Abstract
In recent years, advances in computing power and computational methods have made it possible to perform detailed simulations of the coronary artery stenting procedure and of related virtual tests of performance (including fatigue resistance, corrosion and haemodynamic disturbance). Simultaneously, there has been a growth in systematic computational optimisation studies, largely exploiting the suitability of surrogate modelling methods to time-consuming simulations. To date, systematic optimisation has focussed on stent shape optimisation and has re-affirmed the complexity of the multi-disciplinary, multi-objective problem at hand. Also, surrogate modelling has predominantly involved the method of Kriging. Interestingly, though, optimisation tools, particularly those associated with Kriging, haven’t been used as efficiently as they could have been. This has especially been the case with the way that Kriging predictor functions have been updated during the search for optimal designs. Nonetheless, the potential for future, carefully posed, optimisation strategies has been suitably demonstrated, as described in this review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impressive engineering innovation and clinical expertise have made it possible to routinely deliver stents in narrowed coronary arteries such that these tubular structures can be expanded into atherosclerotic plaques to recover arterial flow area. In clinical terms, the aim is to maximise the minimum lumen area (MLA) by achieving the optimal minimal stent area (MSA). Furthermore, considering that stenting (or percutaneous coronary intervention, PCI) is procedurally successful in the majority of cases, this suggests that state of the art stents and delivery systems may have reached close to design optimality for delivery. Is it possible, or even necessary, therefore, to improve the PCI toolkit, including stents, delivery systems and/or imaging? A key driver in answering these questions is that clinical events, representing later complications (i.e., failures) of the stent, such as stent thrombosis (ST) or restenosis, are more likely in circumstances in which stent expansion is suboptimal. Sub-optimal stent deployment is an independent risk factor for both restenosis and stent thrombosis. Restenosis, an exaggerated inflammatory healing response to the vessel injury inherent to PCI, results in recurrent angina or heart attack. It occurred clinically in around 10% of patients after bare metal stents and the incidence is now a few percent in the days of drug-eluting stents (DES). The minimal stent area is inversely related to the incidence of these complications.8 Given the millions of stent deployment procedures being carried out worldwide, even rates of complications in low single digit percentages of the total represents a large cohort of patients. In this context, there is clearly room for improvement in the precision of stent delivery and optimisation.
If further advances are to be made, how likely is it that computational engineering will be utilised more significantly than it has been in the development of PCI technology to date? Curiously, the earliest simulations of stent expansion performance only began to appear in the literature14,15,32 at the time that the first generation of drug eluting stents were undergoing clinical trials.33,36 These early finite element analysis (FEA) studies focussed on stent structures and neglected the fundamental interactions that occur during deployment between the stent, balloon and vessel wall/tissue. Even the earliest FEA studies that included idealised stenotic artery models, didn’t incorporate balloons to expand the stent, using pressure on the internal surface of the stent, instead.3 It wasn’t until 2008 that patient-specific artery reconstructions were first used in simulations of stent deployment.18 The review of computational structural modelling of coronary stent deployment by Martin and Boyle provided a detailed consideration of this history and there was a review of computational fluid dynamics (CFD) prediction of neo-intimal hyperplasia (or restenosis) in stented arteries by Murphy and Boyle.37 Subsequently, Morlacchi and Migliavacca34 reviewed numerical modelling of stented coronary arteries more generally, including FEA, CFD and drug elution.
At the same time that the first stent deployment studies were appearing in the literature, Stoeckel et al. 43 published a survey of stent designs in which approximately 100 different stents were identified. Whilst commenting that such diversity was largely the result of commercial drivers, they also acknowledged that conflicting design requirements underpinned the competition to optimise scaffolding characteristics, largely in terms of radial strength and flexibility. Why is it that, since that time, there has been an increasing frequency of stent related optimisation studies appearing in the academic literature?
This article focuses on answers to the above questions primarily from the perspectives of what has already been reported on systematic coronary artery stent design optimisation and, more especially, that which might now be possible. There are a number of articles comprising parametric studies (e.g.,10,23,45) but they haven’t been considered in detail here due to the focus on systematic optimisation approaches. It should be acknowledged, however, that these types of study often help to inform more detailed searches for optimal designs.11
Starting with a consideration of clinically optimal stenting, attention is drawn to the causes of PCI failure and poor outcomes. An overview is then presented of measures of performance (or objective functions) that can be evaluated computationally, in preparation for a review of the design optimisation of coronary artery stent systems. The article is concluded with some recommendations for future work.
Clinically Optimal Outcomes
In the 2011 ACCF/AHA/SCAIFootnote 1 PCI guidelines, an angiographic benchmark for stent results was defined by a minimum percent diameter stenosis of <10%, or optimally as close to 0% as possible.27 This is re-iterated in the 2013 update on clinical competencies for PCI but with recognition that angiography provides “an imperfect assessment of coronary structure and stenosis severity”.22 Thus, it is recommended that “other diagnostic modalities such as intravascular ultrasound (IVUS) and fractional flow reserve should be available” during PCI. Indeed, Yoon and Hur48 highlight four criteria for optimal stent deployment when using IVUS:
-
(a)
Complete stent expansion;
-
(b)
Complete stent apposition to the vessel wall;
-
(c)
Avoidance of edge dissection and
-
(d)
Complete lesion coverage.
