Abstract
This paper reviews the current state of the art for coronary stent materials and surface coatings, with an emphasis on new technologies that followed on from first-generation bare metal and drug-eluting stents. These developments have been driven mainly by the need to improve long term outcomes, including late stent thrombosis. Biodegradable drug-eluting coatings aim to address the long term effects of residual durable polymer after drug elution; the SYNERGY, BioMatrix, and Nobori stents are all promising devices in this category, with minimal polymer through the use of abluminal coatings. Textured stent surfaces have been used to attached drug directly, without polymer; the Yukon Choice and BioFreedom stents have some promising data in this category, while a hydroxyapatite textured surface has had less success. The use of drug-filled reservoirs looked promising initially but the NEVO device has experienced both technical and commercial set-backs. However this approach may eventually make it to market if trials with the Drug-Filled Stent prove to be successful. Non-pharmacological coatings such as silicon carbide, carbon, and titanium–nitride-oxide are also proving to have potential to provide better performance than BMS, without some of the longer term issues associated with DES. In terms of biological coatings, the Genous stent which promotes attachment of endothelial progenitor cells has made good progress while gene-eluting stents still have some practical challenges to overcome. Perhaps the most advancement has been in the field of biodegradable stents. The BVS PLLA device is now seeing increasing clinical use in many complex indications while magnesium stents continue to make steady advancements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the introduction of the first stainless steel devices in 1987, the materials used for coronary stents have evolved and diversified rapidly. In the drive to obtain a share of what was becoming a vast and growing market, manufacturers invested heavily in research and development to gain continuous product differentiation and improvements. Materials and surface technologies have been central to all of these developments. This review presents some of the more recent and on-going activities in terms of the many technologies used and the pre-clinical or clinical data being obtained. The clinical drivers behind earlier developments have been previously reviewed.51 This current assessment concentrates more on new and evolving devices and technologies rather than those that already have wider regulatory approvals.
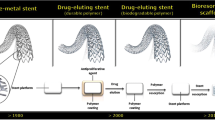
While progressive improvements in stent materials, design, and coatings has resulted in exceptionally low failure and re-intervention rates, the issue of late stent thrombosis (LST) remains. Though LST has been widely associated with drug-eluting stents, it has also been argued that this may be due to use of these devices in increasingly challenging anatomies and patient sub-sets,43 where the use of earlier designs or bare metal devices would not have even been considered. It is however generally accepted that LST is associated with poor endothelialization of devices after implantation.21 The cause of this poor healing has not been precisely elucidated, though the role of polymeric drug-eluting coatings has been widely challenged in light of their potential to elicit localized inflammatory responses.31,50 However, it has also been argued that these polymer coatings themselves are not inherently thrombogenic and that factors such as strut thickness and device malapposition are more significant in terms of causing blood flow disruptions.38 Detailed bench studies of contemporary drug-eluting stents, with permanent polymer coatings, have shown that while coating defects do exist, these are not significantly exacerbated by device over-expansion as is often proposed.2 In any event, while recognizing that correlations between coating characteristics and long term thrombogenic events are largely speculative, there is a drive within the industry to reduce or eliminate permanent polymers.22 The use of biodegradable drug-eluting coatings and methods for attaching drug with no polymer carrier are therefore being widely explored. Similarly, the belief that long term outcomes may be further improved by eliminating the device altogether is driving extensive development in the fields of biodegradable polymeric and metallic stents.
It is also notable that as the use of DES has leveled off, and bare metal stents are seeing wider than expected usage, many of the traditional factors relating to platform stent materials are likely to be again in the spotlight. For example, high strength materials to provide thinner struts and passive surfaces, that do not leach metal ions, are both still important issues.
Surface Coating Technologies
Biodegradable Drug-Eluting Coatings
This will be the first step away from durable polymer layers and already there has been a number of substantial developments, though with varying success. The JACTAX stent (Boston Scientific Corp.) has microdots of biodegradable polymer (polylactide) plus paclitaxel applied to the abluminal surfaces of the device. With each microdot less than 1 μm thick, the overall polymer content is orders of magnitude less than utilized in the equivalent Taxus device. The polymer was designed to fully release the drug in 60 days and be absorbed in 4 months. While studies showed the device to be safe and effective, the failure to demonstrate improved strut endothelialization compared to Taxus,23,24 has ultimately proven to a major hindrance to further development of the concept. The BioMatrix stent (Biosensors International) also contains polylactide, but in this instance it is a continuous abluminal coating, with the Biolimus A9™ drug. The biodegradable layer is 11 μm thick and designed to be fully resorbed within 6–9 months. Optical Coherence Tomography (OCT) has been used to compare strut coverage of this device vs. a Cypher stent.25 Interestingly, while the BioMatrix device showed improved coverage at 9 months, both stents had similar coverage at 24 months. Longer term data would be needed to ascertain if the superior early performance of the BioMatrix device translates to a long term clinical benefit, with reduced LST. The Nobori stent (Terumo Corp.) similarly has an abluminal polylactide layer with Biolimus A9™. However as the stent pattern and coating processes are different, a study has also been undertaken to compare this device against Cypher.32 Endothelialization was not specifically assessed, but at 9 month follow-up both devices were deemed similar with respect to safety and efficacy. The primary endpoint was freedom from target vessel failure (TVF), a composite of cardiac death, myocardial infarction and target vessel revascularization. A study with 5 year follow-up showed favorable outcomes compared to conventional DES but still showed more thrombus formation than BMS.40
Polymer-Free Drug Elution: Textured Surfaces
The concept of attaching drugs to stent surfaces without the use of either permanent or resorbable polymers is an attractive progression on existing drug carrier approaches. The challenge is to obtain and adequate drug load with a controlled and extended release; sudden burst release has hampered a number of initial efforts. One of the concepts that has progressed most in this regard is the Yukon Choice stent (Translumina GmbH) which has a microporous surface for direct drug attachment. The drug is applied to this surface using a custom coating machine, just prior to implantation. A number of promising trials have been completed where this rapamycin-eluting Yukon device has compared well to conventional DES for restenosis rates and re-intervention.45,64 However these trials have not been of sufficient durations to ascertain if the device has an influence on LST. Furthermore, a number of studies have also failed to demonstrate non-inferiority for the Yukon device, including a recent study comparing against Taxus Liberté in patients with diabetes mellitus. Again, the duration of 9 months in this study is insufficient to gain insight to any long term benefits with regard to LST or late catch-up.18 However a 5-year follow-up has demonstrated equivalency for long-term safety and efficacy of the two devices in a randomized trial.36
A variant of the microporous surface is also being incorporated into the BioFreedom stent (Biosensors International), shown in Fig. 1. In this device, the surface is textured predominantly only on the abluminal faces, with the Biolimus A9™ drug applied directly to this, via a solvent coating process during device manufacture.74 While pre-clinical studies have shown reduced neointima formation with the BioFreedom device, it is also noted that the stent struts showed delayed re-endothelialization compared to an equivalent (polished) BMS.78 Thus the contribution of the drug itself to delayed endothelialization and the potential for late thrombosis is still a question for all drug-eluting devices. However the device is progressing well through clinical trials, with recent 2 year data showing outcomes comparable to Taxus Liberté, with neither device being associated with stent thrombosis.6 Enrollment is a US Investigational Device Exemption (IDE) feasibility trial has also commenced.7
BioFreedom DES system: a microscopic view of the stainless steel BioFlex II stent, which has a proprietary surface treatment resulting in a selectively microstructured abluminal surface for loading with drug. (Reprinted with permission from Ref. 1 Copyright 2010 by Wolters Kluwer Health, Inc.).
