Abstract
Implants, including artificial joints, bone fixation devices, and other orthopedic implants, oral implants, and vascular interventional devices, are used to repair or replace human tissues or organs and restore their functions. Since biodegradable implants have advantage of avoiding long-term complications (including bone stress shielding, restenosis, thrombosis, and secondary surgery) while remaining safe and productive, personalized biodegradable implants will be an irresistible trend in the clinic for implantable and interventional medical devices. However, innovation of personalized biodegradable implants faces several challenges, including the interaction between the implant and its surrounding tissues or cells, the coordination of structural strength of implants and its degradation, the topological microstructure of implant and its fatigue properties, reliability, and safety. In this review, we introduced critical progresses achieved in the fields related to implants, including mechanical properties of materials, interaction between implants and host tissue, effect of stress on degradation; furthermore, we highlighted the optimized design and manufacture of implants as well as the evaluation of their reliability.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Implants are used to repair or replace human tissues or organs so that these tissues or organs can restore their functions. Implants include artificial joints, bone fixation devices, and other orthopedic implants, oral implants, and vascular interventions such as vascular stents, artificial heart valves, and vascular grafts. An estimanted 200 million individuals worldwide have osteoporosis, and an osteoporotic fracture occurs every 3 seconds on an average [1]. More than 500 thousand patients need joint replacement surgery each year. The number of patients with cardiovascular diseases is as high as 290 million, and approximately 5 million vascular stents are needed.
Meanwhile, there is also a stable demand for artificial heart valves. With the increase of aging population in China, the continuous improvement of people’s health needs, and the rapid development of domestic medical standards, the market demand for orthopedic, oral, cardiovascular, and other medical implants is also increasing. This will develop a large market of implants in the world. The mechanical property of implants, such as elastic modulus, strength, fatigue, and so forth, is as essential as its components or materials, design, and fabrication. There are some critical unresolved scientific and technical difficulties in designing, manufacturing, and evaluating implants and their clinical applications.
Metal and bio-ceramics are mainly used in orthopedic implants for fixation or defect repair. However, there are often some drawbacks after implantation. For instance, the absorption of the bone tissue around the implants induced by stress shielding typically results in a long-term nonunion of bone trauma, septic loosening, instability, or fixation failure [2,3,4]. Two to twenty-one percent of patients have required a second surgery [5], and 63% of osteoporotic patients experience loosening when pedicle screws are implanted [6]. To improve the implant’s stability after being implanted, previous studies adopted an optimization design (i.e., increasing the friction and contact area between implants and surrounding bone tissue) to improve the stability of implants [7, 8].
Tissue remodeling is frequently present with cell phenotype, morphology, structure, and function changes. Therefore, only structural design cannot balance mechanical strength, stability, stress shielding, and tissue regenerations. Biodegradable implants aroused more attention in the clinic owing to their advantages of avoiding stress shielding, long-term restenosis and thrombosis, and secondary surgeries. Several studies focused on materials composition and crystallinity, molecular weight, pore size or porosity, permeability, pH value, and ion concentration [9, 10]. The control of degradation behavior is a benefit in maintaining enough strength before the healed injury tissue. The degradation process affects mechanical strength and structural parameters as well as influences biological responses of surrounding tissue cells based on in vitro and in vivo experiments [11, 12]. After surgery, the effectiveness of implants highly depended on the mechanical environment, including stretching, compression, shearing, bending, torsion, and compound stress [13]. For polylactic acid (PLA)-glycolic acid polymer, as with biodegradable materials, molecular weight, mass loss, and mechanical strength will be decreased under dynamic compression [14]. The effect of stress on stretch, compression, dynamic compression, dynamic stretch, or combined load significantly affected degradation behavior [15,16,17,18]. Moreover, different degradation behavior occurred in in vitro and in vivo experiments [19]. Therefore, a good structure design can improve the mechanical strength and interaction of implants and host tissues and promote uniform degradation performance and tissue regeneration.
In addition, a personalized implant is fashionable since it meets the specific needs of different patients, especially with severe deformities, although it has a long design and manufacture time as well as high cost. Three-dimensional (3D) printing, also known as additive manufacturing, has provided a good solution for the personalization of implants, which is becoming increasingly popular in the clinic. 3D printing achieved fast, safe, and customized control of complex microstructure, which provides the possibility of applying personalized implant devices. For personalized biodegradable implants, it is essential to control structural strength, its synergy with degradation, and the interaction of the implant and surrounding tissues. The micro-topological structure design is also a factor that is very important on fatigue characteristics, reliability, and safety of the implants as well as on cell organization, which can also change the stress distribution of the surrounding tissue. The cells will subsequently have different biological responses under various stresses, which will lead to tissue remodeling. Then, all of these changes will affect the mechanical strength and reliability of the implant. The mechanical strength of degradable implants or interventional devices can not be well maintained in the complex mechanical environment of the human body, which will result in insufficient strength even to premature collapse and failure, thereby restricting the clinical application of degradable implants. Thus, the influence of degradation behavior under stress should not be neglected.
To date, biomechanical and mechanobiological mechanisms of personalized biodegradable implants’ degradation rules and their interactions with tissues and cells have become critical scientific issues. It is difficult to follow its changes after being implanted in vivo. Experiments and numerical simulations can quantitatively evaluate mechanical strength and reliability or biological response in vitro. Existing theoretical models are difficult to guide the design of implant simulation experimental systems since the human system is very complex and changeable. The porous structure design will reduce the stiffness of the implant and alleviate the stress shielding effect, which mainly improve the osseointegration ability of implants [20, 21]. The porous structure could increase bone growth, prolong the implant’s life, and avoid loosening [22, 23]. Previous studies achieved stress shielding and improve in vivo stability using porous structure optimization design [24,25,26,27]. However, the porous structure resulted in less mechanical strength, thereby increasing the risk of fatigue failure [28]. Passable shape, density, pore size, and others are factors that affect the fatigue of implants [29,30,31]. Different structural parameters are critical for the implant’s performance and its stability and reliability after being implanted [32]. It is crucial for an in-depth understanding of how porous structure parameters affect the interaction of implants and surrounding tissue after implantation. Therefore, it is necessary to establish optimization design and manufacture methods, in vitro quantitative simulation system with high fidelity, evaluation system of degradation behavior and fatigue, and mechanobiology in vitro for biodegradable implants, which will be helpful and act as a key platform for the innovation of various implants.
Therefore, the following critical questions must be answered.
-
1.
What mechanical properties of materials can be used for implants?
-
2.
What is the interaction relationship between host tissue and implants?
-
3.
How does stress affects the degradable behavior of implants, and how does degradation behavior affect the host tissue?
-
4.
What can guide people to perform the optimization design and manufacture of implants to adapt to varied mechanical environments?
-
5.
How can we evaluate for reliability and safety in vitro before implantation?
2 Mechanical property of materials used for implants
Metals, bioceramics, and polymers are the most commonly used materials in orthopedic implants for fixation or repair. Metal fixation implants typically cause surrounding bone tissue absorption due to stress shielding, leading to a prolonged repair process, fixation failure of the implants, or removal difficulty [2,3,4]. Tantalum is a promising biosubstitution material due to its excellent inertness, high corrosion resistance, and exceptional biocompatibility. Porous tantalum medical devices have a higher success rate [33]. Compared with porous titanium, porous tantalum can benefit from the growth of the bone marrow mesenchymal stem cells, with stronger cell adhesion and proliferation in vitro [34]. Porous titanium and porous tantalum have the same exceptional biocompatibility and bone osseointegration capability; however, the elastic modulus of porous tantalum is closer to the human bone and has better biomechanical adaptability [35]. It is generally made of pure titanium metal and has a higher elastic modulus than the bone, resulting in the phenomenon of “stress barrier” of the alveolar bone and inflammation around the implant, and eventually leading to the failure of implant surgery. The porous structure, root-analog implant, tantalum metal implant can reduce the elastic modulus of the implant and weaken the “stress barrier” phenomenon. The titanium implant with a porous structure can effectively reduce the elastic modulus of the titanium metal. As the pore size increases, the elastic modulus of porous titanium decreases, ranging from 1.7 to 3.7 GPa, which is significantly lower than that of dense titanium alloys [36]. As the pore size increases, the friction coefficient between the porous metal and the surrounding bone tissue significantly increased. Decreasing the fretting of the implant-bone tissue interface will improve the initial stability of the implant and promote the growth of new bone tissue [37]. The interconnected porous structure is conducive to cell migration and proliferation, providing reliable long-term stability for the surrounding bone tissue [38]. The porous implant can achieve favorable osseointegration and osteogenesis in the absence of mechanical stimulation during the early healing period. However, the surrounding bone tissue absorption of the implant often causes aseptic loosening, instability, fractures around the implant, and prolapse of the bone screw. Various structural designs improve the stability of the implant by increasing friction or contact area between the implant and the bone tissue in previous studies [7, 8]. However, the above-mentioned structural designs still have not reached perfect balances between mechanical strength, stability, and stress shielding, which are limited by the individuality of human anatomy. Although vascular interventional technology quickly developed, it is far from achieving an ideal therapeutic effect.
Indeed, biomaterials commonly used in the implant included natural, artificial, and hybrid materials. Natural biomaterials are produced based on natural resources such as decellularized extracellular matrix, collagen, fibrin, starch, chitosan, hyaluronic acid derivatives, and fibrin gels. Synthetic polymers, such as polycaprolactone (PCL) and PLA, and alloys are common artificial biomaterials to be applied for the manufacture of biodegradable implants [39]. Hybrid scaffolds are produced through the combination of natural and synthetic materials. Since the 1970s, biodegradable bone implants and vascular stents have been invented and gradually used in orthopedics, cardiovascular, and other clinical treatments [40, 41]. Some biodegradable materials are currently used in clinical practice, including magnesium alloys and PLA-based degradable polymers, such as PLA, polyglycolic acid, and PCL, and so on [42, 43]. So far, studies on biodegradable materials mostly focuses on the composition, molecular weight, and distribution, pore size and porosity, permeability and crystallinity of the material, as well as chemical and biological factors such as the composition of the degradation medium, pH value, ion concentration, temperature, enzymes, etc. [9, 10]. Previous studies carried out much work from the perspective of biomaterial to study the biocompatibility and tissue induction of degradable materials, the influence of degradable material morphology (pores, porosity, etc.), material surface mode on tissue cells, the scale of the material such as the biological effect at the nanoscale, the influence of physical and chemical properties of the material (such as the hardness of the material) on the cell tissue, etc. [44, 45].
3 Interaction mechanism of implants and host tissue
Implants in vivo face a complex interaction between themselves and surrounding host tissue. Under the condition of complicated stress, cells have different biological responses, which lead to the other tissue remodeling process, thereby affecting the mechanical strength and reliability of the implant. In general, cells and tissues are sensitive to stress. Although the relationship between mechanical stimulation and tissue growth has long been proposed, the “stress-growth” relationship has not been thoroughly and quantitatively understood, especially tissue remodeling after implantation. Stent expansion and the subsequent vascular remodeling will affect blood flow, leading to stress on the vascular wall [46, 47], which becomes the initiating factor of internal restenosis [48]. For oral implants, orthodontic mini-screws, maxillofacial implants, and titanium mesh are the standard oral, implantable medical devices. A personalized, root-analog implant can simulate the morphology of natural tooth roots and is consistent with the shape of the extraction socket. The initial stability of personalized root implants is relatively high after implantation. It is unnecessary to prepare implant sockets for the root-analog implant, and the damage to the surrounding bone or soft tissue is minimized in implanted surgery. The bone tissue around the personalized porous root-analog implant is more evenly stressed, and the stress concentration is reduced [49]. Therefore, the root-analog implant is more conducive to bone tissue growth and stress, thereby reducing the “stress barrier” phenomenon. In addition, immediate loading reduced the waiting time from implantation to loading. The biomechanical response of the bone around the implant after immediate implantation adapts to optimize the mode of loading. Progressive loading mode is suitable for direct implantation [50]. The effect of quick static loading on bone osteogenesis around the 3D printed porous implants is also to be evaluated [51]. The quantity, quality, and mechanical properties of the new bone would increase healing time.