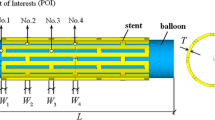
Criteria 1–3 are depicted in Fig. 1 as they might appear in IVUS slices and aligned with a longitudinal cartoon to show where along a stented segment they are likely to occur. In practice, sub-optimal performance in terms of stent under-expansion and malapposition can be addressed by post-dilatation in which a non-compliant balloon is inflated inside the partially deployed stent so as to overcome the failings of the original stenting procedure. Whilst it is important for the interventional cardiologist to have methods such as post-dilatation to correct shortcomings of an initially sub-optimal stent expansion, this can introduce other dangers including tissue dissection, longitudinal stent deformation and changes to stent fatigue resistance. An example of malappostion and post-dilatation is shown in Fig. 2 as obtained using the more recently developed intravascular imaging technique of optical coherence tomography (OCT).
(Fig. 1C in Ref. 24) OCT image of an under-expanded stent (left). The same stent segment seen after post-dilatation, now completely apposing the vessel wall (right). Reprinted with permission from Springer.
Although PCI is now a relatively mature practice, there are two areas in which computational modelling might result in improved stent deployment: (1) preclinical testing of modern iterations of stents and (2) design of novel stent/delivery system characteristics.
Computationally Measurable Optimality
Overview
Procedural optimality as defined above is largely unequivocal and can be measured using intravascular imaging methods. However, there are other metrics of stent performance that are not readily obtained during PCI but which can have a very significant influence on PCI outcome. These metrics include:
-
(a)
Radial (and longitudinal) strength;
-
(b)
Fatigue resistance;
-
(c)
Flexibility;
-
(d)
Stent malapposition;
-
(e)
Tissue damage;
-
(f)
Drug distribution (for DESs) and
-
(g)
Flow metrics, particularly related to flow disturbance and the wall shear stress environment.
Whilst it is possible to selectively combine any of these metrics in research studies, regulatory guidance by the Food and Drugs Administration (FDA) on non-clinical engineering tests provides a long list of recommendations primarily based on mechanical and structural attributes.16 Whilst measures of performance could be defined and simulated for all of the FDA recommended tests, the focus here is primarily on those that have featured in reported optimisation studies. Indeed, some of these (e.g., tissue damage, drug distribution and flow disturbance) don’t appear in the FDA recommendations or in the draft update of 2013.
FEA and CFD are the two principal simulation disciplines that are employed to generate these measures of stent performance. Other physical models have been used (e.g., corrosion modelling by Grogan et al. 19 and drug kinetics by Bozsak et al. 7) but the majority of optimisation studies have employed FEA to obtain structural metrics including recoil, radial strength, foreshortening, flexibility, malapposition, fatigue resistance and tissue stress. Others have focussed solely on CFD simulations to extract and compare wall shear stress metrics. A small number of articles have reported multi-disciplinary optimisations wherein a stent deployment simulation using FEA is followed by a CFD blood flow simulation through the deformed vessel and over the expanded stent and/or by a drug elution simulation using a CFD based scalar transport model.
FEA and Structural Optimality
One way to characterise the various optimisation studies is to consider the level of detail included in the simulation models. For example, the majority of FEA studies have used single unit stent models, completely neglecting interaction with arterial tissue. Others have used high levels of detail including full three-dimensionality and models for a complete balloon delivery system and a diseased artery with contact interactions between balloon, stent and tissue.19,39
In addition to the review by Martin and Boyle,31 Migliavacca et al. 32 provided a succinct overview of early FEA studies of stent behaviour and performance. Notable among them was the two-dimensional study by Rogers et al. 40 who focussed on the need to minimise vascular injury during stenting. This work is particularly pertinent since it addressed vascular injury induced by balloon contact forces combined with stent strut lacerations with the aspiration to optimise long-term outcomes for patients. Whilst Rogers et al. 40 focussed on clinical effects, Migliavacca et al. 32 noted that FEA could be used in the optimisation of coronary stents by investigating the effects of different geometrical parameters on mechanical performance. Indeed, nearly all stent optimisation studies have employed geometry variation to define the optimisation design parameters including strut width, strut thickness, strut length, crown curvature, connector shape and a range of other shape variables set up to generate more complicated cell shapes. A detailed consideration of structural metrics as used in optimisation is provided in Supplementary Material A but the following key elements are noted here for certain metrics that: (i) should be checked globally along the stent and in the tissue but for which, numerically, single values are needed for optimisation; (ii) can be obtained numerically and/or experimentally (e.g., radial strength); (iii) have not been used in optimisation studies since they have been only recently defined (e.g., longitudinal stent deformation); (iv) have been under used (e.g., fatigue resistance) and (v) are difficult to quantify (e.g., tissue damage).