Another polymer-free concept that has been extensively explored is that of textured hydroxyapatite coatings to retain the drug. A sirolimus–lipid mixture is embedded into the porous hydroyapatite with full elution expected within 3 months. It is also expected that the hydroxapatite carrier would be fully resorbed by approximately 12 months—while this has been demonstrated in pre-clinical studies, it is not evident that this has been verified in humans. Results from the initial Vestasync I Trial showed promising safety and efficacy at 9 months follow-up.16 Data from the larger Vestasync II Trial was also positive,17 however commercial issues appear to have hindered further progress, with the original developer (MIV Therapeutics Inc) no longer trading.
Polymer-Free Drug Elution: Drug-Filled Reservoirs
Commercial issues and decisions may also be playing a role in how long the concept of drug-filled reservoirs may take to reach the market. This approach was initially pioneered by Conor MedSystems with the original CoStar stent having laser-cut drug-filled reservoirs along the struts. The concept cannot be considered as a truly polymer-free approach, as the drug (paclitaxel) is still mixed with a biodegradable (PLGA) polymer. However the overall area and duration of contact between the tissue and polymer is therefore substantially reduced compared to conventional DES. Initial data was sufficiently promising to prompt the acquisition of Conor MedSystems by Cordis, though the device subsequently failed to show non-inferiority against Taxus39 and development of this iteration ceased. Cordis did however develop a new generation of the concept as shown in Fig. 2; a different stent design with modified ductile hinges, a change of the drug to sirolimus and use of only partially filled reservoirs to reduce direct contact between the polymer–drug mixture and the vessel wall. An initial study showed superiority for this NEVO device against Taxus Liberté at 6 months, with lower late loss for the NEVO device.55 Again, long term data would be essential but it is also noted that while two cases of probable stent thrombosis were reported for Taxus Liberté, none were observed for NEVO. Though, a 1-year OCT follow-up showed the NEVO stent trending to a higher neointimal response compared to Xience.71 In any event, despite the good prospects for this technology, Cordis canceled the project as they announced their withdrawal from the coronary stent market in 2011.15 It will be interesting to see if the technology is licensed out from Cordis or if similar concepts are further developed.
(a) NEVO open-cell design. Multiple laser-cut reservoirs hold a blend of PLGA and sirolimus in mechanically stable stent struts. Sigmoidal bridge elements connect the circumferential strut rings. (b) Drawing shoes a detailed view of the filled reservoirs. (c) Scanning electron micrograph of a reservoir. (Reprinted with permission from Ref. 55 Copyright 2010 by Wolters Kluwer Health, Inc.).
The Janus Flex stent (CID SpA) also contains reservoirs, though of a different design. This device contains deep abluminal reservoirs running for longer distances along the struts; the struts are locally wider to compensate for this.62 This device also has the unique feature of being carbon coated i.e., over the bare metal surface, before loading of the drug. Carbon coated stents have been available for several years, with the Carbostent from Sorin Biomedica (sister company of CID) being the most popular. It is proposed that the carbon surface is less thrombogenic than bare metal; the device has also been successfully used for interventions after acute myocardial infarction, where stent thrombosis can be a major complication.47 The reservoirs of the current Janus Flex device are loaded with Tacrolimus, thus providing both a thromboresistant carbon coating to luminal surfaces of the devices and drug elution directly into the tissue at the abluminal faces. However, the performance of this device has been somewhat mixed. In a registry comparing the Janus stent against Cypher, the Janus stent had worse clinical outcomes at 24 months.72 Though in a study with dual anti-platlet therapy (DAT) for durations of only 2 and 6 months, the device was proven to be safe and effective at 12 month follow-up.13 As is often noted, this still provides little insight to late thrombotic events.
The Drug-Filled Stent (DFS) technology currently being developed (Medtronic) is a progression on these initial reservoir concepts. In this design, the stent struts are a tubular configuration, with a hollow core; small access holes connect the inner core to the outside surface. Drug is loaded in the inner core and diffuses out through the holes into the vessel wall. Initial animal studies are showing promising results with the ability to control drug release rates at levels similar to Resolute device allowing for controlled, polymer-free drug elution over a desired period of time directly into the arterial wall to potentially avoid chronic inflammation and adverse vascular responses. Medtronic have just recently announced plans to commence clinical trials using this technology.44
Non-pharmacological Stent Coatings
While drug-eluting coatings have been the focus of extensive development for several years, work has also been progressing steadily with a number of non-pharmacological solutions to address device restenosis. These have seen some renewed interest recently as potential alternatives to drug-eluting devices, yet potentially providing superior performance to bare metal stents. Silicon carbide is one these materials and has been investigated for several years. It is proposed that the semi-conductor nature of the coating reduces inflammatory and thrombogenic responses. Early results failed to demonstrate a benefit over stainless steel devices.76 A more recent study in patients with acute coronary syndrome (ACS) showed low rates for major events and re-interventions, despite the potentially higher risk of thrombosis in this group.5 This Prokinetic stent (Biotronik) may also be benefiting from improved designs with thinner struts, compared to earlier coated devices. A drug-coated version is also available which elutes sirolimus from a biodegradable PLLA matrix. It is proposed that once drug elution and coating degradation is completed, the silicon carbide surface continues to offer protection against any potential late thrombotic events. A multi-centre clinical trial on this device has commenced and will include follow-up out to 5 years.10 Biotronik recently announced completion of enrollment in the Bioflow-IV study,9 which aims to verify the efficacy and safety of the Orsiro Hybrid Drug-Eluting Stent (DES) from Biotronik. The quality and efficacy of Orsiro has already been confirmed by the trials Bioflow-I, Bioflow-II and Bioflow-III, which demonstrated the safety and efficacy of Orsiro. The Bioflow-IV study is the first prospective, randomized, controlled, global multi-center study to compare the TVF rate of Orsiro DES and Abbott Vascular’s Xience Prime/Xpedition DES in a non-inferiority setting with the primary endpoint of TVF at 12 months.