Immediate static loading increases new bone volume within 2 weeks after implantation, although with pool quality. Then adverse effects gradually appear with the prolongation of time. A delayed progressive loading scheme is adopted and recommended. Tilted maxillary implants are more likely to increase bone density in the anterior maxillary area; however, the tilt lip of the implant endangers its stability [52]. The microstructure evolution of the implant system is predicted [53]. The success rate of screw implantation exceeds 80% [54].
The failure of micro-screw implantation requires additional surgical intervention and prolonged treatment time. The effects of different bone-implant interfaces on micro-bone and surrounding tissues are explored [55]. When bone fusion is approximately 15%, orthodontic loads are applied to the micro-screws without losing stability. Excessive orthodontic loading leads to root resorption; therefore, it is necessary to avoid excessive loading. Excessive orthodontic loading used for the crown induced microcracks on the root surface; microcracks are one of the critical factors that cause root resorption in orthodontics treatment [56, 57].
4 Effect of stress on implants’ degradation
For biodegradable implants, the local mechanical environment around the implants is an important factor for their degradation [15, 17, 18, 58, 59]. In the case of a bone repair under heavy loading, perhaps the degradation of implants is fast, it will be difficult to maintain enough strength before healing, or a cavity will form inside the bone tissue, which will eventually lead to repair failure. Conversely, it will hinder the formation of bone tissue or induce a stress shielding effect when the degradation process is slow, which will also harm tissue repair. In vitro and in vivo results show that the scaffolds degradation process affects mechanical and structural properties and biological responses of surrounding tissues and cells [11, 12]. Biodegradable implants face a complex biomechanical environment in the human body in vivo. The effectiveness and reliability of implants highly depended on the local mechanical environment after implantation [13]. Under dynamic compression, the decreasing trend of molecular weight, mass, and mechanical strength of poly (L-lactide-co-glycolide) scaffold is more obvious than under static compression [14]. The degradation rate of AZ31 magnesium alloy in a flowing Hank’s solution is approximately 3-8 times faster than that of a static solution [60]. Different stress (tensile, compressive, dynamic compressive, combined stress, or fluid shear stress) on the degradation behavior significantly affects the degradation behavior of the implants [15, 17,18,19, 61, 62] (Fig. 1). A new corrosion model that considers the influence of multi-dimensional corrosion was developed for magnesium (Mg) in our previous study [63]. In addition, the sensitivities to stress and strain for Mg-based implants’ degradation are different in vitro and in vivo [19, 64]. Based on previous studies, we also established an evaluation method combining experiment and simulation of mechanical parameters of implant intervention devices, and applied it to the design and evaluation of degradable implant intervention devices (Fig. 2).
5 Optimized design and manufacture of implants
The design (i.e., morphological, porous microstructure) and manufacture of implants are fundamental, which would affect the reliability after implantation. The micropore and surface microstructure design of personalized 3D printing implants plays an essential role in the success or failure of the implant [65, 66]. A 3D-layered scaffold of Ca-Si with a controllable micropore size was developed using 3D printing [67]. This structure enhances the expression of alkaline phosphatase, osteogenic genes, and angiogenic gene markers. Our previous studies also explored the biological mass transfer and tissue regeneration performance of porous implants [68]. The pore size, shape, interconnection, and surface area parameters of the biodegradable scaffold were analyzed based on multi-scale modeling [40, 69,70,71,72]. The tissue regeneration function of different microstructure scaffolds was also investigated [73].
5.1 Morphological design of implants
Morphological parameters are closely related to the biomechanical properties of implants. Morphological design variables of interbody fusion cage significantly affects stability in the process of intervertebral osseointegration. The design variables are identified by sensitivity analysis based on orthogonal tests, such as the density of spike row distributing on the cage’s surface, spike oblique, spike height, and spike diameter, which are the basis of cage optimization [74,75,76,77,78,79,80,81]. In the cardiovascular system, the geometric configuration of vascular stents is also closely related to its performance. The stent strut’s geometry (e.g., angle, thickness) is associated with adverse clinical outcomes, including stent thrombosis, in-stent restenosis, and inflammation [82]. Moreover, the geometry of the stent is related to endothelialization. Compared with stents with smaller struts, larger strut sizes are not beneficial to vascular healing and therapeutic outcomes [83]. The thicker the support, the more challenging it is to adapt to the uneven and elastic human blood vessels, leading to adverse reactions [84]. Connection modes of struts play roles in longitudinal anti-compression of stents. Longitudinal compression stress significantly increases with the increasing numbers of struts connection, since the increase in the number of connecting struts improves the stiffness of the support and devides external loading [85, 86]. Thus, it is necessary to achieve a better understanding of the biomechanical performance of implants after implantation.
5.2 Microporous design of implants
Since conventional implants used for tissue repair have some drawbacks including insufficient space for tissue growth, limited mass transfer, and the mismatch of biomechanical properties with surrounding tissues, which can be overcome by using porous structure design, the porosity effectively improved tissue regeneration [20], reduce the stiffness of the implant, and alleviate the stress shielding effect [21]. Meanwhile, porous structure promoted surrounding tissue regeneration and avoided implant loosening [22, 23]. The mechanical properties of the absorbent structure were easily modified by varied porous parameters based on optimization methods to balance the mechanical properties of the implant and surrounding tissues [24,25,26,27]. The shape, size, and porosity affected the perception of the cell, which provided more space for cell culture. The geometry of the primary cell unit will affect its final configuration and mechanical properties, which is one of the most critical parameters. Different designs of basic cell units lead to the other spatial distribution of pores and the cell adhesion mode [87]. Curvature is another critical parameter that affected tissue regeneration. The curvature’s porous structure affected the arrangement, proliferation, and differentiation of cells, even tissue formation [88]. The rate of bone tissue formation was positively correlated with the curvature of the porous structure [89,90,91]. The types of curved surfaces, namely concave and convex characters, also play a role in regulating tissue regeneration. The growth of bone tissue is more efficient on open rather than convex and planar surfaces. The density of actin fibers and myosin on concave surfaces is higher, indicating that cell stress stimulation near concave surfaces is higher [92]. The shapes of the basic units can significantly affect cellular response and the rate of bone tissue regeneration [93,94,95]. Significant intracellular differentiation occurs on porous scaffolds with triangular units compared with hexagonal and rectangular units in in vitro cell experiments [24]. Bone tissue was more likely to grow into cubic branches than wavy structure [96]. Compared with the cubic and diagonal units, trigonal units were most suitable for cell survival and migration [87, 97].
The pore size of porous implants also affects the transport and discharge of nutrients and metabolic wastes, thus is closely related to cell proliferation, cell migration, and blood vessel formation. The ideal pore size should facilitate the transport of nutrients and promote cell adhesion. A pore diameter of 100-700 \(\upmu \hbox {m}\) is conducive to osseointegration. The suitable diameter for bone insertion should be \(>100\) \(\upmu \hbox {m}\) [7, 98]. However, a pore diameter of \(<400\) \(\upmu \hbox {m}\) allowes only small blood vessels to form, significantly limiting the formation of large blood vessels. Therefore, some researchers believed that the pore size should be \(>400\) \(\upmu \hbox {m}\) to allow angiogenesis [99, 100]. Taniguchi et al. [101] implanted porous titanium scaffolds with pore sizes of 309 \(\upmu \hbox {m}\), 632 \(\upmu \hbox {m}\), and 956-\(\upmu \hbox {m}\) into rabbit femurs and found that the rate of bone growth in the 632-\(\upmu \hbox {m}\) and 956-\(\upmu \hbox {m}\) groups was significantly higher than that of the 309 \(\upmu \hbox {m}\) group. The bone tissue in the 632-\(\upmu \hbox {m}\) group had the strongest bond to scaffold after 12 weeks [101]. For porous titanium alloy scaffolds with pore sizes of 640 and 1200 \(\upmu \hbox {m}\), alkaline phosphatase and osteocalcin content were higher in the 640-\(\upmu \hbox {m}\) group, indicating a more active process of osteogenesis [102]. Jan et al. [103] found that porous Ti6Al4V scaffolds with a 700-\(\upmu \hbox {m}\) pore size had a favorable osseointegration effect in repairing a 20 mm bone defect in a sheep diaphysis. However, Doernberg et al. [104] conducted an in vivo study of bioceramic porous scaffolds with different pore sizes (150, 260, 510, and 1220 \(\upmu \hbox {m}\)) in sheep. After 24 weeks, histomorphological examination showed no significant difference in bone growth between these groups. Roosa et al. [105] implanted PCL porous scaffolds with 350-, 550-, and 800-\(\upmu \hbox {m}\) pore sizes into mice and found no significant difference in bone regeneration after 8 weeks [96]. Such varing experimental results were induced by the combined factors of porosity, pore connectivity, and pore structure.
On the other hand, the connectivity between pores also affected the mass transfer efficiency between different pores. Porous tantalum implants with 80% porosity effectively facilitated bone regeneration based on mechanical and histological tests [106]. As porosity was closer to cancellous bone, the ability of bone scaffolds to stimulate osteoblast differentiation became stronger [107]. Scaffolds with 65% and 75% porosity had higher protein content than scaffolds with 25% porosity, and high porosity facilitated the entry of oxygen and nutrients [108]. The biological efficiency of porous titanium scaffolds under the static and dynamic cell detection techniques was 51% and 76% porosity, respectively; the 76% porosity scaffold group had more active cell metabolism and migration [97]. Both the diameter and the number of new blood vessels increased with the increase of the porous bone scaffold’s connectivity [109]. A pore size \(>300\) \(\upmu \hbox {m}\) [98] and porosity more significant than 50% [107] can promote cell infiltration, migration, vascularization, and mass transfer efficiency. However, high porosity and pore size will reduce the specific surface area of the scaffold, which is not conducive to cell adhesion [102], and weaken the mechanical strength [110]. Since the porosity directly affects the mechanical properties of the porous structures, high-porosity implants may not possess adequate mechanical strength. Therefore, the balance between these two parameters of porous structure should be considered.
Poisson’s ratio can be used to control the deformation mode of structure in the design of the porous implant, thereby adjusting the loading transfer from the implant to the surrounding tissue. An auxetic system with a negative Poisson’s ratio (NPR) would expand in the transverse direction under stretch (or shrink under contraction) and, thus, possess the unprecedented advantage in unique application [111,112,113,114]. The Poisson’s ratio could be adjusted from positive to negative based on the auxetic structural design [115, 116]. Mechanical properties, including shear strength, impact resistance, fracture toughness, indentation resistance, and crack propagation resistance of the NPR porous structure, are better than those of the ordinary porous structure. Besides, the concave surface configuration of NPR porous structure is favorable for cell adhesion and proliferation, possessing promising application prospects in porous implant design [117]. Six kinds of auxetic unit cells with NPR were designed and used to design bone screws, and then they were manufactured using 3D printing in our previous study [118]. Pullout tests and finite element analyses (FEA) were conducted to analyze the static mechanical and anti-pullout properties of the auxetic bone screws. Varying auxetic structures altered the screw’s mechanical properties, especially its functional properties. Auxetic designs could improve the bone-screw fixation. The biomechanical interaction between the screw and the surrounding bone would affect the expansion deformation of the auxetic screw. An auxetic pedicle screw was proposed by introducing the re-entrant unit cell into the screw body and optimized to obtain excellent anti-pullout ability for a particular bone based on the biomechanical interaction between the screw and the surrounding bone. Appropriate mechanical and anti-pullout properties could be obtained by rationally designing the unit cells’ wall thickness and re-entrant angle, providing a reference for the customized design of pedicle screws with adequate anti-pullout performance [119].