CFD and Transport: Flow and Drug Optimality
CFD based coronary artery stent optimisation has featured in six key studies.1,2,5,20,39,41 Similarly to FEA studies, these can be characterised by simulation detail. Atherton and Bates2 used a simplified model involving steady state 3D CFD for single stent units whilst Blouza et al. 5 and Srinivas et al. 41 applied steady state 2D CFD over displaced strut cross-sections. Gundert et al. 20 and Amirjani et al. 1 employed pulsatile and steady state 3D CFD, respectively, but both used idealised vessels and stents constructed in expanded configurations from a repeating cell unit. With further complexity, Pant et al. 39 performed pulsatile 3D CFD through representative diseased vessels deformed using FEA stent deployment simulations. Further, Atherton and Bates,2 Srinivas et al. 41 and Gundert et al. 20 only considered flow optimality whereas the others adopted a multi-disciplinary approach.
To capture the effect of flow on arterial walls, metrics are needed that can be minimised with respect to the flow disturbance caused by the presence of stent struts embedded in an irregular arterial wall boundary. This is based on the assumption that an optimal flow environment exists for a smooth vessel in the absence of a stenosis. Gundert et al. 20 extracted time averaged wall shear stresses that were averaged over the arterial surface exposed to flow in the central rings of the stents. Blouza et al. 5 and Srinivas et al. 41 considered multi-objective optimisation, respectively, for two metrics (steady state wall shear stress and swirl) and three metrics (vorticity, recirculation distance and reattachment lengths between struts). Atherton and Bates2 calculated power dissipation as a surrogate for wall shear stress.
Pant et al. 39 devised a haemodynamic low and reversed flow index (HLRFI), as a function of regions where wall shear stress was below a prescribed level or reversed relative to the main flow direction. HLRFI was minimised to reflect the fact that strut distribution can influence the extent of disturbed flow on the arterial wall.
Similarly to tissue damage, the efficacy of drug delivery can be defined by a volume averaged concentration, which needs to be maximised. Drug concentration can be calculated within the tissue by solving a CFD-based transport equation for drug concentration or through heat transfer equations in FEA solvers. However, optimisation of drug delivery has been considered in far more significant detail by Bozsak et al. 7 Solely focussing on the drug kinetics of sirolimus and paclitaxel, a single measure of performance was derived to combine drug efficacy in the media with an average toxicity metric across the lumen, sub-endothelial space and the media and penalised by a buffer term to avoid drug concentrations close to the toxicity limit. Notably, optimal paclitaxel-eluting stents were identified with far lower concentrations than existing DESs and designed to release the drug either very rapidly or very slowly (up to 12 months).
Multi-disciplinary Optimality
The procedural and long-term efficacy of PCI is known to be dependent on a wide range of factors related to structural performance, haemodynamics and the bio-chemistry of disease, inflammation, drug delivery and healing. Patient-specificity with respect to anatomy and disease is also important. Although no optimisation study to date has included more than six separate objectives, obtained from multiple disciplines, it is encouraging that a small number of studies have successfully demonstrated that it is possible to conduct high fidelity multi-disciplinary optimisation.
Pant et al. 39 and Amirjani et al. 1 conducted FEA and CFD simulations to generate a range of multi-disciplinary objectives. Amirjani et al. 1 combined stent and tissue stress metrics with stent recoil and a flow induced wall shear stress metric in a single aggregated objective function.
Pant et al. 38 used structural deployment and flexibility objectives with a drug elution metric in a constrained optimisation study in which optimal designs were found for each metric without diminishing any other metric. It was only in Pant et al. 39 that structural (stent recoil and tissue stress), flow and drug elution metrics were used in a fully multi-disciplinary, multi-objective framework.
Although CFD wasn’t included in the study by Grogan et al. 19 , multi-disciplinary optimisation was performed by coupling a corrosion algorithm to FEA of a stent system that was tested for radial collapse strength.
Optmization Framework: The Stent Design Challenge
Overview
Having discussed stent optimality from both clinical and mechanical engineering perspectives, different ways of framing stent optimisation studies is now considered. Whatever method is used, there are four key, common elements:
-
(a)
Design variables which are the inputs (often geometry parameters) to be varied;
-
(b)
The objective function comprising one or more quantified measures of performance that can be used to compare different designs;
-
(c)
Constraints defining regions of the design space that cannot be included—lower and upper bounds are needed for the design variables and it may be necessary to specify values of derived quantities that must satisfy prescribed equality or inequality constraints;
-
d)
An optimisation algorithm in which, simply stated, the optimiser needs to find a combination of design variables that are optimal with respect to the objective function subject to satisfying the specified constraints.
Generally, these separate elements should be considered simultaneously such that the design variables and the objective function(s) are defined appropriately for a given problem and for a particular optimisation algorithm. For example, if considering flexibility, design variables for the connectors should be included. With respect to the optimisation, whilst it might be possible to have many (>10) design variables when optimising a single strut using a direct search method such as a genetic algorithm, it is advisable to reduce the number of inputs when using computationally expensive full stent deployment simulations within a response surface modelling approach.