The potential benefits of passive surfaces like carbon and silicon carbide have long been considered in the context of metal allergies; specifically the ability of these coating to reduce the release of metal ions from stent surfaces. Since publication of an initial study suggesting a link between metal allergies and restenosis,37 there have been several debates, but little clinical evidence implicating metal ion release. However with the realization that bare metal stents will retain a substantial share of the market, there is some renewed interest in passive coated devices. A recent study has again shown a link between the allergic response to nickel and in-stent restenosis.65
Similar to the carbon and silicon carbide coated stents, the Titan devices (Hexacath), with a titanium–nitride-oxide coating have been steadily proving their worth as bare metal alternatives to DES. The long-established biocompatibility of titanium and titanium oxide is the basis for these coatings—it is debatable that the nitride component contributes much to the positive outcomes. Several studies have now been published showing reduced neointimal response, as illustrated in Fig. 3. Five-year follow-up in a study comparing the Titan 2 device against Taxus has shown lower rates of MI, MACE and stent thrombosis for Titan 2.33 The titanium–nitride-oxide devices also required shorter anti-platelet therapy treatments. Data has also been recently published showing positive outcomes at 9-month follow-up in a diabetic population—where risk of stent thrombosis would have been higher.14 However longer duration follow-ups would be needed to fully assess this outcome. While the devices may not offer superiority to DES in terms of late loss or binary restenosis rates,60 they do appear to offer an alternative to DES in some situations.
(a) Control segment with normal three-layer vessel structure. (b) Stented segment (uncoated control) with severe neointimal hyperplasia showing near obliteration of lumen. (c) Stented segment (coated with TiNOX 1) with minimal neointima hyperplasia. (d) Stented segment (coated with TiNOX 2) with mild hyperplasia. (Reprinted with permission from Ref. 80 Copyright 2001 by Wolters Kluwer Health, Inc.).
Biological Stent Coatings
As understanding of the biological interactions between the stent and the in vivo arterial environment increased, a number of device improvement efforts concentrated on optimizing and exploiting these biological responses—without the use of pharmaceutical coatings. Avoiding the use of drug compounds that have a cytostatic or cytotoxic effect on neointimal tissue could provide better stent endothelialization. In addition it is proposed that attraction of circulating endothelial progenitor cells (EPCs) to the stent surface would further enhance re-endothelialization. The Genous stent (OrbusNeich) has been one of the most widely explored devices in this regard. This device is coated with a polysaccharide matrix containing anti-human CD34+ antibodies that are directed against the human CD34 antigen, which is a cell surface marker found on circulating EPCs. The mechanism by which this coating promotes attraction of EPCs and enhances device endothelialization has been demonstrated in a recent human arteriovenous shunt study; inspection of devices showed less strut coverage and the presence of thrombogenic deposits on a bare metal device compared to the Genous stent.42 While a number of clinical studies have been performed, results have been mixed. A single-centre pilot study comparing the Genous against Taxus, in patients with a high risk of restenosis, showed a higher rate of re-intervention, at 1 year, for the Genous device—though Taxus had more stent thromboses.4 Two year follow-up did show less of a difference in re-intervention between the two devices3 but the results of further studies are awaited. Interestingly, a dual therapy version of the device is now in trial; this combines an abluminal dose of sirolimus with the EPC capture technology.54 While this may have the potential to provide a balance of pro-healing and anti-proliferative effects, it does however seem to a complex approach. The novel stent (Combo Dual Therapy stent; OrbusNeich, Fort Lauderdale, FL, USA) combining CD34 antibody technology with the anti-proliferative agent, sirolimus, and a biodegradable polymer coating has recently been approved for use in Europe.
Although there has been significant benchside and pre-clinical research conducted in the area of gene-eluting stents (GES), to date there is no clinical data on these stents. In pre-clinical models, several different genes have been delivered successfully to the blood vessel wall (e.g. nitric oxide synthase, vascular endothelial growth factors, TIMP-3) with accelerated re-endothelialization and reduction of neointima formation post-stenting, at present significant hurdles remain to clinical translation of gene-eluting stents.30,68,69,79 The key issue in the development of GES is final step sterilization of the device without inactivating the gene. Secondary issue is related to the identification of a suitable and acceptable vector for carrying gene into the vascular cells. For vascular gene delivery in general, third generation adenovirus and adeno-associated virus have shown promising results but they remain unacceptable for clinical use.70 Liposome may provide an alternative as a non-viral vector but lacks cell-specificity.69 At present the development of GES faces significant challenges especially development of an ideal vector, optimization of gene elution profile and end process sterilization. We believe further research in the area of gene-eluting stents is required before its translation into clinical medicine.
Stent Substrate Technologies
Bare Metal Stent Materials
Considering that biodegradable drug-eluting coatings will result in an in vivo return to bare metal surfaces, there is likely to be renewed interest in the characteristics of bare metal devices—both surface and mechanical performance aspects. Cobalt–chromium alloys have provided higher strength and therefore have enabled reduced strut profiles compared to earlier generation stainless steel devices. While some stainless steel stents are still available, the shift to cobalt–chromium is nearly complete. One exception to this has been the introduction of a new platinum–chromium alloy in the Taxus Element stent (Boston Scientific Corp).35 This alloy offers an improved balance of strength and radiopacity, enabling thinner strut designs. In addition to the paclitaxel-eluting device, an everolimus-eluting version of the stent has also been successful.73 Both these devices have utilized a permanent polymer for drug elution. With future designs aiming to avoid permanent polymers, the reduced levels of iron and nickel (due to the addition of platinum), may also be a benefit in the context of metal ions and potential allergic responses. The bare metal version of the stent is currently in trial, while promising results are also being obtained for a version with a biodegradable PLGA everolimus-eluting coating.11 In the first successful U.S. pivotal trial of a bioabsorbable polymer stent, the Boston Scientific SYNERGY™ Everolimus-Eluting Bioabsorbable Polymer Platinum Chromium Coronary Stent System met its primary endpoint in this non-inferiority study, which evaluated the 1-year rate of target lesion failure (TLF). The key findings for the SYNERGY Stent from the EVOLVE II Trial 1 year data included that at 12 months, the TLF rate was 6.4% per protocol (p = 0.0003 for non-inferiority) and 6.7% for intent-to-treat (p = 0.0005 for non-inferiority). Stent thrombosis (ST) was rare, with Definite or Probable ST occurring in only 0.4% of patients through 1 year and with no definite ST occurred after 24 h. The HOST-ASSURE randomized trial also showed the platinum–chromium and everolimus stent to be comparable to the zotarolimus-eluting cobalt–chromium device.58
BMS have approximately 30% lower rates of restenosis compared to plain balloon angioplasty.12 Although many efforts have been made to further reduce restenosis by modification of bare stent design and materials, thinning of stent struts has been the only proven modification capable of reducing restenosis of BMS.34,57 There is no clear evidence of a difference between DES and BMS in the risk of stent thrombosis following unplanned disruption of DAPT.46 Although thinner strut designs are desirable to achieve superior deliverability, such new generation stents may have compromised longitudinal strength. Longitudinal compression/crushing or a “concertina effect” appears to be related to guide catheter compression or stent catching during re-crossing of a sub-optimally deployed stent. Procedural technique with optimal post-dilatation has a major impact on the occurrence of longitudinal stent deformation and overall frequency of this event remains low.26,49,61 Initial reports were mainly associated with the Promus Element device, but it has also been observed on the Endeavor, BioMatrix and Taxus stents.41
Polymeric Biodegradable Stents
There is general agreement that stents are needed only for a limited duration after implantation and that the scaffold is unnecessary and potentially disadvantageous beyond the initial period of healing and remodeling. Long term effects can include reduced re-endothelialization, thrombogenicity, local inflammatory responses and mechanical mismatch due to local stiffening of the vessel. The concept of biodegradable devices is therefore attractive, though raises many technological and clinical challenges beyond those of conventional stents. One of the earliest devices developed was the Igaki–Tamai stent (Kyoto Medical Planning Co Ltd) which is made from poly-l-lactic acid (PLLA).75 This was the first fully bioresorbable stent to be implanted in humans, with complete degradation taking 18–24 months. While early trials have been promising and the device did receive CE marking, it has not been widely commercialized. Perhaps the need to warm the device to 50 °C during deployment (to improve expansion and reduce recoil) has hindered interest.