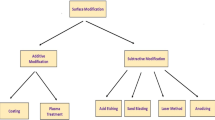
5.3 Manufacture technologies of implants
Implant manufacturing technologies include membrane lamination, particulate leaching, gas foaming, solvent casting, electrospinning (ES), and 3D printing technology. Each method has its advantages and application situation. Membrane lamination is a quick way to produce a two-dimensional (2D) membrane by pressing the bulk biomaterial to form a membrane; however, it is unsuitable for manufacturing scaffolds with a complex 3D structure. For instance, the personalized 3D-printed titanium mesh is suitable for all kinds of alveolar bone defects, especially for the treatment of complex alveolar bone defects with large areas. The personalized 3D-printed titanium mesh has its unique advantages. It serves as spatial support for bone augmentation, which can create enough space to form new bone. Biomechanical optimize 3D titanium mesh scaffolds are used to repair mandibular defects [120]. Particulate leaching, gas foaming, and solvent casting are used to produce 3D scaffolds with a requisite pore structure inside through removing particles, gas, or solvents from bulk and leaving pores in their matrix during the scaffold formation. However, the shape of scaffolds is restricted by the mold shape in all the methods mentioned above, and the morphological properties, such as pore size and porosity, are not easily controlled [121]. As a result, the implants from these methods frequently have irregularly shaped pores and insufficient interconnectivity. Besides, particle retention in the matrix is a considerable challenge for particulate leaching technology. ES technology is a conventional method utilized in tissue engineering research. Under electric loading, charged threads of material solution are drawn to produce fibers with diameters ranging from submicron to nanometer. ES scaffolds have been reported for application in tissue engineering research.
For example, Fasolino et al. [122] tested the behavior of human mesenchymal stem cells (hMSCs) on a PCL fibrous scaffold, which was fabricated by ES (voltage, 15 kV; material feed rate, 0.1 mL/h). They found that the hMSCs differentiated and fused to myotubes. In a preliminary study by Giraud et al. [123], ES PCL-based nanofibers (100-2500 nm) were produced and then treated with methane and ethylene plasma to increase hydrophilicity (water contact angle decreased), which further promoted cell attachment, proliferation, and differentiation. Chen et al. [124] fabricated aligned PCL fibers, which provided topographical cues to guide C2C12 orientation and announced myotube formation. Polyaniline was incorporated into PCL fibers to increase electrical conductivity, enhancing myotube maturation compared with non-conductive aligned PCL scaffolds.
Similarly, graphene oxide nanoparticles were introduced into electrospun PCL nanofibers (\(390 \pm 125\) nm) to improve conductivity (\(\sigma \)) and dielectric permittivity (\(\epsilon \)). Consequently, the enhancement of \(\sigma \) and \(\epsilon \) values provided sound cues for forming superior multinucleated myotubes on the electrospun meshes compared with those without nanoparticles [125]. PCL films produced by ES have been used as platforms for tissue regeneration research for several years. However, PCL films are fragile and straightforward in structure. Although the thickness of electrospun PCL films can be increased by extending the spinning time, the mechanical property is still not strong enough to replace the hard tissue. In addition, this fabrication method could control the orientation of nanofibers; however, it could not maintain the distance between individual fibers. As a result, the pores of PCL scaffolds were heterogeneous, with uncontrolled and random sizes [126], which did not wholly represent the complicated 3D microenvironment in vivo.
3D printing technology shows apparent advantages compared with traditional fabrication methods. The earliest 3D printing technology was first visible in the late 1980s with rapid prototyping technologies. With the continuous advancement of technological applications, 3D printing technology has been applied to fabricate implants for cell proliferation and tissue injury repair in vivo [127]. Precisely, based on a digital mode either by computer-assisted design or 3D remodeling from patient-specific medical images (e.g., X-ray, computerized tomography (CT), and magnetic resonance imaging (MRI) scan), a 3D printer delivers the material to the pre-defined location and builds the scaffolds layer by layer [128]. Compared with other conventional fabrication methods (such as casting, leaching, and ES technology), 3D printing technology can accurately adjust the space between printed fibers in a 100 \(\upmu \hbox {m}\) scale and precisely control the interior architecture pore size and porosity of the scaffold. Therefore, the 3D printing technology is in place to fabricate bespoke 3D structure/scaffolds, and their applications have recently been researched in regenerative medicine. For example, Cai et al. [129] found the porcine chondrocytes proliferated on a 3D PCL scaffold and expressed sulfated glycosaminoglycan (sGAG), the main component of cartilage extracellular matrix (ECM), a marker of porcine chondrogenesis; moreover, sGAG expression increased when the PCL scaffolds were pre-coated with collagen. Marino et al. [130] seeded adipose-derived stem cells (ADSC) into porous PCL scaffolds and evaluated the growth and differentiation during a 14-day incubation period. ADSC proliferation was observed at different time points, and the endothelial formation was monitored by endothelial-specific markers (CD31, vWF, and eNOS). Furthermore, they observed the neuronal differentiation potential of ADSC on the same scaffolds and detected the expression of neuronal-specific markers (nestin, \(\upbeta \)-tubulin-III, and neuron-specific enolase (NSE)) [131].
Indeed, 3D printing technology is capable to accurately control pore size at approximately 100 \(\upmu \hbox {m}\) scales in the scaffold fabrication process. However, this printed filament is also approximately 100 \(\upmu \hbox {m}\), which is influenced by the printer’s nozzle diameter. Visser et al. designed a new technology, melt ES writing (MEM), which combines the advantages of 3D printing and ES technology [132]. In particular, melt PCL was extruded at a rate of \(18~\upmu \hbox {L/h}\) in an electrostatic field of 8-10 kV, and the collector plate moved based on a computer pre-designed program to collect extruded PCL materials. As a result, PCL scaffolds were fabricated with small-diameter fibers, which were approximately 19.4 \(\upmu \hbox {m}\). During the printing process, the accumulation of excess charge distorted scaffold architectures; Wunner et al. [133] applied variable working distances to maintain the electrostatic loading at a constant level between the extruder and the collector. As a result, large-volume scaffold architectures can be produced by MEM technology. MEM-PCL fibrous scaffolds were consistently combined with gelatin to manufacture a layer-specific scaffold for osteochondral regeneration [134]. With the decrease of the filament diameter, the mechanical properties of scaffolds also decrease, which limited the potential application in the clinic. Therefore, balancing compromised mechanical properties and more acceptable filament diameter and architecture will challenge the MEM technology.
Additive manufacturing techniques, including selective laser melting (SLM) [135], selective laser sintering (SLS) [136], fused deposition modeling (FDM) [137, 138], and selective electron beam melting (SEBM) [139], have distinct advantages in the fabrication of porous structures. These advantages involve accurate control over the pore size, cell geometry, and the convenience to combine porous materials with different meso-architecture in one single design, providing the possibility of personalized implants and extensively promoting the application of complex porous implanted medical devices. Personalized porous implants developed by additive manufacturing techniques mainly target patients with special needs, such as severe anatomical deformities or defects. The properties of porous implants largely depend on the features of microstructures, which are significantly affected by the additive manufacturing process [140, 141]. A reasonable 5-mm channel length might provide adequate blood circulation and fluid movement [142]. Ataee et al. [143] design anisotropic porous scaffolds with different unit sizes based on implicit surface modeling and manufactured by electron beam melting (EBM). The elastic modulus and yield strength of as-built platforms were 637-1084 MPa and 13.1-19.2 MPa. The ratio of elastic modulus anisotropy in orthogonal directions was comparable to that of the human cancellous bone, showing good compatibility with bone mechanically and suitability for the application in orthopedic implants. With the help of a modified 3D printing strategy, a biomimetic bone scaffold was inspired by the microstructure of natural plant lotus root. Lotus root-like platform with different channel numbers and directions would significantly improve in vitro cell attachment and in vivo osteogenesis, indicating potential application for cell delivery and bone regeneration [144]. The 3D printed Ca-Si system of 3D-layered scaffolds with controllable micropore size can enhance the expression of alkaline phosphatase, osteogenic genes, and angiogenic genetic markers [67, 145]. Through multi-scale modeling based on images, Hollister et al. found that the mechanical strength of porous biodegradable scaffolds fabricated by additive manufacturing could be controlled by designing structure parameters, including pore size, shape, interconnection, and surface area [69, 70, 146, 147]. The 3D printing process parameters of biodegradable materials were optimized and established a topology optimization method based on the structural design of different microporous scaffolds [71, 72]. The clinical application value of patient-specific porous implanted medical devices fabricated by additive manufacturing technology has been highly appreciated and possesses a broad application prospect [148, 149].
6 Reliability evaluation technologies of implants in vitro
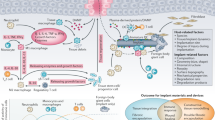
A preclinical in vitro evaluation of implantable and interventional medical devices is required to ensure safety and improve the structural performance of the devices in a well-controlled environment. In addition, in vitro bench testing reduces reliance and the use of animals in the safety and risk assessment of medical devices. Preclinical animal models are time-consuming, expensive, and raise ethical concerns [150, 151]. In vitro simulating and reproducing the complex biomechanical environment in vivo is the key technology to evaluate the function, biomechanical properties, destruction mechanism, and fatigue characteristics of implantable and interventional medical devices. For orthopedic implantation medical devices, different orthopedic implants (knee joint, hip joint, bone fixation instruments for different purposes, etc.) are often in different biomechanical environments in vivo. The implants and their surrounding host tissues are typically in complex dynamic stress environments. For cardiovascular implantable and interventional devices, the complex hemodynamics of the human blood circulation system and the vascular and myocardial mechanical environment are important factors affecting cardiovascular and cerebrovascular physiology and pathology. The development and evaluation of cardiovascular interventional techniques and devices (vascular stents, artificial heart valves, vascular grafts, cardiovascular interventions, etc.) depend on the simulation of real and complex biomechanical conditions. In vitro reproducing the complex biomechanical environment is of great significance in evaluating stent collapse and fracture risks, valve failure, cardiovascular interventional therapy restenosis, or failure. Thus, we developed biomechanics and mechanobiology evaluation technology based on the mechanism of interaction between biodegradable implant interventional medical devices and host tissues after implantation (Figs. 3 and 4 ).
6.1 Reliability evaluation technologies of orthopedic implants
The evaluation of implantable and interventional devices, which is essential to maintain long-term and effective functionality, is not easy to perform after implantation. Fatigue durability is one of the important evaluation indexes. In addition, there are different requirements for fatigue durability, depending on the service environment of different devices. In vitro evaluation equipment cannot satisfy such a long test cycle. Accelerating the loading frequency of implants under approximate mechanical conditions can shorten the test cycle. The accelerated durability testing of prosthetic valves and vascular stents can typically reach 30 Hz, which is dozens of times the normal cardiac cycle, and can shorten the testing time of 10 years to several months. However, the biggest challenge of accelerated durability testing is that the frequency of increased mechanical loads on devices can not remain the same as the actual situation, and the influence of these changed loads on the durability performance has yet to be determined. The compromise method uses the quasi-real-time test to maintain a more realistic load through a lesser acceleration (2-3 times), which may identify failure modes similar to animal tests.
Orthopedic implant evaluation technologies are complex due to different biomechanical characteristics of the musculoskeletal system. At present, imitating human gait biomechanical durability testing systems for the knee and hip joints has made a great breakthrough. However, there is still a significant shortage in the biomedical simulation of the spine and other musculoskeletal system implants, especially the personalized orthopedic implants. In recent years, the role of computational modeling and simulation is becoming increasingly important and evident in the design and development of medical devices [152]. Computational simulations reduce the need to perform expensive preclinical tests to optimize the design and reduce the associated risk factors. In addition, regulatory agencies such as the United States Food and Drug Administration (FDA) and European Medical Device Regulatory (MDR) currently accept validated computational modeling and simulation as scientific evidence in regulatory submissions [153]. In addition, studies and analyses of design, research, and construction of prostheses, implants, and prophylactic internal fixations were performed over the past few years by several researchers using computational simulation.