Design Variables
In the optimisation studies considered here, the largest number of design variables was seven in Grogan et al. 19 and Wu et al. 46 and most reported research has used three or four variables. Strut width is the most commonly included design variable and strut thickness (measured radially), strut length and parameters to control crown shape are also relatively common. More detailed control of stent unit shapes has been considered by Clune et al. 9 using a set of NURBS weights, by Grogan et al. 19 with various strut lengths and heights and by Wu et al. 46 with a variety of strut widths and arc radii. When flexibility has been of interest, design variables have been used for the connectors as in Pant et al. 38,39 In cases when haemodynamic optimality has been sought, Atherton and Bates2 and Gundert et al.,20 the angle of struts to the flow has been included. In contrast to the majority of studies that employ shape optimisation, Bozsak et al. 7 considered only drug kinetics design variables: the initial drug concentration and the drug release time.
Objective Functions (A Multi-objective, Multi-disciplinary Problem)
Whilst most optimisation studies have incorporated multiple objectives, some earlier articles considered a single objective function. Atherton and Bates used power dissipation as a surrogate for wall shear stress and Harewood et al. 21 focussed on radial stiffness of a single ring. More recently, Li et al. 28 sought to just focus on stent dog-boning. When considering multiple objectives, the majority of studies have either combined them in a single weighted objective function1,11,29,44 (Bozsak et al. 7) or have endeavoured to construct and search the Pareto fronts generated by treating each objective separately. One of the earliest attempts to do this by Blouza et al. 5 used the multi-objective evolutionary optimisation algorithm by Deb et al. 13 to analyse the trade-off between wall shear stress and swirl within a two-dimensional flow disturbance model of stent struts. Similarly, Srinivas et al. 41 sought to minimise vorticity and recirculation distances whilst maximising the reattachment length between struts.
More advanced incarnations of this approach, using the non-dominated sorting genetic algorithm, NSGA II by Deb and Agrawal,12 have been adopted by Pant et al. 39 for six objectives (obtained from multi-disciplinary structural, haemodynamic and drug elution simulations) and by Clune et al. 9 for the trade-off between fracture resistance and flexibility. Finally, multiple objectives have also been incorporated in slightly different ways by Wu et al. 46 and Pant et al. 38 In the former, the dual objectives of maximum principal strain and mass of material were treated in a two stage process of maximising mass once the maximum principal strain had been minimised. In contrast, Pant et al. 38 used constrained single objective optimisation to separately minimise one of four objectives in turn, constrained by the requirement for the other objectives not to deteriorate.
A key issue related to the treatment of multiple objectives concerns the trade-off between measures of performance that are in competition. When using a weighted single objective function the balance between objectives can be controlled by the values of the weights. This approach is exemplified by Timmins et al. 44 who assessed different weight combinations to generate stent designs optimised for critical tissue stress, luminal gain or cyclic radial deflection. Further, discussion of “lesion-specific stenting” alluded to the possibility of maximising minimum lumen area at the expense of high wall stress for stiff, calcific plaque by having lower distances between stent rings in contrast to the minimisation of wall stress for softer lipid type lesions by having wider strut spacing.
Various paradigms for stent selection were considered by Pant et al. 39 Figure 18 from that work is reproduced in Fig. 3, depicting the trade-off between recoil and volume averaged stress and how a design based on the Cypher® platform was predicted to be biased towards low recoil at the expense of potential tissue damage. A conservative approach to selection would seek designs closest to the so-called utopia point (located at the lowest values of the respective objectives). However, noting that six objectives were considered (and other important measures of performance were neglected) a more experiential paradigm would suitably bias selection to the specificity of a particular patient and lesion. Indeed, the rigid, closed cell design of the Cypher® platform is emblematic of the fact that minimal recoil and maximal radial strength were likely to have been the prominent considerations when it became the PCI work-horse in the first generation of drug-eluting stents.
(Fig. 18 in Pant et al. 39). Final Pareto front slice showing the trade-off between volume average stress (VAS) and acute recoil (Recoil). Reprinted with permission from Elsevier.
Constraints
All systematic optimisation studies require constraints on the design variables. These constraints are commonly referred to as bounds and act to define the design space of the problem. For example, when varying strut width, the lower and upper bounds define/constrain the range of variation of strut width during optimisation. Other constraints are typically imposed on a problem such that certain requirements aren’t violated. Most constraints used in coronary stent optimisation studies have been based on structural requirements. Harewood et al. 21 applied constraints on the mean magnitude of the principal tensile stresses during pressure loading and bending and the difference between them. In this way, radial stiffness was maximised without compromising fatigue resistance.
The application of constraints can be implied as well as in the two stage process by Wu et al. 46 De Beule et al. 11 sought to reduce foreshortening by 20% whilst maintaining radial stiffness relative to the reference geometry of a self-expandable braided stent.
Only four studies have been identified that applied constraints directly during optimisation. In addition to Pant et al.,38 (i) Wu et al. 47 combined a constraint on the drug holding capacity of a Conor stent (Conor Medsystems Inc.) with manufacturing constraints related to the extrusion of strut geometry and minimum member size control, to optimise strut stiffness; (ii) Azaouzi et al. 4 optimised fatigue resistance of a nitinol stent with constraints on the minimum radial force that it could support and on the maximum strain amplitude when exposed to a physiological pulse and (iii) Bozsak et al. 7 penalised the objective function by introducing a term to keep eluted drug concentrations away from a predefined toxicity level.