Most progress with clinical trials and commercialization has been with the BVS stent (Abbott Vascular). This device is also made from a PLLA material and in addition is coated with a more rapidly degrading layer of poly-d,l-lactic acid (PDLLA) for elution of everolimus. Similar to the Igaki–Tamai device, degradation of the BVS stent is primarily based on hydrolysis of the polymer back-bone. While results of the initial trial were promising it highlighted the challenges with both acute and late recoil with such biodegradable devices. A second generation of the device was designed to improve the scaffolding behavior and prolong mechanical stability, while not delaying overall absorption, which is approximately 2 years.53 Though no major improvements in acute recoil have been observed, OCT studies at 12 months have shown an absence of late recoil with resorption initiated.67 Figure 4 illustrates IVUS and OCT images of the stent at different time points. While larger trials are underway, the device has already obtained the CE Mark. With 3- and 5-year data now available, further trials are exploring use of BVS in more complex lesions and patient categories.20
Corresponding intravascular ultrasound (IVUS) and optical coherence tomography (OCT) images from baseline (BL), 6 months and 2 years. OCT gives better resolution of strut integration within the tissue. (Reprinted with permission from Ref. 56 Copyright 2012 by Wolters Kluwer Health, Inc.).
Also in development is the ReZolve stent (REVA Medical Inc) which is made from a tyrosine polycarbonate. Uniquely, this biodegradable structure also incorporates iodine molecules, which impart an improved level of radiopacity to the device, compared to other absorbable polymers. The stent has a slide-and-lock mechanism which leads to significantly improved radial strength compared to most polymers and also prevents acute and late recoil. The device has gone through a number of iterations to optimize polymer chemistry and the locking mechanism.52 The improved version (ReZolve2) is a lower profile and sheathless version of the first-generation ReZolve scaffold that offers significantly improved deliverability. It provides an approximate 30% increase in scaffold strength to provide increased support to significant coronary artery lesions before being resorbed by the body. A total of 112 patients have been enrolled in the clinical trial RESTORE-II for ReZolve2 to provide the data needed to apply for European CE Marking and an update on data from these patients will be presented at the EUROPCR course in Paris in May 2015.
REVA Medical, Inc. announced recently that it has positioned the Company to fast-track the development of its breakthrough “thin-strut” fully bioresorbable scaffold family, Fantom. The Fantom scaffold is made from a single piece of REVA’s proprietary high performance desaminotyrosine polycarbonate polymer. Fantom is intended to halve the strut thickness of ReZolve without a decrease in radial strength. Thinner scaffold dimensions greatly improve deliverability and healing response, each of which can help to ensure broader adoption. Fantom maintains REVA’s unique attributes of X-ray visibility and a single inflation to expand the device that competitive polymer scaffolds do not offer. Recent early results from the initial clinical feasibility trial have been deemed successful with no adverse events.63
Metallic Biodegradable Stents
Metallic biodegradables offer the possibility of higher initial radial strength and reduced recoil compared to many of the biodegradable polymer devices. Magnesium has been investigated for several years now, with work on the AMS stent (Biotronik), made from magnesium alloy WE 43, having progressed significantly. While initial trials demonstrated safety, it was also established that devices were degrading too fast; this resulted in early loss of radial force and associated vessel recoil, leading to high re-intervention rates.19 Improvements have therefore focused on prolonging the duration for mechanical stability and scaffolding. These changes include a modified stent design, a modified magnesium alloy and improved surface passivation.77 This device is coated with a biodegradable polymer and paclitaxel mixture. The drug-eluting absorbable metal scaffold (DREAMS-1) which eluted paclitaxel (0.07 µg/mm2) for the first 3 months was tested in the BIOSOLVE-1 study showing good safety (one case of MI, no death, and no stent thrombosis) and efficacy at 12 months. The 2-year clinical outcomes presented at EuroPCR 2013 showed 6.8% TLF, including two cases of clinically driven TLR and one target vessel MI.27 A new optimized device DREAMS-2 has a six-crown, two-link design, 150 µm strut thickness, radiopaque marker at both ends, and a thin PLLA-based carrier to deliver a more potent anti-proliferative drug (sirolimus). DREAMS-2 is currently being tested in the BIOSOLVE-II study (n = 120) to get the data needed to apply for CE mark; enrollment for this study has just recently been completed.8 It is interesting to note that there is a vast array of research underway worldwide relating to improved magnesium alloys and surface treatments to control degradation rates; a successful outcome is therefore most likely.
The possibility of using iron as a biodegradable device material has also been explored for some time, though overall this approach has not progressed as far as magnesium alloys. Initial pre-clinical trials have shown that while mechanical properties are in a suitable range, the iron devices were corroding too slowly.59 Thus much effort has since focused on control of material composition and microstructure to obtain increased degradation rates.48,66 Work with an iron–manganese alloy has progressed well; promising in vitro degradation rates have been obtained and prototype stents have been made.28 Many standard processing techniques can be applied as illustrated in Fig. 5. It will be interesting to see animal in vivo data for these devices.
SEM images of the evolution of a Fe–35 Mn stent: (a) initial machined minitube, (b) laser-cut minitube, (c) annealed laser-cut minitube, and (d) descaled stent. (Reprinted with permission from Ref. 29 Copyright 2013 by Elsevier.).