Studies of screws in the spinal vertebrae involving finite element models (FEM) are ideal for examming the effects of isolated parameters that can be easily controlled and modified. FEA based on non-idealized series models or patient-specific models may have more excellent value. It could directly show the mechanical relationship between the bone screw and the surrounding soft tissue and quickly examine the stress distributions. The bone-pullout performance of screws with NPR ratio structure was analyzed by computational simulation in our previous study [154]. The biomechanical performance of the personalized plate for tibiotalocalcaneal arthrodesis was also evaluated based on FEM [155].
The computational simulation method can also be used to evaluate the durability performance of implants. Martin and Sun [156] developed a computational framework to simulate bioprosthetic heart valve leaflet fatigue, which is both efficient and quantitative, making it an attractive alternative to traditional, accelerated wear testing. Since it is inevitable to repeatedly bear complex physiological loads due to a patient’s daily activities, the reliability must be evaluated during the development of implanted medical devices, especially for porous implants. Given the repetitive nature of physiological loads, the static mechanical properties and the fatigue behavior of porous implants, which affects their longevity, need to be correctly adjusted for optimum tissue regeneration performance and reliability [157]. Due to the significant anisotropy of the structure of porous implants, their fatigue properties are significantly anisotropic [158]. The structural characteristics of the implants, material features, and manufacturing process also have significant influences on their fatigue behaviors [159,160,161,162]. Hedayati et al. [159] found that the pores density will affect the fatigue crack propagation behavior of porous implant under tensile cyclic loads. Furthermore, the additive manufacturing process significantly affects the fatigue crack propagation behavior of porous implants, which could be described by the multi-scale modeling method [160]. By optimizing additive manufacturing process parameters, the fatigue performance of porous structures can be improved [161]. The osseointegration, mechanical stability as well as fatigue strength of porous implants could be enhanced by roughening the surface with metal particles of adequate size [162]. In addition, it is an essential function of porous implants to promote tissue regeneration, which has a significant influence on their fatigue properties. Hedayati et al. investigated the effect of bone tissue regeneration degree on the fatigue behaviors of 3D-printed porous scaffolds in vitro [157]. Different stages of bone tissue growth in the implants were simulated by filling porous scaffolds with polymer materials of different mechanical strengths. This study preliminarily showed that the bone tissue regeneration degree had a nonnegligible effect on the fatigue behaviors of porous scaffolds. With the increase of the bone regeneration degree in the implant, the fatigue strength of the porous scaffolds would be significantly improved.
Moreover, Cheong et al. [163] explored the process of bone regeneration and its influence on the fatigue properties of porous scaffolds through FE simulation, which had been verified with in vivo observation and histological results. It was found that the pore size and density would affect the internal stress distribution among porous scaffolds, which in turn act in the osseointegration. Meanwhile, osseointegration could alter the local stress distribution in stands, thus improving the fatigue strength of scaffolds.
In addition to the fatigue behavior of porous orthopedic implants, physiological loads may lead to implant-bone relative micromotions of the order of micrometer [164]. Different amplitudes of implant-bone interface micromotions have distinct effects on osseointegration among porous implants, which might affect its long-term biomechanical reliability [32]. The implant-bone micromotion amplitude, which was a benefit from bone growth, was \(<40~\upmu \hbox {m}\) [165]. When the implant-bone micromotion amplitude is \(<28~\upmu \hbox {m}\), osseointegration could be significantly promoted [166, 167]. However, fibrocartilage tissue will be formed in the implant-bone interface when the implant-bone micromotion amplitude is \(>150~\upmu \hbox {m}\), leading to the wear and fatigue of the implant and inhibiting osseointegration [168, 169]. Once a firm combination can not be established between the implant and the surrounding bone tissue, the loosening or even fatigue fracture of the implant would be inevitable under a long-term physiological load [170].
Therefore, the implant-bone interface micromotion, which affects the reliability of porous bone implants in vivo, is an essential factor to be considered during the development of this kind of implanted medical device. The in vitro measurement methods were adopted to measure the implant-bone micromotion amplitude in most existing studies [168, 169, 171, 172]. However, this kind of measurement is unable to monitor the actual in vivo implant-bone micromotion. Therefore, it is necessary to develop the measurement technique for implant-bone micromotion in vivo monitor to assess whether the design of porous implants can ensure adequate implant-bone micromotion amplitude, promoting the reliability of osseointegration. Fractures healing is a complex biological process. Fractures are generally treated with intramedullary nail fixation [173]. Although this model-making operation is simple and does minor damage to the soft tissue around the fracture, it has its shortcomings, including the inability to control well the axial and rotational stability and the risk of medullary cavity injury [174,175,176]. Despite \(>10\%\) of fixation failure was associated with screw migration and after-implant pullout due to weak bone-screw focus, bone screws are widely used in orthopedics for fracture fixation [177]. The evaluation of the mechanical properties of bone screws is vital for the study of the mechanism of intramedullary screws in the treatment of fractures. Compression stiffness, torsional stiffness, and three-point bending stiffness can be measured by axial compression load test, horizontal torsion test, and three-point bending test.
For artificial joints, take the knee prosthesis as an example. The performance evaluation of knee prosthesis is divided into two parts: routine tribological tests and joint simulation tests. Regular tests of knee prostheses are mainly performed in sliding contact on various types of pin-disk friction and wear testing machines. Conventional test methods are inadequate to test the contention and sports performance of knee prostheses owing to their complex multi-degree-of-freedom motion mode. It is necessary to attempt the clash and wear performance of knee prostheses to simulate the various motion modes of natural knee joints. Researchers use the knee prosthesis simulation test machine to perform long-term large-volume tests. They can detect the friction and wear performance of the knee prosthesis and predict the lifespan of the entire joint to verify whether the geometric shape and structure of the artificial knee joint can be adapted to the patient after surgical replacement. During the test, continuous and periodic extension and flexion, internal and external, and axial movement were performed on the artificial knee joint under different load conditions. Friction torque, deformation, loading, damping, and sliding surface temperature of the prosthesis were measured. Currently, a six-degree-of-freedom (6-DOF) environment knee simulator can provide a patent-pending multi-fiber ligament model, which can capture the 6-DOF inter-axis coupling typical of biological soft tissue systems. It can combine speed, range of motion and loading capability, programmability, and virtual soft tissue models to test real-world implant failure modes, such as adverse edge loading conditions, micro-separation, stem and cup impingement, condylar liftoff, and joint subluxation. Aspherical contact testing machine can test the knee prosthesis ball head material, and the tibial platform prosthesis material and hip prosthesis material (ultra-high molecular weight polyethylene, UHMWPE) can be tested by the prosthetic loading and motion directly controlled testing machine [178,179,180].
The performance evaluation of knee prosthesis should combine with the natural motion law and influence the fundamental physiological environment for the comprehensive assessment through biomechanics and kinematics perspective. At the same time, the effects of material properties and structural design on knee prostheses should be considered.
6.2 Reliability evaluation technologies of cardiovascular interventional devices
The human arterial system is exceptionally complicated. The distribution and configuration of artery branches is complex. The vascular wall elasticity and viscoelasticity are diverse in different segments of the arteries. The complex kinematic characteristics of heart motion and rigid blood vessel wall fluid-solid coupling difficulties result in unique pulse wave characteristics and various mechanical loads in different routes (periodical flow shear stress, tensile stress, swirling flow, pressure, and torsion). Complex flow fields lead to complex and variable interactions between arterial blood vessels and implants (stents, wires, catheters, etc.). The development and evaluation of cardiovascular implantable and interventional medical devices (vascular stents, artificial heart valves, and artificial grafts) are highly dependent on techniques and equipment that mimic the complex biomechanical environment of human arteries. It is difficult for the current gear to fully reproduce the natural environment. Evaluating different kinds of implantable and interventional devices is focused on the risk factors that may cause their failure. For example, vascular stents are mainly used to evaluate the effect of the cyclical stress of the compression and tension of the arterial wall on durability performance. For the evaluation of artificial heart valve function, the pressure and flow wave in the ventricle/aorta were simulated.
Hydrodynamic testing should be performed to verify the hemodynamic performance of heart valve substitutes [181]. At the same time, the risk assessment of heart valves and components, including structural performance testing as well as physical and material properties testing, should also be performed. Leaflet calcification is the highest risk of a bioprosthetic heart valve. Whelan et al. [182] used cyclically loading method to accelerate the evaluation of leaflets calcification. Vascular stents are in direct contact with blood after being implanted and are subjected to the cyclic compression/tensile stress of the blood vessel wall and shear stress of blood flow at the same time. In vitro tests high-frequency cyclic compression stress was loaded to evaluate its fatigue durability [183]. Engels et al. simulated the physiological shear stress of blood flow on the stent and evaluated the stent’s blood compatibility in this state [184]. In addition, we also developed fatigue test devices for vascular stents, which could load both tensile stress and compressive stress at the same time (Fig. 5a and 5b).
For example, the in vitro assessment of heart valve substitutes mainly includes hydrodynamic evaluation and accelerated wear testing. The hydrodynamic evaluation provides information on the substitutes’ fluid mechanical performance and can be divided into continuous flow testing and pulsatile flow testing. Pulsatile flow systems can closely mimic blood flow characteristics within the heart and vasculature system (including ventricular, atrial, aortic pressure, blood flow rate, heartbeat frequency, and vascular compliance).
A programmable piston pump modulates the left ventricular chamber volume to drive cardiac output, and afterload is provided by a system of compliances and resistances analogous to the classical Windkessel circulation model. The performance parameters of the heart valve, such as transvalvular pressure gradient, regurgitation volumes, and the effective orifice area, can be measured or calculated during testing. Serval commercial pulse duplicator systems are available [185,186,187,188].
The implantable and interventional device is affected by the host tissues after being implanted in the human body and involves host tissue remodeling. Generally, numerical simulation and in vitro cell experiments can study the effects and mechanisms of different stress conditions on bone and vascular remodeling after implantation [189]. In vitro cell experiments typically use bioreactors. A bioreactor is a tissue that carries out a biological reaction and is used to culture aerobic cells for conducting cellular or enzymatic immobilization [190]. By simulating the effects of implants on the host tissues, the effects of different stresses on vascular endothelial cells, smooth muscle cells, bone cells, osteoblasts, and bone marrow mesenchymal stem cells can be studied. This evaluation method is significant for studying the interaction between implants and host tissues in physiological/pathological environments as well as for the optimal designing and performance testing of implants. Typical bioreactors can simulate mechanical loads in one or two different modes. However, the mechanical environment of the implant in the host is complex and subject to multiple ways of loads simultaneously. For example, tissues and cells in the human arterial system are under a complex physiological pulsating flow environment that combined periodic shear stress, compressive stress, tensile stress, and torsion, which is quite different from the mechanical environment simulated by bioreactors. Fan et al. [10, 191,192,193] used multi-parameter control technology to couple multiple modes of loads and developed a variety of bioreactors that can independently or simultaneously load quasi-physiological shear stresses, tensile stresses, torsion, and other quasi-physiological loads (Fig. 5c and 5d).
Microreactor is a micromodel with physiological functions. Based on microfabrication technology, 3D bioprinting technology, and multiple detection technologies, cells are cultured in continuously perfused micro-chambers, and physiological environments of tissues and organs are simulated [194, 195]. Functional microreactor allows accurate measurements of multiple physical information with small consumption, and the experimental environment is controllable [196, 197]. Therefore, it is widely applied in biomechanical studies of the musculoskeletal system and tissue engineering, especially for the designing and testing of implantable and interventional medical devices. In terms of vessels, microreactors are mainly applied for the capillary vascular system remodeling.