Optimization Methods
Due to the long computational times needed to simulate stent performance, the majority of coronary artery optimisation studies have adopted a surrogate modelling approach in which response surface models (RSMs) have been constructed to represent the relationship between objective functions and design variables. Simply stated in the current context, a RSM is a surface fit of one or more measures of performance against multiple design variables. Earlier RSM optimisations21,29,46 used polynomial based least squares functions but more recent studies have adopted Gaussian Process Models, commonly referred to as Kriging25 after the South African geo-statistician, Krige.26 Before describing Kriging in more detail below, optimisation using RSMs is described in general, with reference to Fig. 4.
At the start of a study, it is necessary to setup a baseline model (1), the definition of the problem (2) and the simulations that are to be performed (dashed box). Then, an initial RSM is constructed (3) from a sample of design points defined by a design of experiments (DoE). The DoE may be generated randomly but a number of methods have been developed with better space filling properties, e.g., optimised Latin hypercubes17,35 and \(LP_{\tau }\).42 For each point, simulations are performed to evaluate measures of performance (4). The construction of the RSM (5) involves the derivation of a function from the values of the objective function obtained for a set of design variables (defined by the DoE for the initial sample). In a multi-objective problem, separate RSMs are constructed for each objective and, similarly, in a constrained optimisation, separate RSMs can be constructed for each constraint. Importantly, RSMs only provide a prediction of the complete response of the system and, since the goal of the optimisation method is to find optimal designs, it is likely to be necessary to improve the accuracy of the RSM before determining an optimum. RSMs are improved (or updated) by generating new design point data (or updates) at appropriate locations in the design space (6). Updates are generated by searching the current RSM and running further simulations at appropriately selected design points to obtain the value(s) of objective(s) at these new points (7). This process can be repeated until a convergence criterion has been satisfied (8) or a computational budget exhausted. The accuracy/quality of the RSM can be evaluated/validated using cross-validation methods that sequentially compare predictions of at least one data point from RSMs constructed from the data-set with this (these) point(s) excluded. The use of leave one out and standard cross validation residual plots was demonstrated in Pant et al. 38 An alternative, brute force approach can be applied, if affordable, by running additional simulations to generate new validation data. This was done by Harewood et al.21 in which a RSM constructed from a sixty point DoE was validated (and enhanced) by a separate twenty point DoE.
Kriging
There are a number of advantageous features of Kriging that make it particularly suitable for surrogate modelling and optimisation of engineering problems. Given a set of inputs and experimentally obtained outputs, the Kriging predictor:
-
(a)
Comprises a linear combination of tuneable basis functions;
-
(b)
Interpolates the data;
-
(c)
Has a statistical interpretation from which the mean squared error (MSE) of the predictor can be formulated and
-
(d)
Yields additional functions, including the expected improvement (EI), which can be used to enhance the search for optimal designs.
Both the MSE and the EI are particularly useful for defining update points when it is necessary to improve the accuracy of the predictor.
Derivation of the Kriging equations can be found elsewhere25 but the predictor is described in Supplementary material B.
Srinivas et al. 41 performed possibly the first Kriging based optimisation of coronary stents using a simplified 2D, steady-state flow model. With a three-dimensional Latin hypercube DoE for strut width, thickness and spacing, Krigs were constructed for three metrics from which non-dominated optimal designs were found. Evidence for the subsequent use of Kriging for the optimisation of coronary stents is sparse until Pant et al. 39 constructed separate Krigs for six objective which were used in an NSGA II search of the design space. A sequence of three parallel updates was performed in which five designs were selected from the non-dominated Pareto front for each set of updates. New Krigs were constructed following the generation of data for each update. Starting from a 15 point \(LP_{\tau }\) DoE, the three updates produced a total sample size of thirty points.
Gundert et al. 20 determined haemodynamically optimal stent geometries using the MATLAB DACEFootnote 2 implementation of Kriging30 within a pattern search algorithm based on the Surrogate Management Framework described by Booker et al. 6 A single design parameter (the intra-strut angle) was optimised for a single objective (the area of low time averaged wall shear stress) for a range of intra-strut areas and numbers of circumferential units. Starting with a Latin hypercube DoE, most runs converged within 10–15 function evaluations and the optimal intra-strut angle was found to be independent of both vessel size and the intra-strut area of the stent cell.
Update points in Gundert et al. 20 were identified from the predicted optima following a search of the RSM. The equivalent to this in the multi-objective problem is to select non-dominated points on the Pareto front as demonstrated by Pant et al. 39 However, as noted above, Kriging usefully provides alternative means for generating update points. Since the EI function blends exploration and exploitation, used repeatedly, it simultaneously improves the accuracy of the RSM throughout the design space and enhances the search for optimum designs. Grogan et al. 19 and Li et al. 28 used EI updates in their single objective optimisations for maximum radial strength and minimum dog-boning, respectively.
Grogan et al. 19 performed an impressive number of simulations, running five separate optimisations, each starting from a different 28 point Latin hypercube DoE followed by 122 EI updates. It isn’t clear why the separate optimisations were performed or whether the problem warranted so many updates. Multiple runs are often performed when assessing the mean and variance of an optimisation strategy but that wasn’t the case in Grogan et al. 19 Experience suggests that approximately 70 simulations would have been sufficient (i.e., ten times the number of design parameters) even though there was greater than 6% variation in the optimum designs found from the five optimisations. It’s possible that mesh related issues compromised convergence and it may have been advisable to force the Krig to regress the potentially noisy data. The DoE size of 28 points was well judged for seven design variables but it should be possible to run smaller numbers of updates.