Summary and Future Direction
Table 1 presents an overview of the technologies and devices reviewed here. Considering that current commercial devices have indeed high levels of safety and efficacy, this is an impressive array of technologies aimed at eliciting further improvements. With most of these efforts focused on LST, the challenges to developers are immense, considering the low numbers and long durations involved, as well as some uncertainty about underlying mechanisms and contributory factors. It is therefore not surprising that one of the first notable observations is that several programs have now stopped, despite early promise and large investment. New technologies with complex processes and many variables are being used in an equally complex and variable biological environment—uncertain or undesirable outcomes are inevitable. It is also noted that many of the devices are truly in early development with only relatively short-term clinical data available—data out to 5 years will be needed to assess benefits in terms of LST.
Of all the technologies reviewed, biodegradable drug-eluting coatings certainly look to be well positioned to see wider use in the coming years, though the full benefits remain to be determined in different clinical settings. Several of the polymer-free drug-elution approaches have been abandoned, though techniques based on microtextured surfaces may still prove attractive. Reservoir-based concepts have not fared well to-date, for both commercial and technical reasons. Biodegradable devices have many challenges to overcome—both polymeric and metallic—but the polymeric BVS device is certainly gaining momentum and showing good data. Metallic biodegradable devices need much more development and data, with magnesium being the most likely to see regular use in the long term. Devices with carbon, silicon carbide or titanium–nitride-oxide coatings are proving to be of value in certain niche segments, while devices utilizing biological coatings will require much more fundamental and applied development work.
References
Abizaid, A., and J. R. Costa. New drug-eluting stents: an overview on biodegradable and polymer-free next-generation stent systems. Circ. Cardiovasc. Interv. 3:384–393, 2010.
Basalus, M., K. Tandjung, A. Van Apeldoorn, M. J. K. Ankone, and C. Von Birgelen. Effect of oversized partial postdilation on coatings of contemporary durable polymer-based drug-eluting stents. A scanning electron microscopy study. J. Interv. Cardiol. 24:149–161, 2011.
Beijk, M., M. Klomp, N. van Geloven, K. T. Koch, J. P. S. Henriques, J. Baan, M. M. Vis, J. G. P. Tijssen, J. J. Piek, and R. J. de Winter. Two-year follow-up of the genous endothelial progenitor capturing cell capturing stent versus the taxus liberte stent in patients with de novo coronary artery lesions with a risk of restenosis. Catheter. Cardiovasc. Interv. 78:189–195, 2011.
Beijk, M., M. Klomp, N. Verouden, N. van Geloven, K. T. Koch, J. P. S. Henriques, J. Baan, M. M. Vis, E. Scheunhage, J. J. Piek, J. G. P. Tijssen, and R. J. de Winter. Genous™ endothelial progenitor cell capturing stent vs the Taxus Liberte stent in patients with de novo coronary lesions with a high-risk of coronary restenosis: a randomized, single-centred pilot study. Eur. Heart J. 31:1055–1064, 2010.
Berlin, T., E. Rozenbaum, J. Arbel, O. Reges, J. Erel, I. Shetboun, M. Leibovitch, and M. Mosseri. Six- and twelve-month clinical outcomes after implantation of prokinetic BMS in patients with acute coronary syndrome. J. Interv. Cardiol. 23:377–381, 2010.
Biosensors Press Release. BioFreedom™ FIM Trial Demonstrates Comparable Long-Term Safety and Efficacy to Conventional Drug-Eluting Stent, November 10th, 2011.
Biosensors Press Release. Initial Patient Enrolled in BioFreedom USA: First Major American Trial for Biosensors BioFreedom Stent—Polymer-Free, Shorter DAPT, September 4th, 2014.
Biotronik Press Release. BIOTRONIK Advances Clinical Evaluation of Latest Generation DREAMS, World’s First Bioabsorbable Magnesium Scaffold, February 19th, 2015.
Biotronik Press Release. BIOTRONIK Announces Completion of Patient Enrollment in BIOFLOW-IV Study Evaluating Safety and Efficacy of Orsiro, February 4th, 2015.
Biotronik Press Release. Biotronik announces first patients treated in BIOFLOW II clinical study comparing ORSIRO hybrid drug-eluting stent to Abbott’s Xience Prime, July 13th, 2011.
Boston Scientific Corp Press Release. Boston Scientific SYNERGY™ Stent Demonstrates Comparable Safety and Effectiveness Outcomes Versus PROMUS Element™ Platinum Chromium Stent, November 11th, 2011.
Brophy, J. M., P. Belisle, and L. Joseph. Evidence for use of coronary stents. A hierarchical bayesian meta-analysis. Ann. Intern. Med. 138:777–786, 2003.
Cassese, S., G. De Luca, B. Villari, S. Berti, P. Bellone, A. Alfieri, A. Montinaro, G. Quaranta, P. Marraccini, and F. Piscione. Reduced antiplatelet therapy after drug-eluting stenting: multicenter janus Flex Carbostent implantation with short dual antiplatelet treatment for 2 or 6 months—MATRIX study. Catheter. Cardiovasc. Interv. 80:408–416, 2012.
Chavarri, M. V., A. Bethencourt, E. Pinar, A. Gomez, J. F. Portales, F. Pomar, I. Calvo, J. R. Lopez Minguez, R. Valdesuso, J. Moreu, A. Martinez, and W. Nammas. Titanium-nitride-oxide-coated stents multicenter registry in diaBEtic patienTs: the TIBET registry. Heart Vessels 27:151–158, 2012.
Cordis announces discontinuation of NEVO sirolimus-eluting coronary stent. Company to focus on areas of significant patient need in cardiovascular disease. Cordis Press Release, June 15th, 2011.
Costa, J. R., A. Abizaid, R. Costa, F. Feres, L. F. Tanajura, A. Abizaid, G. Maldonado, R. Staico, D. Siqueira, A. Sousa, R. Bonan, and J. E. Sousa. 1-year results of the hydroxyapatite polymer-free sirolimus-eluting stent for the treatment of single de novo coronary lesions. J. Am. Coll. Cardiol. Interv. 2:422–427, 2009.
Costa, J. R., B. A. Oliveira, A. Abizaid, R. Costa, M. Perin, A. Abizaid, D. Chamié, L. F. Tanajura, A. Sousa, and J. E. M. R. Sousa. Clinical, angiographic, and intravascular ultrasound results of the VestaSync II trial. Catheter. Cardiovasc. Interv. 84:1073–1079, 2014.
Desch, S., D. Schloma, S. Möbius-Winkler, S. Erbs, S. Gielen, A. Linke, J. Yu, B. Lauer, K. Kleinertz, W. Danschel, G. Schuler, and H. Thiele. Randomized comparison of a polymer-free sirolimus-eluting stent versus a polymer-based paclitaxel-eluting stent in patients with diabetes mellitus. J. Am. Coll. Cardiol. Interv. 4:452–459, 2011.
Erbel, R., C. Di Mario, J. Bartunek, J. Bonnier, B. de Bruyne, F. R. Eberli, P. Erne, M. Haude, B. Heublin, M. Harrigan, C. Ilsley, D. Boese, J. Koolen, T. F. Luescher, N. Weissman, and R. Waksman. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents; a prospective non-randomized multi-centre trial. Lancet 369:1869–1875, 2007.