Capillaries are composed of a single layer of endothelial cells, with parietal cells scattered around to stabilize the capillary structure. Different schemes are proposed to construct two-dimensional or 3D vascular networks in vitro. A frequently used strategy is to build microfluidic channels with microfabrication technology or 3D printing and culture endothelial cells in the media. Perfusion vessels are then constructed as a result of the cell growth [198, 199]. However, it is difficult to build complex vascular structures or capillaries with small diameters. A scheme is to culture cells (endothelial cells, fibroblasts, endothelial progenitor cells, stem cells, etc.) in hydrogels such as fibrin and collagen [200, 201]. These cells will grow into 3D capillary structures owing to natural assembly characteristics [202]. However, vessels constructed with this scheme are typically immature. Complete monolayers cannot be formed with endothelial cells, and the integrity is poor with large porosity [203]. Thus, it is a significant concern to promote the maturity of the constructed vascular structures. Precise control of growth factors is a crucial point to induce capillary angiogenesis; the fluid shear stress of normal physiological conditions can reduce vascular permeability and promote the maturation of vascular function. Appropriate shear stress should be applied to the new capillaries to promote functional maturity, and the construction of perfusion capillary structures with liquid transport functions is promising to be achieved in microreactors.
In studies of the myocardium, microreactors have been used to explore the influence of implants on different tissues and cells. The myocardial tissue is highly organized; the contractile function is affected by force, chemical, and electrical stimulations. A microreactor platform for the 3D culture of cardiomyocytes should be built combining mature and highly functional microcardiac tissues with microfluidic technology, and then the mechanical environment of in vivo tissues can be simulated [204]. Both physiological functions and drug effects on cardiomyocytes can be explored. It is found that the adhesion and arrangement of cells on topological surfaces can be adjusted through microreactor technology, and the biocompatibility of implantable biomaterials and medical devices is improved.
In terms of orthopedic medical implants, traditional bioreactors are mainly applied in related researches instead of microreactors [205]. However, the application of microreactor technology is conducive to clarify the mechanism of implants to promote bone repair and the influence of biodegradable implants on tissues [17, 18, 58]. The cardiovascular and musculoskeletal systems play an essential role in load-bearing and are responsible for several diseases. The development of relatively new drugs and medical devices is limited by the disadvantage of traditional methods, whereas microreactor offers a possible direction to improve it. However, current research on microreactor technology is still immature, with several challenges to be solved, including how to express the complex relationship among different tissues with appropriate combinations of multiple microreactors. Besides, the flow control in the microreactor is challenging to design and simulate, mainly when it is applied to culture-sensitive cells, such as endothelial cells and hepatocytes.
7 Conclusions
The long-term efficacy of implants heavily depends on the local mechanical environment after surgery. In vitro and in vivo experimental and numerical test techniques are the most critical way to evaluate implant and surrounding host tissue interactions. The biocompatibility and toxicity of implants are necessary to assess (1) the mechanical properties of implants and (2) the interaction between implants and surrounding tissues or cells, given in biomechanics and mechanobiology. As previously mentioned, stress on degradation behavior is important for the personalized degradable implant, which is a frontier trend in the clinic. Therefore, the following critical scientific issues of individualized degradable implants should be paid attention to.
-
A systemic study of both cellular- and tissue-level-cytobiology given in mechanobiology and biomechanics will fill the gap and support the optimization and design of biodegradable implants.
-
Multi-cale modeling/remodeling of the interaction between implants and host tissues in vitro should be compared with that in vivo.
-
New mechanobiology test techniques based on microbioreactors, microenvironment simulation of topology (microporous and interface features), biological activity, and long-term fatigue performance will provide an excellent way to push the applications and further innovation of implants.
-
The biomechanical and mechanobiological adaptation law of biodegradable implants should be explored, which will guide the optimization, design, fabrication, and evaluation of implants. We also believe that it will become the theoretical basis of implant innovation and applications.
References
Pisani, P., Renna, M.D., Conversano, F., et al.: Major osteoporotic fragility fractures: Risk factor updates and societal impact. World J. Orthop. 7(3), 171–181 (2016)
Chanda, S., Mukherjee, K., Gupta, S., et al.: A comparative assessment of two hip stem designs using rule-based simulation of combined osseointegration and remodeling. Proc. Inst. Mech. Eng. Part H 234(1), 118–128 (2020)
Wu, C., Zheng, K.K., Fang, J.G., et al.: Time-dependent topology optimization of bone plates considering bone remodeling. Methods Appl. Mech. Eng. 359, 112702 (2020)
Saeidi, M., Gubaua, J.E., Kelly, P., et al.: The influence of an extra-articular implant on the bone remodeling of the knee joint. Biomech. Model. Mechanobiol. 19(1), 37–46 (2020)
Claessen, F.M., Braun, Y., Peters, R.M., et al.: Plate and screw fixation of bicolumnar distal humerus fractures: factors associated with loosening or breakage of implants or nonunion. J. Hand Surg. 40(10), 2045–2051.e2042 (2015)
El Saman, A., Meier, S., Sander, A., et al.: Reduced loosening rate and loss of correction following posterior stabilization with or without PMMA augmentation of pedicle screws vertebral fractures in the elderly. Eur. J. Trauma Emerg. S. 39(5), 455–460 (2013)
Tsai, P.I., Chen, C.Y., Huang, S.W., et al.: Improvement of bone-tendon fixation by porous titanium interference screw: a rabbit animal model. J. Orthop. Res. 36(10), 2633–2640 (2018)
Deb, P., Deoghare, A.B., Borah, A., et al.: Scaffold development using biomaterials: a review. Mater. Today. Proc. 5(5), 12909–12919 (2018)
Manavitehrani, I., Le, T.Y.L., Daly, S., et al.: Formation of porous biodegradable scaffolds based on poly(propylene carbonate) using gas foaming technology. Mater. Sci. Eng. C. 96, 824–830 (2019)
Gong, X., Liu, H., Ding, X., et al.: Physiological pulsatile flow culture conditions to generate functional endothelium on a sulfated silk fibroin nanofibrous scaffold. Biomaterials 35(17), 4782–4791 (2014)
Lam, C.X., Hutmacher, D.W., Schantz, J.T., et al.: Evaluation of polycaprolactone scaffold degradation for six months in vitro and in vivo. J. Biomed. Mater. Res. Part A 90(3), 906–919 (2009)
Schimke, M.M., Paul, S., Tillmann, K., et al.: Hard tissue augmentation of aged bone by means of a tin-free plla-pcl co-polymer exhibiting in vivo anergy and long-term structural stability. Gerontology 65(2), 174–185 (2019)
Haider, T.P., Völker, C., Kramm, J., et al.: Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. Engl. 58(1), 50–62 (2019)
Yang, Y.F., Zhao, Y.H., Tang, G.W., et al.: In vitro degradation of porous poly(L-lactide-co-glycolide)/beta-tricalcium phosphate (PLGA/beta-TCP) scaffolds under dynamic and static conditions. Polym. Degrad. Stabil. 93(10), 1838–1845 (2008)
Fan, Y.B., Li, P., Zeng, L., et al.: Effects of mechanical load on the degradation of poly(D, L-lactic acid) foam. Polym. Degrad. Stabil. 93(3), 677–683 (2008)
Fan, Y.B., Xiu, K.H., Dong, X., et al.: The influence of mechanical loading on osseointegration: an animal study. Sci. China Ser. C 52(6), 579–586 (2009)
Guo, M., Chu, Z.W., Yao, J., et al.: The effects of tensile stress on the degradation of biodegradable PLGA membranes: a quantitative study. Polym. Degrad. Stabil. 124, 95–100 (2016)
Chu, Z.W., Li, X.M., Li, Y., et al.: Effects of different fluid shear stress patterns on the in vitro degradation of poly(lactide-co-glycolide) acid membranes. J. Biomed. Mater. Res. Part A 105(1), 23–30 (2017)
Gao, Y.M., Wang, L.Z., Li, L.H., et al.: Effect of stress on corrosion of high-purity magnesium in vitro and in vivo. Acta. Biomater. 83, 477–486 (2019)
Fraser, D., Funkenbusch, P., Ercoli, C., et al.: Biomechanical analysis of the osseointegration of porous tantalum implants. J. Prosthet. Dent. 123(6), 811–820 (2020)
Shi, J.P., Liang, H.X., Jiang, J., et al.: Design and performance evaluation of porous titanium alloy structures for bone implantation. Math. Probl. Eng. 2019, 5268280 (2019)
Wang, Y., Sun, N., Zhang, Y., et al.: Enhanced osteogenic proliferation and differentiation of human adipose-derived stem cells on a porous n-HA/PGS-M composite scaffold. Sci. Rep. 9(1), 7960 (2019)
Chen, X., Fan, H., Deng, X., et al.: Scaffold structural microenvironmental cues to guide tissue regeneration in bone tissue applications. Nanomaterials 8(11), 960 (2018)
Van Bael, S., Chai, Y.C., Truscello, S., et al.: The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds. Acta. Biomater. 8(7), 2824–2834 (2012)
Hu, J., Wang, J.H., Wang, R., et al.: Analysis of biomechanical behavior of 3D printed mandibular graft with porous scaffold structure designed by topological optimization. 3D Print. Med. 5(1), 5 (2019)
Ataee, A., Li, Y.C., Fraser, D., et al.: Anisotropic Ti-6Al-4V gyroid scaffolds manufactured by electron beam melting (EBM) for bone implant applications. Mater. Des. 137, 345–354 (2018)
Li, Y.H., Yang, C., Zhao, H.D., et al.: New developments of Ti-based alloys for biomedical applications. Materials 7(3), 1709–1800 (2014)
Zadpoor, A.A.: Mechanical performance of additively manufactured meta-biomaterials. Acta. Biomater. 85, 41–59 (2019)
Yavari, S.A., Ahmadi, S.M., Wauthle, R., et al.: Relationship between unit cell type and porosity and the fatigue behavior of selective laser melted meta-biomaterials. J. Mech. Behav. Biomed. 43, 91–100 (2015)
Hedayati, R., Yavari, S.A., Zadpoor, A.A.: Fatigue crack propagation in additively manufactured porous biomaterials. Mater. Sci. Eng. C 76, 457–463 (2017)
San Cheong, V., Fromme, P., Coathup, M.J., et al.: Partial bone formation in additive manufactured porous implants reduces predicted stress and danger of fatigue failure. Ann. Biomed. Eng. 48(1), 502–514 (2020)
Grzeskowiak, R.M., Schumacher, J., Dhar, M.S., et al.: Bone and cartilage interfaces with orthopedic implants: a literature review. Front. Surg. 7, 601244 (2020)