More modest numbers of EI updates were used by Li et al. 28 for four slotted tube design parameters in four deployment simulation scenarios, the maximum number of updates being 22. Despite using a simplified stent model, shape optimisation using Kriging successfully led to designs with reduced dog-boning.
Similarly to Gundert et al.,20 Bozsak et al. 7 used Kriging in a surrogate modelling framework but, during the search steps, update points were identified by maximising the probability of improving a current optimum by a prescribed margin.
In contrast to the aforementioned approaches to RSM updating, two other studies, both with a focus on shape optimisation of a single crown unit for the maximisation of fatigue resistance, have avoided using updates. Azaouzi et al. 4 adopted a trust-region strategy in which successive RSMs were constructed for increasingly smaller design space samples centred on optimal locations found from each search. Starting from a very large volume design of a Nitinol strut, five iterations were needed to reduce strut volume by 78% whilst satisfying constraints on the minimum outward force of the complete structure and the maximum value of the strain amplitude for all elements. As one of the few examples of RSM-based coronary artery stent optimisation studies to directly apply constraints, it is useful to note that separate Krigs were constructed for each constraint.
Updates can also be completely avoided by committing to an exhaustive number of points as undertaken by Clune et al. 9 in a randomly generated Latin hypercube DoE for six geometry design variables. A Pareto front was successfully generated to represent the trade-off between fatigue resistance and flexibility. Using the MATLAB implementation of NSGA II, a range of designs was depicted along the front. Although very high accuracy was demonstrated for the respective RSMs using cross-validation, it would be interesting to determine the minimum number of designs that would actually be needed to achieve a similar level of predictive accuracy.
From this review of the literature, it would appear that, despite the increasing use of Kriging in coronary artery stent design, Krig tuning is hidden from and/or overlooked by many users. Also, there is limited evidence for the efficient use of updating strategies.
Future Challenges and Opportunities
The emergence over the last 10 years of systematic numerical optimisation of coronary artery stent design has been catalysed by advances in:
-
(a)
Surrogate modelling using response surface models, particularly Kriging;
-
(b)
Numerical modelling of structural performance using FEA and
-
(c)
Computing power and resources.
Taken together, these three elements have made it possible to perform multiple, detailed (and computationally expensive) simulations of stent behaviour as described by Pant et al.,38 Grogan et al. 19 and Bozsak et al. 7 However, the majority of other reported studies have introduced significant simplifications into the numerical models, often involving the simulation of single crown units, that don’t necessarily require high performance computing resources. Therefore, although it might be technically feasible to design bespoke, patient-specific coronary stents using detailed 3D simulations, the required computational run-times are likely to render such an approach unusable in the catheter-laboratory for the foreseeable future. Further, even if simplified models that can be solved quickly could be used in this way, regulatory approval is likely to act as a significant barrier. What remains to be seen is how detailed and simplified approaches to stent optimisation could be used to address the low percentage of PCI cases that have sub-optimal outcomes. Potentially, novel stent characterisations could be developed that are optimised for sub-sets of challenging patient cases. Another area to explore concerns optimisation of the delivery system wherein, for example, balloon unpressurised diameter and inflation pressure could be optimised to balance strut malapposition against tissue damage. Other biological endpoints could also be targeted through pre-clinical trials, for example, aiming to minimise inflammation and/or restenosis. One of the biggest challenges in these areas concerns the need and value of validating computational predictions with in vitro experiments, pre-clinical and clinical findings and, ultimately, with clinical practice. Finally, since Kriging appears to be becoming a favoured optimisation technology, the knowledge gained as applied to coronary artery stents should be applicable to the design of bifurcation stents and bifurcation stenting protocols, heart valve frames, peripheral stents and other biomedical devices.
Conclusions
Common to the design optimisation of coronary artery stent systems considered here are the facts that:
-
(a)
The great majority of design variables have been geometric;
-
(b)
Only a subset of performance measures have been considered in each case;
-
(c)
Host vessel geometry has been, at best, idealised and often neglected completely;
-
(d)
Surrogate modelling using Kriging has become the dominant optimisation framework.
It is also clear that the growth in optimisation studies, often using Kriging, is a relatively recent phenomenon. Consequently, despite a range of weaknesses and limitations, the work to date has revealed a large array of opportunities for further systematic optimisation of coronary artery stenting, including enhanced accuracy of computational modelling, more efficient surrogate modelling, patient-specific device optimisation and the challenges of solving a complex, multi-disciplinary, multi-objective problem. Using these methods it will be possible to design new iterations of stents and/or novel stent/delivery system characteristics. Ultimately, the aim of computational modelling applied in these ways is to facilitate clinical optimality for more patients in all interventional procedures.
Notes
ACCF/AHA/SCAI: American College of Cardiology Foundation/American Heart Association/Society for Cardiovascular Angiography and Interventions.
DACE: Design and analysis of computer experiments.
