus transplantation in primates and issues for clinical application. Fertil. Steril. 100:280–294, 2013.
Lesieur, O., M. Leloup, F. Gonzalez, and M.-F. Mamzer. Eligibility for organ donation following end-of-life decisions: a study performed in 43 French intensive care units. Intensive Care Med. 40:1323–1331, 2014.
Li, X., H. Sun, N. Lin, X. Hou, J. Wang, B. Zhou, P. Xu, Z. Xiao, B. Chen, J. Dai, and Y. Hu. Regeneration of uterine horns in rats by collagen scaffolds loaded with collagen-binding human basic fibroblast growth factor. Biomaterials 32:8172–8181, 2011.
Lin, P., W. C. W. Chan, S. F. Badylak, and S. N. Bhatia. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 10:1046–1053, 2004.
Lin, N., X. Li, T. Song, J. Wang, K. Meng, J. Yang, X. Hou, J. Dai, and Y. Hu. The effect of collagen-binding vascular endothelial growth factor on the remodeling of scarred rat uterus following full-thickness injury. Biomaterials 33:1801–1807, 2012.
Lü, S.-H., H.-B. Wang, H. Liu, H.-P. Wang, Q.-X. Lin, D.-X. Li, Y.-X. Song, C.-M. Duan, L.-X. Feng, and C.-Y. Wang. Reconstruction of engineered uterine tissues containing smooth muscle layer in collagen/matrigel scaffold in vitro. Tissue Eng. Part A 15:1611–1618, 2008.
MacKintosh, S. B., L. P. Serino, P. D. Iddon, R. Brown, R. S. Conlan, C. J. Wright, T. G. G. Maffeis, M. J. Raxworthy, and I. M. Sheldon. A three-dimensional model of primary bovine endometrium using an electrospun scaffold. Biofabrication 7:25010, 2015.
Mano, J., G. Silva, H. Azevedo, P. Malafaya, R. Sousa, S. Silva, L. Boesel, J. Oliveira, T. Santos, A. Marques, N. Neves, and R. Reis. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J. R. Soc. Interface 4:999–1030, 2007.
Matzuk, M. M., and D. J. Lamb. The biology of infertility: research advances and clinical challenges. Nat. Med. 14:1197–1213, 2008.
Meng, C.-X., K. L. Andersson, U. Bentin-Ley, K. Gemzell-Danielsson, and P. G. L. Lalitkumar. Effect of levonorgestrel and mifepristone on endometrial receptivity markers in a three-dimensional human endometrial cell culture model. Fertil. Steril. 91:256–264, 2009.
Milliez, J. Uterine transplantation: FIGO Committee for the ethical aspects of human reproduction and women’s health. Int. J. Gynecol. Obstet. 106:270, 2009.
Miyazaki, K., and T. Maruyama. Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials 35:8791–8800, 2014.
Ott, H. C., T. S. Matthiesen, S.-K. Goh, L. D. Black, S. M. Kren, T. I. Netoff, and D. A. Taylor. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat. Med. 14:213–221, 2008.
Park, D. W., D. S. Choi, H.-S. Ryu, H. C. Kwon, H. Joo, and C. K. Min. A well-defined in vitro three-dimensional culture of human endometrium and its applicability to endometrial cancer invasion. Cancer Lett. 195:185–192, 2003.
Patra, S., and V. Young. A review of 3D printing techniques and the future in biofabrication of bioprinted tissue. Cell Biochem. Biophys. 74:93–98, 2016.
Petit-Zeman, S. Regenerative medicine. Nat. Biotechnol. 19:201–206, 2001.
Ramakrishna, V., P. B. Janardhan, and L. Sudarsanareddy. Stem cells and regenerative medicine a review. Ann. Rev. Res. Biol. 1(4):79–110, 2011. http://imsear.li.mahidol.ac.th/handle/123456789/162194
Raya-Rivera, A. M., D. Esquiliano, R. Fierro-Pastrana, E. López-Bayghen, P. Valencia, R. Ordorica-Flores, S. Soker, J. J. Yoo, and A. Atala. Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet 384:329–336, 2014.
Rubin, R. Why the first American uterus transplant failed. http://www.forbes.com/sites/ritarubin/2016/04/08/cleveland-clinic-doctors-say-an-infection-forced-them-to-remove-the-first-u-s-transplanted-uterus/
Sánchez, P. L., M. E. Fernández-Santos, S. Costanza, A. M. Climent, I. Moscoso, M. A. Gonzalez-Nicolas, R. Sanz-Ruiz, H. Rodríguez, S. M. Kren, G. Garrido, J. L. Escalante, J. Bermejo, J. Elizaga, J. Menarguez, R. Yotti, C. Pérez del Villar, M. A. Espinosa, Guillem, MS, J. T. Willerson, A. Bernad, R. Matesanz, D. A. Taylor, and F. Fernández-Avilés. Acellular human heart matrix: a critical step toward whole heart grafts. Biomaterials 61:279–289, 2015.