Bergemann, C., Klinkenberg, E.D., Lüthen, F., et al.: Proliferation and migration of human osteoblasts on porous three dimensional scaffolds. Mater. Sci. Forum 2010, 506–511 (2010)
Balla, V.K., Banerjee, S., Bose, S., et al.: Direct laser processing of a tantalum coating on titanium for bone replacement structures. Acta. Biomater. 6(6), 2329–2334 (2010)
Wang, H., Su, K., Su, L., et al.: Comparison of 3D-printed porous tantalum and titanium scaffolds on osteointegration and osteogenesis. Mater. Sci. Eng. C 104, 109908 (2019)
Li, G., Wang, L., Pan, W., et al.: In vitro and in vivo study of additive manufactured porous Ti6Al4V scaffolds for repairing bone defects. Sci. Rep. 6(1), 340 (2016)
Shirazi-Adl, A., Dammak, M., Paiement, G.: Experimental determination of friction characteristics at the trabecular bone/porous-coated metal interface in cementless implants. J. Biomed. Mater. Res. 27(2), 167–175 (1993)
Mour, M., Das, D., Winkler, T., et al.: Advances in porous biomaterials for dental and orthopaedic applications. Materials 3(5), 2947–2974 (2010)
Gunatillake, P.A., Adhikari, R.: Biodegradable synthetic polymers for tissue engineering. Eur. Cell Mater. 5, 1–16 (2003). discussion 16
Hollister, S.J.: Paediatric devices that grow up. Nat. Biomed. Eng. 1(10), 777–778 (2017)
Huang, S.B., Wang, B.J., Zhang, X.Z., et al.: High-purity weight-bearing magnesium screw: translational application in the healing of femoral neck fracture. Biomaterials 238, 19829 (2020)
Da Silva, D., Kaduri, M., Poley, M., et al.: Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 340, 9–14 (2018)
Rogina, A., Antunovic, M., Milovac, D.: Biomimetic design of bone substitutes based on cuttlefish bone-derived hydroxyapatite and biodegradable polymers. J. Biomed. Mater. Res. B 107(1), 197–204 (2019)
Langer, R., Tirrell, D.A.: Designing materials for biology and medicine. Nature 428(6982), 487–492 (2004)
Conoscenti, G., Pavia, F.C., Ciraldo, F.E., et al.: In vitro degradation and bioactivity of composite poly-l-lactic (PLLA)/bioactive glass (BG) scaffolds: comparison of 45S5 and 1393BG compositions. J. Mater. Sci. 53(4), 2362–2374 (2018)
Seo, T., Schachter, L.G., Barakat, A.I.: Computational study of fluid mechanical disturbance induced by endovascular stents. Ann. Biomed. Eng. 33(4), 444–456 (2005)
Liu, D.X., Hu, S.W., Yin, X.Y., et al.: Degradation mechanism of magnesium alloy stent under simulated human micro-stress environment. Mater. Sci. Eng. C. Mater. Biol. Appl. 84, 263–270 (2018)
Korshunov, V.A., Schwartz, S.M., Berk, B.C.: Vascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov’s phenomenon. Arterioscler. Thromb. Vasc. Biol. 27(8), 1722–1728 (2007)
Liu, T., Chen, Y., Apicella, A., et al.: Effect of porous microstructures on the biomechanical characteristics of a root analogue implant: an animal study and a finite element analysis. Acs. Biomater. Sci. Eng. 6(11), 6356–6367 (2020)
Han, J., Hou, J., Zhou, G., et al.: A histological and biomechanical study of bone stress and bone remodeling around immediately loaded implants. Sci. China. Life. Sci. 57(6), 618–626 (2014)
Yu, T., Gao, H., Liu, T., et al.: Effects of immediately static loading on osteointegration and osteogenesis around 3D-printed porous implant: a histological and biomechanical study. Mat. Sci. Eng. C 108, 1873–10191 (2020)
Wang, C., Zhang, W., Ajmera, D.H., et al.: Simulated bone remodeling around tilted dental implants in the anterior maxilla. Biomech. Model. Mech. 15(3), 701–712 (2016)
Wang, C., Wang, L., Liu, X., et al.: Numerical simulation of the remodelling process of trabecular architecture around dental implants. Comput. Method. Biomech. 17(3), 286–295 (2014)
Reynders, R., Ronchi, L., Bipat, S.: Mini-implants in orthodontics: a systematic review of the literature. Am. J. Orthod. Dentofac. 135(5), 564 (2009). e561–564. e519
Tan, F., Wang, C., Yang, C., et al.: Biomechanical effects of various bone-implant interfaces on the stability of orthodontic miniscrews: a finite element study. J. Healthcare Eng. 2017, 7495606 (2017)
Xiao, S., Li, L., Wang, L., et al.: Root surface microcracks induced by orthodontic force as a potential primary indicator of root resorption. J. Biomech. 110, 109938 (2020)
Xiao, S.A.-O., Li, L.A.-O., Yao, J., et al.: Microcracks on the rat root surface induced by orthodontic force, crack extension simulation, and proteomics study. Ann. Biomed. Eng. (2021). https://doi.org/10.1007/s10439-021-02733-y
Chu, Z.W., Zheng, Q., Guo, M., et al.: The effect of fluid shear stress on the in vitro degradation of poly(lactide-co-glycolide) acid membranes. J. Biomed. Mater. Res. Part A 104(9), 2315–2324 (2016)
Li, P., Feng, X.L., Jia, X.L., et al.: Influences of tensile load on in vitro degradation of an electrospun poly(L-lactide-co-glycolide) scaffold. Acta. Biomater. 6(8), 2991–2996 (2010)
Grogan, J.A., Gastaldi, D., Castelletti, M., et al.: A novel flow chamber for biodegradable alloy assessment in physiologically realistic environments. Rev. Sci. Instrum. 84(9), 094301 (2013)
Fan, Y.B., Xiu, K.H., Duan, H., et al.: Biomechanical and histological evaluation of the application of biodegradable poly-L-lactic cushion to the plate internal fixation for bone fracture healing. Clin. Biomech. 23, S7–S16 (2008)
Fan, Y.B., Xiu, K.H., Dong, X., et al.: Sci. China Ser. C Life Sci. 52, 1186–1191 (2009)
Gao, Y.M., Wang, L.Z., Gu, X.N., et al.: A quantitative study on magnesium alloy stent biodegradation. J. Biomech. 74, 98–105 (2018)
Chen, K., Lu, Y., Tang, H.Y., et al.: Effect of strain on degradation behaviors of WE43. Fe and Zn wires. Acta. Biomater. 113, 627–645 (2020)
Murr, L.E.: Metallurgy principles applied to powder bed fusion 3D printing/additive manufacturing of personalized and optimized metal and alloy biomedical implants: an overview. J. Mater. Res. Technol. 9(1), 1087–1103 (2020)
Kang, J.F., Dong, E.C., Li, D.C., et al.: Anisotropy characteristics of microstructures for bone substitutes and porous implants with application of additive manufacturing in orthopaedic. Mater. Des. 191, 108608 (2020)
Zhu, M., Zhang, J.H., Zhao, S.C., et al.: Three-dimensional printing of cerium-incorporated mesoporous calcium-silicate scaffolds for bone repair. J. Mater. Sci. 51(2), 836–844 (2016)
Li, J., Chen, D.S., Luan, H.Q., et al.: Numerical evaluation and prediction of porous implant design and flow performance. Biomed. Res. Int. 2018, 1215021 (2018)
Hollister, S.J., Hollister, M.P., Hollister, S.K.: Computational modeling of airway instability and collapse in tracheomalacia. Resp. Res. 18, 62 (2017)
Jeong, C.G., Zhang, H.N., Hollister, S.J.: Three-dimensional poly(1,8-octanediol-co-citrate) scaffold pore shape and permeability effects on sub-cutaneous in vivo chondrogenesis using primary chondrocytes. Acta. Biomater. 7(2), 505–514 (2011)
Zopf, D.A., Hollister, S.J., Nelson, M.E., et al.: Bioresorbable airway splint created with a three-dimensional printer. N. Engl. J. Med. 368(21), 2043–2045 (2013)
Zopf, D.A., Flanagan, C.L., Mitsak, A.G., et al.: Pore architecture effects on chondrogenic potential of patient-specific 3-dimensionally printed porous tissue bioscaffolds for auricular tissue engineering. Int. J. Pediatr. Otorhinolaryngol. 114, 170–174 (2018)
Jia, X.L., Yang, J.Y., Song, W., et al.: Involvement of large conductance Ca2+-activated K+ channel in laminar shear stress-induced inhibition of vascular smooth muscle cell proliferation. Pflugers Arch. 465(2), 221–232 (2013)
Hsu, W.H., Chao, C.K., Hsu, H.C., et al.: Parametric study on the interface pullout strength of the vertebral body replacement cage using FEM-based Taguchi methods. Med. Eng. Phys. 31(3), 287–294 (2009)
El Baz, E.A., Sultan, A.M., Barakat, A.S., et al.: The use of anterior cervical interbody spacer with integrated fixation screws for management of cervical disc disease. Sicot J 5, 8 (2019)
Lan, T., Lin, J.-Z., Hu, S.-Y., et al.: Comparison between zero-profile spacer and plate with cage in the treatment of single level cervical spondylosis. J. Back Musculoskelet. Rehabil. 31(2), 299–304 (2018)
Lonjon, N., Favreul, E., Huppert, J., et al.: Clinical and radiological outcomes of a cervical cage with integrated fixation. Medicine 98(3), e14097 (2019)
Rong, Y., Luo, Y., Liu, W., et al.: Clinical effects of the bridge-type ROI-C interbody fusion cage system in the treatment of cervical spondylosis with osteoporosis This. Clin. Interv. Aging 13, 2543–2551 (2018)
De Leo-Vargas, R.A., Munoz-Romero, I., Mondragon-Soto, M.G., et al.: Locking stand-alone cage constructs for the treatment of cervical spine degenerative disease. Asian Spine J. 13(4), 630–637 (2019)
Noh, S.H., Zhang, H.Y.: Comparison among perfect-C (R), zero-P (R), and plates with a cage in single-level cervical degenerative disc disease. BMC Musculoskelet. Disord. 19, 33 (2018)
Wang, H.-R., Li, X.-L., Dong, J., et al.: Skip-level anterior cervical discectomy and fusion with self-locking stand-alone PEEK cages for the treatment of 2 noncontiguous levels of cervical spondylosis. J. Spinal Disord. Tech. 26(7), E286–E292 (2013)
Yue, Y.A., Wang, L.L., Yang, N., et al.: Effectiveness of biodegradable magnesium alloy stents in coronary artery and femoral artery. J. Interv. Cardiol. 28(4), 358–364 (2015)
Katsikis, A., Serruys, P.W.: Bioresorbable scaffolds versus metallic stents in routine PCI: the plot thickens. J. Thorac. Dis. 9(8), 2296–2300 (2017)
Tanigawa, J., Barlis, P., Di Mario, C.: Intravascular optical coherence tomography: optimisation of image acquisition and quantitative assessment of stent strut apposition. EuroIntervention 3(1), 128–136 (2007)
Hsiao, T.C., Jaques, P.A., Gao, P.F.: A multidomain magnetic passive aerosol sampler: development and experimental evaluation. Aerosol. Sci. Technol. 47(1), 37–45 (2013)
Amirjani, A., Yousefi, M., Cheshmaroo, M.: Parametrical optimization of stent design; a numerical-based approach. Comput. Mater. Sci. 90, 210–220 (2014)
Wang, Z.H., Wang, C.Y., Li, C., et al.: Analysis of factors influencing bone ingrowth into three-dimensional printed porous metal scaffolds: a review. J. Alloy. Compd. 717, 271–285 (2017)
Callens, S.J.P., Uyttendaele, R.J.C., Fratila-Apachitei, L.E., et al.: Substrate curvature as a cue to guide spatiotemporal cell and tissue organization. Biomaterials 232, 119739 (2020)
Bianchi, M., Edreira, E.R.U., Wolke, J.G.C., et al.: Substrate geometry directs the in vitro mineralization of calcium phosphate ceramics. Acta. Biomater. 10(2), 661–669 (2014)
Bidan, C.M., Kommareddy, K.P., Rumpler, M., et al.: How linear tension converts to curvature: geometric control of bone tissue growth. PLoS ONE 7(5), e36336 (2012)
Sun, P., Chen, C., Wu, C., et al.: Assignment and verification on mechanical parameters of soft tissue in finite element analysis. J. Med. Biomech. 1, 006 (2012)
Ripamonti, U., Roden, L.C., Renton, L.F.: Osteoinductive hydroxyapatite-coated titanium implants. Biomaterials 33(15), 3813–3823 (2012)