Felix, C., B. Everaert, R. Diletti, N. Van Mieghem, J. Daemen, M. Valgimigli, P. P. de Jaegere, F. Zijlstra, E. Regar, C. Simsek, Y. Onuma, and R. J. M. van Geuns. Current status of clinically available bioresorbable scaffolds in percutaneous coronary interventions. Neth. Heart J. 23:153–160, 2015.
Finn, A. V., M. Joner, G. Nakazawa, F. Kolodgie, J. Newell, M. C. John, H. K. Gold, and R. Virmani. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 115:2435–2441, 2007.
Granada, J. F., and J. W. Moses. Assessing the durability of durable stent polymers: will they pass the test of time? J. Interv. Cardiol. 24:162–164, 2011.
Grube, E., J. Schofer, K. E. Hauptmann, G. Nickenig, N. Curzen, D. J. Allocco, and K. D. Dawkins. A novel paclitaxel-eluting stent with an ultrathin abluminal biodegradable polymer. J. Am. Coll. Cardiol. Interv. 3:431–438, 2010.
Guagliumi, G., V. Sirbu, G. Musumeci, H. G. Bezerra, A. Aprile, H. Kyono, L. Fiocca, A. Matiashvili, N. Lortkipanidze, A. Vassileva, J. J. Popma, D. J. Allocco, K. D. Dawkins, O. Valsecchi, and M. A. Costa. Strut coverage and vessel wall response to a new-generation paclitaxel-eluting stent with an ultrathin biodegradable abluminal polymer. Circ. Cardiovasc. Interv. 3:367–375, 2010.
Gutiérrez-Chico, J. L., P. Jüni, H. M. García-García, E. Regar, E. Nuesch, F. Borgia, W. J. van der Giessen, S. Davies, R. J. van Geuns, G. G. Secco, S. Meis, S. Windecker, P. W. Serruys, and C. di Mario. Long-term tissue coverage of a biodegradable polylactide polymer-coated biolimus-eluting stent: comparative sequential assessment with optical coherence tomography until complete resorption of the polymer. Am. Heart J. 162:922–931, 2011.
Hanratty, C. G., and S. J. Walsh. Longitudinal compression: a “new” complication with modern coronary stent platforms—time to think beyond deliverability. EuroIntervention 7:872–877, 2011.
Haude, M. Two-Year Clinical Data and Multi-modality Imaging Results up to 1-year Follow-Up of the BIOSOLVE-I Study with the Paclitaxel-Eluting Bioabsorbable Magnesium Scaffold (DREAMS). Paris: EuroPCR, 2013.
Hermawan H, Mantovani D. New generation of medical implants: metallic biodegradable coronary stent. In: International Conference on Instrumentation, Communication, Information Technology and Biomedical Engineering, Indonesia, November 8–9, 2011.
Hermawan, H., and D. Mantovani. Process of prototyping coronary stent from biodegradable Fe-Mn alloys. Acta Biomater. 9:8585–8592, 2013.
Johnson, T. W., Y. X. Wu, C. Herdeg, A. Baumbach, A. C. Newby, K. R. Karsch, and M. Oberhoff. Stent-based delivery of tissue inhibitor metalloproteinase-3 adenovirus inhibits neointimal formation in porcine coronary arteries. Arterioscler. Thromb. Vasc. 25:754–759, 2005.
Joner, M., A. V. Finn, A. Farb, E. K. Mont, F. D. Kolodgie, E. Ladich, R. Kutys, K. Skorija, H. K. Gold, and R. Virmani. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 48:193–202, 2006.
Kadota, K., T. Muramatsu, M. Iwabuchi, S. Saito, Y. Hayashi, Y. Ikari, S. Nanto, K. Fujii, N. Inoue, A. Namiki, T. Kimura, and K. Mitsudo. Randomized comparison of the nobori Biolimus A9-eluting stent with the sirolimus-eluting stent in patients with stenosis in native coronary arteries. Catheter. Cardiovasc. Interv. 80:789–796, 2012.
Karjalainen, P. P., A. Ylitalo, J. K. Airaksinen, and W. Nammas. Five-year clinical outcome of titanium-nitride-oxide-coated bioactive stent implantation in a real-world population. J. Interv. Cardiol. 24:1–8, 2011.
Kastrati, A., J. Mehilli, J. Dirschinger, F. Dotzer, H. Schuhlen, F. J. Neumann, M. Fleckenstein, C. Pfafferott, M. Seyfarth, and A. Schomig. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation 103:2816–2821, 2001.
Kereiakes, D. J., L. A. Cannon, R. L. Feldman, J. J. Popma, R. Magorien, R. Whitbourn, I. M. Dauber, A. C. Rabinowitz, M. W. Ball, B. Bertolet, A. Kabour, M. C. Foster, J. C. Wang, P. Underwood, and K. D. Dawkins. Clinical and angiographic outcomes after treatment of de novo coronary stenoses with a novel platinum chromium thin-strut stent. J. Am. Coll. Cardiol. 56:264–271, 2010.
King, L., R. A. Byrne, J. Mehilli, A. Schömig, A. Kastrati, and J. Pache. Five-year clinical outcomes for a polymer-free sirolimus-eluting stent versus a permanent polymer paclitaxel-eluting stent. Catheter. Cardiovasc. Interv. 81:E23–E28, 2013.
Koster, R., D. Vieluf, M. Kiehn, M. Sommerauer, J. Kaehler, S. Baldus, T. Meinertz, and C. W. Hamm. Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet 365:1895–1897, 2000.
Kovandaivelu, K., R. Swaminathan, W. J. Gibson, V. B. Kolachalama, K. L. Nguyen-Ehrenreich, V. L. Giddings, L. Coleman, G. K. Wong, and E. R. Edelman. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation 123:1400–1409, 2011.
Krucoff, M. W., D. J. Kereiakes, J. L. Petersen, R. Mehran, V. Hasselblad, A. J. Lansky, P. J. Fitzgerald, J. Garg, M. A. Turco, C. A. Simonton, S. Verheye, C. L. Dubois, R. Gammon, W. B. Batchelor, C. D. O’Shaughnessy, J. B. Hermiller, J. Schofer, M. Buchbinder, and W. Wijns. A novel bioresorbable polymer paclitaxel-eluting stent for the treatment of single and multivessel coronary disease. J. Am. Coll. Cardiol. 51:1543–1552, 2008.
Kuramitsu, S., S. Sonodo, H. Yokoi, M. Iwabuchi, Y. Nishizaki, T. Shinozaki, T. Domei, M. Hyodo, K. Inoue, S. Shirai, K. Ando, and M. Nobuyoshi. Long-term cornary arterial response to biodegradable polymer biolimus-eluting stents in comparison with durable polymer sirolimus-eluting stents and bare-metal stents: five-year follow-up optical coherence tomorgraphy study. Atherosclerosis 237:23–29, 2014.