References
Amirjani, A., M. Yousefi, and M. Cheshmaroo. Parametrical optimization of stent design; a numerical-based approach. Comput. Mat. Sci. 90:210–220, 2014.
Atherton, M. A., and R. A. Bates. Robust optimization of cardiovascular stents: a comparison of methods. Eng. Optimiz. 36(2):207–217, 2004.
Auricchio, F., M. Di Loreto, and E. Sacco. Finite element analysis of a stenotic artery revascularisation through a stent insertion. Comput. Methods Biomech. Biomed. Eng. 4:249–263, 2001.
Azaouzi, M., N. Lebaal, A. Makradi, and S. Belouettar. Optimization based simulation of self-expanding Nitinol stent. Mater. Des. 50:917–928, 2013.
Blouza, A., L. Dumas and I. M’Baye. Multiobjective optimization of a stent in a fluid-structure context. Proceedings of the 2008 GECCO Conference Companion on Genetic and Evolutionary Computation, pp. 2055–2060, 2008.
Booker, A. J., J. E. Dennis, P. D. Frank, D. B. Serafini, and V. Torczon. A rigorous framework for optimization of expensive functions by surrogates. Struct. Optim. 17:1–13, 1999.
Bozsak, F., D. Gonzalez-Rodriguez, Z. Sternberger, P. Belitz, T. Bewley, J-M. Chomaz, and A. I. Barakat. Optimization of drug delivery by drug-eluting stents. PLoS One 10(6):e0130182, 2015. doi:10.1371/journal.pone.0130182.
Caixeta, A., P. Genereux, G. Dangas, and R. Mehran. In-stent restenosis in the drug-eluting stent era. In: Chapter 28 in Oxford Textbook of Interventional Cardiology, edited by S. Redwood, N. Curzen, and M. Thomas. Oxford: Oxford University Press, 2010.
Clune, R., D. Kelliher, J. C. Robinson, and J. S. Campbell. NURBS modeling and structural shape optimization of cardiovascular stents. Struct. Multidisc. Optim. 50:159–168, 2014.
Conway, C., F. Sharif, J. McGarry, and P. McHugh. A computational test-bed to assess coronary stent implantation mechanics using a population-specific approach. Cardiovasc. Eng. Technol. 3(4):374–387, 2012.
De Beule, M., S. Van Cauter, P. Mortier, D. Van Loo, R. Van Impe, and P. Verdonck. Virtual optimization of self-expandable braided wire stents. Med. Eng. Phys. 31:448–453, 2009.
Deb, K., and R. B. Agrawal. A fast and elitist multi-objective genetic algorithm: NSGA-II. IEEE Trans. Evol. Comput. 6(2):182–197, 2002.
Deb, K., M. Mohan and S. Mishra. A fast multiobjective evolutionary algorithm for finding well-spread pareto-optimal solutions. KanGAL Report Number 2003002, 2003.
Dumoulin, C., and B. Cochelin. Mechanical behaviour modelling of balloon-expandable stents. J. Biomech. 33:1461–1470, 2000.
Etave, F., G. Finet, M. Boivin, J. Boyer, G. Rioufol, and G. Thollet. Mechanical properties of coronary stents determined by using finite element analysis. J. Biomech. 34:1065–1075, 2001.
FDA. Non-clinical engineering tests and recommended labeling for intravascular stents and associated delivery systems. http://www.fda.gov/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm071863.htm. 2010. Accessed 1st April 2015.
Forrester, A. I. J., A. Sóbester, and A. J. Keane. Engineering Design via Surrogate Modelling: A Practical Guide. Chichester: Wiley, 2008.
Gijsen, F., F. Migliavacca, S. Schievano, L. Socci, L. Petrini, A. Thury, J. Wentzel, A. van der Steen, P. Serruys, and G. Dubini. Simulation of stent deployment in a realistic human coronary artery. BioMed. Eng. OnLine 7:23, 2008.
Grogan, J. A., S. B. Leen, and P. E. McHugh. Optimizing the design of a bioabsorbable metal stent using computer simulation methods. Biomaterials 34(33):8049–8060, 2013.
Gundert, T. J., A. L. Marsden, W. Yang, and J. F. LaDisa, Jr. Optimization of cardiovascular stent design using computational fluid dynamics. J. Biomech. Eng. 134:011002-1–011002-8, 2012.
Harewood, F., R. Thornton and P. Sharp. Step change in design: exploring sixty stent design variations overnight. www.altairproductdesign.com, 2011.
Harold, J. G., T. A. Bass, T. M. Bashore, et al. ACCF/AHA/SCAI, Update of the clinical competence statement on coronary artery interventional procedures. J. Am. Coll. Cardiol. 128:436–472, 2013.
He, Y., N. Duraiswamy, A. O. Frank, and J. E. Moore, Jr. Blood flow in stented arteries: a parametric comparison of strut design patterns in three dimensions. ASME J. Biomech. Eng. 127:637–647, 2005.
Johnson, P. M., J. Patel, M. Yeung, and P. Kaul. Intra-coronary imaging modalities. Curr. Treat. Options Cardiovasc. Med. 16:304, 2014.