Campoli, G., Borleffs, M.S., Yavari, S.A., et al.: Mechanical properties of open-cell metallic biomaterials manufactured using additive manufacturing. Mater. Des. 49, 957–965 (2013)
Peng, H., Gao, F., Hu, W.: Design, modeling and characterization of triply periodic minimal surface heat exchangers with additive manufacturing. Proceedings of the 30th Annual International Solid Freeform Fabrication Symposium-An Additive Manufacturing Conference, 2325–2337 (2019)
Guyot, Y., Papantoniou, I., Chai, Y.C., et al.: A computational model for cell/ECM growth on 3D surfaces using the level set method: a bone tissue engineering case study. Biomech. Model. Mechanobiol. 13(6), 1361–1371 (2014)
Biemond, J.E., Aquarius, R., Verdonschot, N., et al.: Frictional and bone ingrowth properties of engineered surface topographies produced by electron beam technology. Arch. Orthop. Trauma Surg. 131(5), 711–718 (2011)
Markhoff, J., Wieding, J., Weissmann, V., et al.: Influence of different three-dimensional open porous titanium scaffold designs on human osteoblasts behavior in static and dynamic cell investigations. Materials 8(8), 5490–5507 (2015)
Karageorgiou, V., Kaplan, D.: Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26(27), 5474–5491 (2005)
Bai, F., Zhang, J.K., Wang, Z., et al.: The effect of pore size on tissue ingrowth and neovascularization in porous bioceramics of controlled architecture in vivo. Biomed. Mater. 6(1), 015007 (2011)
Fukuda, A., Takemoto, M., Saito, T., et al.: Osteoinduction of porous Ti implants with a channel structure fabricated by selective laser melting. Acta. Biomater. 7(5), 2327–2336 (2011)
Taniguchi, N., Fujibayashi, S., Takemoto, M., et al.: Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: an in vivo experiment. Mat. Sci. Eng. C-Mater. 59, 690–701 (2016)
Lv, J., Jia, Z.J., Li, J., et al.: Electron beam melting fabrication of porous Ti6Al4V scaffolds: cytocompatibility and osteogenesis. Adv. Eng. Mater. 17(9), 1391–1398 (2015)
Wieding, J., Lindner, T., Bergschmidt, P., et al.: Biomechanical stability of novel mechanically adapted open-porous titanium scaffolds in metatarsal bone defects of sheep. Biomaterials 46, 35–47 (2015)
von Doernberg, M.C., von Rechenberg, B., Bohner, M., et al.: In vivo behavior of calcium phosphate scaffolds with four different pore sizes. Biomaterials 27(30), 5186–5198 (2006)
Roosa, S.M.M., Kemppainen, J.M., Moffitt, E.N., et al.: The pore size of polycaprolactone scaffolds has limited influence on bone regeneration in an in vivo model. J. Biomed. Mater. Res. A 92a(1), 359–368 (2010)
Wauthle, R., van der Stok, J., Yavari, S.A., et al.: Additively manufactured porous tantalum implants. Acta. Biomater. 14, 217–225 (2015)
Cheng, M.Q., Wahafu, T.E.H.J., Jiang, G.F., et al.: A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration. Sci. Rep. 6, 24134 (2016)
Kasten, P., Beyen, I., Niemeyer, P., et al.: Porosity and pore size of beta-tricalcium phosphate scaffold can influence protein production and osteogenic differentiation of human mesenchymal stem cells: an in vitro and in vivo study. Acta. Biomater. 4(6), 1904–1915 (2008)
Bai, F.: The correlation between the internal structure and vascularization of controllable porous bioceramic materials in vivo: a quantitative study. Tissue Eng. Part A 16(12), 3791–3803 (2010)
Arjunan, A., Demetriou, M., Baroutaji, A., et al.: Mechanical performance of highly permeable laser melted Ti6Al4V bone scaffolds. J. Mech. Behav. Biomed. 102, 103517 (2020)
Ma, Y.H., Scarpa, F., Zhang, D.Y., et al.: A nonlinear auxetic structural vibration damper with metal rubber particles. Smart Mater. Struct. 22(8), 084012 (2013)
Li, Y., Luo, S.D., Yang, M.C., et al.: Poisson ratio and piezoresistive sensing: a new route to high-performance 3d flexible and stretchable sensors of multimodal sensing capability. Adv. Funct. Mater. 26(17), 2900–2908 (2016)
Bhullar, S.K., Rana, D., Lekesiz, H., et al.: Design and fabrication of auxetic PCL nanofiber membranes for biomedical applications. Mat. Sci. Eng. C-Mater. 81, 334–340 (2017)
Yan, Y.W., Li, Y., Song, L.Q., et al.: Pluripotent stem cell expansion and neural differentiation in 3-D scaffolds of tunable Poisson’s ratio. Acta Biomater. 49, 192–203 (2017)
Grima, J.N., Alderson, A., Evans, K.E.: Auxetic behaviour from, rotating rigid units. Phys. Status Solidi. B 242(3), 561–575 (2005)
Grima, J.N., Farrugia, P.S., Gatt, R., et al.: On the auxetic properties of rotating rhombi and parallelograms: a preliminary investigation. Phys. Status Solidi. B 245(3), 521–529 (2008)
Kolken, E.: The rational design of meta-implants using a combination of auxetic and conventional microstructures. Mater. Horiz. 5, 28–35 (2017)
Yao, Y., Wang, L.Z., Li, J., et al.: A novel auxetic structure based bone screw design: tensile mechanical characterization and pullout fixation strength evaluation. Mater. Des. 188, 108424 (2020)
Yao, Y., Yuan, H., Huang, H., et al.: Biomechanical design and analysis of auxetic pedicle screw to resist loosening. Comput. Biol. Med. 133, 104386 (2021)
Gao, H., Li, X., Wang, C., et al.: Mechanobiologically optimization of a 3D titanium-mesh implant for mandibular large defect: a simulated study. Mat. Sci. Eng. C 104, 109934 (2019)
Jing, X., Mi, H.-Y., Cordie, T., et al.: Fabrication of porous poly(\(\epsilon \)-caprolactone) scaffolds containing chitosan nanofibers by combining extrusion foaming, leaching, and freeze-drying methods. Ind. Eng. Chem. Res. 53(46), 17909–17918 (2014)
Fasolino, I., Guarino, V., Cirillo, V., et al.: 5-Azacytidine-mediated hMSC behavior on electrospun scaffolds for skeletal muscle regeneration. J. Biomed. Mater. Res. A 105(9), 2551–2561 (2017)
Giraud, M.N., Keller, D., Balazs, D., et al.: Selection of tuning PCL nanofiber non-wovens for muscle tissue engineering. Eur. Cell. Mater. 14, 68 (2007)
Chen, M.C., Sun, Y.C., Chen, Y.H.: Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater. 9(3), 5562–5572 (2013)
Chaudhuri, B., Bhadra, D., Moroni, L., et al.: Myoblast differentiation of human mesenchymal stem cells on graphene oxide and electrospun graphene oxide-polymer composite fibrous meshes: importance of graphene oxide conductivity and dielectric constant on their biocompatibility. Biofabrication 7(1), 015009 (2015)
Murugan, R., Ramakrishna, S.: Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng. 13(8), 1845–1866 (2007)
Chia, H.N., Wu, B.M.: Recent advances in 3D printing of biomaterials. J. Biol. Eng. 9, 4 (2015)
Pati, F., Jang, J., Ha, D.H., et al.: Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 5, 3935 (2014)
Cai, Y., Li, J., Poh, C.K., et al.: Collagen grafted 3D polycaprolactone scaffolds for enhanced cartilage regeneration. J. Mater. Chem. B 1(43), 5971 (2013)
Marino, G., Rosso, F., Ferdinando, P., et al.: Growth and endothelial differentiation of adipose stem cells on polycaprolactone. J. Biomed. Mater. Res. A 100(3), 543–548 (2012)
Barbarisi, M., Marino, G., Armenia, E., et al.: Use of polycaprolactone (PCL) as scaffolds for the regeneration of nerve tissue. J. Biomed. Mater. Res. A 103(5), 1755–1760 (2015)
Visser, J., Melchels, F.P., Jeon, J.E., et al.: Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 6, 6933 (2015)
Wunner, F.M., Wille, M.L., Noonan, T.G., et al.: Melt electrospinning writing of highly ordered large volume scaffold architectures. Adv. Mater. 30(20), e1706570 (2018)
Qiao, Z., Lian, M., Han, Y., et al.: Bioinspired stratified electrowritten fiber-reinforced hydrogel constructs with layer-specific induction capacity for functional osteochondral regeneration. Biomaterials 266, 120385 (2021)
Warnke, P.H., Douglas, T., Wollny, P., et al.: Rapid prototyping: porous titanium alloy scaffolds produced by selective laser melting for bone tissue engineering. Tissue Eng. Part C-Methods 15(2), 115–124 (2009)
Traini, T., Mangano, C., Sammons, R.L., et al.: Direct laser metal sintering as a new approach to fabrication of an isoelastic functionally graded material for manufacture of porous titanium dental implants. Dent. Mater. 24(11), 1525–1533 (2008)
Hedayati, R., Sadighi, M., Mohammadi-Aghdam, M., et al.: Mechanical properties of additively manufactured thick honeycombs. Mater. Sci. Eng. C 69, 1307–1317 (2016)
Hedayati, R., Sadighi, M., Mohammadi-Aghdam, M., et al.: Analytical relationships for the mechanical properties of additively manufactured porous biomaterials based on octahedral unit cells. Appl. Math. Model. 46, 408–422 (2017)
Heinl, P., Muller, M., Korner, C., et al.: Cellular Ti-6Al-4V structures with interconnected macro porosity for bone implants fabricated by selective electron beam melting. Acta Biomater. 4(5), 1536–1544 (2008)
Murr, L.E.: Metallurgy principles applied to powder bed fusion 3D printing/additive manufacturing of personalized and optimized metal and alloy biomedical implants: an overview. J. Mater. Res. Technol. (2019). https://doi.org/10.1016/j.jmrt.2019.12.015
Kang, J., Dong, E., Li, D., et al.: Anisotropy characteristics of microstructures for bone substitutes and porous implants with application of additive manufacturing in orthopaedic. Mater. Des. 191, 108608 (2020)
Fukuda, A., Takemoto, M., Saito, T., et al.: Osteoinduction of porous Ti implants with a channel structure fabricated by selective laser melting. Acta Biomater. 7(5), 2327–2336 (2011)
Ataee, A., Li, Y., Fraser, D., et al.: Anisotropic Ti-6Al-4V gyroid scaffolds manufactured by electron beam melting (EBM) for bone implant applications. Mater. Des. 137, 345–354 (2018)
Feng, C., Zhang, W.J., Deng, C.J., et al.: 3D printing of lotus root-like biomimetic materials for cell delivery and tissue regeneration. Adv. Sci. 4, 12 (2017)
Hess, T.A., Drinkhouse, M.E., Prey, J.D., et al.: Analysis of platinum content in biodegradable carboplatin-impregnated beads and retrospective assessment of tolerability for intralesional use of the beads in dogs following excision of subcutaneous sarcomas: 29 cases (2011–2014). J. Am. Vet. Med. A 252(4), 448–456 (2018)
Hollister, S.J.: Chapter 4: Computational Design and Modeling of Linear and Nonlinear Elastic Tissue Engineering ScaffoldTriply Periodic Minimal Surface (TPMS) Porous Architecture. Biofabrication and 3D Tissue Modeling, Cambridge (2019)
Hollister, S.J.: Paediatric devices that grow up. Nat. Biomed. Eng. 1(10), 777–778 (2017)
Ah, N.J., Sheha, E.D., Gandhi, S.D., et al.: Three-dimensional printing and models: current applications, limitations, and trends in spinal surgery. Semin. Spine. Surg. 32(2), 100790 (2020)
Xiu, P., Jia, Z., Lv, J., et al.: Tailored surface treatment of 3d printed porous Ti6Al4V by microarc oxidation for enhanced osseointegration via optimized bone in-growth patterns and interlocked bone/implant interface. ACS Appl. Mater. Interfaces 8(28), 17964 (2016)
Salerno, C.T., Droel, J., Bianco, R.W.: Current state of in vivo preclinical heart valve evaluation. J. Heart Valve Dis. 7(2), 158–162 (1998)