Kwok, O. H. Stent “Concertina”: stent design does matter. J. Invasive Cardiol. 25(6):E114–E119, 2013.
Larsen, K., C. Cheng, D. Tempel, S. Parker, S. Yazdani, W. K. den Dekker, J. H. Houtgraaf, R. De Jong, S. Swager-ten Hoor, E. Ligtenberg, S. R. Hanson, S. Rowland, F. Kolodgie, P. W. Serruys, R. Virmani, and H. J. Duckers. Capture of circulatory endothelial progenitor cells and accelerated re-endothelialization of a bio-engineered stent in human ex vivo shunt and rabbit denudation model. Eur. Heart J. 33:120–128, 2012.
Mauri, L., W. Hsieh, J. M. Massaro, K. K. L. Ho, R. D’Agostino, and D. E. Cutlip. Stent thrombosis in randomized clinical trials of drug-eluting stents. N. Engl. J. Med. 356:1020–1029, 2007.
Medtronic Press Release. Medtronic to initiate clinical study of Drug-Filled Stent following successful preclinical results, March 14th, 2015.
Mehilli, J., A. Kastrati, R. Wessely, A. Dibra, J. Hausleiter, B. Jaschke, J. Dirschinger, and A. Schomig. Randomized trial of a non-polymer based rapamycin-eluting stent versus a polymer-based paclitaxel-eluting stent for the reduction of late Lumen loss. Circulation 113:273–279, 2006.
Mehran, R., U. Baber, P. G. Steg, C. Ariti, G. Weisz, B. Witzenbichler, T. D. Henry, A. S. Kini, T. Stuckey, D. J. Cohen, P. B. Berger, I. Iakovou, G. Dangas, R. Waksman, D. Antoniucci, S. Sartori, M. W. Krucoff, J. B. Hermiller, F. Shawl, C. M. Gibson, A. Chieffo, M. Alu, D. J. Moliterno, A. Colombo, and S. Pocock. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet 382:1714–1722, 2013.
Migliorini, A., R. Valenti, G. Moschi, G. Parodi, G. Cerisano, P. Buonamici, N. Carrabba, and D. Antoniucci. Predictor of stent thrombosis in patients treated with turbostratic carbon-coated stent implantation for acute myocardial infarction. J. Interv. Cardiol. 23:554–559, 2010.
Moravej, M., and D. Mantovani. Biodegradable metals for cardiovascular stent applications: interests and new opportunities. Int. J. Mol. Sci. 12:4250–4270, 2011.
Mortier, P., and M. De Beule. Stent design back in the picture: an engineering perspective on longitudinal stent compression. EuroIntervention 7:773–775, 2011.
Nakazawa, G. Stent thrombosis of drug eluting stent: pathological perspective. J. Cardiol. 58:84–91, 2011.
O’Brien, B., and W. Carroll. The evolution of cardiovascular stent materials and surfaces in response to clinical drivers: a review. Acta Biomater. 5:945–958, 2009.
Onuma, Y., and P. W. Serruys. Bioresorbable scaffold: the advent of a new era in percutaneous coronary and peripheral revascularization. Circulation 123:779–797, 2011.
Onuma, Y., P. W. Serruys, J. Gomez, B. de Bruyne, D. Dudek, L. Thuesen, P. Smits, B. Chevalier, D. McClean, J. Koolen, S. Windecker, R. Whitbourn, I. Meredith, H. Garcia-Garcia, and J. A. Ormiston. Comparison of in-vivo acute stent recoil between the bioresorbable everolimus-eluting coronary scaffolds (revision 1.0 and 1.1) and the metallic everolimus-eluting stent. Catheter. Cardiovasc. Interv. 78:3–12, 2011.
OrbusNeich Press Release. OrbusNeich Introduces the Combo Dual Therapy Stent at TCT 2011, Nov 10th, 2011.
Ormiston, J. A., A. Abizaid, J. Spertus, J. Fajadet, L. Mauri, J. Schofer, S. Verheye, J. Dens, L. Thuesen, C. Dubois, R. Hoffmann, W. Wijns, P. J. Fitzgerald, J. J. Popma, N. Macours, A. Cebrian, H. P. Stoll, C. Rogers, and C. Spaulding. Six-month results of the NEVO RES-ELUTION I (NEVO RES I) trial. Circ. Cardiovasc. Interv. 3:556–564, 2010.
Ormiston, J. A., P. W. Serruys, Y. Onuma, R. J. van Geuns, B. de Bruyne, D. Dudek, L. Thuesen, P. C. Smits, B. Chevalier, D. McClean, J. Koolen, S. Windecker, R. Whitbourn, I. Meredith, C. Dorange, S. Veldhof, K. M. Hebert, R. Rapoza, and H. M. Garcia-Garcia. First serial assessment at 6 months and 2 years for the second generation of absorb everolimus-eluting bioresorbable vascular scaffold. Circ. Cardiovasc. Interv. 5:620–632, 2012.
Pache, J., A. Kastrati, J. Mehilli, H. Schuhlen, F. Dotzer, J. Hausleiter, M. Fleckenstein, F. J. Neumann, U. Sattelberger, C. Schmitt, M. Muller, J. Dirschinger, and A. Schomig. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J. Am. Coll. Cardiol. 41:1283–1288, 2003.
Park, K. W. A randomized comparison of platinum chromium-based everolimus-eluting stents versus cobalt chromium-based zotarolimus-eluting stents in all-comers receiving percutaneous coronary intervention. J. Am. Coll. Cardiol. 63:2805–2816, 2014.
Peuster, M., C. Hesse, T. Schloo, C. Fink, P. Beerbaum, and C. von Schnakenburg. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 27:4955–4962, 2006.
Pilgrim, T., L. Raeber, A. Limacher, L. Loeffel, P. Wenaweser, S. Cook, J. C. Stauffer, M. Togni, R. Vogel, A. Garachemani, A. Moschovitis, A. A. Khattab, C. Seiler, B. Meier, P. Juni, and C. Windecker. Comparison of titanium-nitride-oxide-coated stents with zotarolimus-eluting stents for coronary revascularization. J. Am. Coll. Cardiol. Interv. 4:672–682, 2011.
Prabhu, S., T. Schikorr, T. Mahmoud, J. Jacobs, A. Potgieter, and C. Simonton. Engineering assessment of the longitudinal compression behaviour of contemporary coronary stents. EuroIntervention 8:275–281, 2012.
Prunotto, M., C. Vignolini, V. Lolli, A. Black, S. Gaggianesi, A. Santarelli, and M. Galloni. Short-, mid-, and long-term effects of a polymer-free tacrolimus-eluting stent in a porcine coronary model. J. Biomed. Mater. Res. A 88:872–879, 2009.