Santoso, E. G., K. Yoshida, Y. Hirota, M. Aizawa, O. Yoshino, A. Kishida, Y. Osuga, S. Saito, T. Ushida, and K. S. Furukawa. Application of detergents or high hydrostatic pressure as decellularization processes in uterine tissues and their subsequent effects on in vivo uterine regeneration in murine models. PLoS ONE 9:e103201, 2014.
Schenke-Layland, K., and R. M. Nerem. In vitro human tissue models—moving towards personalized regenerative medicine. Adv. Drug Deliv. Rev. 63:195–196, 2011.
Schmidt, C. E., and J. M. Baier. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials 21:2215–2231, 2000.
Schutte, S. C., C. O. James, N. Sidell, and R. N. Taylor. Tissue-engineered endometrial model for the study of cell-cell interactions. Reprod. Sci. 22:308–315, 2015.
Schutte, S. C., and R. N. Taylor. A tissue engineered human endometrial stroma that responds to cues for secretory differentiation, decidualization and menstruation. Fertil. Steril. 97:997–1003, 2012.
Shenfield, F., G. Pennings, J. Cohen, P. Devroey, G. de Wert, B. Tarlatzis, and ESHRE Task Force on Ethics and Law. ESHRE task force on ethics and law 10: surrogacy. Hum. Reprod. Oxf. Engl. 20:2705–2707, 2005.
Shi, C., W. Chen, Y. Zhao, B. Chen, Z. Xiao, Z. Wei, X. Hou, J. Tang, Z. Wang, and J. Dai. Regeneration of full-thickness abdominal wall defects in rats using collagen scaffolds loaded with collagen-binding basic fibroblast growth factor. Biomaterials 32:753–759, 2011.
Song, T., X. Zhao, H. Sun, X. Li, N. Lin, L. Ding, J. Dai, and Y. Hu. Regeneration of uterine horns in rats using collagen scaffolds loaded with human embryonic stem cell-derived endometrium-like cells. Tissue Eng. Part A 21:353–361, 2015.
Stoltz, J.-F., N. de Isla, Y. P. Li, D. Bensoussan, L. Zhang, C. Huselstein, Y. Chen, V. Decot, J. Magdalou, N. Li, L. Reppel, and Y. He. Stem cells and regenerative medicine: myth or reality of the 21th century. Stem Cells Int. 2015:734731, 2015.
Taveau, J. W., M. Tartaglia, D. Buchannan, B. Smith, G. Koenig, K. Thomfohrde, B. Stouch, S. Jeck, and C. H. Greene. Regeneration of uterine horn using porcine small intestinal submucosa grafts in rabbits. J. Investig. Surg. Off. J. Acad. Surg. Res. 17:81–92, 2004.
Walters, N. J., and E. Gentleman. Evolving insights in cell-matrix interactions: elucidating how non-soluble properties of the extracellular niche direct stem cell fate. Acta Biomater. 11:3–16, 2015.
Wang, H., F. Pilla, S. Anderson, S. Martínez-Escribano, I. Herrer, J. M. Moreno-Moya, S. Musti, S. Bocca, S. Oehninger, and J. A. Horcajadas. A novel model of human implantation: 3D endometrium-like culture system to study attachment of human trophoblast (Jar) cell spheroids. Mol. Hum. Reprod. 2011. doi:10.1093/molehr/gar064.
Weimar, C. H. E., E. D. Post Uiterweer, G. Teklenburg, C. J. Heijnen, and N. S. Macklon. In-vitro model systems for the study of human embryo–endometrium interactions. Reprod. Biomed. Online 27:461–476, 2013.
Yamauchi, N., O. Yamada, T. Takahashi, K. Imai, T. Sato, A. Ito, and K. Hashizume. A three-dimensional cell culture model for bovine endometrium: regeneration of a multicellular spheroid using ascorbate. Placenta 24:258–269, 2003.
Young, R. C., and G. Goloman. Allo- and xeno-reassembly of human and rat myometrium from cells and scaffolds. Tissue Eng. Part A 19:2112–2119, 2013.
Zegers-Hochschild, F., G. D. Adamson, J. de Mouzon, O. Ishihara, R. Mansour, K. Nygren, E. Sullivan, and S. Vanderpoel. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil. Steril. 92:1520–1524, 2009.
Acknowledgment
This work was supported by SAF/2012-31017 (PI: CS), PROMETEOII/2013/018 (PI: CS) and by GRISOLIA/2015/002.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Christiani Amorim oversaw the review of this article.
Irene Cervelló and Carlos Simón have contributed equally.
Rights and permissions
About this article
Cite this article
Campo, H., Cervelló, I. & Simón, C. Bioengineering the Uterus: An Overview of Recent Advances and Future Perspectives in Reproductive Medicine. Ann Biomed Eng 45, 1710–1717 (2017). https://doi.org/10.1007/s10439-016-1783-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-016-1783-3