Bianco, R.W., Gallegos, R.P., Rivard, A.L., et al.: Handbook of Cardiac Anatomy, Physiology, and Devices. Humana Press, Totowa, NJ (2009)
Oberkampf, W.L., Trucano, T.G., Hirsch, C.: Verification, validation, and predictive capability in computational engineering and physics. Appl. Mech. Rev. 57(5), 345–384 (2004)
FDA: Reporting of computational modeling studies in medical device submissions draft, Guidance for industry and food and Drug administration staff only. Food and Drug Administration, Silver Springs (2014)
Yao, Y., Wang, L.Z., Li, J., et al.: A novel auxetic structure based bone screw design: tensile mechanical characterization and pullout fixation strength evaluation. Mater. Des. 188, 108424 (2020)
Yao, Y., Mo, Z., Wu, G., et al.: A personalized 3D-printed plate for tibiotalocalcaneal arthrodesis: design, fabrication, biomechanical evaluation and postoperative assessment. Comput. Biol. Med. 133, 104368 (2021)
Martin, C., Sun, W.: Simulation of long-term fatigue damage in bioprosthetic heart valves: effects of leaflet and stent elastic properties. Biomech Model Mechanobiol 13, 759–770 (2014)
Hedayati, R., Janbaz, S., Sadighi, M., et al.: How does tissue regeneration influence the mechanical behavior of additively manufactured porous biomaterials? J. Mech. Behav. Biomed. Mater. 65, 831–841 (2017)
Lipinski, P., Barbas, A., Bonnet, A.S.: Fatigue behavior of thin-walled grade 2 titanium samples processed by selective laser melting. Application to life prediction of porous titanium implants. J. Mech. Behav. Biomed. 28, 274–290 (2013)
Hedayati, R., Yavari, S., Zadpoor, A.A.: Fatigue crack propagation in additively manufactured porous biomaterials. Mater. Sci. Eng. C Mater. 76, 457 (2017)
Hedayati, R., Hosseini-Toudeshky, H., Sadighi, M., et al.: Multiscale modeling of fatigue crack propagation in additively manufactured porous biomaterials. Int. J. Fatigue 76, 457 (2018)
Benedetti, M., Plessis, A.D., Ritchie, R.O., et al.: Architected cellular materials: a review on their mechanical properties towards fatigue-tolerant design and fabrication. Mater. Sci. Eng. R Rep. 144, 100606 (2021)
Barriuso, S., Chao, J., Jiménez, J., et al.: Fatigue behavior of Ti6Al4V and 316 LVM blasted with ceramic particles of interest for medical devices. J. Mech. Behav. Biomed. 30, 30–40 (2014)
Cheong, V.S., Fromme, P., Coathup, M.J., et al.: Partial bone formation in additive manufactured porous implants reduces predicted stress and danger of fatigue failure. Ann. Biomed. Eng. 48(1), 1–13 (2019)
Viceconti, M., Muccini, R., Bernakiewicz, M., et al.: Large-sliding contact elements accurately predict levels of bone-implant micromotion relevant to osseointegration. J. Biomech. 33(12), 1611–1618 (2000)
Engh, C.A., O’Connor, D., Jasty, M., et al.: Quantification of implant micromotion, strain shielding, and bone resorption with porous-coated anatomic medullary locking femoral prostheses. Clin. Orthop. Relat. Res. 285, 13–29 (1992)
Søballe, K., Hansen, E.S., Rasmussen, H., et al.: Tissue ingrowth into titanium and hydroxyapatite-coated implants during stable and unstable mechanical conditions. J. Orthop. Res. 10(2), 28599 (1992)
Justy, M.: In vivo skeletal responses to porous-surfaced implants subjected to small induced motions. J. Bone Joint Surg. Am. 79, 707 (1997)
Trisi, P., Berardini, M., Falco, A., et al.: Effect of implant thread geometry on secondary stability, bone density, and bone-to-implant contact. Implant Dent. 24, 384 (2015)
Trisi, P., Berardini, M., Falco, A., et al.: Validation of value of actual micromotion as a direct measure of implant micromobility after healing (secondary implant stability). An invivo histologic and biomechanical study. Clin. Oral Implant. Res. 27(11), 1423–1430 (2016)
Chowdhary, R., Jimbo, R., Thomsen, C., et al.: Biomechanical evaluation of macro and micro designed screw-type implants: an insertion torque and removal torque study in rabbits. Clin. Oral Implant. Res. 24(3), 342–346 (2013)
Trisi, P., Falco, A., Berardini, M.: Single-drill implant induces bone corticalization during submerged healing: an in vivo pilot study. Int. J. Implant. Dent. 6(1), 2 (2020)
Overmann, A.L., Aparicio, C., Richards, J.T., et al.: Orthopaedic osseointegration: implantology and future directions. J. Orthop. Res. 38(7), 1445–1454 (2020)
Manigrasso, M.B., O’Connor, J.P.: Characterization of a closed femur fracture model in mice. J. Orthop. Trauma 18(10), 687–695 (2004)
Krischak, G.D., Augat, P., Blakytny, R., et al.: The non-steroidal anti-inflammatory drug diclofenac reduces appearance of osteoblasts in bone defect healing in rats. Arch. Orthop. Trauma Surg. 127(6), 453–458 (2007)
Krischak, G.D., Augat, P., Sorg, T., et al.: Effects of diclofenac on periosteal callus maturation in osteotomy healing in an animal model. Arch. Orthop. Trauma Surg. 127(1), 3–9 (2007)
Histing, T., Holstein, J.H., Garcia, P., et al.: Ex vivo analysis of rotational stiffness of different osteosynthesis techniques in mouse femur fracture. J. Orthop. Res. 27(9), 1152–1156 (2009)
Stahel, P.F., Alfonso, N.A., Henderson, C., et al.: Introducing the “Bone-Screw-Fastener” for improved screw fixation in orthopedic surgery: a revolutionary paradigm shift? Patient Saf. Surg. 11, 6 (2017)
Saikko, V., Ahlroos, T., Calonius, O.: A three-axis knee wear simulator with ball-on-flat contact. Wear 249(3–4), 310–315 (2001)
Muratoglu, O.K., Bragdon, C.R., Jasty, M., et al.: Knee-simulator testing of conventional and cross-linked polyethylene tibial inserts. J. Arthroplasty 19(7), 887–897 (2004)
Onate, J.I., Comin, M., Braceras, I., et al.: Wear reduction effect on ultra-high-molecular-weight polyethylene by application of hard coatings and ion implantation on cobalt chromium alloy, as measured in a knee wear simulation machine. Surf. Coat. Technol. 142, 1056–1062 (2001)
Standard, I.: ISO 5840: Cardiovascular implants: cardiac valve prostheses. ISO Copyright Office (2015)
Whelan, A., Williams, E., Fitzpatrick, E., et al.: Collagen fibre-mediated mechanical damage increases calcification of bovine pericardium for use in bioprosthetic heart valves. Acta Biomater. (2021). https://doi.org/10.1016/j.actbio.2021.04.046
Standard, I.: ISO 25539-2: Cardiovascular implants-Endovascular devices ISO Copyright Office (2020)
Engels, G.E., Blok, S.L.J., van Oeveren, W.: In vitro blood flow model with physiological wall shear stress for hemocompatibility testing-An example of coronary stent testing. Biointerphases 11(3), 031004 (2016)
Azadani, A.N., Jaussaud, N., Matthews, P.B., et al.: Aortic valve-in-valve implantation: impact of transcatheter-bioprosthesis size mismatch. J. Heart. Valve. Dis. 18(4), 367–373 (2009)
Azadani, A.N., Jaussaud, N., Matthews, P.B., et al.: Transcatheter aortic valves inadequately relieve stenosis in small degenerated bioprostheses. Interact. Cardiov. Thorac. Surg. 11(1), 70–77 (2010)
Vahidkhah, K., Barakat, M., Abbasi, M., et al.: Valve thrombosis following transcatheter aortic valve replacement: significance of blood stasis on the leaflets. Eur. J. Cardio-Thorac. 51(5), 927–935 (2017)
Ducci, A., Tzamtzis, S., Mullen, M.J., et al.: Hemodynamics in the valsalva sinuses after transcatheter aortic valve implantation (TAVI). J. Heart Valve Dis. 22(5), 688–696 (2013)
Helmholz, H., Luthringer-Feyerabend, B.J.C., Willumeit-Romer, R.: Elemental mapping of biodegradable magnesium-based implants in bone and soft tissue by means of mu X-ray fluorescence analysis. J. Anal. Atom. Spectrom 34(2), 356–365 (2019)
Bhatia, S., Sharma, K., Dahiya, R., et al.: Modern applications of plant biotechnology in pharmaceutical sciences. Academic Press, New York (2015)
Huang, Y., Niu, X.F., Wang, L.Y., et al.: Effects of hydroxyapatite/collagen composite on osteogenic differentiation of rat bone marrow derived mesenchymal stem cells. J. Compos. Mater. 48(16), 1971–1980 (2014)
Li, X.M., Huang, Y., Zheng, L.S., et al.: Effect of substrate stiffness on the functions of rat bone marrow and adipose tissue derived mesenchymal stem cells. J. Biomed. Mater. Res. A 102(4), 1092–1101 (2014)
Huang, Y., Niu, X.F., Song, W., et al.: Combined effects of mechanical strain and hydroxyapatite/collagen composite on osteogenic differentiation of rat bone marrow derived mesenchymal stem cells. J. Nanomater. 2013, 343909 (2013)
Péter, P., Ferenc, E., Márta, R.: Geometric optimization of microreactor chambers to increase the homogeneity of the velocity field. J. Micromech. Microeng. 28(6), 064002 (2018)
Li, J., Zhang, Y., Gao, T., et al.: A confined ’microreactor’ synthesis strategy to three dimensional nitrogen-doped graphene for high-performance sodium ion battery anodes. J. Power Sources 378, 105–111 (2018)
Mason, B.P., Price, K.E., Steinbacher, J.L., et al.: Greener approaches to organic synthesis using microreactor technology. Chem. Rev. 107(6), 2300–2318 (2007)
Ahmed-Omer, B., Brandt, J.C., Wirth, T.: Advanced organic synthesis using microreactor technology. Org. Biomol. Chem. 5(5), 733–740 (2007)
Miller, J.S., Stevens, K.R., Yang, M.T., et al.: Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11(9), 768–774 (2012)
Morgan, J.P., Delnero, P.F., Ying, Z., et al.: Formation of microvascular networks in vitro. Nat. Protoc. 8(9), 1820–1836 (2013)
Sebastian, U., Andreas, W., Hubert, F., et al.: Electrochemical multisensor system for monitoring hydrogen peroxide, hydrogen and oxygen in direct synthesis microreactors. Sens. Actuators B (2018). https://doi.org/10.1016/j.snb.2018.07.014
Khong, D., Li, M., Singleton, A., et al.: Stromalized microreactor supports murine hematopoietic progenitor enrichment. Biomed. Microdev. 20(1), 13 (2018)
Mirabella, T., Macarthur, J.W., Cheng, D., et al.: 3D-printed vascular networks direct therapeutic angiogenesis in ischaemia. Nat. Biomed. Eng. 1(6), 0083 (2017)
Zhou, W., Wei, Y.U., Pei, P., et al.: Performances of a methanol reforming microreactor with gradient porosity fiber support for hydrogen production. Journal of Automotive Safety and Energy 9(1), 85–92 (2018) (in Chinese)
Marsano, A., Conficconi, C., Lemme, M., et al.: Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip 16, 599 (2016)
Mitra, D., Whitehead, J., Yasui, O.W., et al.: Bioreactor culture duration of engineered constructs influences bone formation by mesenchymal stem cells. Biomaterials 146, 29–39 (2017)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants U20A20390 and 11827803) and the National Key Technology R&D Program (2016YFC1102202).
Author information
Authors and Affiliations
Corresponding author
Additional information
Executive Editor: Yuejie Wei.
Rights and permissions
About this article
Cite this article
Wang, L., Ding, X., Feng, W. et al. Biomechanical study on implantable and interventional medical devices. Acta Mech. Sin. 37, 875–894 (2021). https://doi.org/10.1007/s10409-021-01116-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10409-021-01116-9