REVA Medical Press Release. REVA releases initial clinical results from FANTOM scaffold, May 21st, 2015.
Ruef, J., H. Störger, F. Schwarz, and J. Haase. Comparison of a polymer-free rapamycin-eluting stent (YUKON) with a polymer-based paclitaxel-eluting stent (TAXUS) in real world coronary artery lesions. Catheter. Cardiovasc. Interv. 71:333–339, 2008.
Saito, T., S. Hokomito, S. Oshima, K. Noda, Y. Kojyo, and K. Matsunaga. Metal allergic reaction in chronic-refractory in-stent restenosis. Cardiovasc. Revasc. Med. 10:17–22, 2009.
Schinhammer, N., A. C. Hänzi, J. F. Löffler, and P. J. Uggowitzer. Design strategy for biodegradable Fe-based alloys for medical applications. Acta Biomater. 6:1705–1713, 2010.
Serruys, P. W., Y. Onuma, D. Dudek, P. C. Smits, J. Koolen, B. Chevalier, B. de Bruyne, L. Thuesen, D. McClean, R. J. van Geuns, S. Windecker, R. Whitbourn, I. Meredith, C. Dorange, S. Veldhof, K. M. Hebert, K. Sudhir, H. M. Garcia-Garcia, and J. A. Ormiston. Evaluation of the second generation of a bioresorbable everolimus-eluting vascular scaffold for the treatment of de novo coronary artery stenosis. J. Am. Coll. Cardiol. 58:1578–1588, 2011.
Sharif, F., S. Hynes, R. Cooney, L. Howard, J. McMahon, K. Daly, J. Crowley, and T. O’Brien. Gene eluting stents: delivery of adenovirus mediated endothelial nitric oxide synthase to the blood vessel wall accelerates re-endothelialization and inhibits restenosis in rabbit iliac arteries. Mol. Ther. 16:1674–1680, 2008.
Sharif, F., S. O. Hynes, K. McCullagh, S. Ganley, U. Geiser, P. McHugh, J. Crowley, F. Barry, and T. O’Brien. Gene-eluting stents: non-viral, liposome-based gene delivery of eNOS to the blood vessel wall in vivo results in enhanced endothelialisation but does not reduce restenosis in a hypercholesterolemic model. Gene Ther. 19:321–328, 2012.
Sharif, F., S. Hynes, J. McMahon, R. Cooney, S. Conroy, P. Dockery, G. Duffy, K. Daly, J. Crowley, J. S. Bartlett, and T. O’Brien. Gene eluting stents: a comparison of adenoviral and adeno-associated viral gene-delivery to the blood vessel wall in vivo. Hum. Gene Ther. 17:741–750, 2006.
Shiratori, Y., S. Brugaletta, L. Allvarez-Contreras, Y. Azpeitia, N. Ospino, S. Gaido, A. Delahanty, A. Santos, V. Martin-Yuste, M. Masotti, P. W. Serruys, S. Windecker, and M. Sabaté. One-year head to head comparison of the neointimal response between sirolimus eluting stent with reservoir technology and everolimus eluting stent. Catheter. Cardiovasc. Interv. 82:428–436, 2013.
Siller-Matula, J. M., I. Tentzeris, B. Vogel, S. Schacherl, R. Jarai, A. Geppert, G. Unger, and K. Huber. Tacrolimus-eluting carbon-coated stents versus sirolimus-eluting stents for prevention of symptom-driven clinical end points. Clin. Res. Cardiol. 99:645–650, 2010.
Stone, G. W., P. S. Teirstein, I. T. Meredith, B. Farah, C. L. Dubois, R. L. Feldman, J. Dens, N. Hagiwara, D. J. Allocco, and K. D. Dawkins. A prospective, randomized evaluation of a novel everolimus-eluting coronary stent. J. Am. Coll. Cardiol. 57:1700–1708, 2011.
Tada, N., R. Virmani, G. Grant, L. Bartlett, A. Black, C. Clavijo, U. Christians, R. Betts, D. Savage, S. H. Su, J. Shulze, and S. Kar. Polymer-free Biolimus A9-coated stent demonstrates more sustained intimal inhibition, improved healing, and reduced inflammation compared with a polymer-coated sirolimus-eluting cypher stent in a porcine model. Circ. Cardiovasc. Interv. 3:174–183, 2010.
Tamai, H., K. Igaki, E. Kyo, K. Kosuga, A. Kawashima, S. Matsui, H. Komori, T. Tsuji, S. Motohara, and H. Uehata. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation 102:399–404, 2000.
Unverdorben, M., B. Sippel, R. Degenhardt, K. Sattler, R. Fries, B. Abt, E. Wagner, H. Koehler, G. Daemgen, M. Scholz, H. Ibrahim, K. H. Tews, B. Hennen, H. K. Berthold, and C. Vallbracht. Comparison of a silicon-carbide coated stent versus a non-coated stent in human beings. Am. Heart J. 145:e17, 2003.
Waksman, R. Update on Absorbable Magnesium Stent. Tel Aviv: Innovation in Cardiovascular Interventions, 2009.
Waksman, R., R. Pakala, R. Baffour, R. Seabron, D. Hellinga, R. Chan, S. H. Su, F. Kolodgie, and R. Virmani. In vivo comparison of a polymer-free Biolimus A9-eluting stent with a biodegradable polymer-based Biolimus A9 eluting stent and a bare metal stent in balloon denuded and radiated hypercholesterolemic rabbit iliac arteries. Catheter. Cardiovasc. Interv. 80:429–436, 2012.
Walter, D. H., M. Cejna, L. Diaz-Sandoval, S. Willis, P. W. Stratford, A. B. Tietz, R. Kirchmair, M. Silver, C. Curry, A. Wecker, Y. S. Yoon, R. Heidenreich, A. Hanley, M. Kearney, F. O. Tio, P. Kuenzler, J. M. Isner, and D. W. Losordo. Local gene transfer of phVEGF-2 plasmid by gene-eluting stents: an alternative strategy for inhibition of restenosis. Circulation 110:36–45, 2004.
Windecker, S., I. Mayer, G. De Pasquale, W. Maier, O. Dirsch, P. De Groot, Y. P. Wu, G. Noll, B. Leskosek, B. Meier, and O. M. Hess. Stent coating with titanium-nitride-oxide for reduction of neointimal hyperplasia. Circulation 104:928–933, 2001.
Acknowledgments
We thank the Health Service Executive (Ireland) that provides all of Ireland’s public health services in hospitals and communities across the country.
Conflict of interest
We declare that there is no conflict of interest for any author.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Sean McGinty oversaw the review of this article.
Rights and permissions
About this article
Cite this article
O’Brien, B., Zafar, H., Ibrahim, A. et al. Coronary Stent Materials and Coatings: A Technology and Performance Update. Ann Biomed Eng 44, 523–535 (2016). https://doi.org/10.1007/s10439-015-1380-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1380-x