Jones, D. R. A taxonomy of global optimization methods based on response surfaces. J. Glob. Optim. 21(4):345–383, 2001.
Krige, D. G. A statistical approach to some basic mine valuation problems on the Wit-watersrand. J. Chem. Metall. Miner. Soc. SA 52(6):119–139, 1951.
Levine, G. N., E. R. Bates, J. C. Blankenship, et al. ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J. Am. Coll. Cardiol. 58:e44–e122, 2011.
Li, H., T. Qiu, B. Zhu, J. Wu, and X. Wang. Design optimization of coronary stent based on finite element models. Sci. World J. 2013:630243, 2013.
Li, N., H. Zhang, and H. Ouyang. Shape optimization of coronary artery stent based on a parametric model. Finite Elem. Anal. Des. 45:468–475, 2009.
Lophaven, S., H. Nielsen and J. Søndergaard. DACE—A MATLAB Kriging Toolbox Version 2.0, Technical University of Denmark, Copenhagen, Technical Report IMM-TR-2002-12, 2002.
Martin, D., and F. J. Boyle. Computational structural modelling of coronary stent deployment: a review. Comput. Methods Biomech. Biomed. Eng. 14(4):331–348, 2011.
Migliavacca, F., L. Petrini, M. Colombo, F. Auricchio, and R. Pietrabissa. Mechanical behavior of coronary stents investigated through the finite element method. J. Biomech. 35:803–811, 2002.
Morice, M.-C., P. Serruys, J. Sousa, J. Fajadet, E. Ban Hayashi, M. Perin, A. Colombo, G. Schuler, P. Barragan, G. Guagliumi, et al. The RAVEL Study Group. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 346:1773–1780, 2002.
Morlacchi, S., and F. Migliavacca. Modeling stented coronary arteries: where we are, where to go? Ann. Biomed. Eng. 41(7):1428–1444, 2013.
Morris, M. D., and T. J. Mitchell. Exploratory designs for computational experiments. J. Stat. Plan. Inference 43:381–402, 1995.
Moses, J., M. Leon, J. Popma, P. Fitzgerald, D. Holmes, C. O’Shaughnessy, R. Caputo, D. Kereiakes, D. Williams, P. Teirstein, et al. The SIRIUS Investigators. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349:1315–1323, 2003.
Murphy, J., and F. J. Boyle. Predicting neointimal hyperplasia in stented arteries using time-dependant computational fluid dynamics: a review. Comput. Biol. Med. 40:408–418, 2010.
Pant, S., N. W. Bressloff, and G. Limbert. Geometry parameterization and multidisciplinary constrained optimisation of coronary stents. Biomech. Model Mechanobiol. 11(1):61–82, 2012.
Pant, S., G. Limbert, N. Curzen, and N. W. Bressloff. Multi-objective design optimisation of coronary stents. Biomaterials 32:7755–7773, 2011.
Rogers, C., D. Y. Tseng, J. C. Squere, and E. R. Edelman. Balloon-artery interactions during stent placement. A finite element analysis approach to pressure, compliance, and stent design as contributors to vascular injury. Circ. Res. 84:378–383, 1999.
Srinivas, K., T. Nakayama, M. Ohta, S. Obayashi, and T. Yamaguchi. Studies on design optimization of coronary stents. ASME J. Med. Devices 2(1):011004, 2008.
Statnikov, R., and J. Matusov. Multicriteria Analysis in Engineering: Using the PSI Method with MOVI 1.0. Dordrecht: Kluwer Academic Publications, 2002.
Stoeckel, D., C. Bonsignore, and S. Duda. A survey of stent designs. Minim. Invasive Ther. Allied Technol. 11(4):137–147, 2002.
Timmins, L. H., M. R. Moreno, C. A. Meyer, J. C. Criscione, A. Rachev, and J. E. Moore, Jr. Stented artery biomechanics and device design optimization. Med. Biol. Eng. Comput. 45(5):505–513, 2007.
Wang, W.-Q., D.-K. Liang, D.-Z. Liang, and M. Qi. Analysis of the transient expansion behavior and design optimization of coronary stents by finite element method. J. Biomech. 29:21–32, 2006.
Wu, W., L. Petrini, D. Gastaldi, T. Villa, M. Vedani, E. Lesma, B. Previtali, and F. Migliavacca. Finite element shape optimization for biodegradable magnesium alloy stents. Ann. Biomed. Eng. 38(9):2829–2840, 2010.
Wu, W., D. Z. Yang, Y. Y. Huang, M. Qi, and W. Q. Wang. Topology optimization of a novel stent platform with drug reservoirs. Med. Eng. Phys. 30(9):1177–1185, 2008.
Yoon, H.-J., and S.-H. Hur. Optimization of stent deployment by intravascular ultrasound. Korean J. Int. Med. 27:30–38, 2012.
Acknowledgments
The authors would like to thank Medtronic Inc. (Minnesota, USA) for their unrestricted support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Peter E. McHugh oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bressloff, N.W., Ragkousis, G. & Curzen, N. Design Optimisation of Coronary Artery Stent Systems. Ann Biomed Eng 44, 357–367 (2016). https://doi.org/10.1007/s10439-015-1373-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1373-9